Introduction
With changes in lifestyle, the incidence (23.57% in
2018) and mortality rate (298.42/100000 in 2018) of cardiovascular
diseases are still rising (1).
Among them, coronary heart disease (CHD) has become a disease with
a high mortality rate (128.24/100000 in 2018) worldwide (2). Early revascularization is the
preferred treatment strategy for CHD; however, the resulting
myocardial ischaemia-reperfusion (I/R) injury (MIRI) is a main
factor leading to ventricular dysfunction and affects long-term
prognosis of patients (3).
MIRI has a complicated pathological mechanism.
Studies have shown that oxidative stress and the inflammatory
response play important roles (4,5). The
increase in reactive oxygen species (ROS) during I/R cannot only
affect the functions of mitochondria and promote the occurrence of
apoptosis but also mediate inflammatory cascades and aggravate
myocardial damage (6). Previous
studies have found that a single treatment for oxidative stress or
inflammation can alleviate MIRI to varying degrees (7,8).
However, since MIRI is a pathophysiological process mediated by
multiple signalling pathways and multiple transcription factors, a
single intervention with a regulatory factor cannot fully exert its
protective effect on I/R in the myocardium (9). Oxidative stress and the inflammatory
response are critical in regulating MIRI through multiple genes.
Effectively relieving myocardial inflammation and oxidative stress
simultaneously could provide a new prevention and treatment
strategy for fully and effectively reducing MIRI.
The chemical components of ginseng are very complex.
Ginsenoside is the main component in ginseng and has attracted the
attention of researchers (10). As
a monomer isolated from red ginseng, Ginsenoside Rh2 (GRh2) has
high biological safety and antitumour, antiasthma and antiallergy
functions (11). Due to its wide
range of pharmacological effects, GRh2 has gained interest due to
its protective effects in diseases (12,13).
Hsieh et al (12) found
that GRh2 could alleviate the oxidative stress caused by
lipopolysaccharide-induced acute lung injury by activating the
nuclear factor E2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1)
signalling pathway. Ma et al (13) also revealed that GRh2 could slow
the development of inflammatory diseases by inhibiting the
activation of NLRP3 inflammasomes. Therefore, based on the
pharmacological properties of GRh2, it was hypothesized that GRh2
can protect the myocardium against I/R through antioxidation and
anti-inflammatory effects. However, the role and mechanism of GRh2
in MIRI have not yet been elucidated. In the present study, the
effect of GRh2 on I/R in the myocardium was observed and its
molecular mechanism was further explored to provide more effective
prevention and treatment strategies for MIRI.
Materials and methods
Reagents
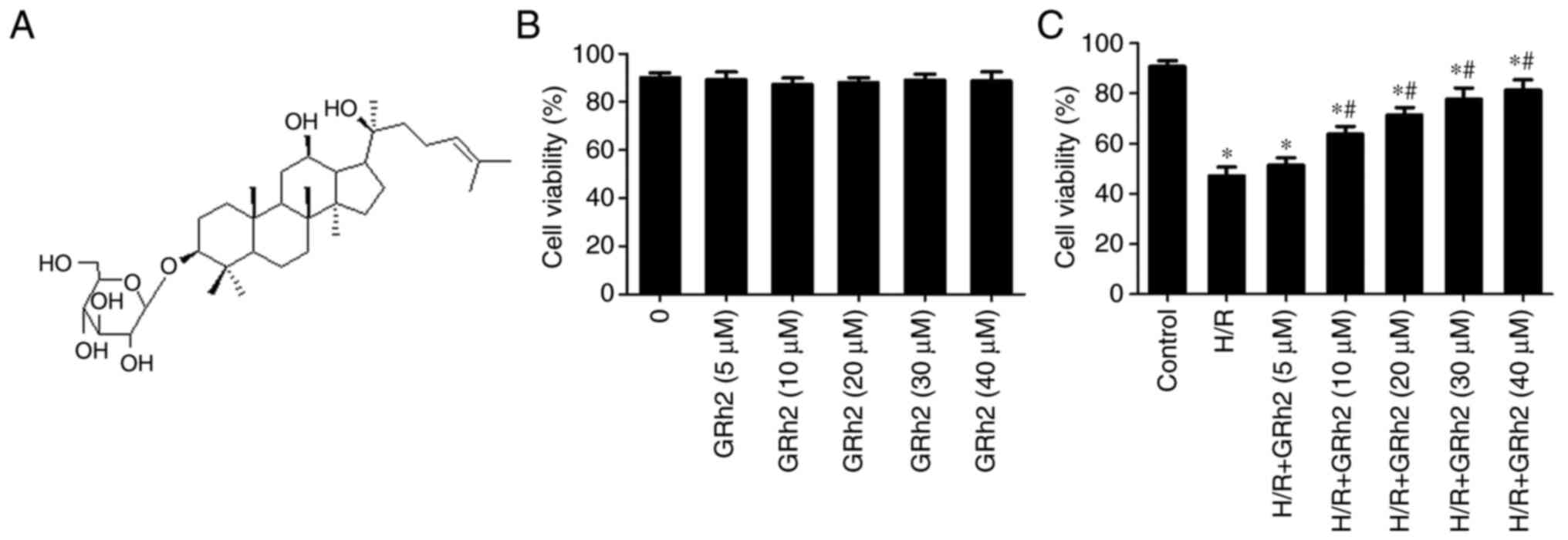
GRh2 (Fig. 1A) was
purchased from Chengdu Must Bio-Technology Co., Ltd. (purity
>98%; cat. no. A0241). Lactate dehydrogenase (LDH; cat. no.
A020-1-2), creatine kinase (CK; cat. no. A032-1-1) and creatine
kinase-myocardial band (CK-MB; cat. no. E006-1-1) biochemical kits
were purchased from the Nanjing Jiancheng Bioengineering Institute.
2,3,5-Triphenyltetrazolium chloride reagents (TTC; cat. no. T8877)
were purchased from Sigma-Aldrich (Merck KGaA). Cell Counting Kit-8
(CCK-8; product code CK04) was purchased from Dojindo Laboratories,
Inc. Malondialdehyde (MDA; cat. no. S0131S), superoxide dismutase
(SOD; cat. no. S0101S) and glutathione peroxidase (GSH-Px; cat. no.
S0056) kits were purchased from Beyotime Institute of
Biotechnology. Dimethyl sulfoxide (DMSO) was purchased from MP
Biomedicals, LLC (MPBIO; cat. no. MP0219605591). An intracellular
ROS detection kit with DCFH-DA (cat. no. MAK144) was purchased from
Sigma-Aldrich; Merck KGaA. The fluorescent probe dihydroethidium
(DHE) was purchased from Invitrogen; Thermo Fisher Scientific, Inc.
The protein primary antibodies against Nrf2 (1:800; cat. no.
ab92946), HO-1 (1:600 dilution, product code ab13243), NOD-like
receptor family pyrin domain-containing 3 (NLRP3; 1:1,000 dilution,
product code ab214185), apoptosis-associated speck-like protein
(ASC; 1:1,000; cat. no. ab180799), caspase-1 (1:1,000 dilution,
product code ab179515), interleukin (IL)-1β (1:500; cat. no.
ab9722), IL-18 (1:500 dilution, product code ab191860) and tumor
necrosis factor (TNF)-α (1:200 dilution, product code ab205587)
were purchased from Abcam. The protein secondary antibody goat
anti-rabbit IgG (1:2,000 dilution, product code ab205718) was also
purchased from Abcam.
Neonatal rat cardiomyocyte (NRCM)
culture and establishment of a hypoxia/reoxygenation (H/R)
model
NRCMs were isolated from ~2-day-old Sprague-Dawley
(SD) rats (six rats) provided by the Experimental Animal Center of
China Three Gorges University (Yichang, China). The SD rats were
anaesthetized, and their hearts were quickly removed. The obtained
heart tissues were rinsed with ice-cold phosphate-buffered saline
(PBS) solution to remove any residual blood. Then, 0.08%
collagenase type II and 0.125% trypsin were used to digest the
tissues at 37˚C for 7 min. Finally, the NRCMs were centrifuged
(1,000 x g at 4˚C for 10 min) and resuspended in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc.) with 10% foetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin at 37˚C with 5%
CO2 and 95% O2. Cells no older than passage 5
were used in the H/R experiments. Briefly, cultured NRCMs were
preserved in serum-free DMEM at 37˚C for 12 h. Then, the NRCMs were
incubated in an anaerobic chamber (95% N2-5%
CO2) at 37˚C for 4 h. The NRCMs were moved into a normal
incubator (37˚C) for an additional 4 h to induce reoxygenation. The
primary cardiomyocytes were randomly separated into the following
groups: Control, H/R, and H/R+GRh2 (with different concentrations
of GRh2 pre-treatment 24 h before H/R). Each experiment was
repeated ≥5 times.
