Introduction
Synovial hemangioma is a rare benign tumor that
occurs most frequently in the knee in children and young adults
(1-3).
There are four histological subtypes: venous, venous vascular
malformation, cavernous, and capillary hemangiomas (4). Since the clinical presentation and
radiological findings of synovial hemangiomas are nonspecific,
there is often a long period from the onset to the diagnosis. In
some cases, the diagnosis is made after osteoarthritis and
cartilage damage have already occurred (5). After the diagnosis, an open or
arthroscopic resection is performed in most cases, and good
postoperative outcomes have been reported (6). Because of the rarity of synovial
hemangiomas, most reports described a single clinical case, and
they highlighted the rarity of this tumor. In this report, we
describe a series of nine patients with synovial hemangiomas and
the results of our retrospective analysis of the correlation
between patients' pathological and radiological characteristics.
Moreover, the usefulness of diffusion-weighted image (DWI) in the
differentiation of synovial hemangioma from diffuse-type
tenosynovial giant cell tumor (D-TSGCT) (formerly named pigmented
villonodular synovitis) is also discussed.
Patients and methods
After obtaining institutional review board approval,
we identified the nine patients (five males and four females) who
were each diagnose with synovial hemangioma of a knee and treated
at Fukushima Medical University Hospital (Fukushima, Japan) or
Fukushima Red Cross Hospital (Fukushima, Japan) during the period
between January 1998 and December 2021. The patients' median age at
surgery was 22 years (range 1-43 yrs). We retrospectively reviewed
the patients' clinical presentations, radiological findings,
surgical procedures, postoperative courses, and pathological
findings.
Results
Clinical presentations and tumor
locations
Table I summarizes
the patients' clinical characteristics. All nine patients had
persistent knee pain. Three patients also had a swollen knee with
intra-articular hemorrhage. The duration of symptoms from their
onset to the patient's initial visit varied, ranging from 1 day to
10 years. One patient had multiple lesions (Patient #6). Among the
other eight cases, the synovial hemangioma was located in the
suprapatellar bursa in two patients (#2 and #4), in the subcapsular
fat body in four (Patients #1, #5, #7, and #9), and anterior to the
femoral condyle localized in two (Patients #3 and #8).
 | Table IPatients' clinical
characteristics. |
Table I
Patients' clinical
characteristics.
| Patient no. | Age, years | Sex | Symptom | Intra-articular
hemorrhage | Symptom duration
(until first visit) | Affected side | Location |
|---|
| 1 | 1 | F | Pain, swelling | + | 3 months | Right | Infrapatella and
suprapatellar fat-pad |
| 2 | 11 | F | Pain | - | 3 months | Right | Suprapatellar
bursa |
| 3 | 14 | M | Pain | - | 1 month | Left | In front of the
medial condyle of femur |
| 4 | 17 | F | Pain | - | 0.5 months | Left | Suprapatellar
bursa |
| 5 | 22 | M | Pain, Swelling | + | 1 day | Right | Infra patella
fat-pad |
| 6 | 24 | M | Pain | - | 10 years | Left | Inter condylar fossa,
infra patellar fat-pad and posterolateral capsule |
| 7 | 28 | M | Pain | - | 1 year | Right | Suprapatella
fat-pad |
| 8 | 34 | F | Pain | - | 6 months | Left | In front of the
medial condyle of femur |
| 9 | 43 | M | Pain, swelling | + | 1 months | Left | Infrapatellar
fat-pad |
Radiological findings
On plain radiographs, intra-articular calcification
consistent with phleboliths was observed in two patients.
T1-weighted magnetic resonance imaging (MRI) showed low signal
intensity with and without small signal voids in six patients and
two patients, respectively, and high signal intensity without
signal voids in the other patient (Patient #7). In all nine
patients, T2-weighted imaging (WI) showed high signal intensity
containing small signal voids and intra-tumoral septum comparable
to joint fluid and fat. In the present cases, the synovial
hemangiomas showed a small honeycomb pattern with a thin septum and
a lobulated pattern. T2*-WI showed high intensity, containing low
intensity area that could be post-hemorrhagic changes. Gadolinium
enhancement was performed for four patients, with heterogeneous
staining of the tumor (Figs. 1 and
2). The maximum tumor diameter
ranged from 1.5 to 7.2 cm (Table
II). MRI findings of D-TSGCT show nodular and extensive
synovial proliferation, joint effusion, and bone erosion.
Hemosiderin deposition in the proliferative synovial tissue results
in point-like low signal on T1-weighted and T2-weighted images and
extensive low-signal changes within the proliferative synovium.
These may be differentiation from synovial hemangioma.
