Introduction
Intestinal ischemia/reperfusion (I/R) injury is a
serious clinical complication, associated with abdominal and
thoracic vascular surgery, small bowel transplantation, traumatic
hemorrhagic shock, cardiopulmonary bypass and strangulated
intestinal obstruction, that increases the incidence rate and
mortality of intestinal mucosa injury and intestinal necrosis
(1,2). Intestinal ischemia is caused by local
or systemic factors, such as mechanical vascular obstruction,
hypovolemia, hypotension, hypoxia or sepsis, which lead to
cytopathy and death due to oxygen and nutrient deprivation
(3). Blood reperfusion following
intestinal ischemia also leads to cell damage and death, mainly due
to the accumulation of oxygen free radicals and other cytotoxic
substances resulting in lipid peroxidation of the cell membrane
(4).
Several drugs have been proposed to decrease or
prevent the cellular dysfunctions caused by intestinal I/R injury,
including drugs that interfere with adenosine (ADO) receptors (ARs)
(5,6). In mammals, ATP degradation produces
ADO that blocks potentially destructive inflammatory cascades and
decreases activation of platelets, leukocytes and endothelial cells
by mediating four subtypes of AR: A1, A2A,
A2B and A3 (7,8). A
previous study concluded that ADO blocks intestinal I/R injury and
an A1R agonist (CPA) ameliorated intestinal contractile
dysfunction induced by I/R. It was shown that intestinal I/R injury
was limited by decreasing oxidative stress, lowering neutrophil
infiltration and increasing reduced glutathione content (9,10).
In addition, activation of A1R has been shown to have
cardioprotective, renal and pulse protective effects on I/R injury
(11-14).
Upon activation of A1R, specific signal
transduction pathways are modulated, including the
phosphatidylinositol 3-kinase (PI3K)/activated protein kinase B
(Akt) signaling pathways. The activation of A1R in cells
and tissue leads to increased expression levels of Akt, resulting
in an anti-apoptotic effect (15,16).
Studies have shown that pharmacological phosphorylation of select
reperfusion survival promoting kinases, such as PI3K and Akt,
protects the heart from I/R injury following ischemia (17,18).
Therefore, it was hypothesized that A1R agonism may
protect against intestinal I/R injury, not only by ameliorating
intestinal contractile dysfunction but also by activating the
PI3K/Akt signaling pathway.
In the present study, two selective A1R
agonists were used with different residence times. CPA
(N6-cyclopentyl-adenosine) and LUF6941
[2-amino-6-((2-(4-chlorophenyl)
thiazol-4-yl)methylthio)4-(4-methoxyphenyl)pyridine-3,5-dicarbonitrile]
were examined to assess their protective effects on enterocytes in
an I/R model. Copeland et al (19) defined residence time as the period
when the drug and receptor form a complex after the drug binds.
Previous investigations have shown that A1R agonists
with different residence times could produce different
anti-lipolytic effects (20,21).
The present study aimed to determine whether A1R
agonists CPA and LUF6941 protect cells by activating the PI3K/Akt
signaling pathway.
Materials and methods
Cell culture
The human colon carcinoma cell line Caco-2 cell line
(Jiangsu Laisen Institute of Biotechnology Co, Ltd.) was used for
this study. The cell line was grown in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 20% FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% HEPES in a humidified atmosphere at 5%
CO2 and 37˚C. Cells were used at 60-70% confluence and
plated at the density of 1x106 cells per ml in a 96-well
flat-bottomed plate 1 day before assay.
Oxygen-glucose
deprivation/reoxygenation (OGD/R) model construction and
experimental groups
To simulate intestinal I/R injury, a cell OGD/R
model was established as previously described (22,23).
Briefly, Caco-2 cells cultured in glucose-free Earle balanced salt
solution (Leagene Biotechnology; Beijing Regen Biotechnology Co.,
Ltd.) in a microaerophilic system at 37˚C, 5% CO2, 1%
O2 and 94% N2 for 8 h to achieve OGD. Media
was then replaced with culture medium EBSS containing high sugar
(glucose 4.5 g/l)(Leagene Biotechnology; Beijing Regen
Biotechnology Co., Ltd.) and cells were returned to normal oxygen
culture conditions (5% CO2, 37˚C) for 20 h to achieve
reoxygenation. The control group was kept in normal oxygen
conditions (5% CO2, 37˚C) for the same period time. A
previous study showed that 1x10-6 M agonists CPA and
LUF6941 produces strong biological activity (20). Agonists were diluted in EBSS to
1x10-6 M concentration from the stock solution and added
at the beginning of OGD/R. ADO deaminase at 1 U/ml was used to
remove endogenously released adenosine from Caco-2 cells during
OGD/R before the addition of the agonists (24). Cells were divided into following
groups: i) Control; ii) OGD/R; iii) CPA (OGD/R + 1x10-6
M CPA) and iv) LUF6941 (OGD/R + 1x10-6 M LUF6941).