Cell viability assay
A Cell Counting Kit-8 (CCK-8) kit was used to detect
the viability of NRCMs according to standard procedures. The cell
density was 5x103 cells/well. The volume of CCK-8 was 10
µl for each well (incubation for 2 h at 37˚C). The CCK-8 optical
density (OD) at 450 nm was recorded.
Animal experimental design
A total of 40 male SD rats (aged ~8 weeks) were
provided by the Experimental Animal Center of China Three Gorges
University (Yichang, China). The rats were housed at 23±2˚C with
50% relative humidity, 12-h light/dark cycles and free access to
water. The rats were divided into the following four groups: The
sham group, the I/R group, the I/R+GRh2 (10 mg/kg) group and the
I/R+GRh2 (20 mg/kg) group. Ten days before the construction of the
I/R models, continuous intragastric administration was initiated in
the GRh2 group. In the sham and I/R groups, an equal volume of DMSO
was administered by intragastric administration. For the
establishment of the I/R model, the rats were administered
abdominal anaesthesia (30 mg/kg pentobarbital sodium) and fixed.
Ventilation was provided through the assistance of a ventilator.
The chest of each rat was opened between the third and fourth ribs
of the left sternum to fully expose the heart. A 4-0 silk suture
covered with a latex tube at a diameter of 0.5 cm was used to pass
through the initial segment of the left anterior descending artery
(LAD). After tightening the silk suture to block the LAD for 0.5 h,
the silk suture was cut, and then, reperfusion was performed for 24
h. Changes in the electrocardiogram (ECG) of each rat were observed
using a small animal ECG machine; the changes in the ST-T elevation
(tightening the silk suture) and ST-T decrease (cutting the silk
suture) indicated that the models were constructed successfully.
All experimental procedures were approved (approval no. 2020090B)
by the Ethics Committee of Experimental Animals of China Three
Gorges University (Yichang, China).
Ultrasonography
After reperfusion, the rats were fixed on insulation
pads. Then, the anterior chests of the rats were depilated, and the
rats were subjected to cardiac ultrasonography. The left ventricle
ejection fraction (LVEF), left ventricle fractional shortening
(LVFS), left ventricular end-diastolic diameter (LVEDD) and left
ventricular end-systolic diameter (LVESD) were measured and
recorded.
Collection of samples
Following ultrasonography, the peripheral blood and
hearts of the rats were collected. Briefly, 2 ml of blood were
obtained from the jugular vein and then centrifuged at 500 x g for
8 min at 4˚C. The serum was collected and stored at -80˚C. The rats
were euthanized by injecting 10% potassium chloride (75 mg/kg) into
the right jugular vein, and their heart tissues were removed and
quickly rinsed with normal saline. The left ventricular tissues
were cut, and certain myocardial tissue was frozen or fixed with 4%
paraformaldehyde for 48 h at room temperature for sectioning. Then,
immunofluorescence (IF) staining and haematoxylin/eosin (H&E)
staining were performed as described below in the histological
analysis. Another portion of the tissue was stored in a freezer at
-80˚C for the protein and genetic testing.
Measurement of the infarct area
Following I/R, the abdominal cavity of each rat was
reopened, and 1 ml of TTC solution was injected from the inferior
vena cava; 10 min later, the heart was removed. After washing the
surface of the heart, the heart was frozen at -80˚C for 20 min.
Slices were cut along the long axis of the heart at a width of 2
mm. After cleaning, the slices were fixed in 4% paraformaldehyde
for 24 h at room temperature. The white area indicates myocardial
infarction, and the red area indicates normal myocardial tissue.
The white and red areas of the myocardial tissue after the TTC
staining were measured by Image-Pro Plus 5.0 software (Media
Cybernetics, Inc.). The infarct area was calculated as follows:
[White area/(white area + red area) x100%].
Histological analysis
The heart tissues were fixed using 4%
paraformaldehyde for 48 h at room temperature. The tissues were
embedded in paraffin, cut into 4-µm sections, and subjected to
H&E (room temperature, hematoxylin staining, 5 min; eosin
staining, 2 min) and IF staining. For the IF staining, the sections
were blocked with 1% goat serum (cat. no. 31873; Thermo Fisher
Scientific, Inc.) for 30 min at room temperature. Sections were
then incubated with a TNF-α antibody (1:200 dilution) at 4˚C for 12
h and subsequently incubated with anti-Rabbit HRP secondary
antibody (1:200 dilution, product code ab150079, abcam) at 37˚C for
1 h. After washing with PBS, the sections were counterstained with
DAPI (100 ng/ml) at room temperature for 5 min. The sections were
examined using a fluorescence microscope and a digital camera (Axio
Observer Al; Carl Zeiss AG).
Biochemical index analysis
The activities of the antioxidant enzymes SOD and
GSH-Px and the levels of MDA in the heart tissues or NRCM
homogenates were determined following the manufacturer's protocol.
The activities of LDH, CK and CK-MB in both serum and culture
supernatant were assessed using kits according to the
instructions.
Detection of ROS
For the detection of ROS in cells, after completing
the H/R treatment of the NRCMs, trypsin was added to digest the
cells until 95% of the cells were shed as assessed under a light
microscope. The digested cells were transferred to a 15-ml
Eppendorf tube and centrifuged at 500 x g for 5 min at 4˚C. The
supernatant was discarded; under dark conditions, DCFH-DA (cat. no.
MAK144) was added to each group and the samples were incubated at
37˚C in the dark for 30 min. After the incubation, the samples were
centrifuged at 500 x g for 5 min at 4˚C to discard the supernatant,
and then, the cells in each group were gently washed with PBS
solution once. After centrifugation at 500 x g for 5 min at 4˚C,
the supernatant was discarded, and PBS was added again to resuspend
the cells for detection. For the detection of ROS in the tissues,
the myocardial tissues were washed with PBS solution, and frozen
sectioning was performed immediately. The myocardial slices were
incubated with the fluorescent probe DHE (10 µM) for 30 min at 37˚C
under dark and humid conditions. After washing twice with PBS
solution, the samples were observed under a fluorescence
microscope.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed to detect the mRNA levels.
Briefly, TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract the total RNA from the heart
tissues. The obtained RNA (~4.0 µg) was then reverse transcribed
into cDNA using SuperScript IV Reverse Transcriptase (Thermo Fisher
Scientific, Inc.) at 37˚C for 60 min. Then, qPCR was performed
using a SYBR Green Master Mix kit (Thermo Fisher Scientific, Inc.)
on a 7500 ABI Prism system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The qPCR thermocycling conditions were as
follows: 45˚C for 2 min and 95˚C for 10 min, immediately followed
by 45 cycles at 95˚C for 30 sec and 60˚C for 30 sec. The mRNA
expression levels were normalized to that of GAPDH. The
2-ΔΔCq method was used to calculate the changes in mRNA
expression (14). The following
primers were used: IL-1β forward, 5'-CCTGTGTGATGAAAGACGGC-3' and
reverse, 5'-TATGTCCCGACCATTGCTGT-3'; IL-18 forward,
5'-CTACCAGCAAACATCTCACTTCAG-3' and reverse,
5'-CAACTGAGAGGCTGTGCCCT-3'; TNF-α forward,
5'-CCGATTTGCCATTTCATACCAG-3' and reverse,
5'-TCACAGAGCAATGACTCCAAAG-3'; and GAPDH forward,
5'-GAACGGGAAGCTCACTGG-3' and reverse,
5'-GCCTGCTTCACCACCTTCT-3'.
Western blot analysis (WB)
The protein levels of Nrf-2, HO-1, NLRP3, ASC,
caspase-1, IL-1β, IL-18 and TNF-α were detected by WB. First, the
obtained cells or tissues were homogenized. Then, the proteins were
extracted using a protein extraction kit (cat. no. P0028; Beyotime
Institute of Biotechnology), and the concentrations were determined
by the BCA method. To prepare the gels for WB, glass plates were
aligned and clamped tightly. After a 12% separation gel solution
was prepared, the gel was slowly poured and sealed with water.
After the separation gel was solidified, a 4% spacer gel solution
was prepared and poured into glass plates, and a comb was placed.
After the spacer gel was solidified, the comb was removed. For each
group, 50 µg of total protein were used for electrophoresis. After
electrophoresis, the gel blocks of the protein bands were cut and
transferred to a polyvinylidene difluoride (PVDF) membrane. After
the transfer, the PVDF membrane was blocked with 5% skim milk
powder at room temperature for 1 h and incubated with a protein
primary antibody overnight at 4˚C. On the following day, the PVDF
membrane was incubated with a secondary antibody for 1 h at 37˚C
and developed according to the relevant instructions of the
developing and fixing kit. An enhanced chemiluminescence detection
kit (Thermo Fisher Scientific, Inc.) was used for visualization and
GAPDH served as a loading control. Odyssey® Infrared
Imaging system (model 9120; LI-cOR Biosciences) was used to capture
images of the membranes and Quantity One 1-D software (version
4.6.9; Bio-Rad Laboratories, Inc.) was used to quantify the protein
bands.