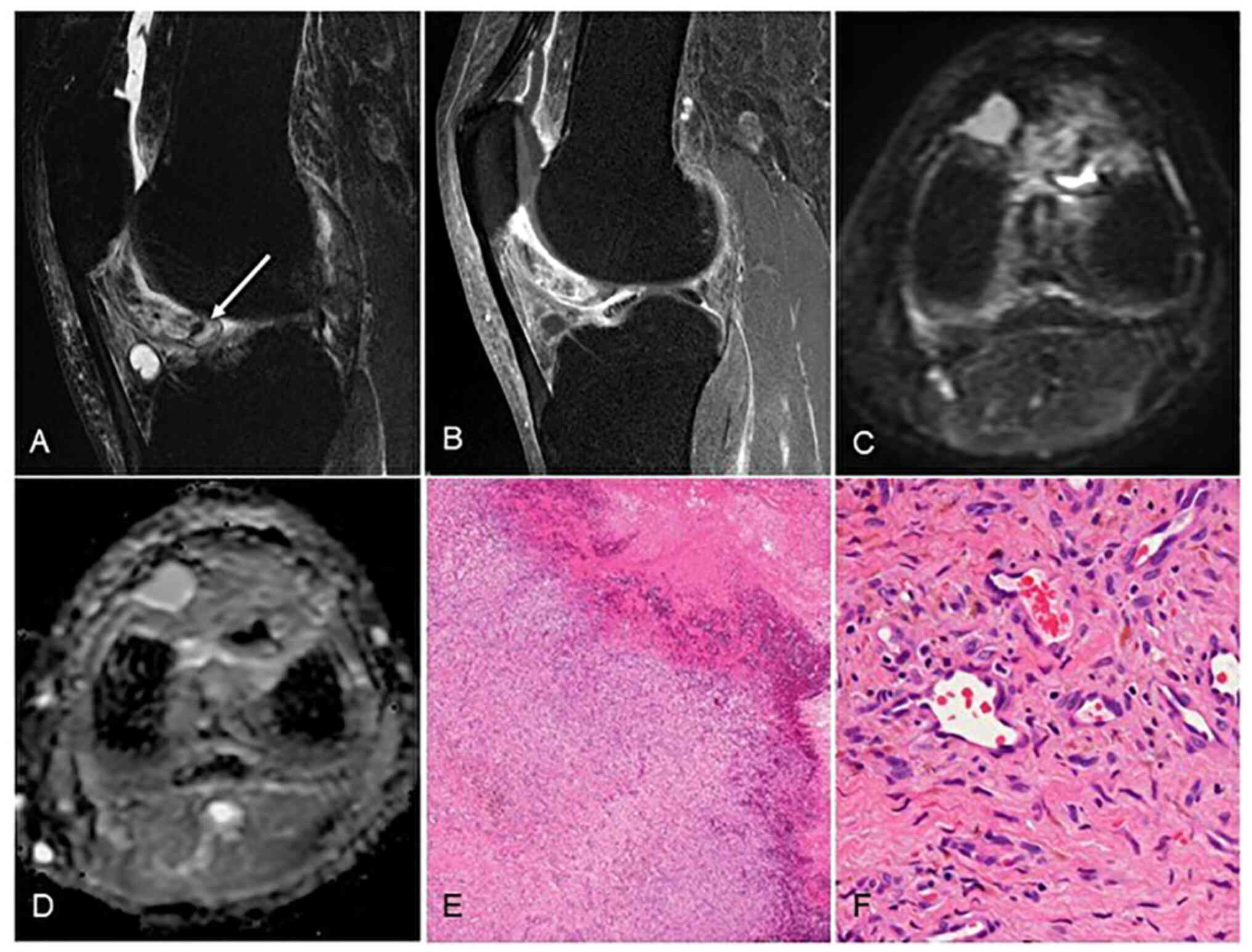 | Figure 2Capillary hemangioma (patient #9). (A)
On magnetic resonance imaging, T2-weighed fat-suppression imaging
showed high signal intensity containing small signal void (arrow).
(B) Gadolinium enhancement showed heterogeneous staining of the
tumor. (C) DWI showed low-signal intensity in the tumor and (D)
high apparent diffusion coefficient (ADC) value of 2,184/mm2/sec.
Microscopic findings show small vascular lumen, which was less
likely to form thrombus or phleboliths, and this tumor was
difficult to diagnose without microscopic confirmation: (E)
low-power field (magnification, x20), H-E staining; (F) high-power
field, (magnification, x200), H-E staining. H-E, hematoxylin and
eosin. |
 | Table IIRadiological characteristics of the
tumor. |
Table II
Radiological characteristics of the
tumor.
