In neurosurgical setting, intracranial hematomas
(ICH) are a frequent condition with acute intracranial expansive
effect (1), that lead to secondary
injuries and complications, such as intracranial hypertension (IHT)
(2,3), which may become the cause of death
for these patients (4). In the
last decades, spontaneous ICH prevalence has remained stable, with
an average of 24.6 cases per 100,000 inhabitants per year in
developed countries (5). Systemic
arterial hypertension is the most important risk factor for
non-traumatic bleeding, especially among subjects no adherent to
antihypertensive treatment (4).
Other causes for intracranial hemorrhages are aneurism rupture,
arteriovenous malformation, vasculitis, coagulopathies, venous
thrombosis, cocaine use, amyloid angiopathy and hemorrhagic
complications after ischemic stroke thrombolysis (4).
ICH may present with a wide variety of signs and
symptoms depending on the location and severity of the bleeding.
Patients can be asymptomatic or have mild local deficits, whereas
others present with IHT syndromes and complete loss of
consciousness (4). Spontaneous ICH
has an overall mortality rate of 50% after 30 days (6,7), and
approximately half of deaths occur within the first 24 h of the
initial bleeding (8). The
functional prognosis for survivors is poor, and only 20% of
patients are expected to be functionally independent at 6 months
(9).
ICH may promote cerebral hemodynamic impairment with
a shift from aerobic to anaerobic metabolism that leads to: i)
Lactate and free radicals accumulation (10); ii) activation of inflammatory
cascades (interleukin 1β and tumor necrosis factor) promoted by the
complement, microglia, macrophages and neutrophils (11); iii) immune responses (and systemic
immunosuppression) that contribute to blood-brain barrier
impairment; and iv) additional swelling of the brain tissue,
leading to IHT, which adds even more damage to the brain tissue
(11,12).
When therapeutic strategies such as hypertonic
saline or mannitol infusions, hyperventilation and mild hypothermia
fail to control IHT, the spreading ischemia may contribute to brain
death unless an emergency neurosurgical procedure as decompressive
craniectomy is performed (13).
Therefore, animal experimentation and testing remains an important
tool to improve understanding of the different pathophysiological
mechanisms of injury in order to investigate the techniques of
intervention and neuroprotection improvement. Currently, to the
best of our knowledge, the available literature has few models of
experimental ICH in animals, which are characterized by
heterogeneity and varied methodology. The aim of the present review
was to discuss the main animal models for ICH investigation,
emphasizing the advantages and disadvantages of each method.
The present comprehensive review included
experimental original studies published in English. Reviews were
assessed to build the body of evidence. The present study's aim was
to identify relevant studies on animal models of ICH. The search
was effectuated in October 2021 and updated in May 2022 through the
electronic databases PubMed/MEDLINE (https://pubmed.ncbi.nlm.nih.gov/), Google Scholar
(https://scholar.google.com.br/), LILACS
(https://lilacs.bvsalud.org/en/) and
EMBASE (https://www.embase.com/landing?status=grey), by two
investigators (WSP and SB). The following terms were applied to
identify potential eligible articles: ‘Animal model’ OR
‘experimental model’ AND ‘intracranial hematoma’ OR ‘cerebral
hematoma’ AND ‘intracranial hypertension’, and were then selected
by title and abstract. In addition, a manual search was done using
the reference lists of included studies to identify others relevant
papers, as the ‘Related Articles’ tool for the selection of
additional relevant articles. Inclusion criteria included
experimental studies of any animal species with the purpose of
assessing the effects of hematomas and/or intracranial hypertension
over the brain. Exclusion criteria comprised of experimental
interventional studies for the assessment of systemic effects of
ICH and studies not published in English. The funneling process for
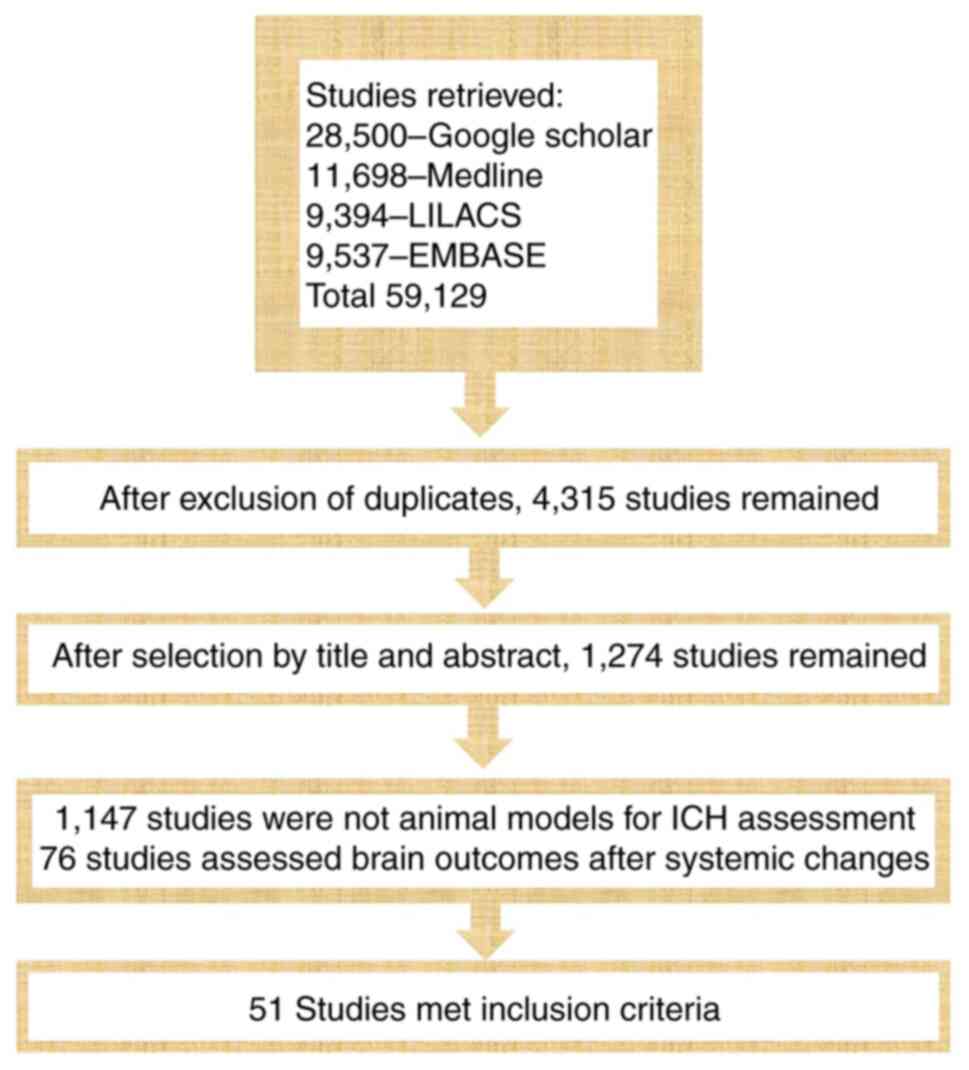
selection of studies is presented in Fig. 1.