Cell viability assay and imaging of
Caco-2 cells
PI assay was used to detect non-viable cells in all
groups. At the end of OGD/R, 5 mM PI (Sigma-Aldrich; Merck KGaA)
was added to each well (1x106 cells) and incubated for
15 min at room temperature in the dark. Cells were imaged using an
inverted fluorescence microscope (10x magnification) connected to a
SPOT RT camera (Axio Imager A2; Zeiss GmbH) and DG-4 lamp box
(Sutter Instruments, Novato, CA) at 488 nm excitation wavelength
and 617 nm emission filter. From each well, two images were taken
and PI-positive cells were quantified using Image J 1.44 software
(National Institutes of Health). Data were normalized to the OGD/R
treatment group. Duplicate wells were used for each experiment and
each experiment was repeated 3 times.
Cell counting kit-8 (CCK-8) assay
CCK-8 assay (Beyotime Institute of Biotechnology)
was used to determine cell viability according to the
manufacturer's protocols. Briefly, Caco-2 cells were seeded in
96-well plates (150 µl/well, 1x104 cells) and pretreated
with 1x10-6 M CPA or LUF6941, as aforementioned.
Following OGD/R, 10% CCK-8 reagent was added to each well and cells
were incubated for 2 h at 37˚C. Absorbance was recorded at 450 nm
using a microplate reader (Varioskan LUX; Thermo Fisher Scientific,
Inc.). Data were normalized to the control group.
Western blotting
Caco-2 cell protein was obtained using protein lysis
buffer (RIPA lysis buffer; Beyotime) containing 1 mmol/l
phenylmethylsulfonyl fluoride (PMSF; Beyotime) and phosphatase
inhibitors (Beyotime). Protein concentrations of cell lysates were
determined using a BCA protein assay kit (Beyotime). Equal amounts
of protein (20 µg/lane) were electrophoresed on 10%
SDS-polyacrylamide gel and transferred to a nitrocellulose
membrane. The membranes were blocked with 5% BSA (Cell Signaling
Technology) for 2 h at room temperature, before incubation at 4˚C
overnight with the following primary antibodies (all from Cell
Signaling Technology) at a 1:1,000 dilution: PI3K (cat. no. 3811S),
Akt (cat. no. 9272S), phosphorylated (p)-Akt (cat. no. 9275S), p53
(cat. no. 9282S), p-p53 (cat. no. 9284S) and GAPDH (cat. no. 5174S;
Cell Signaling Technology, Inc.). Membranes were washed by PBS
buffer and incubated with horseradish peroxidase-conjugated second
antibody (1:2,000; cat. no. ab97051; Abcam) at room temperature for
1 h. Immunoreactive protein bands were observed with a
chemiluminescence kit (Sigma-Aldrich; Merck KGaA) and quantified
using Image J software (National Institutes of Health). GAPDH was
used as the loading control.
Flow cytometry
Apoptosis was assessed using the PE Annexin V
Apoptosis Detection kit I (cat. no. 559763; Becton, Dickinson and
Company) according to the manufacturer's protocols. Briefly, Caco-2
cells were suspended in binding buffer at a concentration of
1x106 cells/ml. Aliquots of 100 µl cell suspension
(1x105 cells) were transferred to 5 ml culture tubes and
5 µl PE Annexin V and 5 µl 7-AAD were added to each tube. The cells
were gently vortex and incubated in darkness at room temperature
(25˚C) for 15 min. An additional 400 µl binding buffer was added to
each tube. Finally, cell early and late apoptosis were analyzed
using a CytoFLEX Flow Cytometer (Beckman Coulter, Inc.) with FlowJo
8.7.1 software (FlowJo LLC).