Statistical analysis
SPSS 22.0 software (IBM Corp.) was used for the data
analysis. The data are presented as the mean ± SD (n=5). Student's
unpaired t-test and one-way ANOVA were used for the comparisons
between the groups. If interactions were significant, a Tukey's
post hoc test was used for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
GRh2 promotes NRCM survival
The effect of different concentrations of GRh2 on
the survival rate of NRCMs was evaluated by CCK-8 assay. The
results showed that when the concentration of GRh2 was <40 µM,
GRh2 had no obvious cytotoxicity (Fig.
1B). After the establishment of the H/R model, it was
demonstrated that GRh2 at a concentration ≥10 µM could improve the
survival rate following H/R. When the concentration was ≥30 µM, the
cell protective effect was obvious and tended to be stable
(Fig. 1C). Therefore, 10 and 30 µM
GRh2 were selected as the low and high concentrations,
respectively, in subsequent in vitro experiments.
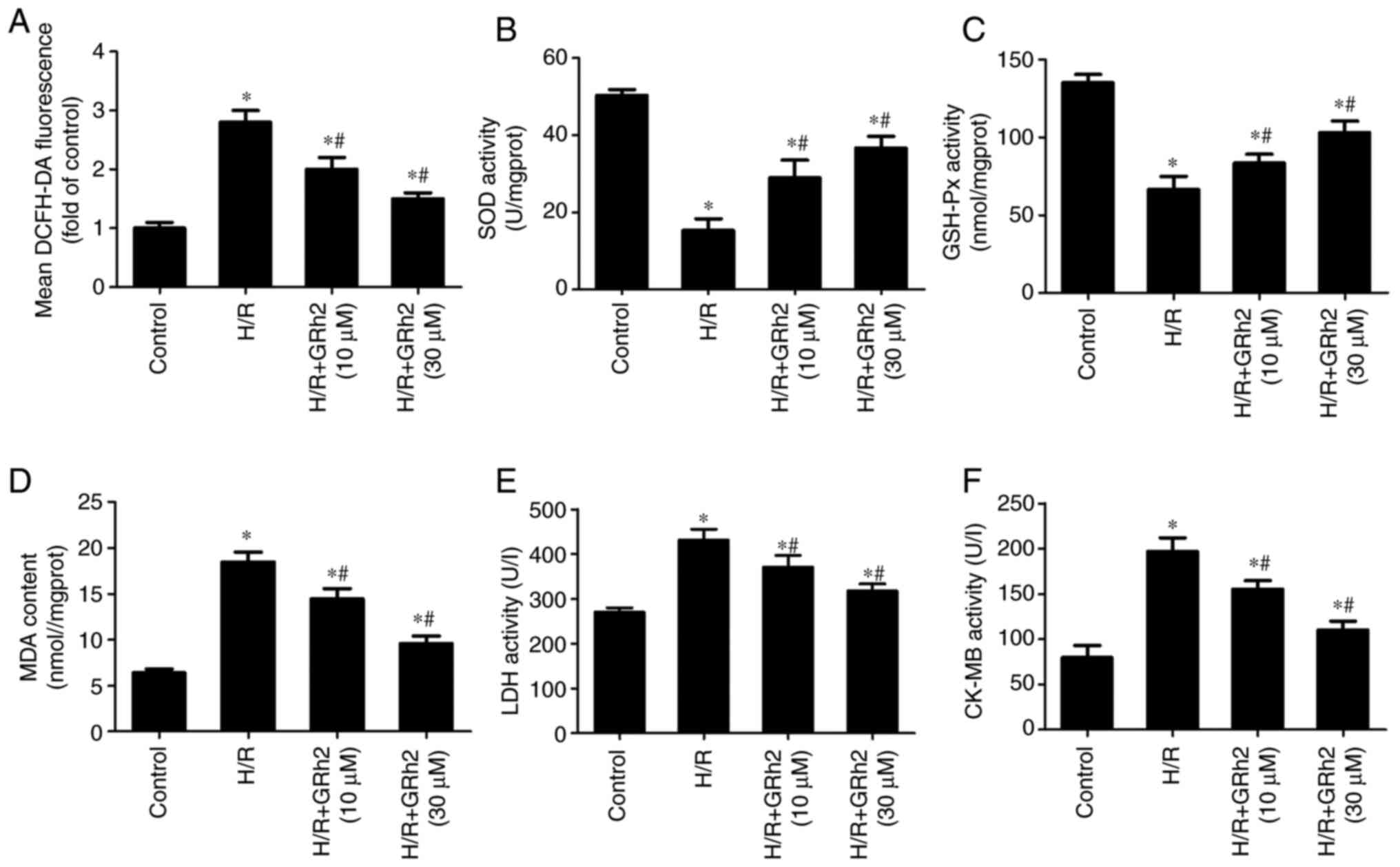
GRh2 inhibits H/R-induced oxidative
stress in vitro
After the pre-treatment with GRh2, H/R models were
established, and indicators related to oxidative stress and
myocardial damage were evaluated. Compared with the control group,
the levels of ROS (Fig. 2A), MDA
(Fig. 2D), LDH (Fig. 2E) and CK-MB (Fig. 2F) were increased in the H/R group.
GRh2 reduced the levels of ROS, MDA, LDH and CK-MB, and the
reduction in the H/R+GRh2 (30 µM) group was more significant than
that in the H/R+GRh2 (10 µM) group. Compared with the control
group, the activities of SOD (Fig.
2B) and GSH-Px (Fig. 2C) were
decreased in the H/R group. GRh2 increased the activities of SOD
and GSH-Px, and the increase in the H/R+GRh2 (30 µM) group was more
significant than that in the H/R+GRh2 (10 µM) group.
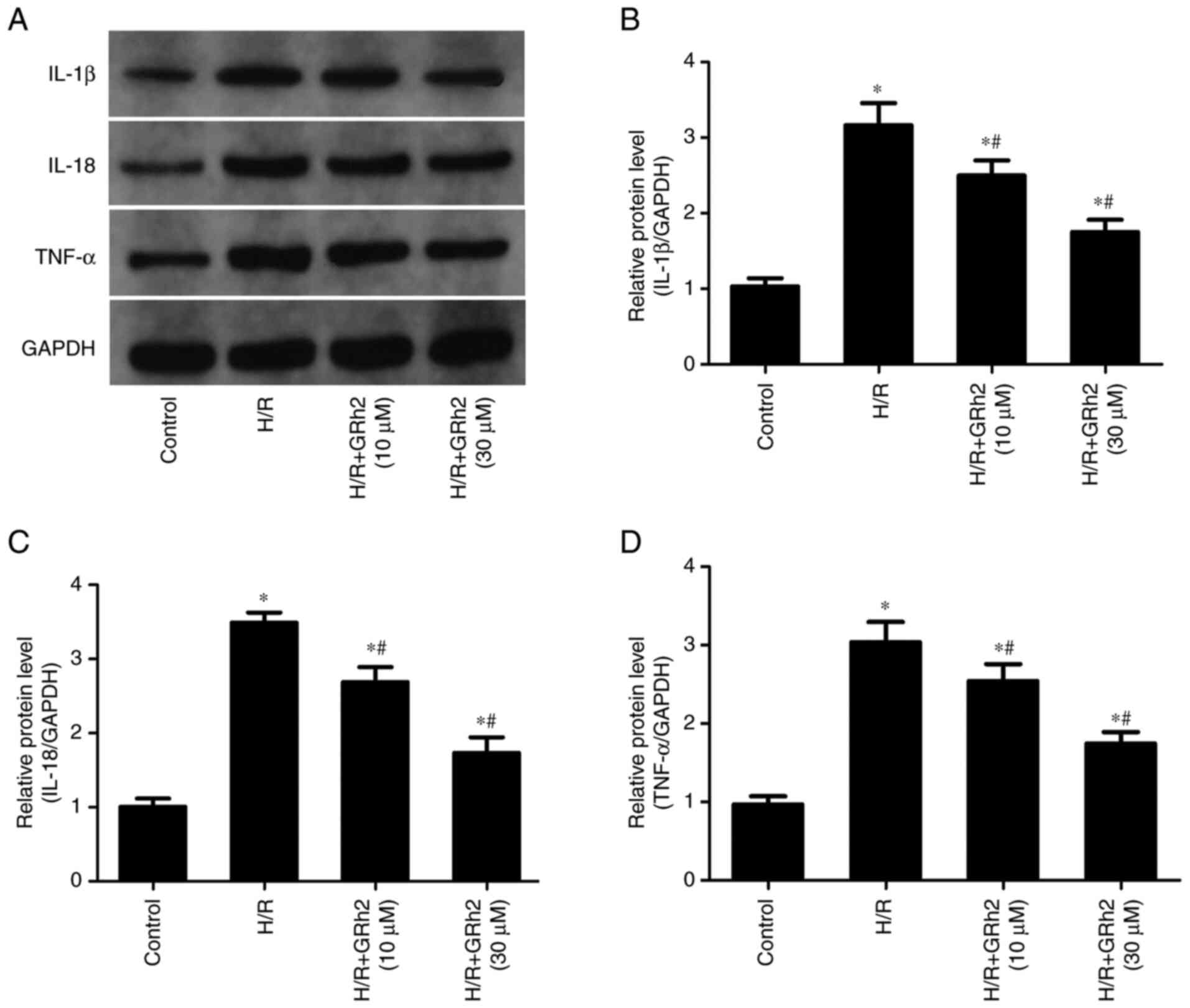
GRh2 inhibits H/R-induced inflammation
in vitro
The results revealed that compared with the control
group, the protein expression levels of IL-1β, IL-18 and TNF-α were
significantly increased in the H/R group (Fig. 3). The pre-treatment with GRh2
reduced the expression levels of IL-1β, IL-18 and TNF-α, and the
decrease in the H/R+GRh2 (30 µM) group was more significant than
that observed in the H/R+GRh2 (10 µM) group.