Regarding the animals used for modeling ICH, the
following different species were used: Mice or rats (14-33),
pigs (34-40),
dogs (41-43),
monkeys (44), sheep (45), cats (46) and rabbits (47,48).
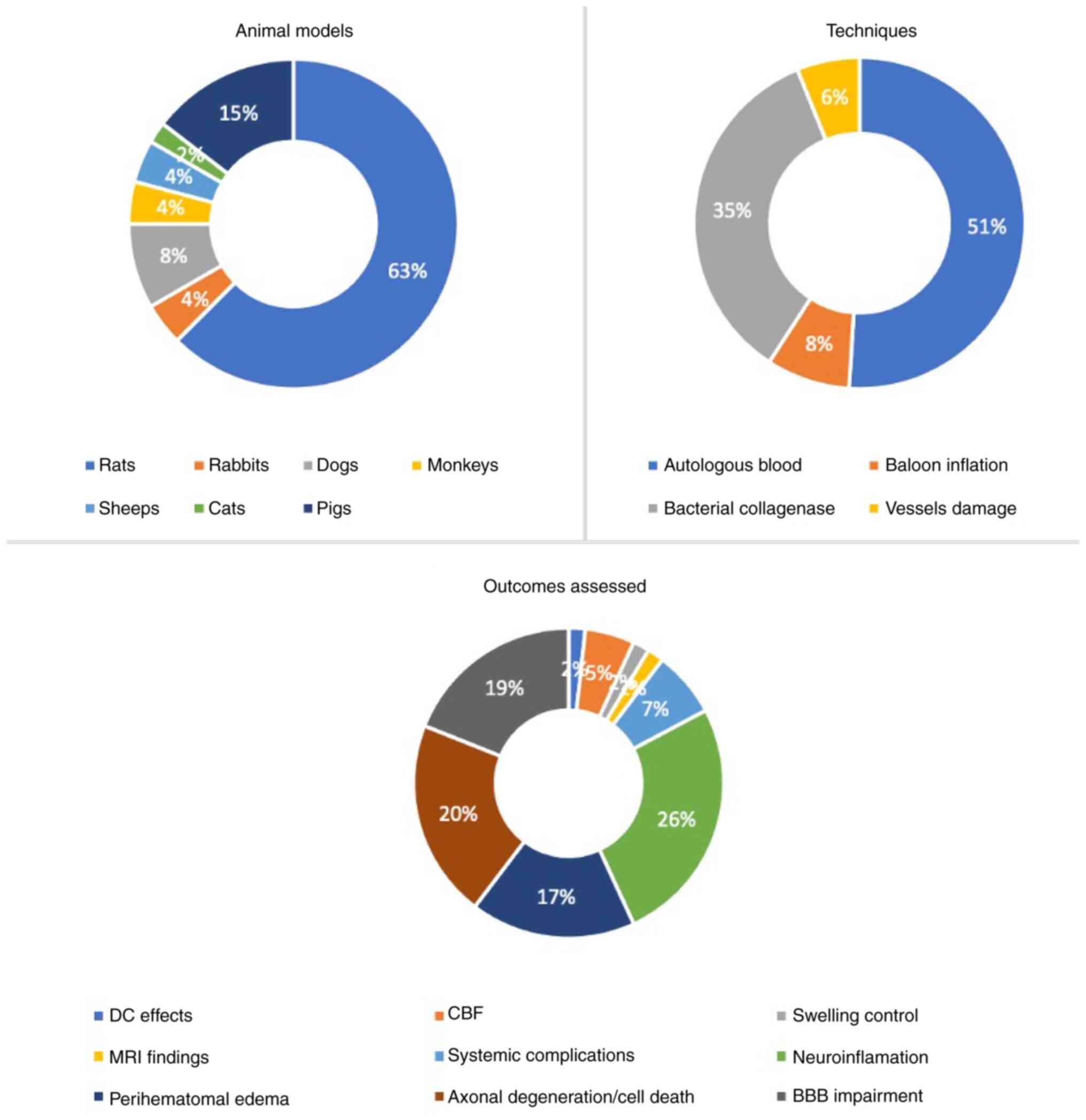
The majority of studies (63%) utilized rats, whereas the model most
used is autologous blood intracranial injection (51%). The
assessment of neuroinflammation cascades was the outcome studied in
26% of the studies. Fig. 2
summarizes the representation of each animal type, model and
outcomes assessed in reproducing intracranial hematomas.
Although each model recreates the fundamentals of
human hematoma with good precision, they differ in ways that
influence outcome. The present study described the technique
details of ICH induction and compared the technical and
pathological advantages and disadvantages of the existing models
(Table I). Each model is presented
in further detail bellow.
Blood injection models allow the observation of
hemolysis-induced toxicity assessment, as the following
immune-inflammatory responses (49). This model also allows the
assessment of interventions to reduce swelling, hematoma expansion
and the IHT effects on brain hemodynamics (49). Experimental models of brain
hematomas have been described since the 1960's, and typically
involve the intracerebral injection of autologous blood, which is a
simple and effective technique for the production of brain
parenchymal hematoma (50). This
type of model has been developed for larger animals (cats, dogs,
pigs, sheep and monkeys) (36,37,41-43,45,49,51,52)
through the injection of blood in the frontal lobe or basal
ganglia. There are several variations within studies, such as blood
source, the amount of blood injected and depth of injection.
Whisnant et al (43)
induced an intracerebral collection in dogs by infusing venous
blood in the deep white matter. Sussman et al (41), by contrast, injected arterial blood
superficially into the right frontal lobe (0.5 cm beneath the
cortex) in dogs. A similar technique was presented by Wagner et
al in 1996, 1998 and 1999 (49,50),
who produced lobar hematomas in pigs by infusions of arterial blood
into right frontal white matter.
For smaller animals (rats and mice) blood is
injected into the caudate nucleus in the majority of studies
(53-60).
Yang et al (53) injected
autologous blood into the caudate nuclei of rats in order to study
the formation and resolution of brain edema. As an alternative for
basal ganglia, Bullock et al (61) injected autologous blood into the
lateral ventricles to compare the consequences of contained and
uncontained hemorrhage. They also evaluated the impact of the
intracerebral collection on the IHT.
The autologous injection of blood method has a major
advantage, which is allowing the production of hematomas with
homogeneous volumes. It also mimics the rapid accumulation of blood
noted to occur in the clinical setting (62). However, it does not reproduce the
rupture of blood vessel present in human brain hematomas and can
lead to intraventricular hemorrhage and/or subarachnoid hemorrhage
by ruptures in the ventricular and subdural spaces (50). Moreover, there is a risk for the
infused blood to back flow along the needle track (62). Nevertheless, it can be used for the
study of biochemical and pathophysiological effects in patients
with acute ICH and IHT as it permits the infused blood volume to be
controlled, enabling the generation of hematomas sizes and mass
effects (45).
Autologous blood injection allows the observation of
hematoma capsule formation at the boundary tissue around the blood
clot (53). It is composed by a
necrotic layer of brain tissue, fibrin deposits, secondary
capillary hemorrhages and white matter vacuolation in the first
week (42), up to 1 cm from the
ICH site. Impregnation of metalloproteinases and oxidative stress
induced by ischemia contribute to these hemorrhagic phenomena and
disruption of the blood-brain barrier (BBB) (63). Microglia and astrocytes seem to be
more resistant to ICH effects, otherwise, neurons and axons are
more sensitive to hypoxic-ischemic changes (45). At 3 days after ICH, red blood cell
(RBC) morphology is significantly changed to spheric, with
complement system activation responsible for RBC lysis following
phagocytosis by macrophages and microglia (37).