Statistical analysis
All data are presented as the mean ± SEM and all
experiments were performed three times. Statistical differences
between groups were determined by one-way ANOVA followed by post
hoc Dunnett's test. Statistical analysis was performed using SPSS
22.0 (IBM, USA) and GraphPad Prism 7.0 (GraphPad software, San
Diego, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
CPA and LUF6941 increase cell
viability after OGD/R
To investigate whether A1R agonists have
a protective effect on intestinal I/R injury, an OGD/R model was
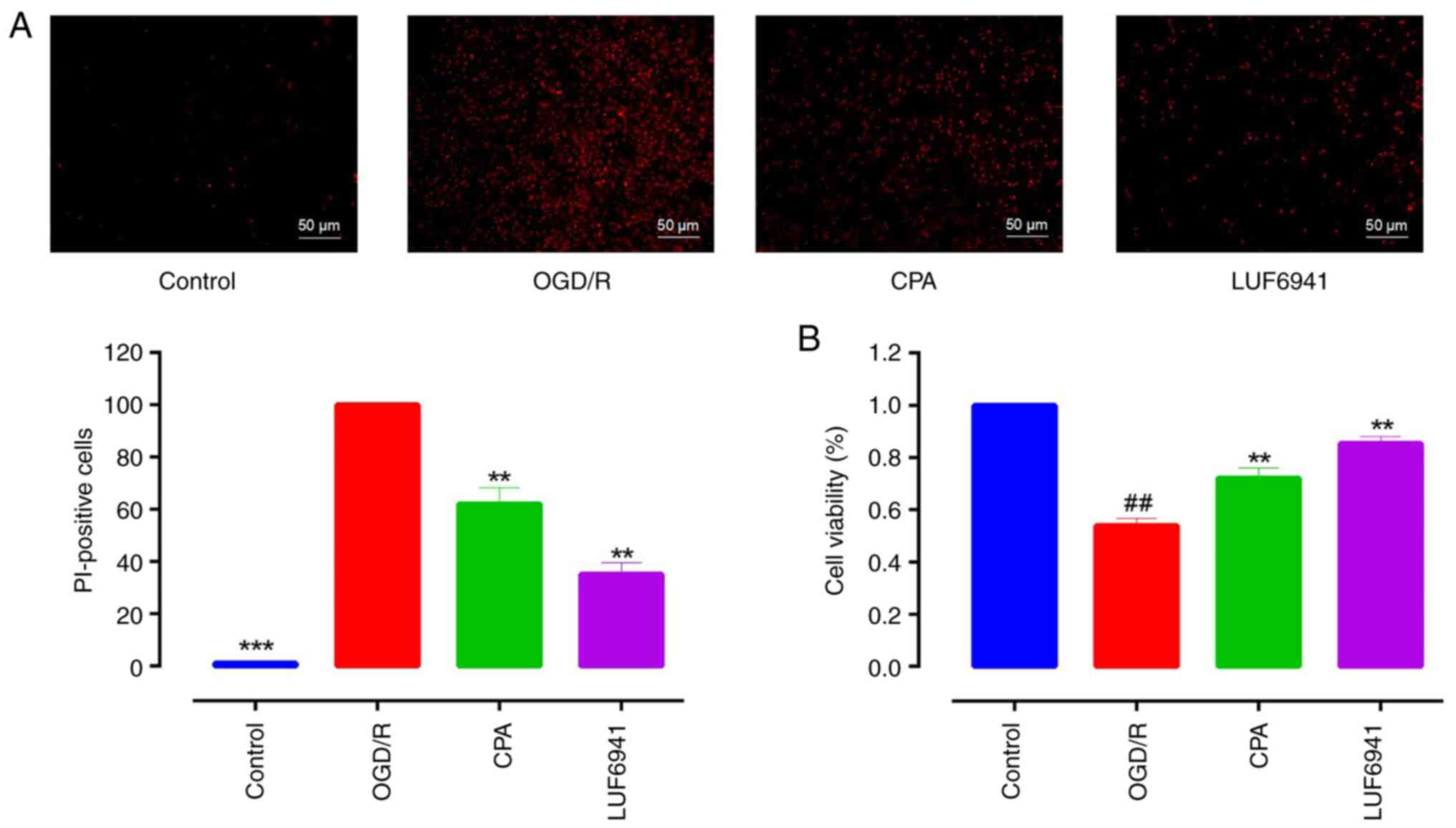
established and the cell viability was examined using PI assay.
Incubation of Caco-2 cells in a microaerophilic system for 8 h
significantly increased the proportion of non-viable cells. The
OGD/R group was assigned as the control to which other treatment
groups were normalized and compared. CPA and LUF6941 significantly
decreased the proportion of non-viable cells (Fig. 1A; 62.04±6.20 and 35.14±4.43%,
respectively; both P<0.01). To evaluate the level of cell
injury, CCK-8 assay was used to determine cell viability.