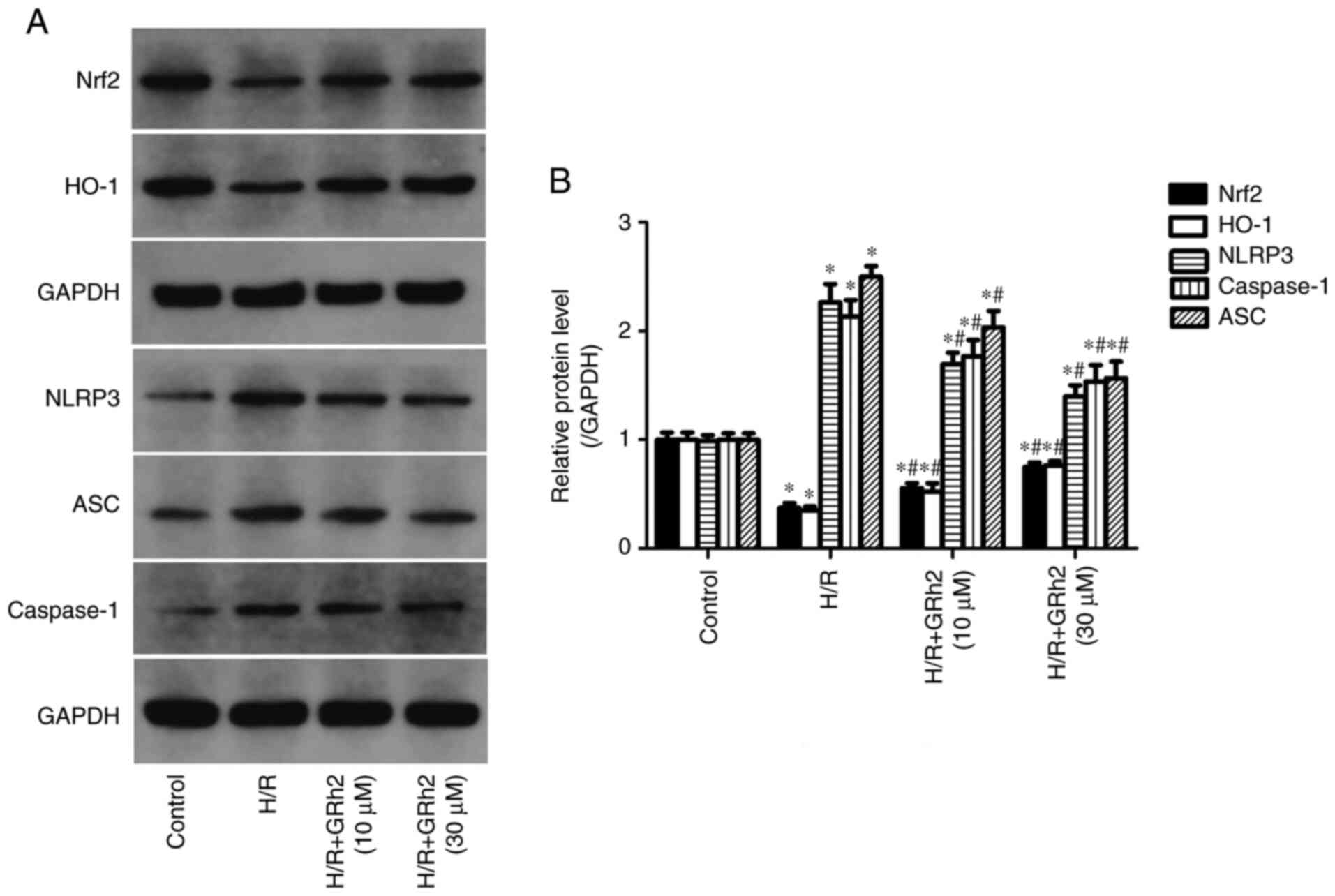
GRh2 participates in the regulation of
the Nrf2/HO-1/NLRP3 signalling pathway in vitro under H/R
stimulation
Compared with the control group, the protein
expression levels of NLRP3, ASC and caspase-1 in the H/R group were
significantly increased. GRh2 reduced the expression levels of
NLRP3, ASC and caspase-1 and the reduction in the H/R+GRh2 (30 µM)
group was more significant than that observed in the H/R+GRh2 (10
µM) group. Compared with the control group, the expression levels
of Nrf2 and HO-1 were decreased in the H/R group. The pre-treatment
with GRh2 increased the expression levels of Nrf2 and HO-1, and the
increase in the H/R+GRh2 (30 µM) group was more significant than
that observed in the H/R+GRh2 (10 µM) group (Fig. 4).
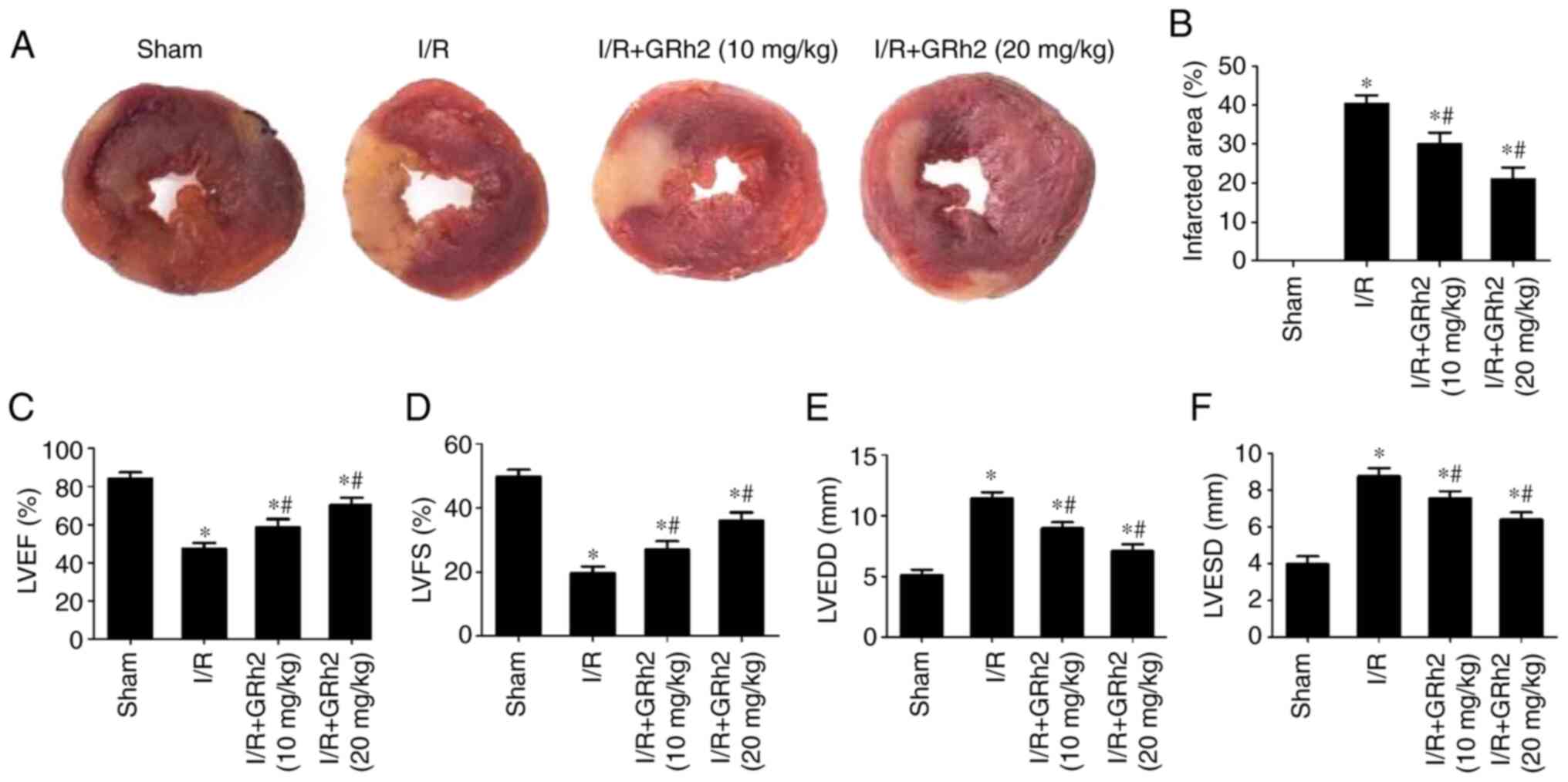
GRh2 reduces the area of myocardial
infarction and improves heart function in vivo after I/R
Compared with the sham group, the area of myocardial
infarction increased after I/R. GRh2 reduced the infarction area,
and the reduction in the I/R+GRh2 (20 mg/kg) group was more
significant than that observed in the I/R+GRh2 (10 mg/kg) group
(Fig. 5A and B). In addition, following I/R, the
cardiac ultrasonography data showed that the LVEF (Fig. 5C) and LVFS (Fig. 5D) of the rats in the I/R group were
significantly lower than those in the sham group, while the LVEDD
(Fig. 5E) and LVESD (Fig. 5F) in the I/R group were
significantly higher than those in the sham group. GRh2 improved
the heart function of the rats, and the improvement in the I/R+GRh2
(20 mg/kg) group was more significant than that observed in the
I/R+GRh2 (10 mg/kg) group.