Bacterial collagenase is a protease that damages the
extracellular matrix around the brain capillaries, weakening them
and causing rupture of the vessel and consequent extravasation of
blood (64). This model produces
spontaneous intracerebral bleeding, which develops over several
hours, as shown in ~30% of patients with ICH (65). It is considered appropriate for
studying hematoma expansion and vasogenic edema, anticoagulation,
axonal degeneration, iron-induced apoptosis, endothelial
disturbances and BBB impairment, in addition to systemic
complications following brain aggression (62,64,70).
The injection of bacterial collagenase in the basal
ganglia, leading to the breakdown of the basement membrane of blood
vessels was first introduced in the early 1990s using mice
(71,72) and has been widely used ever since
(21,73-77).
Collagenases spread and penetrate into the brain parenchyma instead
of remaining on the site of infusion; therefore, although the
pathophysiologic mechanisms of injury are the same of those
disclosed in the section of autologous blood infusion, they seem to
be enhanced in this model (76).
Adjustments to procedural parameters have been seen
between multiple studies. The majority of authors inject bacterial
collagenase type IV (78), but
some have adopted bacterial type VI (71), VII S (17,79-81)
or XI (38) collagenase. The
infusion period also varies, ranging from 2 to 16 min (71,78,80).
This technique is preferably used in small animals (rats and mice).
Mun-Bryce et al (38) was
one of the few to try this model in larger animals by injecting
collagenase into the primary somatosensory cortex of swine. It is
considered a simple and reproducible method since it evokes a
dose-dependent hemorrhage size and can be used in multiple species.
As it can mimic hematoma expansion and vasogenic edema (51,70,82),
it is commonly used to investigate the mechanisms of increased
bruising and for developing treatments that affect cerebral
homeostasis (23,83,84).
Using this model, it is possible to imitate vessel
rupture and represent hematoma expansion. It allows to assess long
term outcomes. Even though it evokes an inflammatory response
earlier and for a prolonged period (when compared with the blood
injection model), it seems that the inflammatory reaction is too
severe and is different from the observed in the human brain
(85,86). Furthermore, extensive bleeding
resulting from the intracerebral injection of collagenase may
produce an unplanned ischemic brain injury (87). As expected from the above, this
technique leads to significantly increased severe neurological
deficits with poorer recovery compared with blood infusion
(88).
In this model, rats have their cortical veins
exposed via a craniotomy and damaged using a curved needle,
resulting in cortical hemorrhages (89,90).
This model has been very little used in the recent research as it
has a large variability of brain injury created due to ischemic
infarction, which limits the reliability of the experimental
results (85). Xue and Del Bigio
(90) compared the three models
and observed that the relative magnitude of the inflammatory
phenomena, molecular and cellular changes may differ between the
models, although within similar patterns. These authors described
damaged DNA neurons and CD8α immunoreactive lymphocytes to be
maximal at 3 days after injury, but because the
microglial/macrophage reaction peaked early between 3 to 7 days and
persisted for weeks, dying neurons were seen in small quantities
after 21 to 28 days. Neutrophils were substantially lower in this
cortical vessel avulsion model compared with the blood and
collagenases infusion models (90)
Alternative to this procedure is rupturing the
vessels using a laser in order to produce microbleeds and assess
coagulation outcomes (91). Zhou
et al (86) induced an
intracerebral hematoma by puncturing the middle cerebral artery.
This procedure has been performed in 12 dogs under the ultrasound
guidance with a high success rate. The main limitations of this