Consistent with PI assay results, CPA and LUF6941 significantly
increased the viability of OGD/R-treated Caco-2 cells (Fig. 1B; 72.02±2.38 and 85.40±1.51%,
respectively; both P<0.01).
Effect of CPA and LUF6941 pretreatment
on PI3K/Akt signaling
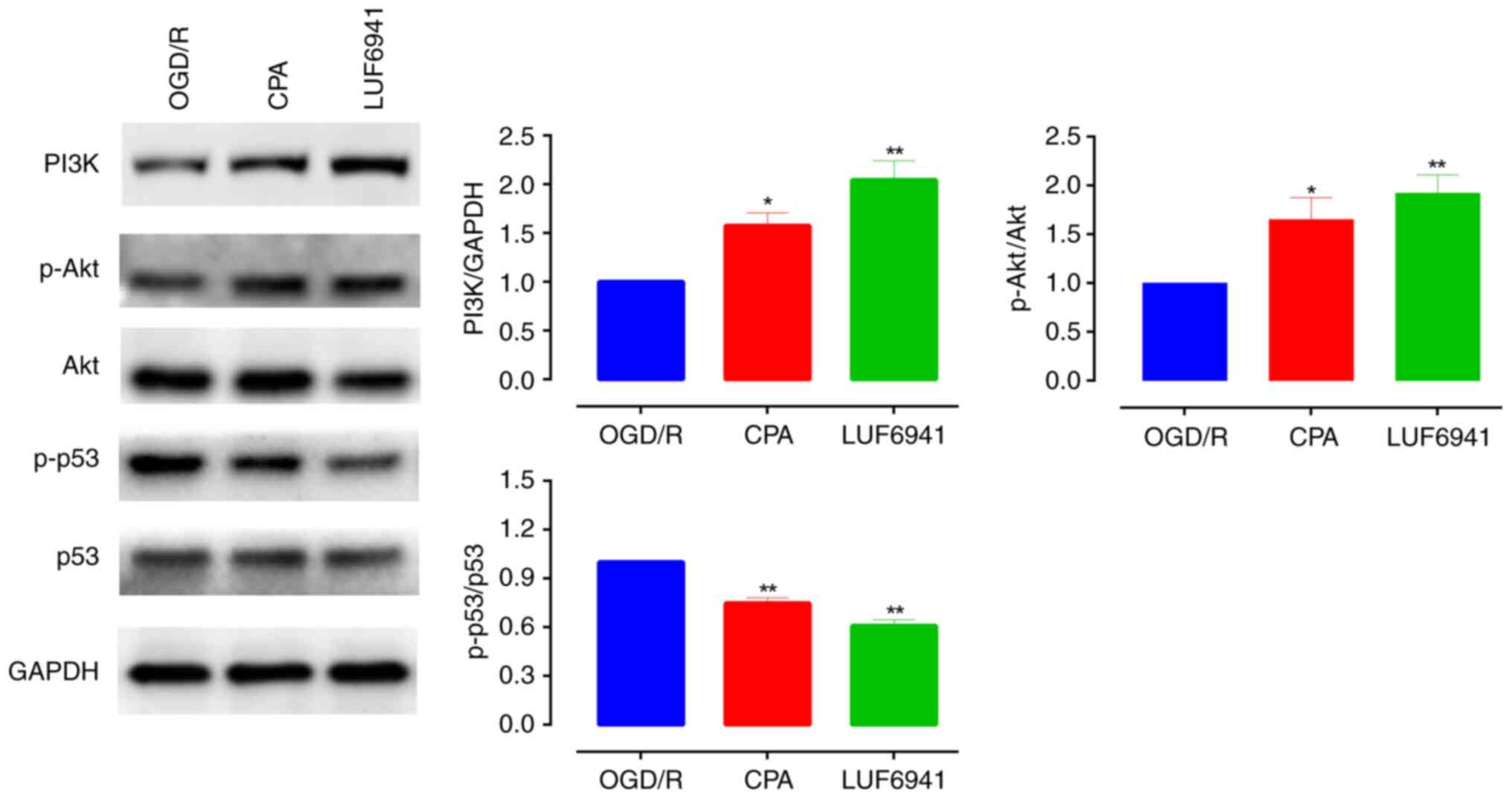
A previous study demonstrated that the PI3K/Akt
signaling pathway is activated following ischemia to promote
proliferation (17). Here,
A1R agonists activated PI3K/Akt signaling pathway
members in intestinal I/R injury (Fig.