| Patient no. | Size, cm | Plain
radiography | T1-weighted
image | T2*-weighted
image | Gadolinium-
enhanced, T1- weighted fat- suppression image | DWI
ADC-mapping |
|---|
| 1 | 3.7x2.4x1.9 | Phlebolith | Low intensity | High intensity,
containing low intensity area of hemorrhage | - | - |
| 2 | 3.0x2.8x1.5 | Normal | Low intensity,
containing small signal voids | - | - | - |
| 3 | 7.2x4.1x0.9 | Cortical erosion,
phlebolith | Low intensity,
containing small signal voids | - | Heterogeneous
enhancement | - |
| 4 | 3.1x2.1x1.1 | Normal | Low intensity | - | Heterogeneous
enhancement | - |
| 5 | 1.5x0.8 | Normal | Low intensity,
containing small signal voids | - | - | - |
| 6 |
Diffuse/multiple | Normal | Low intensity,
containing small signal voids | - | - | - |
| 7 | 3.8x3.3x1.1 | Normal | High intensity,
containing small signal voids | - | Heterogeneous
enhancement | - |
| 8 | 4x3.5x1.1 | Normal | Low intensity,
containing small signal voids | - | - | - |
| 9 | 4.7x5.9x2.9 | Normal | Low intensity,
containing small signal voids | High intensity,
containing low intensity area of hemorrhage | Heterogeneous
enhancement | ADC value 2184 |
Surgical procedures and postoperative
courses
The surgery was performed without a preoperative
biopsy in all nine patients. The duration from the patient's
initial visit to the surgery ranged from 1 to 9 months. Open
resection (n=2 patients), arthroscopic resection (n=3), and
conversion to open resection after arthroscopy (n=4) were
performed. In one of the two patients who underwent an open
resection (Patient #6) and in one of the patients who underwent
arthroscopic resection (Patient #4), additional resections for
local recurrence were performed.
The average postoperative follow-up period from the
final surgery was 17.2 months, with the longest follow-up period
being 60 months. After the final surgery, none of the patients
developed postoperative recurrence, and their knee pain had
disappeared at the final follow-up (Table III).
 | Table IIISurgical procedure, pathological
diagnoses and oncological prognosis. |
Table III
Surgical procedure, pathological
diagnoses and oncological prognosis.
| Patient no. | Duration from
initial visit to surgery, months | Procedure | Pathological
sub-type | Follow-up period,
months | Prognosis |
|---|
| 1 | 45 | Open resection
after arthroscopy | Cavernous
hemangioma | 11 | NED |
| 2 | 5 | Arthroscopic
resection | Capillary
hemangioma | 12 | NED |
| 3 | 9 | Open resection | Cavernous
hemangioma | 1 | NED |
| 4 | 2 | 1st surgery:
Arthroscopic resection 2nd surgery: Open resection | Venous
hemangioma | 9 | NEDa |
| 5 | 3 | Arthroscopic
resection | Capillary
hemangioma | 6 | NED |
| 6 | 1 | 1st surgery: Open
resection 2nd surgery: Arthroscopic resection | Venous
hemangioma | 41 | NEDa |
| 7 | 3 | Open resection
after arthroscopy | Cavernous
hemangioma | 13 | NED |
| 8 | 7 | Open resection | Venous
hemangioma | 2 | NED |
| 9 | 2 | Open resection
after arthroscopy | Capillary
hemangioma | 60 | NED |
Pathological findings
Based on the pathological findings of surgical
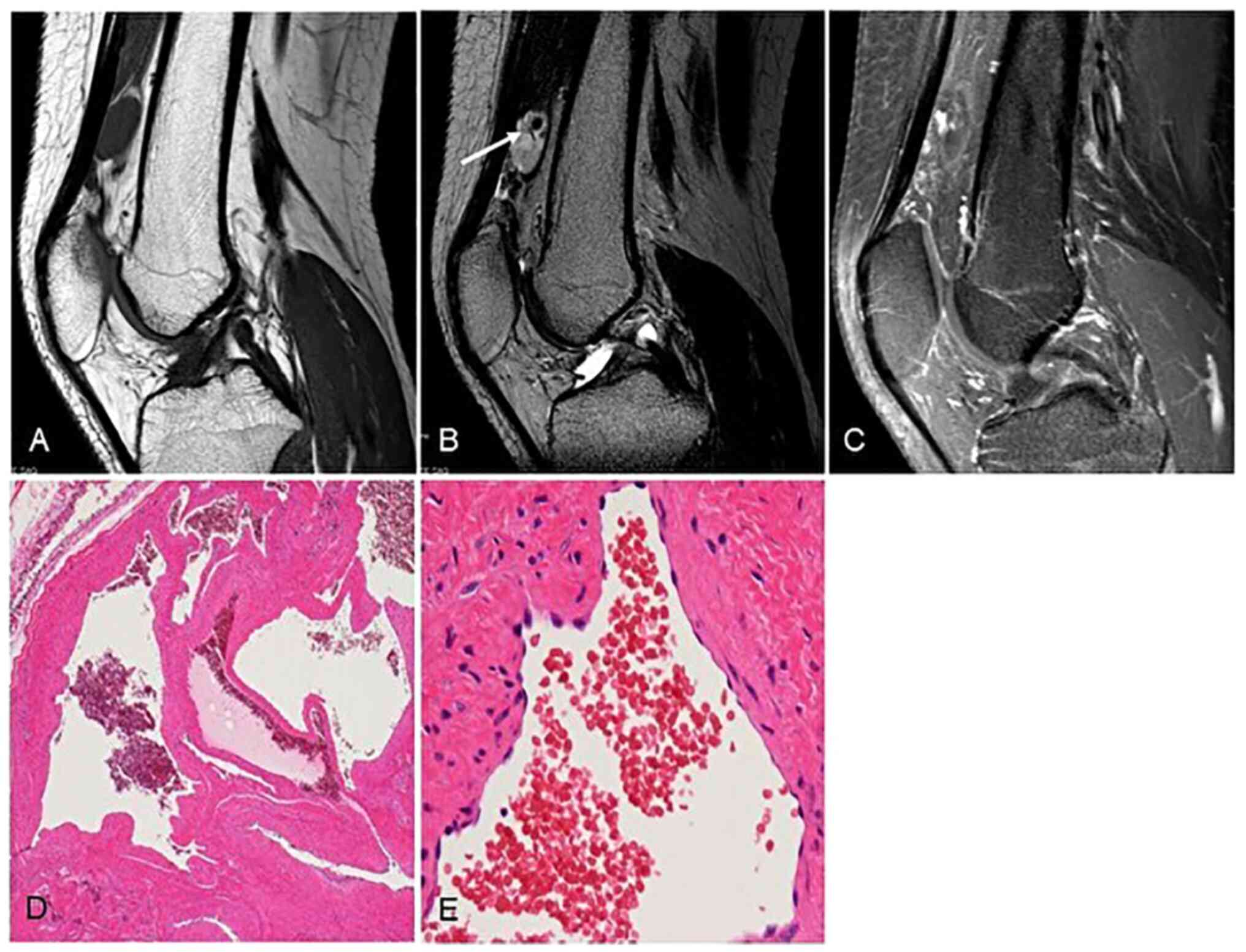
specimens, the diagnoses were venous hemangioma (Fig. 1), capillary hemangioma (Fig. 2), cavernous hemangioma (Fig. 3), in three patients each. Venous
hemangiomas have thickened vascular smooth muscle and a dilated
vascular lumen. Because of slow blood flow, they are prone to
forming thrombi and phleboliths. Capillary hemangiomas have a small
vascular lumen and are less likely to form thrombus or phleboliths.
Microscopy should be used for diagnosis. In cavernous hemangiomas
unlike the venous hemangiomas, vascular smooth muscle is absent.
The size of the intra-tumoral vascular lumen differs depending on
the subtype of hemangioma.
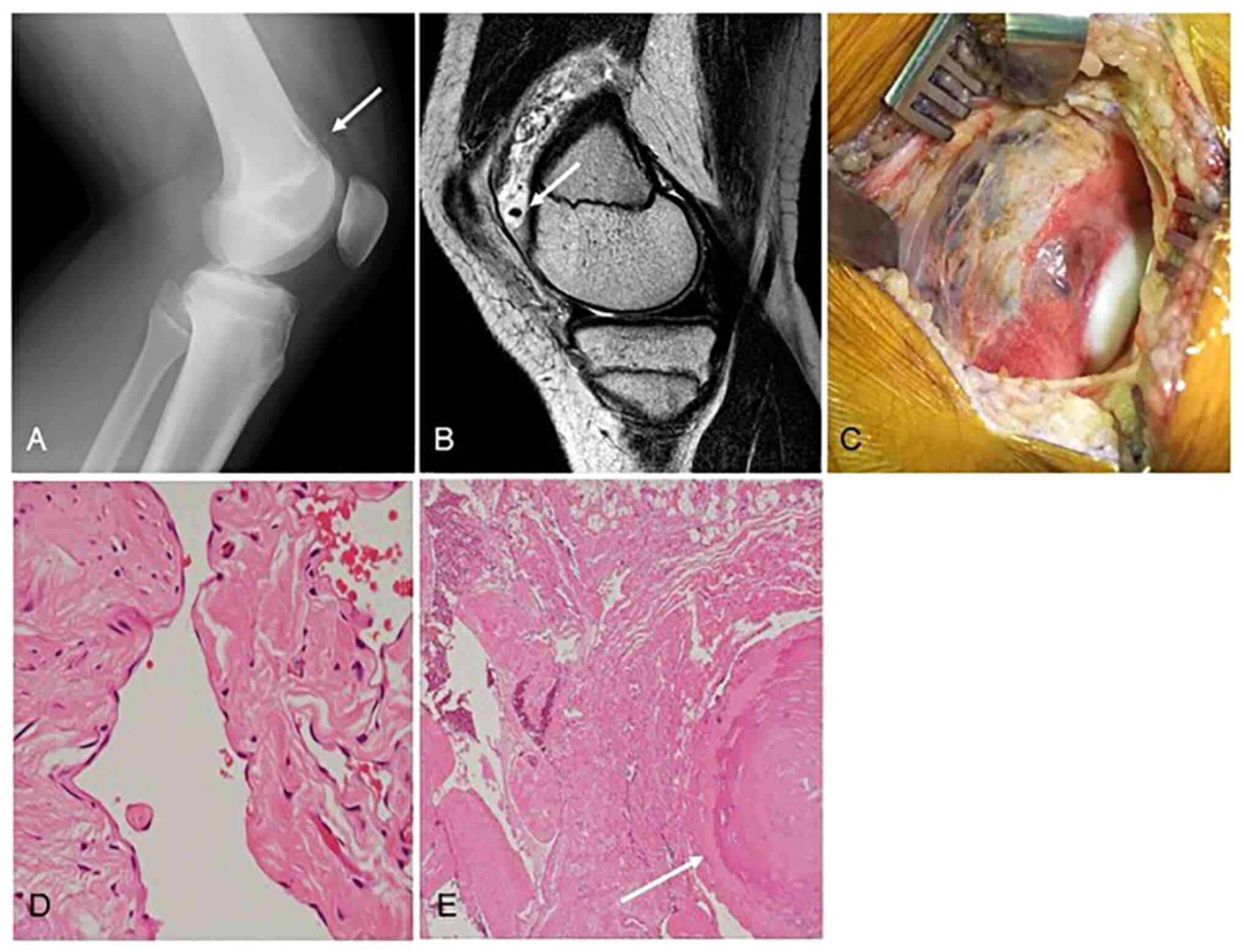
Representative case (Patient #3)
A 14-year-old boy presented with a 1-month history
of continuous left knee pain. Plain radiographs of his left knee
joint showed an intra-articular calcification consistent with
phlebolith (Fig. 3A). MRI revealed
a mass with high signal intensity containing a small signal void on
T2-WI (sagittal plane) (Fig. 3B).
From the radiological findings, we initially diagnosed a synovial
hemangioma of the knee. Since the patient's left knee pain did not
improve, we decided to perform the surgical resection 9 months
after the initial visit. Because of the size of the tumor, open
resection was selected. Intraoperative gross findings showed a dark
red tumor covered by the synovium on the surface of lateral condyle
of the femur (Fig. 3C), and the
tumor was completely resected. The microscopic findings of the
surgical specimen showed expanded blood lumens lacking vascular
smooth muscle (Fig. 3D). The
formation of thromboses and phleboliths caused by slow blood flow
was easily identified (Fig. 3E).
The tumor was diagnosed as a cavernous SH. At 1 month after the
operation, the patient's symptom had completely resolved.
Discussion
Synovial hemangioma has accounted for 0.07% of all
soft tissue tumors and 0.78% of resected hemangiomas (7). Although it can occur in a variety of