model are that it can only be performed using an open bone window,
which can underestimate the effects of intracranial hypertension
and, as discussed above, it seems to produce a less severe
histological damage.
Piglets were separated into 3 groups: First group
with mild intracranial hypertension, a second group with severe
intracranial hypertension and for the third group a cerebral
rebleeding model. Prior to surgery, the animals fasted for 12 h but
had free access to water. Intramuscular ketamine was
co-administered at a dose of 15 mg/kg and xylazine at a dose of 2
mg/kg as a preanesthetic. Once intravenous (IV) access was
obtained, anesthesia was induced with propofol at a dose of 5
mg/kg. The animals also received an initial IV volume of 20 ml/kg
physiological saline (NaCl 0.9%) to compensate for volume loss due
to fasting, and fluid support was continued throughout at a rate of
5 ml/kg/h. Anesthesia was maintained with IV propofol. In this
protocol, ICP, direct brain oxygen pressure and results of
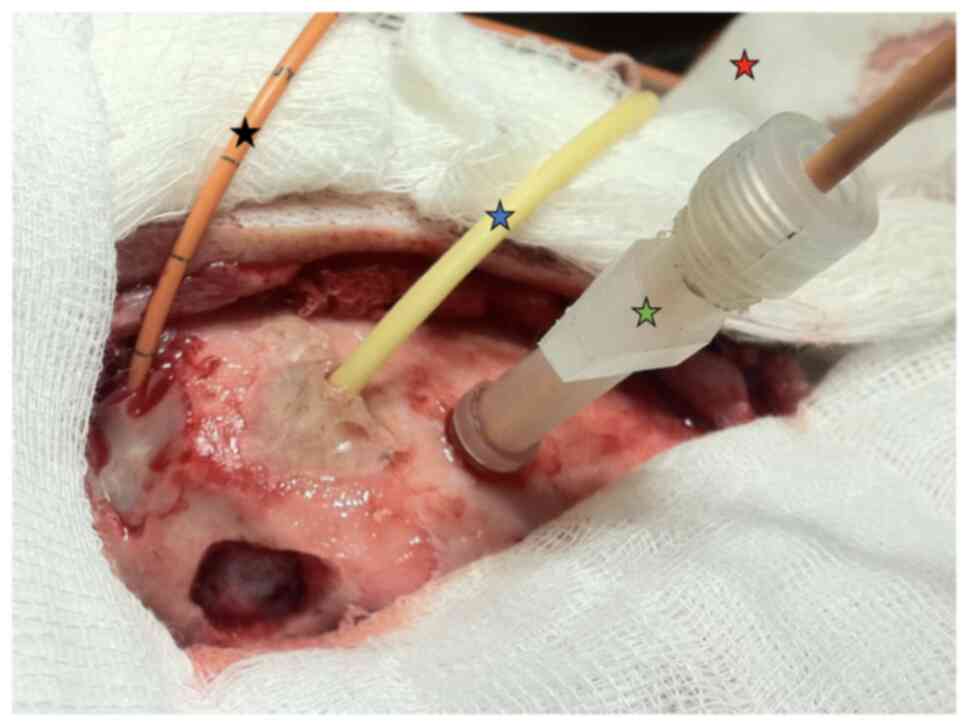
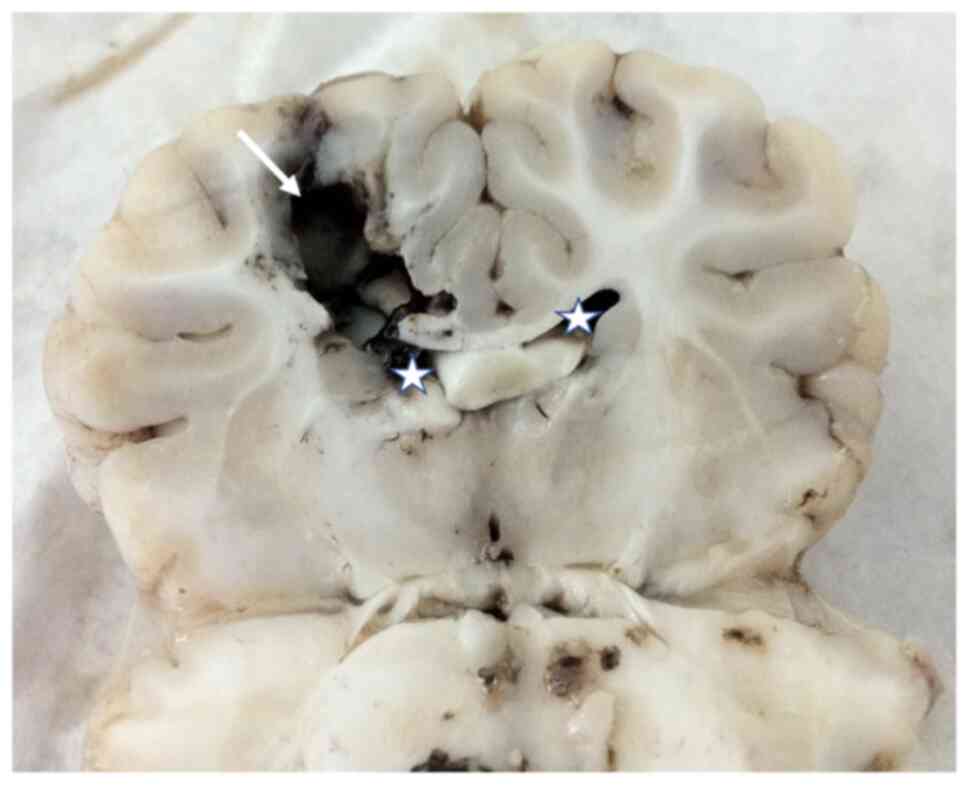
transcranial Doppler exams were performed (Fig. 3). To induce IHT, a balloon 8Fr was
used (Fig. 4) in piglets (average
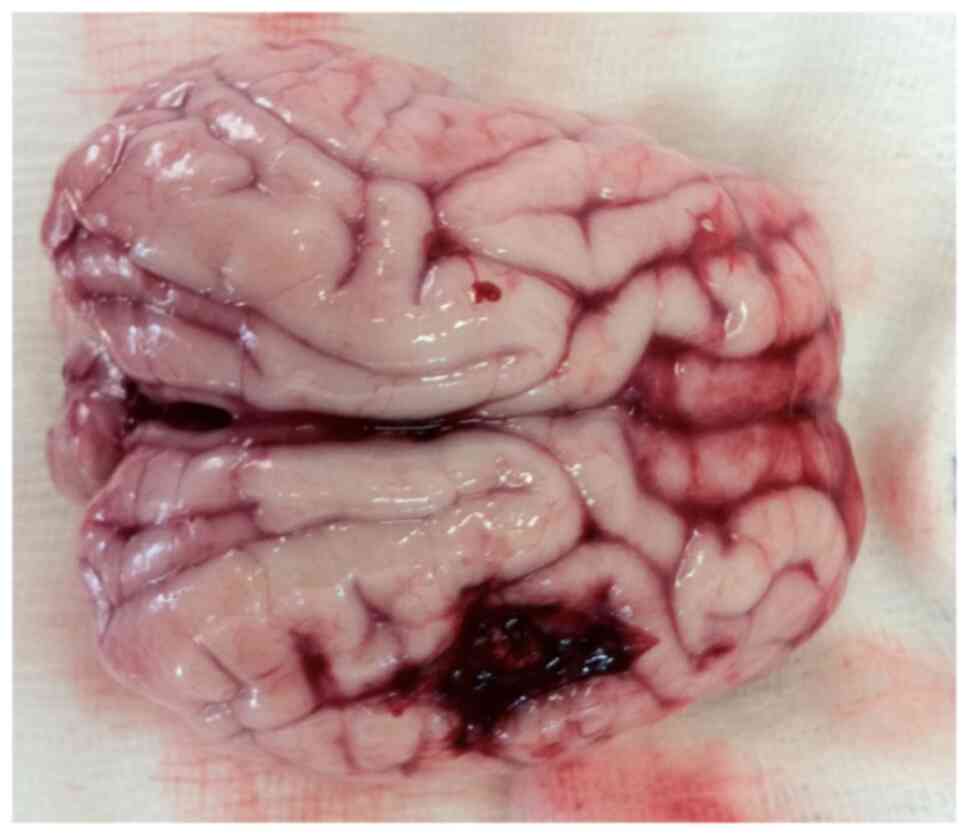
weight, 20 kg). The injury caused by balloon produces IHT with
local expansion (Fig. 5).
There are numerous advantages of using mice and rats
as models for ICH, such as the excellent cost effectiveness
(70), accurate paradigms for
testing and outcomes (95) and an
extensive sample of reagents for immunohistochemistry and molecular
biology (96). Moreover, the
availability of transgenic systems in the mouse allows for genetic
studies on ICH and its mechanisms of lesion (96). The main disadvantage of rodents is
the small size of the brain, which limits the clot volume that can
be created and complicate its use in surgical studies (70,96).
Furthermore, the lack of brain gyrus and the small amount of white
matter limits the correlation with the human brain.
Piglets have well developed white matter, a
relatively low cost and no major difficulties with protective
animal societies, being an excellent species for ICH studies
(93). The possibility of using
hematoma volumes 20 to 30 times higher in this model compared with
rodent species also allows the test of hematoma removal (surgery
simulation) in addition to providing the rebleeding simulation
(93). Shi et al (97) described the balloon technique in
the subcortical white matter, instead of blood injected into the
gray matter or the basal ganglia. The use of this method turns
obtaining a more uniform and reproducible hematoma volume possible,
facilitating the extrapolation of information to humans, especially
because of the greater volume of white matter found in larger
animals and the developing edema adjacent to the hematoma.. This
model is clinically relevant since the ICP information can be
extrapolated from models to clinical treatment.
The difficulties in transferring information from
experimental models to clinical settings derive from specific
aspects of each species, as well as limitations of the models and
methods. For the most part, experiments use younger animals with
greater functional reserve and hemodynamics. Moreover, rodents
disclose an outstanding capacity of regeneration and rehabilitation
that is not comparable with humans (11). The complex pathophysiology of brain
hematomas involving vascular injury as well as apoptosis and
molecular aspects involved hinder the translation of information.
The majority of studies are conducted in murine models and there
are important differences in cerebral hemodynamic changes on the
acute phase of intracranial hypertension for these animals compared
to humans. Regarding the studies, a wide methodologic heterogeneity
for injury inducing was found, likewise, appropriate controlled
study designs are lacking since a few studies used suitable
controls to evaluate their endpoints.