2). Western blotting showed that PI3K and p-Akt/Akt levels
increased significantly when cells were treated with CPA (PI3K,
1.58±0.13; p-Akt/Akt, 1.65±0.22; both P<0.05) and LUF6941 (PI3K,
2.05±0.19; p-Akt/Akt, 1.92±0.88; both P<0.01). In addition,
A1R agonists significantly decreased levels of p-p53/p53
compared with the OGD/R group (CPA, 0.75±0.03; LUF6941, 0.60±0.03;
both P<0.01). These results indicated that the PI3K/Akt
signaling pathway, activated by A1R agonists, exhibited
protective effects during intestinal I/R injury and A1R
agonists significantly inhibited the degree of phosphorylation of
p53 protein.
CPA and LUF6941 decrease Caco-2
apoptosis after OGD/R
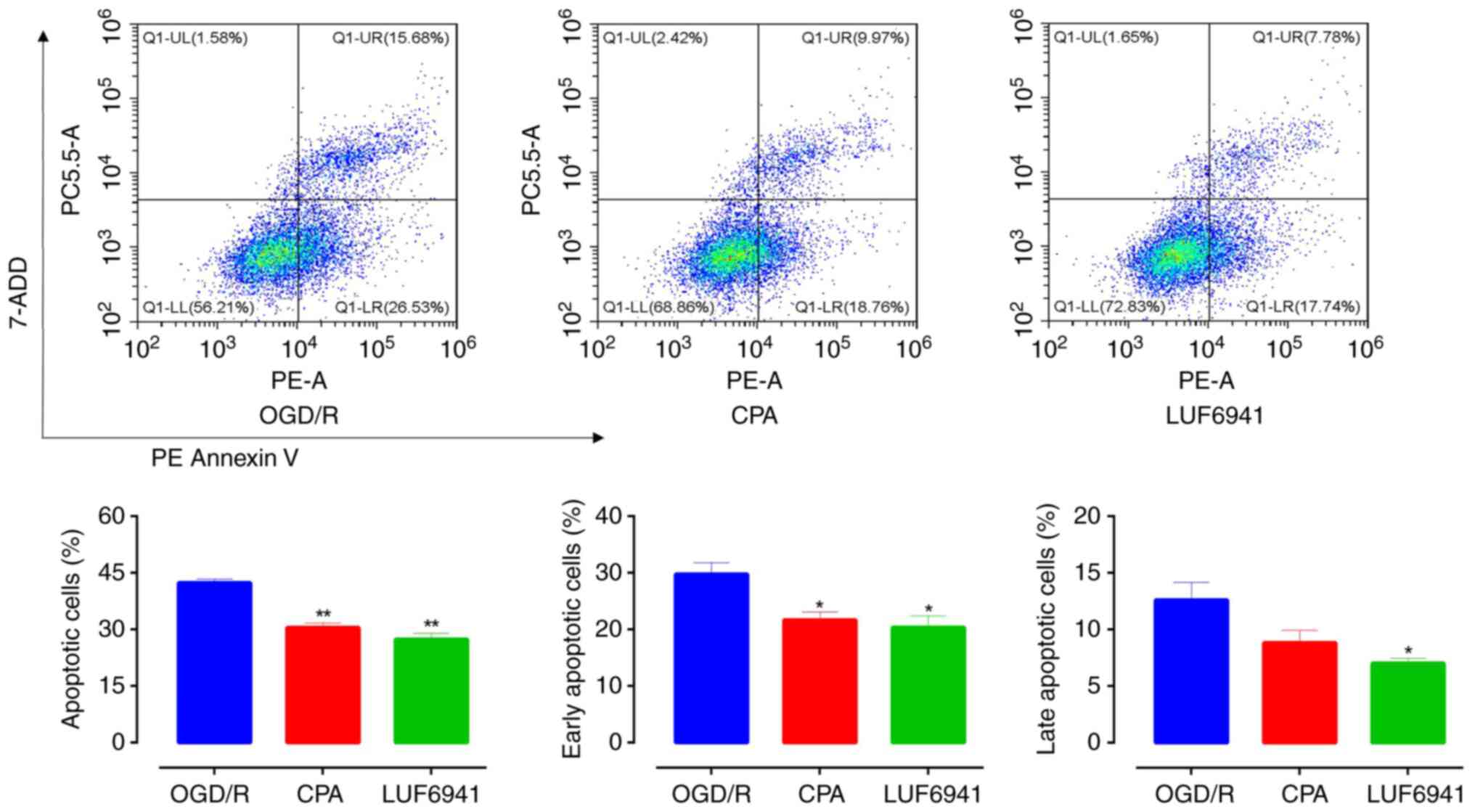
To investigate the effect of A1R agonists
on the physiological function of Caco-2 cells after OGD/R, an
Annexin V-PE/7-AAD double staining assay was performed to assess
the apoptotic activity of Caco-2 cells after CPA and LUF6941
treatment (Fig. 3). Flow cytometry
showed that, compared with the OGD/R group, Caco-2 cell apoptosis
significantly decreased in the CPA and LUF6941 groups (30.48±1.20
and 27.37±1.59%, respectively; both P<0.01). Early apoptotic
cells were also significantly decreased in the CPA (21.66±1.45%;
P=0.0312) and LUF6941 (20.36±2.03%; P=0.0306) groups compared with
the OGD/R group. Late apoptotic cells were observed in all groups
but there was no significant difference between the CPA group and
OGD/R group (P=0.1198). However, there was a significant difference
(P<0.05) between the LUF6941 and OGD/R group.