joints, the most common location is the knee (1). Synovial hemangioma occurs most
frequently in children and young adults (1-3),
with male predominance. Since there is no specific clinical
presentation, there is often a long period between the onset and
the diagnosis (8). The most common
symptom is long-term persistent knee pain; other symptoms include a
limited range of motion, joint swelling, and nontraumatic
intra-articular hematoma. However, except in phases of
exacerbation, the presence of a synovial hemangioma does not limit
activities of daily living, and patients with this benign tumor
rarely visit a medical facility.
The clinical findings of synovial hemangioma are
similar to those of D-TSGCT and hemophilic arthropathy. Both may
cause sudden and non-traumatic intra-articular hemorrhage, and a
delayed diagnosis can thus lead to osteoarthritis and joint
function disuse (9). There are two
case reports of synovial hemangioma in which >40 years passed
from the onset of the disease until the final diagnosis (10,11).
In another report, a 67-year-old patient was diagnosed with a
synovial hemangioma after experiencing severe osteoarthritis; the
patient finally underwent a total knee arthroplasty (12). As a characteristic physical
examination finding, cutaneous hemangiomas have been reported to be
present in approx. 40% of patients with synovial hemangiomas in the
knee (1), but in the present
series there were no coexisting cutaneous lesions. A reported
average duration from the onset of symptoms until the diagnosis of
synovial hemangioma was 4.5 years (13). We were able to diagnose the disease
earlier, with an average duration of 4.18 months from onset to
diagnosis. When clinicians are diagnosing knee pain and/or
intra-articular hemorrhage in young patients, the possibility of
synovial hemangioma should be considered. Synovial hemangiomas are
expected to have good surgical outcomes with resection, but if left
undiagnosed for a long period of time, they can lead to
osteoarthritis and functional disability of the knee. Preoperative
imaging and intraoperative arthroscopic findings should confirm the
extent of the tumor and if adequate resection is possible,
arthroscopic resection should be used. If the tumor is difficult to
resect due to its location or size, the approach should be switched
to open. The present patient series includes a 1-year-old patient,
and surgery is recommended as soon as the diagnosis and
perioperative management are possible.