Animal models have the potential to enhance our
understanding of the pathophysiology and treatment of intracranial
hypertension and brain hematomas, being essential to develop and
evaluate new therapeutic strategies in preclinical settings. To
decide which is the best model for each research, some points must
be considered: The purpose of each model, the primary outcome of
the study, considering whether hematoma is in the acute or chronic
phase and molecular vs. hemodynamic analysis.
Not applicable.
Funding: No funding was received.
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
WSP, SB and IN conceptualized and planned the
execution of the study, collected references and prepared the
manuscript. GCP, EZ, DAG, AFDA and MJT collected and compiled data
and prepared the manuscript. SB, CM and RD performed manuscript
review and online search. WSP, AFDA and SB confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
This study was approved by the Institutional Animal
Care and Use Committee of the School of Medicine at the University
of São Paulo (USP) (approval no. 019/14; being developed according
to the recommendations of the National Council for the Control of
Animal Experimentation and the Ethics Committee on Animal Use.
Not applicable.
The authors declare that they have no competing
interests.
Dr Sérgio Brasil, ORCID 0000-0003-2397-9947.
|
1
|
Bor-Seng-Shu E, Kita WS, Figueiredo EG,
Paiva WS, Fonoff ET, Teixeira MJ and Panerai RB: Cerebral
hemodynamics: Concepts of clinical importance. Arq Neuropsiquiatr.
70:352–356. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Andrade AF, Paiva WS, Amorim RL,
Figueiredo EG, Almeida AN, Brock RS, Bor-Seng-Shu E and Teixeira
MJ: Continuous ventricular cerebrospinal fluid drainage with
intracranial pressure monitoring for management of posttraumatic
diffuse brain swelling. Arq Neuropsiquiatr. 69:79–84.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Paiva WS, de Andrade AF, de Amorim RL,
Muniz RK, Paganelli PM, Bernardo LS, Figueiredo EG and Teixeira MJ:
The prognosis of the traumatic subarachnoid hemorrhage: A
prospective report of 121 patients. Int Surg. 95:172–176.
2010.PubMed/NCBI
|
|
4
|
Qureshi AI, Tuhrim S, Broderick JP, Batjer
HH, Hondo H and Hanley DF: Spontaneous intracerebral hemorrhage. N
Engl J Med. 344:1450–1460. 2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
van Asch CJ, Luitse MJ, Rinkel GJ, van der
Tweel I, Algra A and Klijn CJ: Incidence, case fatality, and
functional outcome of intracerebral haemorrhage over time,
according to age, sex, and ethnic origin: A systematic review and
meta-analysis. Lancet Neurol. 9:167–176. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Broderick JP, Brott TG, Duldner JE,
Tomsick T and Huster G: Volume of intracerebral hemorrhage. A
powerful and easy-to-use predictor of 30-day mortality. Stroke.
24:987–993. 1993.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fogelholm R, Murros K, Rissanen A and
Avikainen S: Long term survival after primary intracerebral
haemorrhage: A retrospective population based study. J Neurol
Neurosurg Psychiatry. 76:1534–1538. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hemphill JCI III, Bonovich DC, Besmertis
L, Manley GT and Johnston SC: The ICH score: A simple, reliable
grading scale for intracerebral hemorrhage. Stroke. 32:891–897.
2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Broderick J, Connolly S, Feldmann E,
Hanley D, Kase C, Krieger D, Mayberg M, Morgenstern L, Ogilvy CS,
Vespa P, et al: Guidelines for the management of spontaneous
intracerebral hemorrhage in adults: 2007 Update: A guideline from
the American heart association/American stroke association stroke
council, high blood pressure research council, and the quality of
care and outcomes in research interdisciplinary working group.
Stroke. 38:2001–2023. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Brasil S, Paiva WS, de Carvalho Nogueira
R, Macedo Salinet A and Teixeira MJ: Letter to the editor.
Decompressive craniectomy in TBI: What is beyond static evaluations
in terms of prognosis? J Neurosurg. 129:845–847. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zille M, Farr TD, Keep RF, Römer C, Xi G
and Boltze J: Novel targets, treatments, and advanced models for
intracerebral haemorrhage. EBioMedicine. 76(103880)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Godoy DA, Núñez-Patiño RA, Zorrilla-Vaca
A, Ziai WC and Hemphill JC III: Intracranial hypertension after
spontaneous intracerebral hemorrhage: A systematic review and
meta-analysis of prevalence and mortality rate. Neurocrit Care.
31:176–187. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Brasil S, Bor-Seng-Shu E, de-Lima-Oliveira
M, Taccone FS, Gattás G, Nunes DM, Gomes de Oliveira RA, Martins
Tomazini B, Tierno PF, Becker RA, et al: Computed tomography
angiography accuracy in brain death diagnosis. J Neurosurg: Sep 27,
2019 (Epub ahead of print).
|
|
14
|
Wagner KR, Hua Y, de Courten-Myers GM,
Broderick JP, Nishimura RN, Lu SY and Dwyer BE: Tin-mesoporphyrin,
a potent heme oxygenase inhibitor, for treatment of intracerebral
hemorrhage: In vivo and in vitro studies. Cell Mol Biol
(Noisy-le-grand). 46:597–608. 2000.PubMed/NCBI
|
|
15
|
Goulay R, Naveau M, Gaberel T, Vivien D
and Parcq J: Optimized tPA: A non-neurotoxic fibrinolytic agent for
the drainage of intracerebral hemorrhages. J Cereb Blood Flow
Metab. 38:1180–1189. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sinar EJ, Mendelow AD, Graham DI and
Teasdale GM: Experimental intracerebral hemorrhage: Effects of a
temporary mass lesion. J Neurosurg. 66:568–576. 1987.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fang Y, Tian Y, Huang Q, Wan Y, Xu L, Wang
W, Pan D, Zhu S and Xie M: Deficiency of TREK-1 potassium channel
exacerbates blood-brain barrier damage and neuroinflammation after
intracerebral hemorrhage in mice. J Neuroinflammation.
16(96)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kane PJ, Modha P, Strachan RD, Cook S,
Chambers IR, Clayton CB and Mendelow AD: The effect of
immunosuppression on the development of cerebral oedema in an
experimental model of intracerebral haemorrhage: Whole body and
regional irradiation. J Neurol Neurosurg Psychiatry. 55:781–786.
1992.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fei X, Dou YN, Wang L, Wu X, Huan Y, Wu S,
He X, Lv W, Wei J and Fei Z: Homer1 promotes the conversion of A1
astrocytes to A2 astrocytes and improves the recovery of transgenic
mice after intracerebral hemorrhage. J Neuroinflammation.