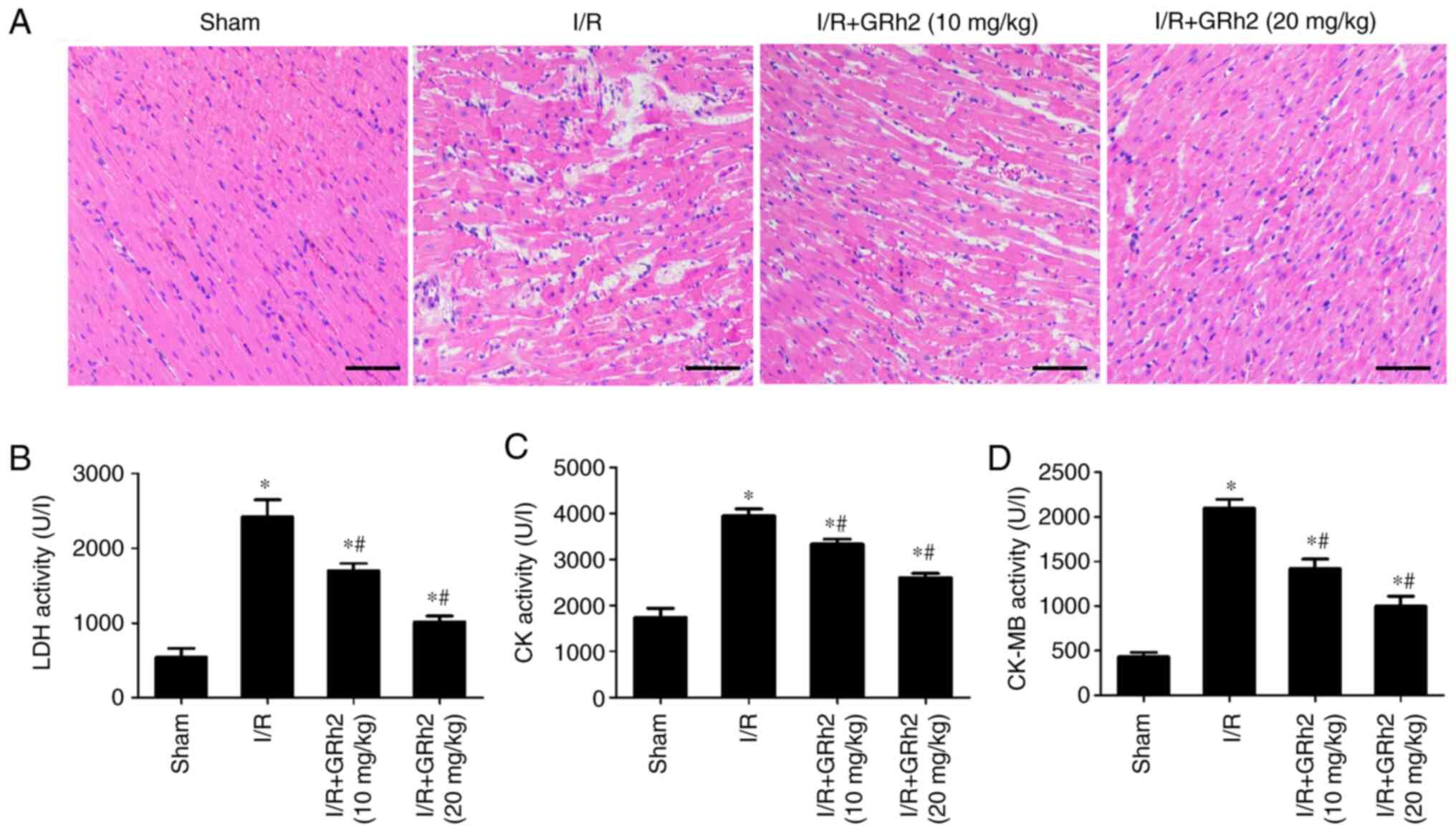
GRh2 reduces MIRI in vivo
Compared with the sham group, disorder and oedema of
the myocardial tissues and inflammatory cell infiltration increased
after I/R. GRh2 improved the degeneration and necrosis of
myocardial cells, and the improvement in the I/R+GRh2 (20 mg/kg)
group was more significant than that in the I/R+GRh2 (10 mg/kg)
group (Fig. 6A). Compared with the
sham group, the levels of LDH, CK and CK-MB in the I/R group were
increased. GRh2 decreased the levels of LDH, CK and CK-MB, and the
decrease in the I/R+GRh2 (20 mg/kg) group was more significant than
that observed in the I/R+GRh2 (10 mg/kg) group (Fig. 6B-D).
GRh2 inhibits I/R-induced oxidative
stress in vivo
Indicators related to oxidative stress were
evaluated in I/R myocardial tissues of rats. The results revealed
that GRh2 significantly reduced the proportion of ROS-positive
cells (Fig. 7A). Compared with the
sham group, the activities of SOD and GSH-Px were decreased, and
the activity of MDA was increased in the I/R group. GRh2 increased
the activities of SOD and GSH-Px and reduced the activity of MDA,
and the effects in the I/R+GRh2 (20 mg/kg) group were more
significant than those in the I/R+GRh2 (10 mg/kg) group (Fig. 7B-D).