Plain radiography is usually the first radiological
examination conducted in patients who are suspected to have knee
pain and/or intra-articular hemorrhage. Plain radiography of a
synovial hemangioma typically shows no abnormality, but periosteal
reactions, osteolysis, osteopenia, intra-articular phleboliths,
osteoarthritis, or soft tissue swelling may be observed (1,2,14-18).
In the present patient series, we identified cortical erosion in
one case and intra-articular phleboliths in two cases, but these
findings were not specific for synovial hemangioma and could not be
used as diagnostic evidence. As in other soft tissue tumors, MRI
thus plays a more important role than plain radiography.
Compared to muscle tissue, an MRI evaluation of a
synovial hemangioma reveals low to iso-intensity on T1-WI and high
intensity on T2-WI, but no characteristic findings are seen on
fat-suppression imaging (19,20).
In the present patients, the synovial hemangiomas showed a small
honeycomb pattern with a thin septum and a lobulated pattern. Small
signal voids were also observed within the tumor in all nine
patients. Contrast-enhanced MRI was performed in four cases, and
gadolinium contrast showed heterogeneous enhancement only of the
septum and tumor margins, indicating a cystic-like structure. In
addition, a fibrous septum separated the tortuous vascular
component, which has been reported as one of the specific findings
of synovial hemangioma (21,22).
The size of the intra-tumoral vascular lumen differs
depending on the subtype of hemangioma. In a larger vascular lumen,
the blood flow becomes slower, and the contrast will be partial. It
was reported that large venous synovial hemangiomas showed high
signal intensity on T1-WI due to slow blood flow (20). Since synovial hemangiomas are
blood-rich tumors and are usually heterogeneously enhanced on
gadolinium contrast, the use of which helps distinguish these
tumors from cystic synovial hyperplasia (23), intra-articular hematoma, and joint
effusion (5,9,24,25).
However, it has been claimed that synovial hemangioma was correctly
diagnosed preoperatively in only 22% of cases (9,14).
The usefulness of contrast MRI had been supported for confirming
the localization and the extension of this tumor and detecting
local recurrence in the postoperative follow-up (5).
The pathological diagnosis of synovial hemangioma is
classified into the venous, arteriovenous, cavernous, and capillary
subtypes (4). Venous synovial
hemangiomas have thick vascular smooth muscle and a dilated
vascular lumen. Cavernous synovial hemangiomas also have a dilated
lumen, but unlike the venous subtype, they are differentiated by
the absence of smooth muscle in the vessel wall. In both of these
subtypes, the dilated lumen slows the blood flow and increases the
formation of a thrombus and/or phleboliths compared to the
capillary subtype, which has a smaller lumen. In the present
patient series, the presence of phleboliths was confirmed by plain
radiography and MRI in only two cases, both of which were the
cavernous subtype (Fig. 2). On the
other hand, capillary subtypes do not form a thrombus or
phleboliths, because of their narrow lumen. It is thus difficult to
diagnose capillary subtypes as hemangiomas without microscopic
confirmation.
An important differential diagnosis for synovial
hemangioma is D-TSGCT. Because of these two tumors' similarity in
age of onset and clinical presentation, D-TSGCT can be difficult to
distinguish in clinical practice. We noted that all nine of the
present patients' cases showed high signal intensity on T2-WI,
similar to circumferential fatty tissue and joint fluid. D-TSGCTs
usually show low-signal changes on T2-WI due to hemosiderin
deposition (26), whereas synovial
hemangiomas show high-signal changes on T2-WI (23,27,28).
This radiological difference in MRI between D-TSGCT and synovial
hemangioma is thought to be useful. However, T2-WI shows various
signal changes according to the presence of intra-articular
hemorrhage or fatty degeneration, leading to potential diagnostic
difficulty for synovial hemangioma on MRI.
In the present series, diffusion-weighted imaging
(DWI) was performed in one of the capillary subtype cases. The DWI
showed low signal intensity in the tumor and the high apparent
diffusion coefficient (ADC) value of 2,184/mm2/sec,
excluding the area with high signal intensity that was thought to
be post-hemorrhage changes (Fig.