Discussion
The present study showed that A1R
agonists CPA and LUF6941 protected against OGD/R in intestinal
epithelial Caco-2 cells, which was associated with activation of
the PI3K/Akt signaling pathway. To simulate clinical intestinal I/R
injury, an OGD/R model was established to evaluate the survival
rate of Caco-2 cells after intestinal I/R injury. The proportion of
apoptotic Caco-2 cells pretreated with A1R agonists
significantly compared with the OGD/R group. CPA and LUF6941 were
able to significantly decrease apoptosis in Caco-2 cells after
OGD/R.
Although the two agonists showed significant
anti-apoptotic effects in early apoptosis, there were differences
in late apoptosis. LUF6941 inhibited late apoptosis, while CPA had
no significant effect. It was hypothesized that this result may be
associated with residence time of the drug (25). Previous research suggests that
enduring drug-target interaction produces sustained functional
response, while short drug-target engagement leads to a more
short-lived duration of drug action (26,27).
Previous investigation has shown that A1R agonists with
longer residence times sustain an anti-lipolytic effect (20). LUF6941 has a longer residence time
than CPA and this longer residence time produces a sustained
wash-resistant anti-lipolytic effect in rat adipocytes (20). Thus, it was hypothesized that
differences in the anti-apoptotic effect of the A1R
agonists may be associated with residence time of the drug during
OGD/R. With a long residence time, LUF6941 had an inhibitory effect
throughout apoptosis, while CPA, with a shorter residence time,
appeared to only play a role in the early stages of apoptosis.
Therefore, CPA and LUF6941 improve survival of Caco-2 cells;
however, the degree of improvement was different.
To determine the underlying mechanisms involved in
inhibition of Caco-2 cell apoptosis induced by A1R
agonists, protein expression levels of PI3K, Akt and p53 were
analyzed using western blotting. Levels of activated Akt and the
expression of upstream PI3K were significantly increased following
OGD/R in the A1R agonist-treated cells. A1R
agonists were also shown to inhibit intestinal cells apoptosis by
downregulating phosphorylation level of p53 protein. Previous
studies have shown that activated Akt promotes survival of
intestinal epithelial cells and increases tolerance of the heart,
brain and other organs to I/R damage by inhibiting apoptotic
pathways (17,28-30).
Apoptosis is the main mechanism of intestinal cell death during I/R
(31). p53 protein serves a key
role in mediating apoptosis and blocking p53-mediated Akt
activation in response to DNA damage results in decreased cell
viability (32). In the present
study, A1R agonist pretreatment significantly enhanced
the activation of Akt and decreased cell apoptosis in the
intestinal I/R model. Therefore, activation of Akt may be involved
in the improvement of recovery from I/R injury induced by
A1R agonism.
There are, however, some limitations to the design
of the current study. Only the mechanisms of A1R agonist
protection at the cellular level following I/R in vitro were
studied, however, Taha et al (33) showed that ADO was able to attenuate
motor and neural dysfunctions of small bowel caused by ischemia,
but not by reperfusion, in rabbits. Meanwhile, Lee et al
(12) showed that A1R
activation or blockade is associated with decreased and enhanced
inflammatory responses, respectively, following renal I/R injury in
mice. After intestinal I/R, A1R may have
anti-inflammatory effects in addition to inhibiting cell apoptosis
and the association between these mechanisms requires further
study. Mandl and Depping (34)
reported that low oxygen also activates the hypoxia-inducible
factor (HIF) pathway. However, to the best of our knowledge, no
studies have reported that CPA and LUF6941 affect the HIF pathway.