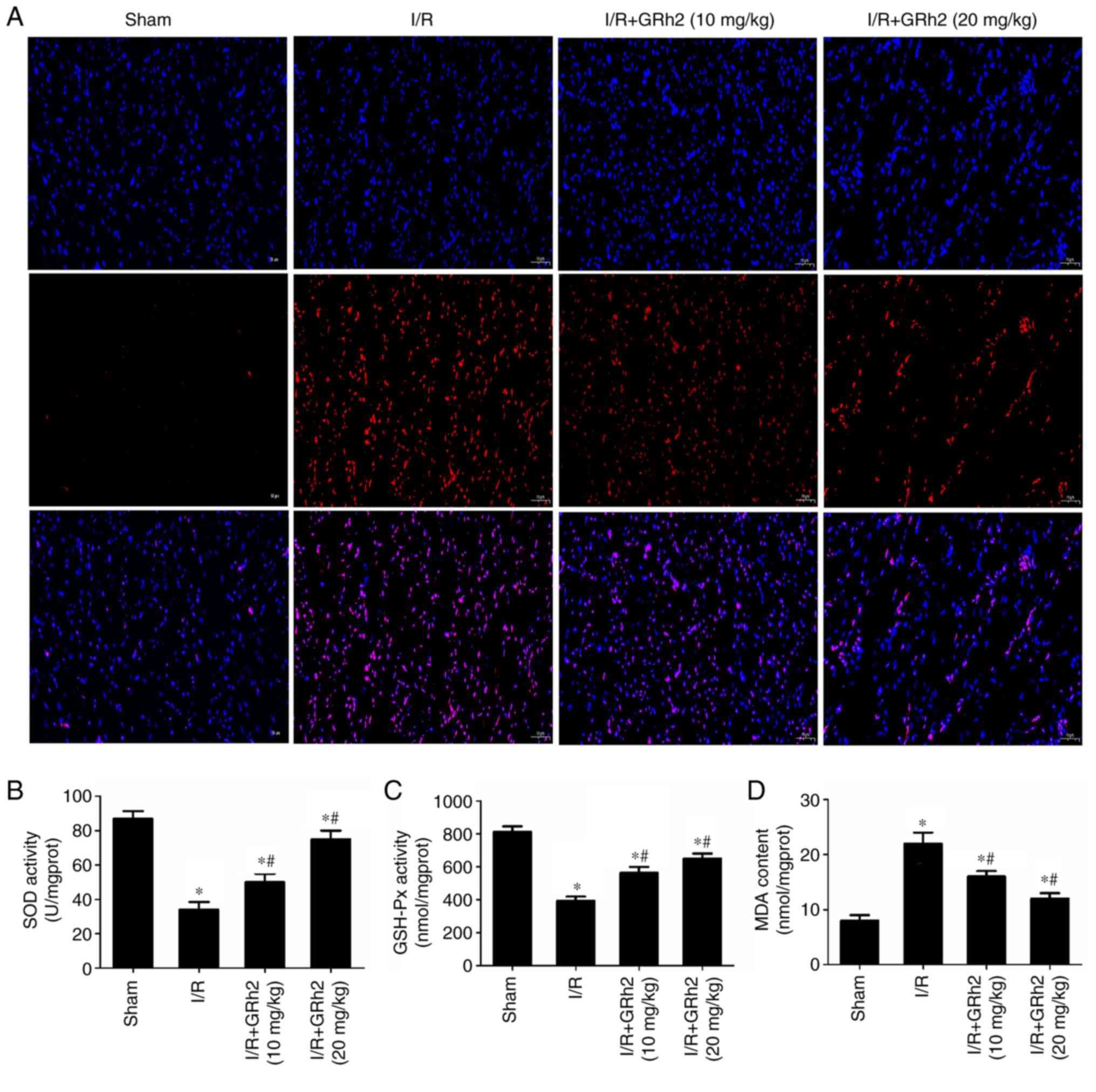 | Figure 7GRh2 inhibits I/R-induced oxidative
stress in vivo. (A) ROS in I/R myocardial tissues of rats
was detected by the fluorescent probe DHE (red, DHE; blue, DAPI;
magnification, x100). (B) SOD activity. (C) GSH-Px activity. (D)
MDA content. The data are expressed as the mean ± SD (n=5).
*P<0.05 vs. the sham group; and #P<0.05
vs. the I/R group. GRh2, ginsenoside Rh2; I/R,
ischaemia/reperfusion; ROS, reactive oxygen species; DHE,
dihydroethidium; SOD, superoxide dismutase; GSH-Px, glutathione
peroxidase; MDA, malondialdehyde. |
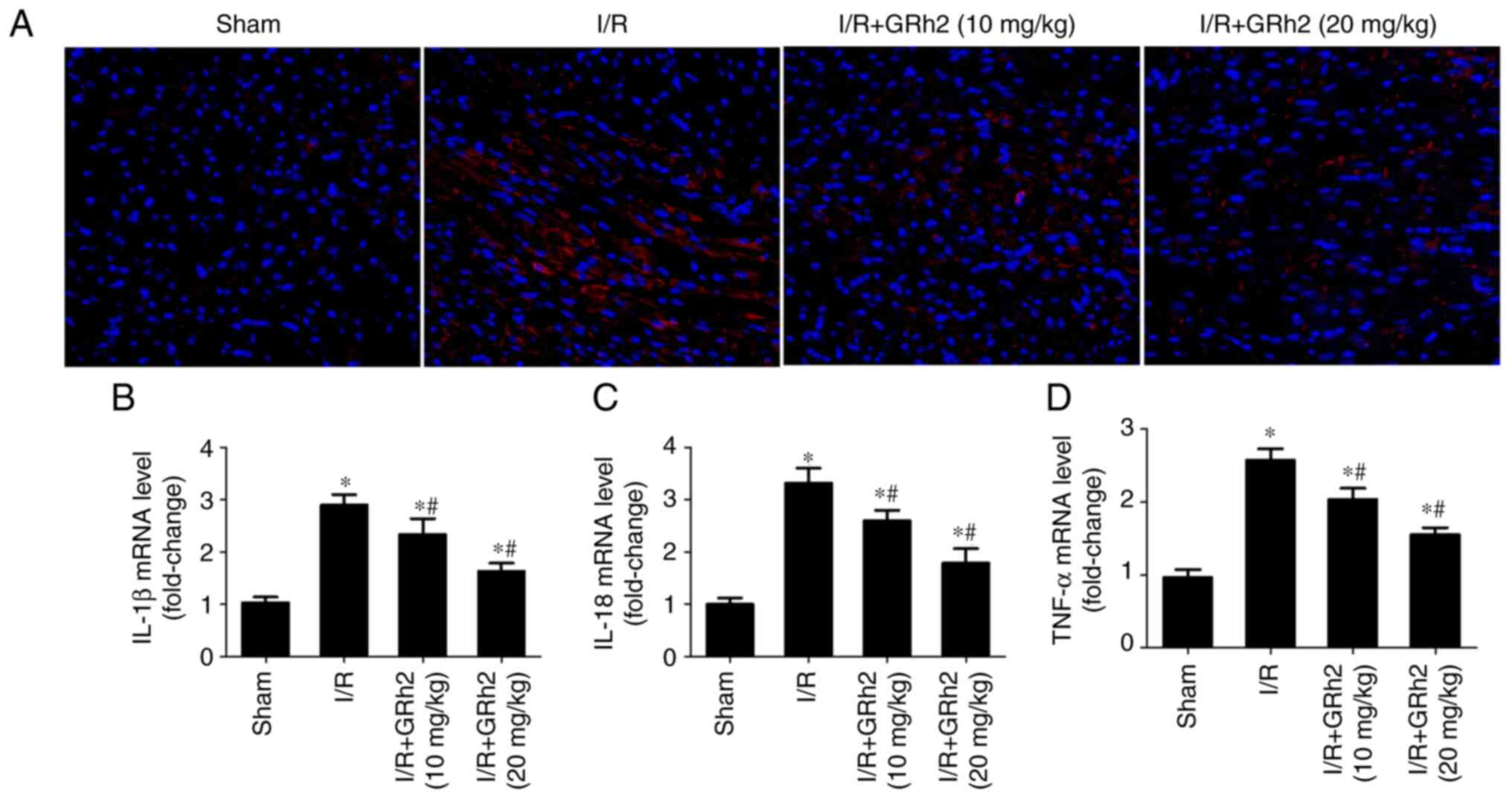
GRh2 inhibits I/R-induced inflammation
in vivo
The expression of TNF-α in I/R myocardial tissues of
rats was detected by IF. The results revealed that GRh2
significantly reduced the expression level of TNF-α (Fig. 8A). Compared with the sham group,
the expression levels of IL-1β, IL-18 and TNF-α in the I/R group
were significantly increased. GRh2 reduced the expression levels of
IL-1β, IL-18 and TNF-α, and the reduction in the I/R+GRh2 (20
mg/kg) group was more significant than that observed in the
I/R+GRh2 (10 mg/kg) group (Fig.
8B-D).