3). Our review of eight cases of D-TSGCT of the knee that were
treated at our institute between 2006 and 2021 revealed that the
MRI findings revealed high signal intensity in DWI and the low mean
ADC value of 770/mm2/sec. Usually, in the case of a
solid or malignant tumor, cell proliferation causes an increase in
intracellular structures and a narrowing of the stroma, which
restricts the movement of water molecules, resulting in a low ADC
value. D-TSGCT is a synovial tumor with a high proliferation of
tumor cells and restricted movement of water molecules. Conversely,
synovial hemangioma is an angioproliferative disease in the
subsynovial layer, and thus the movement of water molecules in the
lumen and stroma is not as restricted as in D-TSGCT, and its ADC
may be higher than that in D-TSGCT. The present case series raises
the possibility that DWI and ADC values may be important
differentiators between synovial hemangioma and D-TSGCT. On the
other hand, the importance of enhanced MRI in diagnosing synovial
hemangioma was not observed in this series.
Despite their similar clinical findings, synovial
hemangiomas and D-TSGCT have different postoperative outcomes.
Although two of the nine patients in our present series had
postoperative recurrence because of an uncomplete resection,
synovial hemangiomas have been reported to have a postoperative
recurrence rate of 3.4% and a symptom persistence rate of 11.4%
(29). From our experience both
careful preoperative radiological examination and intraoperative
attention may reduce the risk of recurrence. D-TSGCT has a high
recurrence rate of 25-46% (30-32),
even when open resection is performed. When D-TSGCT is strongly
suspected from the MRI findings, surgeons should anticipate
circumferential invasion and perform a more aggressive resection of
the tumor. During the surgery, the identification of the
differences in gross findings between synovial hemangioma and
D-TSGCT is also useful in the final decision regarding the
aggressiveness of resection.
A limitation of the present study is that the
imaging conditions used for the MRI examinations were not
standardized, and DWI and ADC values were measured in only one
case. When we encounter patients with intra-articular tumors
including synovial hemangioma and D-TSGCT in the future, we will
obtain MRI findings under uniform conditions for the purpose of
increasing the number of cases for an additional review.
Acknowledgements
Not applicable.
Funding
Funding: This work was partially supported by JSPS KAKENHI
(grant no. JP21K09304).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TS and MH participated in the design of study,
interpreted clinical data and drafted the manuscript. YK and HY
participated in the design of the clinical part of this study and
provided clinical data. OH analyzed and contributed to the
interpretation of the radiological findings. SY analyzed and
contributed to the interpretation of the pathological findings. SK
participated in the design of study and supervised the project. MH
and SK confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This retrospective chart review study involving
human participants was in accordance with the ethical standards of
the institutional and national research committee and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. This study was approved by the Ethical Review Committee
of Fukushima Medical University (approval no. 2022-36).
Patient consent for publication
This study is retrospective study. Patients were not
required to give informed consent to the study because the analysis
used anonymous clinical data that were obtained after each patient
agreed to treatment by written consent. We applied Opt-out method
to obtain consent on this study. The Opt-out was approved by the
Ethical Review Committee of Fukushima Medical University.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Moon NF: Synovial hemangioma of the knee
joint. A review of previously reported cases and inclusion of two
new cases. Clin Orthop Relat Res. 90:183–190. 1973.PubMed/NCBI
|
|
2
|
Dalmonte P, Granata C, Fulcheri E,
Vercellino N, Gregorio S and Magnano G: Intra-articular venous
malformations of the knee. J Pediatr Orthop. 32:394–398.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Abe T, Tomatsu T and Tazaki K: Synovial
hemangioma of the knee in young children. J Pediatr Orthop B.
11:293–297. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Devaney K, Vinh TN and Sweet DE: Synovial
hemangioma: A report of 20 cases with differential diagnostic
considerations. Hum Pathol. 24:737–745. 1993.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Muramatsu K, Iwanaga R and Sakai T:
Synovial hemangioma of the knee joint in pediatrics: Our case
series and review of literature. Eur J Orthop Surg Traumatol.
29:1291–1296. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cotton A, Flipo RM, Herbaux B, Gougeon F,
Lecomte-Houcke M and Chastanet P: Synovial haemangioma of the knee:
A frequently misdiagnosed lesion. Skeletal Radiol. 24:257–261.
1995.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Price NJ and Cundy PJ: Synovial hemangioma
of the knee. J Pediatr Orthop. 17:74–77. 1997.PubMed/NCBI
|
|
8
|
Derzsi Z, Gurzu S, Jung I, László I, Golea
M, Nagy Ö and Pop TS: Arteriovenous synovial hemangioma of the
popliteal fossa diagnosed in an adolescent with history of
unilateral congenital clubfoot: Case report and a
single-institution retrospective review. Rom J Morphol Embryol.
56:549–552. 2015.PubMed/NCBI
|
|
9
|
Lopez-Oliva CL, Wang EH and Cañal JP:
Synovial haemangioma of the knee: An under recognised condition.