19(67)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mello TG, Rosado-de-Castro PH, Vasques JF,
Pinhão C, Santos TM, de Lima RR, Foerster BU, Paiva FF,
Mendez-Otero R and Pimentel-Coelho PM: Hyperacute transplantation
of umbilical cord mesenchymal stromal cells in a model of severe
intracerebral hemorrhage. Future Sci OA. 8(FSO793)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang G, Li T, Duan SN, Dong L, Sun XG and
Xue F: PPAR-γ promotes hematoma clearance through
haptoglobin-hemoglobin-CD163 in a rat model of intracerebral
hemorrhage. Behav Neurol. 2018(7646104)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xu J, Chen Z, Yu F, Liu H, Ma C, Xie D, Hu
X, Leak RK, Chou SHY, Stetler RA, et al: IL-4/STAT6 signaling
facilitates innate hematoma resolution and neurological recovery
after hemorrhagic stroke in mice. Proc Natl Acad Sci USA.
117:32679–32690. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jing C, Bian L, Wang M, Keep RF, Xi G and
Hua Y: Enhancement of hematoma clearance with CD47 blocking
antibody in experimental intracerebral hemorrhage. Stroke.
50:1539–1547. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhao X, Ting SM, Liu CH, Sun G, Kruzel M,
Roy-O'Reilly M and Aronowski J: Neutrophil polarization by IL-27 as
a therapeutic target for intracerebral hemorrhage. Nat Commun.
8(602)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fu X, Zhou G, Zhuang J, Xu C, Zhou H, Peng
Y, Cao Y, Zeng H, Li J, Yan F, et al: White matter injury after
intracerebral hemorrhage. Front Neurol. 12(562090)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xu F, Shen G, Su Z, He Z and Yuan L:
Glibenclamide ameliorates the disrupted blood-brain barrier in
experimental intracerebral hemorrhage by inhibiting the activation
of NLRP3 inflammasome. Brain Behav. 9(e01254)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tschoe C, Bushnell CD, Duncan PW,
Alexander-Miller MA and Wolfe SQ: Neuroinflammation after
intracerebral hemorrhage and potential therapeutic targets. J
Stroke. 22:29–46. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang Y, Chen X, Feng Z, Cai X, Zhu X, Cao
M, Yang L, Chen Y, Wang Y and Feng H: MEC17-induced α-tubulin
acetylation restores mitochondrial transport function and
alleviates axonal injury after intracerebral hemorrhage in mice. J
Neurochem. 160:51–63. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhu W, Gao Y, Chang CF, Wan JR, Zhu SS and
Wang J: Correction: Mouse models of intracerebral hemorrhage in
ventricle, cortex, and hippocampus by injections of autologous
blood or collagenase. PLoS One. 16(e0261640)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liesz A, Middelhoff M, Zhou W, Karcher S,
Illanes S and Veltkamp R: Comparison of humoral neuroinflammation
and adhesion molecule expression in two models of experimental
intracerebral hemorrhage. Exp Transl Stroke Med.
3(11)2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hijioka M, Anan J, Matsushita H, Ishibashi
H, Kurauchi Y, Hisatsune A, Seki T and Katsuki H: Axonal
dysfunction in internal capsule is closely associated with early
motor deficits after intracerebral hemorrhage in mice. Neurosci
Res. 106:38–46. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bahader GA, Nash KM, Almarghalani DA,
Alhadidi Q, McInerney MF and Shah ZA: Type-I diabetes aggravates
post-hemorrhagic stroke cognitive impairment by augmenting
oxidative stress and neuroinflammation in mice. Neurochem Int.
149(105151)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zheng J, Shi L, Liang F, Xu W, Li T, Gao
L, Sun Z, Yu J and Zhang J: Sirt3 ameliorates oxidative stress and
mitochondrial dysfunction after intracerebral hemorrhage in
diabetic rats. Front Neurosci. 12(414)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jeng BCP, de Andrade AF, Brasil S,
Bor-Seng-Shu E, Belon AR, Robertis M, de-Lima-Oliveira M, Rubiano
AM, Godoy DA, Teixeira MJ and Paiva WS: Estimation of intracranial
pressure by ultrasound of the optic nerve sheath in an animal model
of intracranial hypertension. J Clin Neurosci. 86:174–179.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Soares MS, Andrade AF, Brasil S,
DE-Lima-Oliveira M, Belon AR, Bor-Seng-Shu E, Nogueira RC, Godoy DA
and Paiva WS: Evaluation of cerebral hemodynamics by transcranial
Doppler ultrasonography and its correlation with intracranial
pressure in an animal model of intracranial hypertension. Arq
Neuropsiquiatr. 80:344–352. 2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu R, Cao S, Hua Y, Keep RF, Huang Y and
Xi G: CD163 expression in neurons after experimental intracerebral
hemorrhage. Stroke. 48:1369–1375. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cao S, Zheng M, Hua Y, Chen G, Keep RF and
Xi G: Hematoma changes during clot resolution after experimental
intracerebral hemorrhage. Stroke. 47:1626–1631. 2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mun-Bryce S, Wilkerson AC, Papuashvili N
and Okada YC: Recurring episodes of spreading depression are
spontaneously elicited by an intracerebral hemorrhage in the swine.
Brain Res. 888:248–255. 2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Rohde V, Rohde I, Thiex R, Ince A, Jung A,
Dückers G, Gröschel K, Röttger C, Küker W, Müller HD and Gilsbach
JM: Fibrinolysis therapy achieved with tissue plasminogen activator
and aspiration of the liquefied clot after experimental
intracerebral hemorrhage: Rapid reduction in hematoma volume but
intensification of delayed edema formation. J Neurosurg.
97:954–962. 2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xie Q, Gu Y, Hua Y, Liu W, Keep RF and Xi
G: Deferoxamine attenuates white matter injury in a piglet
intracerebral hemorrhage model. Stroke. 45:290–292. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sussman BJ, Barber JB and Goald H:
Experimental intracerebral hematoma. Reduction of oxygen tension in
brain and cerebrospinal fluid. J Neurosurg. 41:177–186.
1974.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Takasugi S, Ueda S and Matsumoto K:
Chronological changes in spontaneous intracerebral hematoma-an
experimental and clinical study. Stroke. 16:651–658.
1985.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Whisnant JP, Sayre GP and Millikan CH:
Experimental Intracerebral Hematoma. Arch Neurol. 9:586–592.
1963.
|
|
44
|
Symon L, Pasztor E, Branston NM and Dorsch
NW: Effect of supratentorial space-occupying lesions on regional
intracranial pressure and local cerebral blood flow: An
experimental study in baboons. J Neurol Neurosurg Psychiatry.
37:617–626. 1974.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Boltze J, Ferrara F, Hainsworth AH,
Bridges LR, Zille M, Lobsien D, Barthel H, McLeod DD, Gräßer F,
Pietsch S, et al: Lesional and perilesional tissue characterization
by automated image processing in a novel gyrencephalic animal model
of peracute intracerebral hemorrhage. J Cereb Blood Flow Metab.
39:2521–2535. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lin X, Tang Y, Sun B, Hou Z, Meng H, Li Z,
Liu Q and Liu S: Cerebral glucose metabolism: Influence on
perihematomal edema formation after intracerebral hemorrhage in cat
models. Acta Radiol. 51:549–554. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kaufman HH, Pruessner JL, Bernstein DP,
Borit A, Ostrow PT and Cahall DL: A rabbit model of intracerebral
hematoma. Acta Neuropathol. 65:318–321. 1985.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhang C, Qian X, Zheng J, Ai P, Cao X, Pan
X, Chen T and Wang Y: Controlled decompression alleviates brain
injury via attenuating oxidative damage and neuroinflammation in
acute intracranial hypertension. Biomed Res Int.