In future studies, the effect of CPA and LUF6491 treatment on this
pathway will be investigated.
In summary, pretreatment with A1R
agonists had a protective effect against intestinal I/R injury by
activating PI3K/Akt signaling. A1R agonists inhibited
Caco-2 cell apoptosis by downregulating phosphorylation of p53
protein in vitro. These results indicated that the
intestinal I/R protection induced by A1R agonists may,
at least in part, be attributed to an anti-apoptotic effect
mediated by activating the PI3K/Akt/p53 signaling pathway.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Suqian Sci&Tech
Program (grant no. K202106).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY conceived the study and designed the experiments.
YY and QX performed the experiments. YY, QX and ZM were responsible
for the acquisition, analysis and interpretation of the data. QX
and ZM wrote and revised the article. YY and QX confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barie PS: Schemes against ischemia;
solutions for reperfusion (injury)? Crit Care Med. 27:684–685.
1999.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Corcos O and Nuzzo A: Gastro-intestinal
vascular emergencies. Best Pract Res Clinl Gastroenterol.
27:709–725. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schoenberg MH and Beger HG: Reperfusion
injury after intestinal ischemia. Crit Care Med. 21:1376–1386.
1993.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Horton JW and Walker PB: Oxygen radicals,
lipid peroxidation, and permeability changes after intestinal
ischemia and reperfusion. J Appl Physiol (1985). 74:1515–1520.
1993.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Taha MO, Fraga MM, Fagundes DJ, Jurkiewicz
A and Caricati-Neto A: Effect of allopurinol on autonomic
dysfunction in rat jejunal segments exposed to cold ischemic
preservation for transplantation. Transplant Proc. 36:293–295.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Taha MO, Fraga MM, Fagundes DJ, Jurkiewicz
A and Caricati-Neto A: Ascorbic acid prevents autonomic dysfunction
in rat jejunal submitted to cold ischemic preservation for
transplantation. Transplant Proc. 36:289–292. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Abbracchio MP and Burnstock G: Purinergic
signalling: Pathophysiological roles. Jpn J Pharmacol. 78:113–145.
1998.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Burnstock G: Purinergic signaling. B J
Pharmacol. 147 (Suppl 1):S172–S181. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kaminski PM and Proctor KG: Attenuation of
no-reflow phenomenon, neutrophil activation, and reperfusion injury
in intestinal microcirculation by topical adenosine. Circ Res.
65:426–435. 1989.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ozacmak VH and Sayan H: Pretreatment with
adenosine and adenosine A1 receptor agonist protects against
intestinal ischemia-reperfusion injury in rat. World J
Gastroenterol. 13:538–547. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Magata S, Taniguchi M, Suzuki T, Shimamura
T, Fukai M, Furukawa H, Fujita M and Todo S: The effect of
antagonism of adenosine A1 receptor against ischemia and
reperfusion injury of the liver. J Surg Res. 139:7–14.
2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee HT, Xu H, Nasr SH, Schnermann J and
Emala CW: A1 adenosine receptor knockout mice exhibit increased
renal injury following ischemia and reperfusion. Am J Physiol Renal
Physiol. 286:F298–F306. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gazoni LM, Walters DM, Unger EB, Linden J,
Kron IL and Laubach VE: Activation of A1, A2A, or A3 adenosine
receptors attenuates lung ischemia-reperfusion injury. J Thorac
Cardiovasc Surg. 140:440–446. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang Z, Cerniway RJ, Byford AM, Berr SS,
French BA and Matherne GP: Cardiac overexpression of A1-adenosine
receptor protects intact mice against myocardial infarction. Am J
Physiol Heart Circ Physiol. 282:H949–H955. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shackelford RE, Alford PB, Xue Y, Thai SF,
Adams DO and Pizzo S: Aspirin inhibits tumor necrosis factor-α gene
expression in murine tissue macrophages. Mol Pharmacol. 52:421–429.
1997.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jacobson KA and Gao ZG: Adenosine
receptors as therapeutic targets. Nat Rev Drug Discov. 5:247–264.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Hausenloy DJ and Yellon DM: New directions
for protecting the heart against ischaemia-reperfusion injury:
Targeting the reperfusion injury salvage kinase (RISK)-pathway.