Int Orthop. 39:2037–2040. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Holzapfel BM, Geitner U, Diebold J, Glaser
C, Jansson V and Dürr HR: Synovial hemangioma of the knee joint
with cystic invasion of the femur: A case report and review of the
literature. Arch Orthop Trauma Surg. 129:143–148. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Tohma Y, Mii Y and Tanaka Y: Undiagnosed
synovial hemangioma of the knee: A case report. J Med Case Rep.
13(231)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Suh JT, Cheon SJ and Choi SJ: Synovial
hemangioma of the knee. Arthroscopy. 19:E27–E30. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De Gori M, Galasso O and Gasparini G:
Synovial hemangioma and osteoarthritis of the knee: A case report.
Acta Orthop Traumatol Turc. 48:607–610. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Akgün I, Kesmezacar H, Oğüt T and
Dervişoğlu S: Intra-articular hemangioma of the knee. Arthroscopy.
19(E17)2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ramseier LE and Exner GU: Arthropathy of
the knee joint caused by synovial hemangioma. J Pediatr Orthop.
24:83–86. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Maeyama A, Saeki K, Hamasaki M, Kato Y and
Naito M: Deformation of the patellofemoral joint caused by synovial
hemangioma: A case report. J Pediatr Orthop B. 23:346–349.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pinar H, Bozkurt M, Baktiroglu L and
Karaoğlan O: Intra-articular hemangioma of the knee with meniscal
and bony attachment. Arthroscopy. 13:507–510. 1997.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Watanabe S, Takahashi T, Fujibuchi T,
Komori H, Kamada K, Nose M and Yamamoto H: Synovial hemangioma of
the knee joint in a 3-year-old girl. J Pediatr Orthop B.
19:515–520. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Llauger J, Monill JM, Palmer J and Clotet
M: Synovial hemangioma of the knee: MRI findings in two cases.
Skeletal Radiol. 24:579–581. 1995.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hospach T, Langendörfer M, Kalle TV,
Tewald F, Wirth T and Dannecker GE: Mimicry of lyme arthritis by
synovial hemangioma. Rheumatol Int. 31:1639–1643. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Larbi A, Viala P, Cyteval C, Snene F,
Greffier J, Faruch M and Beregi JP: Imaging of tumors and
tumor-like lesions of the knee. Diagn Interv Imaging. 97:767–777.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Narváez JA, Narváez J, Aguilera C, De Lama
E and Portabella F: MR imaging of synovial tumors and tumor-like
lesions. Eur Radiol. 11:2549–2560. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Barakat MJ, Hirehal K, Hopkins JR and
Gosal HS: Synovial hemangioma of the knee. J Knee Surg. 20:296–298.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
De Filippo M, Rovani C, Sudberry JJ, Rossi
F, Pogliacomi F and Zompatori M: Magnetic resonance imaging
comparison of intra-articular cavernous synovial hemangioma and
cystic synovial hyperplasia of the knee. Acta Radiol. 47:581–584.
2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Greenspan A, Azouz EM, Matthews J II and
Décarie JC: Synovial hemangioma: Imaging features in eight
histologically proven cases, review of the literature, and
differential diagnosis. Skeletal Radiol. 24:583–590.
1995.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sanghi AK, Ly JQ, McDermott J and Sorge
DG: Synovial hemangioma of the knee: A case report. Radiol Case
Rep. 2:33–36. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mandelbaum BR, Grant TT, Hartzman S,
Reicher MA, Flannigan B, Bassett LW, Mirra J and Finerman GA: The
use of MRI to assist in diagnosis of pigmented villonodular
synovitis of the knee joint. Clin Orthop Relat Res. 231:135–139.
1988.PubMed/NCBI
|
|
28
|
Greenspan A, McGahan JP, Vogelsang P and
Szabo RM: Imaging strategies in the evaluation of soft-tissue
hemangiomas of the extremities: Correlation of the findings of
plain radiography, angiography, CT, MRI, and ultrasonography in 12
histologically proven cases. Skeletal Radiol. 21:11–18.
1992.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Staals EL, Ferrari S, Donati DM and
Palmerini E: Diffuse-type tenosynovial giant cell tumour: Current
treatment concepts and future perspectives. Eur J Cancer. 63:34–40.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Schwartz HS, Unni KK and Pritchard DJ:
Pigmented villonodular synovitis. A retrospective review of
affected large joints. Clin Orthop Relat Res. 247:243–255.
1989.PubMed/NCBI
|
|
31
|
Johansson JE, Ajjoub S, Coughlin LP, Wener
JA and Cruess RL: Pigmented villonodular synovitis of joints. Clin
Orthop Relat Res. 163:159–166. 1982.PubMed/NCBI
|
|
32
|
Byers PD, Cotton RE, Deacon OW, Lowy M,
Newman PH, Sissons HA and Thomson AD: The diagnosis and treatment
of pigmented villonodular synovitis. J Bone Joint Surg Br.
50:290–305. 1968.PubMed/NCBI
|