2022(1936691)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wagner KR, Xi G, Hua Y, Kleinholz M, de
Courten-Myers GM, Myers RE, Broderick JP and Brott TG: Lobar
intracerebral hemorrhage model in pigs: Rapid edema development in
perihematomal white matter. Stroke. 27:490–497. 1996.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wagner KR, Xi G, Hua Y, Kleinholz M, de
Courten-Myers GM and Myers RE: Early metabolic alterations in
edematous perihematomal brain regions following experimental
intracerebral hemorrhage. J Neurosurg. 88:1058–1065.
1998.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Chen J, Koduri S, Dai S, Toyota Y, Hua Y,
Chaudhary N, Pandey AS, Keep RF and Xi G: Intra-hematomal white
matter tracts act as a scaffold for macrophage infiltration after
intracerebral hemorrhage. Transl Stroke Res. 12:858–865.
2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
MacLellan CL, Silasi G, Auriat AM and
Colbourne F: Rodent models of intracerebral hemorrhage. Stroke. 41
(Suppl 10):S95–S98. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Yang GY, Betz AL, Chenevert TL, Brunberg
JA and Hoff JT: Experimental intracerebral hemorrhage: Relationship
between brain edema, blood flow, and blood-brain barrier
permeability in rats. J Neurosurg. 81:93–102. 1994.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Xi G, Keep RF and Hoff JT: Erythrocytes
and delayed brain edema formation following intracerebral
hemorrhage in rats. J Neurosurg. 89:991–996. 1998.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Xi G, Wagner KR, Keep RF, Hua Y, de
Courten-Myers GM, Broderick JP, Brott TG and Hoff JT: Role of blood
clot formation on early edema development after experimental
intracerebral hemorrhage. Stroke. 29:2580–2586. 1998.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Hua Y, Xi G, Keep RF and Hoff JT:
Complement activation in the brain after experimental intracerebral
hemorrhage. J Neurosurg. 92:1016–1022. 2000.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Xi G, Hua Y, Bhasin RR, Ennis SR, Keep RF
and Hoff JT: Mechanisms of edema formation after intracerebral
hemorrhage: Effects of extravasated red blood cells on blood flow
and blood-brain barrier integrity. Stroke. 32:2932–2938.
2001.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Belayev L, Saul I, Curbelo K, Busto R,
Belayev A, Zhang Y, Riyamongkol P, Zhao W and Ginsberg MD:
Experimental intracerebral hemorrhage in the mouse: Histological,
behavioral, and hemodynamic characterization of a double-injection
model. Stroke. 34:2221–2227. 2003.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Nakamura T, Keep RF, Hua Y, Schallert T,
Hoff JT and Xi G: Deferoxamine-induced attenuation of brain edema
and neurological deficits in a rat model of intracerebral
hemorrhage. J Neurosurg. 100:672–678. 2004.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Liu L, Wang S, Xu R, Zheng J, Tang J, Tang
X and Zhang D: Experimental intracerebral haemorrhage: Description
of a semi-coagulated autologous blood model in rats. Neurol Res.
37:874–879. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Bullock R, Mendelow AD, Teasdale GM and
Graham DI: Intracranial haemorrhage induced at arterial pressure in
the rat. Part 1: Description of technique, ICP changes and
neuropathological findings. Neurol Res. 6:184–188. 1984.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Manaenko A, Chen H, Zhang JH and Tang J:
Comparison of different preclinical models of intracerebral
hemorrhage. Acta Neurochir Suppl. 111:9–14. 2011.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wakisaka Y, Chu Y, Miller JD, Rosenberg GA
and Heistad DD: Spontaneous intracerebral hemorrhage during acute
and chronic hypertension in mice. J Cereb Blood Flow Metab.
30:56–69. 2010.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Bai Q, Sheng Z, Liu Y, Zhang R, Yong VW
and Xue M: Intracerebral haemorrhage: From clinical settings to
animal models. Stroke Vasc Neurol. 5:388–395. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Deinsberger W, Vogel J, Kuschinsky W, Auer
LM and Böker DK: Experimental intracerebral hemorrhage: Description
of a double injection model in rats. Neurol Res. 18:475–477.
1996.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Deinsberger W, Hartmann M, Vogel J, Jansen
O, Kuschinsky W, Sartor K and Böker DK: Local fibrinolysis and
aspiration of intracerebral hematomas in rats. An experimental
study using MR monitoring. Neurol Res. 20:349–352. 1998.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Orakcioglu B, Becker K, Sakowitz OW,
Herweh C, Köhrmann M, Huttner HB, Steiner T, Unterberg A and
Schellinger PD: MRI of the perihemorrhagic zone in a rat ICH model:
Effect of hematoma evacuation. Neurocrit Care. 8:448–455.
2008.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Orakcioglu B, Becker K, Sakowitz OW,
Unterberg A and Schellinger PD: Serial diffusion and perfusion MRI
analysis of the perihemorrhagic zone in a rat ICH model. Acta
Neurochir Suppl. 103:15–18. 2008.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Deng S, Feng S, Wang W, Zhao F and Gong Y:
Biomarker and drug target discovery using quantitative proteomics
post-intracerebral hemorrhage stroke in the rat brain. J Mol
Neurosci. 66:639–648. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
James ML, Warner DS and Laskowitz DT:
Preclinical models of intracerebral hemorrhage: A translational
perspective. Neurocrit Care. 9:139–152. 2008.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Rosenberg GA, Mun-Bryce S, Wesley M and
Kornfeld M: Collagenase-induced intracerebral hemorrhage in rats.
Stroke. 21:801–807. 1990.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Clark W, Gunion-Rinker L, Lessov N and
Hazel K: Citicoline treatment for experimental intracerebral
hemorrhage in mice. Stroke. 29:2136–2140. 1998.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Wang J, Wang G, Yi J, Xu Y, Duan S, Li T,
Sun XG and Dong L: The effect of monascin on hematoma clearance and
edema after intracerebral hemorrhage in rats. Brain Res Bull.
134:24–29. 2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Fu P, Liu J, Bai Q, Sun X, Yao Z, Liu L,
Wu C and Wang G: Long-term outcomes of monascin-a novel dual
peroxisome proliferator-activated receptor γ/nuclear
factor-erythroid 2 related factor-2 agonist in experimental
intracerebral hemorrhage. Ther Adv Neurol Disord: May 14, 2020.
|
|
75
|
Wasserman JK, Yang H and Schlichter LC:
Glial responses, neuron death and lesion resolution after
intracerebral hemorrhage in young vs aged rats. Eur J Neurosci.
28:1316–1328. 2008.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Liddle L, Reinders R, South S, Blacker D,
Knuckey N, Colbourne F and Meloni B: Poly-arginine-18 peptides do
not exacerbate bleeding, or improve functional outcomes following
collagenase-induced intracerebral hemorrhage in the rat. PLoS One.