Cardiovasc Res. 61:448–460. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ma XJ, Yin HJ, Guo CY, Jiang YR, Wang JS
and Shi DZ: Ischemic postconditioning through percutaneous
transluminal coronary angioplasty in pigs: Roles of PI3K
activation. Coron Artery Dis. 23:245–250. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Copeland RA, Pompliano DL and Meek TD:
Drug-target residence time and its implications for lead
optimization. Nat Rev Drug Discov. 5:730–739. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Yun Y, Chen J, Liu R, Chen W, Liu C, Wang
R, Hou Z, Yu Z, Sun Y, IJzerman AP, et al: Long residence time
adenosine A1 receptor agonists produce sustained
wash-resistant antilipolytic effect in rat adipocytes. Biochem
Pharmacol. 164:45–52. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Guo D, Heitman LH and IJzerman AP: The
added value of assessing ligand-receptor binding kinetics in drug
discovery. ACS Med Chem Lett. 7:819–821. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun S, Hu F, Wu J and Zhang S: Cannabidiol
attenuates OGD/R-induced damage by enhancing mitochondrial
bioenergetics and modulating glucose metabolism via
pentose-phosphate pathway in hippocampal neurons. Redox Biol.
11:577–585. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lu Y, An J, Liu Y, Ren L and Zhang L: MMP9
is involved in HO-1-mediated upregulation of apical junctional
complex in Caco-2 cells under oxygen-glucose deprivation. Biochem
Biophys Res Commun. 498:125–131. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Maggirwar SB, Dhanraj DN, Somani SM and
Ramkumar V: Adenosine acts as an endogenous activator of the
cellular antioxidant defense system. Biochem Biophys Res Commun.
201:508–515. 1994.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Copeland RA: The drug-target residence
time model: A 10-year retrospective. Nat Rev Drug Discov. 15:87–95.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Vauquelin G, Bostoen S, Vanderheyden P and
Seeman P: Clozapine, atypical antipsychotics, and the benefits of
fast-off D2 dopamine receptor antagonism. Naunyn Schmiedebergs Arch
Pharmacol. 385:337–372. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vauquelin G and Charlton SJ: Long-lasting
target binding and rebinding as mechanisms to prolong in vivo drug
action. Br J Pharmacol. 161:488–508. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Harnois C, Demers MJ and Vachon PH, Vallée
K, Gagné D, Fujita N, Tsuruo T, Vézina A, Beaulieu JF, Côté A and
Vachon PH: Human intestinal epithelial crypt cell survival and
death: Complex modulations of Bcl-2 homologs by Fak, PI3-K/Akt-1,
MEK/Erk, and p38 signaling pathways. J Cell Physiol. 198:209–222.
2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Baines CP, Wang L, Cohen MV and Downey JM:
Myocardial protection by insulin is dependent on
phospatidylinositol 3-kinase but not protein kinase C or KATP
channels in the isolated rabbit heart. Basic Res Cardiol.
94:188–198. 1999.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yano S, Morioka M, Fukunaga K, Kawano T,
Hara T, Kai Y, Hamada J, Miyamoto E and Ushio Y: Activation of
Akt/protein kinase B contributes to induction of ischemic tolerance
in the CA1 subfield of gerbil hippocampus. J Cereb Blood Flow
Metab. 21:351–360. 2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ikeda H, Suzuki Y, Suzuki M, Koike M,
Tamura J, Tong J, Nomura M and Itoh G: Apoptosis is a major mode of
cell death caused by ischaemia and ischaemia/reperfusion injury to
the rat intestinal epithelium. Gut. 42:530–537. 1998.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fang L, Li G, Liu G, Lee SW and Aaronson
SA: p53 induction of heparin-binding EGF-like growth factor
counteracts p53 growth suppression through activation of MAPK and
PI3K/Akt signaling cascades. EMBO J. 20:1931–1939. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Taha MO, Miranda-Ferreira R, Simões RS,
Abrão MS, Oliveira-Junior IS, Monteiro HP, Santos JM, Rodrigues PH,
Rodrigues JV, Alves AE, et al: Role of adenosine on intestinal
ischemia-reperfusion injury in rabbits. Transplant Proc. 4:454–456.
2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mandl M and Depping R: Hypoxia-inducible
aryl hydrocarbon receptor nuclear translocator (ARNT) (HIF-1β): Is
it a rare exception? Mol Med. 20:215–220. 2014.PubMed/NCBI View Article : Google Scholar
|