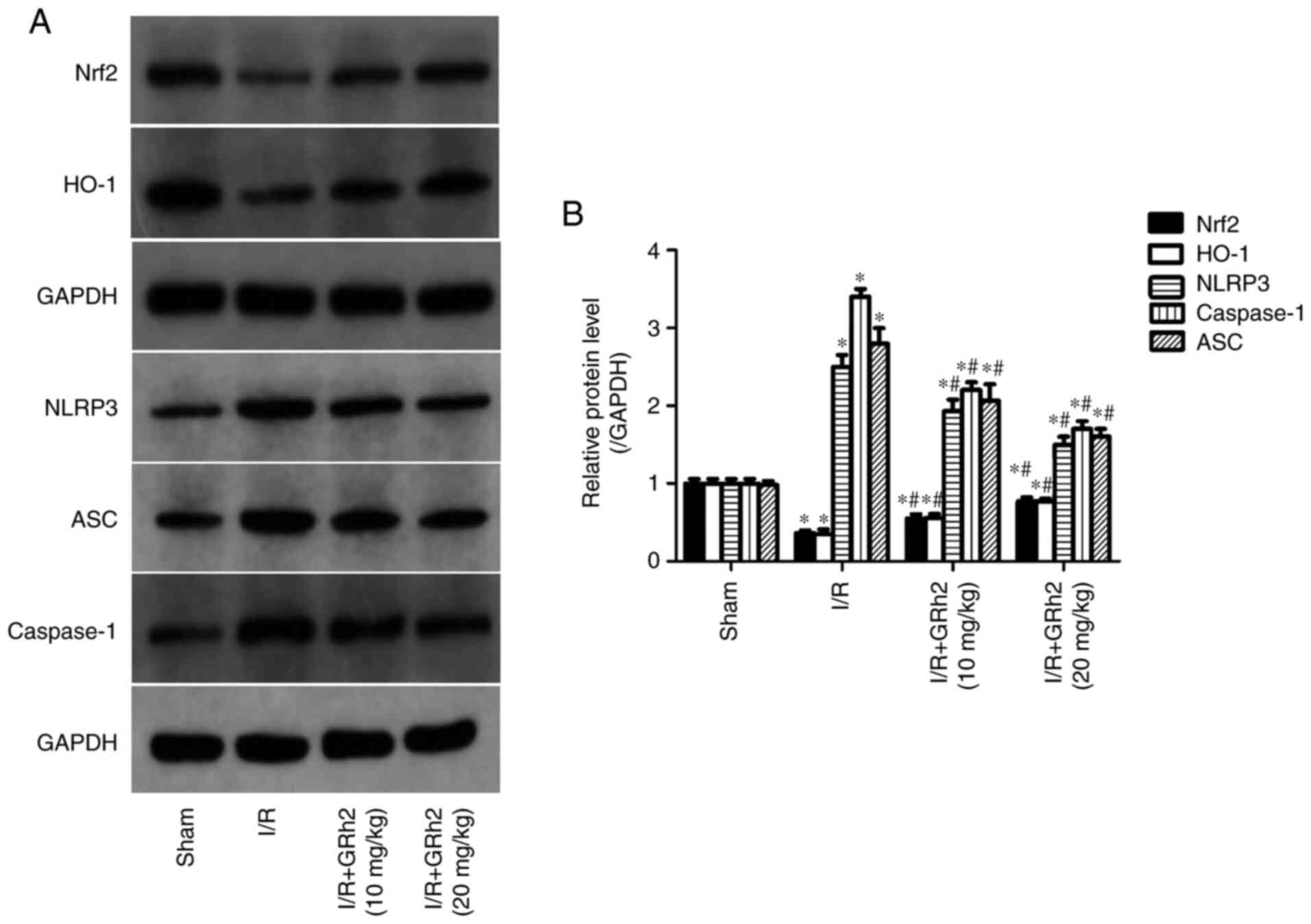
GRh2 participates in the regulation of
the Nrf2/HO-1/NLRP3 signalling pathway in vivo
Compared with the sham group, the protein expression
levels of NLRP3, ASC and caspase-1 in the I/R group were
significantly increased. GRh2 reduced the expression levels of
NLRP3, ASC and caspase-1, and the reduction in the H/R+GRh2 (20
mg/kg) group was more significant than that in the H/R+GRh2 (10
mg/kg) group. Compared with the sham group, the expression levels
of Nrf2 and HO-1 in the I/R group were decreased. The pre-treatment
with GRh2 increased the expression levels of Nrf2 and HO-1, and the
increase in the H/R+GRh2 (20 mg/kg) group was more significant than
that observed in the H/R+GRh2 (10 mg/kg) group (Fig. 9).
Discussion
Percutaneous coronary intervention, coronary artery
bypass grafting and thrombolysis are the most effective treatment
options for CHD (15). However,
reperfusion after ischaemia can aggravate myocardial damage and
cause further damage to the myocardial structure, function and
electrophysiological state, which may seriously impact the effects
of these treatments (16). Several
pharmacological studies have shown that ginsenosides have
pharmacological activities improving ischaemic injury and
anti-inflammatory and antioxidant properties, with great potential
for further development (17-19).
In the present study, it was found that GRh2 enhanced the
antioxidation and anti-inflammatory ability of myocardial cells by
regulating the Nrf2/HO-1/NLRP3 signalling pathway, thereby reducing
the infarction area and improving heart function.
Oxidative stress is an important mechanism that
causes MIRI (7). Under normal
circumstances, the generation and elimination of ROS in the body
are in a dynamic balance (7).
During I/R, ROS are generated, and the antioxidant system is
impaired. ROS and antioxidants, such as SOD and GSH-Px, are
out-of-balance (20). ROS cannot
be eliminated efficiently, which causes cell damage. Nrf2 is an
important antioxidant factor that can reduce oxidative stress
through the regulation of phase II detoxifying enzymes (21). The antioxidant response element is
a molecule downstream of Nrf2. Keap1 is an important regulatory
factor in the oxidation reaction (22). Under physiological conditions, Nrf2
binds Keap-1 and stably exists in the cytoplasm. When oxidative
stress occurs, the conformation of Keap1 changes, which leads to
the dissociation of Nrf2 from Keap1, and then, Nrf2 enters the
nucleus to initiate the expression of the protective gene HO-1 and
regulate the activities of SOD and GSH-Px (23). As one of the most important
pharmacologically active ingredients in ginseng, ginsenoside has
strong antioxidant functions. Our unpublished data (Fan et
al, unpublished data) indicated that GRh2 can bind to the Nrf2
inhibitory protein Keap-1 with a high energy (-9.45 kcal/mol)
according to molecular docking, causing Nrf2 to dissociate from
Keap1. In the present study, the data revealed that pre-treatment
with GRh2 activated the Nrf2/HO-1 signalling pathway, reduced the
levels of ROS and MDA and increased the activity of SOD and GSH-Px,
thereby exerting a protective effect.
MIRI-induced oxidative stress can intensify the
inflammatory responses that play an important role in MIRI
(4). After MIRI, the myocardium
accumulates neutrophils, which adhere to the vascular endothelia,
block the blood vessels and aggravate ischaemia. The myocardia and
neutrophils also release inflammatory mediators to stimulate the
inflammatory response of the organism and affect cell functions
(24). As pattern recognition
receptors, NLRPs can recognize pathogen-related and risk-related
molecular patterns and promote the release of inflammatory factors
by activating the immune system (25). There are 14 members of the NLRP
protein family named NLRP1-14. NLRP3 is a multiprotein complex that
exists in the cytoplasm of cells (23). It is mainly formed by the
combination of NLRP3, ASC and caspase-1 and is an important part of
the natural immune system (26).
After NLRP3 is activated, the NLRP3 inflammation complex is formed
with ASC and inactive caspase-1, which then activates caspase-1.
Active caspase-1 (cle-caspase-1) then cleaves the precursors of
IL-18 and IL-1β and triggers their release in their mature forms
(27). Recent studies have
confirmed that the large amount of ROS produced by MIRI can
activate the NLRP3 inflammasome, amplify the inflammatory response
and mediate tissue damage, which is an important component in the
development of disease (28,29).
In the present study, it was demonstrated that pre-treatment with
GRh2 not only improved oxidative stress damage in myocardial cells
but also inhibited the activation of the NLRP3/caspase-1/IL-1β
signalling pathway and reduced the inflammatory response.
Zeng et al (30) found that ROS could promote the
activation of NLRP3 inflammasomes and promote the inflammatory
response during brain injury. Recent studies have confirmed that
the Nrf2-mediated antioxidant system is a key component that
regulates the activity of NLRP3 inflammasomes (28,29).
The antioxidant system mediated by the increased expression of Nrf2
can inhibit the ROS-mediated activation of NLRP3 inflammasomes to
alleviate damage. Notably, many Chinese herbal medicines, such as
formononetin and maslinic acid have been shown to promote
resistance to I/R injury by upregulating Nrf2 and inhibiting the
activation of NLRP3 in a targeted manner (31,32).
In the present study, it was also observed that GRh2 decreased the
high expression levels of NLRP3 inflammasome-related molecules
after I/R while reducing oxidative stress. However, notably, the
association between the antioxidant system induced by Nrf2
activation and the NLRP3 inflammasome pathway still requires
further elucidation. In a future study our aim will be to directly
silence the Nrf2 gene to further clarify the mechanism by which
GRh2 protects the myocardium and the association between Nrf2 and
NLRP3 in MIRI.
The present study was the first, to the best of our
knowledge, to investigate the role of GRh2 in MIRI by regulating
the Nrf2/HO-1/NLRP3 signalling pathway. Its main limitation is that
due to the different components of ginsenoside which may have
different protective effects on the myocardium, certain other
ginsenoside monomers have also been shown to reduce MIRI. Wang
et al (33) indicated that
GRg3 reduced MIRI via Akt/eNOS signalling and the Bcl-2/Bax
pathway. Zeng et al (34)
suggested that GRd mitigated MIRI via the Nrf2/HO-1 signalling
pathway. Qin et al (35)
indicated that GRb1 inhibited cardiomyocyte autophagy via the
PI3K/Akt/mTOR signalling pathway and reduced MIRI. In the present
study, the results were not compared with a major ginsenoside shown
to be cardioprotective and the protection efficiencies between
different ginsenoside monomers should be observed in-depth.