14(e0224870)2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Akhter M, Qin T, Fischer P, Sadeghian H,
Kim HH, Whalen MJ, Goldstein JN and Ayata C: Rho-kinase inhibitors
do not expand hematoma volume in acute experimental intracerebral
hemorrhage. Ann Clin Transl Neurol. 5:769–776. 2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Lee ST, Chu K, Sinn DI, Jung KH, Kim EH,
Kim SJ, Kim JM, Ko SY, Kim M and Roh JK: Erythropoietin reduces
perihematomal inflammation and cell death with eNOS and STAT3
activations in experimental intracerebral hemorrhage. J Neurochem.
96:1728–1739. 2006.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Wu CH, Shyue SK, Hung TH, Wen S, Lin CC,
Chang CF and Chen SF: Genetic deletion or pharmacological
inhibition of soluble epoxide hydrolase reduces brain damage and
attenuates neuroinflammation after intracerebral hemorrhage. J
Neuroinflammation. 14(230)2017.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Kinoshita K, Ohtomo R, Takase H, Hamanaka
G, Chung KK, Lok J, Katsuki H and Arai K: Different responses after
intracerebral hemorrhage between young and early middle-aged mice.
Neurosci Lett. 735(135249)2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Li W, Chopp M, Zacharek A, Yang W, Chen Z,
Landschoot-Ward J, Venkat P and Chen J: SUMO1 deficiency
exacerbates neurological and cardiac dysfunction after
intracerebral hemorrhage in aged mice. Transl Stroke Res.
12:631–642. 2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Kirkman MA, Allan SM and Parry-Jones AR:
Experimental intracerebral hemorrhage: Avoiding pitfalls in
translational research. J Cereb Blood Flow Metab. 31:2135–2151.
2011.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Chang CC, Huang KH, Hsu SP, Lee YG, Sue YM
and Juan SH: Simvastatin reduces the carcinogenic effect of
3-methylcholanthrene in renal epithelial cells through histone
deacetylase 1 inhibition and RhoA reactivation. Sci Rep.
9(4606)2019.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Wang M, Hua Y, Keep RF, Wan S, Novakovic N
and Xi G: Complement inhibition attenuates early erythrolysis in
the hematoma and brain injury in aged rats. Stroke. 50:1859–1868.
2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Strbian D, Durukan A and Tatlisumak T:
Rodent models of hemorrhagic stroke. Curr Pharm Des. 14:352–358.
2008.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Zhou X, Chen L, Feng C, Li B, Tang J, Liu
A, Lv F and Li T: Establishing an animal model of intracerebral
hemorrhage under the guidance of ultrasound. Ultrasound Med Biol.
39:2116–2122. 2013.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Lei B, Sheng H, Wang H, Lascola CD, Warner
DS, Laskowitz DT and James ML: Intrastriatal injection of
autologous blood or clostridial collagenase as murine models of
intracerebral hemorrhage. J Vis Exp. (51439)2014.PubMed/NCBI View Article : Google Scholar
|
|
88
|
MacLellan CL, Silasi G, Poon CC, Edmundson
CL, Buist R, Peeling J and Colbourne F: Intracerebral hemorrhage
models in rat: Comparing collagenase to blood infusion. J Cereb
Blood Flow Metab. 28:516–525. 2008.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Funnell WR, Maysinger D and Cuello AC:
Three-dimensional reconstruction and quantitative evaluation of
devascularizing cortical lesions in the rat. J Neurosci Methods.
35:147–156. 1990.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Xue M and Del Bigio MR: Comparison of
brain cell death and inflammatory reaction in three models of
intracerebral hemorrhage in adult rats. J Stroke Cerebrovasc Dis.
12:152–159. 2003.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Lauer A, Cianchetti FA, Van Cott EM,
Schlunk F, Schulz E, Pfeilschifter W, Steinmetz H, Schaffer CB, Lo
EH and Foerch C: Anticoagulation with the oral direct thrombin
inhibitor dabigatran does not enlarge hematoma volume in
experimental intracerebral hemorrhage. Circulation. 124:1654–1662.
2011.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Alharbi BM, Tso MK and Macdonald RL:
Animal models of spontaneous intracerebral hemorrhage. Neurol Res.
38:448–455. 2016.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Andrade AF, Soares MS, Patriota GC, Belon
AR, Paiva WS, Bor-Seng-Shu E, Oliveira Mde L, Nascimento CN, Noleto
GS, Alves Junior AC, et al: Experimental model of intracranial
hypertension with continuous multiparametric monitoring in swine.
Arq Neuropsiquiatr. 71:802–806. 2013.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Azevedo MR, de-Lima-Oliveira M, Belon AR,
Brasil S, Teixeira MJ, Paiva WS and Bor-Seng-Shu E: Assessing
ultrasonographic optic nerve sheath diameter in animal model with
anesthesia regimens. Acta Cir Bras. 37(e370308)2022.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Wagner T, Fregni F, Fecteau S, Grodzinsky
A, Zahn M and Pascual-Leone A: Transcranial direct current
stimulation: A computer-based human model study. Neuroimage.
35:1113–1124. 2007.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Wagner KR: Modeling intracerebral
hemorrhage: Glutamate, nuclear factor-kappa B signaling and
cytokines. Stroke. 38 (2 Suppl):S753–S758. 2007.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Shi Y, Li Z, Zhang S, Xie M, Meng X, Xu J,
Liu N and Tang Z: Establishing a model of supratentorial hemorrhage
in the piglet. Tohoku J Exp Med. 220:33–40. 2010.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Küker W, Thiex R, Rohde I, Rohde V and
Thron A: Experimental acute intracerebral hemorrhage. Value of MR
sequences for a safe diagnosis at 1.5 and 0.5 T. Acta Radiol.
41:544–552. 2000.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Wagner KR, Packard BA, Hall CL, Smulian
AG, Linke MJ, De Courten-Myers GM, Packard LM and Hall NC: Protein
oxidation and heme oxygenase-1 induction in porcine white matter
following intracerebral infusions of whole blood or plasma. Dev
Neurosci. 24:154–160. 2002.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Wagner KR, Sharp FR, Ardizzone TD, Lu A
and Clark JF: Heme and iron metabolism: Role in cerebral
hemorrhage. J Cereb Blood Flow Metab. 23:629–652. 2003.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Zuccarello M, Andaluz N and Wagner KR:
Minimally invasive therapy for intracerebral hematomas. Neurosurg
Clin N Am. 13:349–354. 2002.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Wagner KR, Xi G, Hua Y, Zuccarello M, de
Courten-Myers GM, Broderick JP and Brott TG: Ultra-early clot
aspiration after lysis with tissue plasminogen activator in a
porcine model of intracerebral hemorrhage: Edema reduction and
blood-brain barrier protection. J Neurosurg. 90:491–498.
1999.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Gu Y, Hua Y, Keep RF, Morgenstern LB and
Xi G: Deferoxamine reduces intracerebral hematoma-induced iron
accumulation and neuronal death in piglets. Stroke. 40:2241–2243.
2009.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Friess SH, Ralston J, Eucker SA, Helfaer
MA, Smith C and Margulies SS: Neurocritical care monitoring
correlates with neuropathology in a swine model of pediatric
traumatic brain injury. Neurosurgery. 69:1139–1147. 2011.PubMed/NCBI View Article : Google Scholar
|