In conclusion, the present study confirmed that GRh2
has antioxidant and anti-inflammatory effects on the myocardium
following I/R that occur through the regulation of the
Nrf2/HO-1/NLRP3 signalling pathway. Thus, it provided a basis for
the clinical application of GRh2-related drugs.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81800258) and the Natural
Science Foundation of Yichang (grant no. A20-2-004).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZXF and CJY wrote the manuscript, interpreted the
data and performed the experiments. YHL analyzed the data. CXH
performed the literature search, designed the study and revised the
manuscript. JY performed the literature search, designed the study
and analyzed the data. ZXF and CJY confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved (approval
no. 2020090B) by the Ethics Committee of Experimental Animals of
China Three Gorges University (Yichang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao D, Liu J, Wang M, Zhang X and Zhou M:
Epidemiology of cardiovascular disease in China: current features
and implications. Nat Rev Cardiol. 16:203–212. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bauersachs R, Zeymer U, Brière JB, Marre
C, Bowrin K and Huelsebeck M: Burden of coronary artery disease and
peripheral artery disease: A literature review. Cardiovasc Ther.
2019(8295054)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Heusch G: Myocardial ischaemia-reperfusion
injury and cardioprotection in perspective. Nat Rev Cardiol.
17:773–789. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sun W, Wang Z, Sun M, Huang W and Wang Y
and Wang Y: Aloin antagonizes stimulated
ischemia/reperfusion-induced damage and inflammatory response in
cardiomyocytes by activating the Nrf2/HO-1 defense pathway. Cell
Tissue Res. 384:735–744. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Meng X, Zhang L, Han B and Zhang Z: PHLDA3
inhibition protects against myocardial ischemia/reperfusion injury
by alleviating oxidative stress and inflammatory response via the
Akt/Nrf2 axis. Environ Toxicol. 36:2266–2277. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Xiang M, Lu Y, Xin L, Gao J, Shang C,
Jiang Z, Lin H, Fang X, Qu Y, Wang Y, et al: Role of oxidative
stress in reperfusion following myocardial ischemia and its
treatments. Oxid Med Cell Longev. 2021(6614009)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Abouzaki NA, Christopher S, Trankle C, Van
Tassell BW, Carbone S, Mauro AG, Buckley L, Toldo S and Abbate A:
Inhibiting the inflammatory injury after myocardial ischemia
reperfusion with plasma-derived Alpha-1 Antitrypsin: A post hoc
analysis of the VCU-α1RT study. J Cardiovasc Pharmacol. 71:375–379.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Heusch G: Molecular basis of
cardioprotection: Signal transduction in ischemic pre-, post-, and
remote conditioning. Circ Res. 116:674–699. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shi ZY, Zeng JZ and Wong AST: Chemical
structures and pharmacological profiles of ginseng saponins.
Molecules. 24(2443)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kim JH, Yi YS, Kim MY and Cho JY: Role of
ginsenosides, the main active components of Panax ginseng, in
inflammatory responses and diseases. J Ginseng Res. 41:435–443.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hsieh YH, Deng JS, Chang YS and Huang GJ:
Ginsenoside Rh2 ameliorates lipopolysaccharide-induced acute lung
injury by regulating the TLR4/PI3K/Akt/mTOR, Raf-1/MEK/ERK, and
Keap1/Nrf2/HO-1 signaling pathways in mice. Nutrients.
10(1208)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ma R, Tian JH, Jiang J and Jin X:
Inhibitory activities of ginsenosides on the activation of NLRP3
inflammasome. J China Pharm Univ. 47:614–618. 2016.
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tian Y, Deng P, Li B, Wang J, Li J, Huang
Y and Zheng Y: Treatment models of cardiac rehabilitation in
patients with coronary heart disease and related factors affecting
patient compliance. Rev Cardiovasc Med. 20:27–33. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kakavand H, Aghakouchakzadeh M, Coons JC
and Talasaz AH: Pharmacologic prevention of myocardial
ischemia-reperfusion injury in patients with acute coronary
syndrome undergoing percutaneous coronary intervention. J
Cardiovasc Pharmacol. 77:430–449. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Im DS: Pro-resolving effect of
ginsenosides as an anti-inflammatory mechanism of panax ginseng.
Biomolecules. 10(444)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li L, Wang Y, Guo R, Li S, Ni J, Gao S,
Gao X, Mao J, Zhu Y, Wu P, et al: Ginsenoside Rg3-loaded, reactive
oxygen species-responsive polymeric nanoparticles for alleviating
myocardial ischemia-reperfusion injury. J Control Release.
317:259–272. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu X, Jin L, Jiang T, Lu Y, Aosai F, Piao
HN, Xu GH, Jin CH, Jin XJ, Ma J and Piao LX: Ginsenoside Rh2
attenuates microglial activation against toxoplasmic encephalitis
via TLR4/NF-κB signaling pathway. J Ginseng Res. 44:704–716.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shi X, Tao G, Ji L and Tian G: Sappanone a
protects against myocardial ischemia reperfusion injury by
modulation of Nrf2. Drug Des Devel Ther. 14:61–71. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shen Y, Liu X, Shi J and Wu X: Involvement
of Nrf2 in myocardial ischemia and reperfusion injury. Int J Biol
Macromol. 125:496–502. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cheng Y, Cheng L, Gao X, Chen S, Wu P,
Wang C and Liu Z: Covalent modification of Keap1 at Cys77 and
Cys434 by pubescenoside a suppresses oxidative stress-induced NLRP3
inflammasome activation in myocardial ischemia-reperfusion injury.
Theranostics. 11:861–877. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang CY, Deng JS, Huang WC, Jiang WP and
Huang GJ: Attenuation of lipopolysaccharide-induced acute lung
injury by hispolon in mice, through regulating the
TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 pathways, and suppressing
oxidative stress-mediated er stress-induced apoptosis and
autophagy. Nutrients. 12(1742)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yang CJ and Yang J, Fan ZX and Yang J:
Activating transcription factor 3-an endogenous inhibitor of
myocardial ischemia-reperfusion injury (Review). Mol Med Rep.
13:9–12. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tschopp J, Martinon F and Burns K: .
NALPs: A novel protein family involved in inflammation. Nat Rev Mol
Cell Biol. 4:95–104. 2003.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Irrera N, Russo M, Pallio G, Bitto A,
Mannino F, Minutoli L, Altavilla D and Squadrito F: The role of
NLRP3 inflammasome in the pathogenesis of traumatic brain injury.
Int J Mol Sci. 21(6204)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kelley N, Jeltema D, Duan Y and He Y: The
NLRP3 inflammasome: An overview of mechanisms of activation and
regulation. Int J Mol Sci. 20(3328)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jun JH, Shim JK, Oh JE, Shin EJ, Shin E
and Kwak YL: Protective effect of ethyl pyruvate against myocardial
ischemia reperfusion injury through regulations of ros-related
NLRP3 inflammasome activation. Oxid Med Cell Longev.
2019(4264580)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shen S, He F, Cheng C, Xu B and Sheng J:
Uric acid aggravates myocardial ischemia-reperfusion injury via
ROS/NLRP3 pyroptosis pathway. Biomed Pharmacother.
133(110990)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zeng J, Chen Y, Ding R, Feng L, Fu Z, Yang
S, Deng X, Xie Z and Zheng S: Isoliquiritigenin alleviates early
brain injury after experimental intracerebral hemorrhage via
suppressing ROS- and/or NF-κB-mediated NLRP3 inflammasome
activation by promoting Nrf2 antioxidant pathway. J
Neuroinflammation. 14(119)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wang F, Wang H, Liu X, Yu H, Huang X,
Huang W and Wang G: Neuregulin-1 alleviate oxidative stress and
mitigate inflammation by suppressing NOX4 and NLRP3/caspase-1 in
myocardial ischaemia-reperfusion injury. J Cell Mol Med.
25:1783–1795. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang DS, Yan LY, Yang DZ, Lyu Y, Fang LH,
Wang SB and Du GH: Formononetin ameliorates myocardial
ischemia/reperfusion injury in rats by suppressing the
ROS-TXNIP-NLRP3 pathway. Biochem Biophys Res Commun. 525:759–766.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang Y, Hu Z, Sun B, Xu J, Jiang J and Luo
M: Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion
injury via Akt/endothelial nitric oxide synthase signaling and the
B-cell lymphoma/B-cell lymphoma-associated X protein pathway. Mol
Med Rep. 11:4518–4524. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zeng X, Li J and Li Z: Ginsenoside Rd
mitigates myocardial ischemia-reperfusion injury via Nrf2/HO-1
signaling pathway. Int. J Clin Exp Med. 8:14497–14504.
2015.PubMed/NCBI
|
|
35
|
Qin GW, Lu P, Peng L and Jiang W:
Ginsenoside rb1 inhibits cardiomyocyte autophagy via pi3k/akt/mtor
signaling pathway and reduces myocardial ischemia/reperfusion
injury. Am J Chin Med. 49:1913–1927. 2021.PubMed/NCBI View Article : Google Scholar
|