Introduction
Atrial fibrillation (AF) is one of the most common
types of tachyarrhythmia and its prevalence increases with age
(1,2). AF is characterized by high morbidity,
disability and mortality. AF predicts prolonged hospitalization and
increases long-term mortality and major adverse cardiovascular
events in patients with acute myocardial infarction (3). Therefore, interventions for AF,
either early and timely diversion therapy or anticoagulation for
persistent AF, are necessary. According to epidemiological surveys,
the number of patients with AF in the United States is expected to
reach 7 million by 2050(4).
Currently, the two main modalities for the treatment
of AF are antiarrhythmic drugs and radiofrequency ablation
(5). Radiofrequency ablation is
increasingly used to treat drug-refractory symptomatic paroxysmal
or persistent AF, as it significantly improves postoperative
survival in patients with paroxysmal AF compared with
antiarrhythmic drugs. However, during actual radiofrequency
ablation, antiarrhythmic drugs or Electrical cardioversion are
usually selected to improve the success rate of AF conversion
(6). In addition, the mechanism of
early recurrence of AF, especially following AF ablation, differs
from that of conventional atrial arrhythmias and the efficacy of
conventional drugs for early AF recurrence needs to be evaluated
(7).
The antiarrhythmic drugs now recommended for
cardioversion in AF by the European Resuscitation Council
guidelines and the International Consensus on Cardiopulmonary
Resuscitation are propafenone, amiodarone and lidocaine (8). However, all drugs have certain
drawbacks. For example, propafenone is only efficient for AF within
the first 48-72 h, while amiodarone takes longer to convert the
abnormal heartbeat to sinus rhythm (SR) than propafenone and is
prone to adverse effects, even malignant arrhythmias (9).
Nifekalant is a class III antiarrhythmic drug
approved by the European Resuscitation Council guidelines and the
International Consensus on Cardiopulmonary Resuscitation for the
treatment of ventricular arrhythmias (10,11).
It suppresses atrial or ventricular tachycardia by inhibiting
potassium channels and prolonging effective atrial inactivity.
Available studies indicate that nifekalant can inhibit cardiac
potassium current rectification in patients with delayed heartbeats
by prolonging effective cardiac inactivity, which not only helps
localize areas for radiofrequency ablation, but may also improve
the success of cardiac resuscitation (12,13).
To the best of our knowledge, there is no
meta-analysis on PubMed on the efficacy of nifekalant for the
treatment of AF. Given the superiority of nifekalant in the
treatment of AF, this drug not only compensates for some of the
disadvantages of classical drugs, such as propafenone and
amiodarone, but also improves the patient's experience of treatment
(14). In addition, to the best of
our knowledge, there are limited data on the pharmacological
conversion of AF by intravenous nifekalant administration during
radiofrequency ablation (15). The
present study aimed to review the available evidence and assess the
efficacy and safety of nifekalant in the conversion of AF by
performing statistical analysis on conversion indicators.
Materials and methods
Search strategy
PubMed (pubmed.ncbi.nlm.nih.gov/), Cochrane Library
(cochranelibrary.com/) and China National
Knowledge Infrastructure (kns.cnki.net)
data published between 1999 and 2022 were searched, based on the
recommendations of The Cochrane Collaboration and the Preferred
Reporting Items for Systematic Reviews and Meta-Analyses
guidelines, to select single studies receiving nifekalant for AF
reversal (16). The search keyword
was ‘nifekalant’. Two independent researchers (KL and PL) searched
and reviewed the titles, abstracts and full text to identify them
for inclusion.
Data selection inclusion criteria
The published studies included in the present study
had to be randomized controlled trials, retrospective or
prospective studies with patients that received nifekalant alone.
The control group is defined as treated with either lidocaine or
amiodarone. The inclusion criteria were as follows: i) Paroxysmal
AF for ≤48 h or persistent AF for ≥7 days; ii) discontinuation of
other antiarrhythmic drugs after >5 half-lives before
radiofrequency ablation; and iii) no other antiarrhythmic drugs
applied during radiofrequency ablation. The study outcomes included
the success rate of conversion, the success rate of conversion
following radiofrequency ablation, the mean time to conversion and
the incidence of adverse events. The study endpoint was the
conversion of AF to SR and the secondary efficacy endpoint was the
termination of AF. Adverse events included bradycardia, ventricular
arrhythmias, hypotension or gastrointestinal adverse reactions.
Reviews, case reports, animal studies, editorials, studies with
unclear study types and studies with insufficient data were
excluded.
Data extraction and quality
assessment
The following data were collected from eligible
studies screened by both authors: i) The first author; ii) year of
publication; iii) description; iv) sample size; v) follow-up time;
vi) clinical characteristics; and vii) outcomes. The Cochrane
risk-of-bias tool was used to assess the quality of the included
studies by both authors. Any disagreements between the two authors
were discussed and solutions were found, or a third researcher (SW)
was consulted. Publication bias checks were analysed using Stata 16
software (StataCorp LP).
Statistical analysis
The present meta-analysis followed the guidelines of
the Cochrane Handbook for Systematic Reviews of Interventions and
study endpoint parameters (including success rate of conversion,
success rate of conversion following radiofrequency ablation, mean
time to conversion and incidence of adverse events) were collected
to assess the treatment effects (17). Statistical data analysis was
performed using RevMan 5.4 (The Cochrane Collaboration) and Stata
16 (StataCorp LP). Once these data had been extracted, the risk
ratio (RR), mean difference (MD) and 95% confidence interval (CI)
were calculated using the two software packages aforementioned.
P<0.05 was considered to indicate a statistically significant
difference. Egger's test was used to verify the presence of
publication bias. Heterogeneity was assessed using the
I2 statistic and Cochrane's Q test. I2
statistics of 0, <25, 25-49 and >50% were considered to
indicate no, low, moderate and high heterogeneity, respectively. To
ensure the accuracy and stability of the results, a random effects
model was selected directly for statistical analysis when there was
moderate or high heterogeneity in the study.
Results
Results of the article search
A preliminary search of PubMed (160 articles), the
Cochrane Library (two articles) and China National Knowledge
Infrastructure (255 articles) yielded a total of 417 articles.
After reading the titles and abstracts and excluding duplicate
publications, a total of 368 articles were excluded, and 49
articles were selected and further screened by full-text reading. A
total of 37 of these were further excluded for various reasons,
such as insufficient data and inappropriate controls. Finally, 405
articles were excluded and 12 research studies met all the
inclusion criteria (10,18-28).
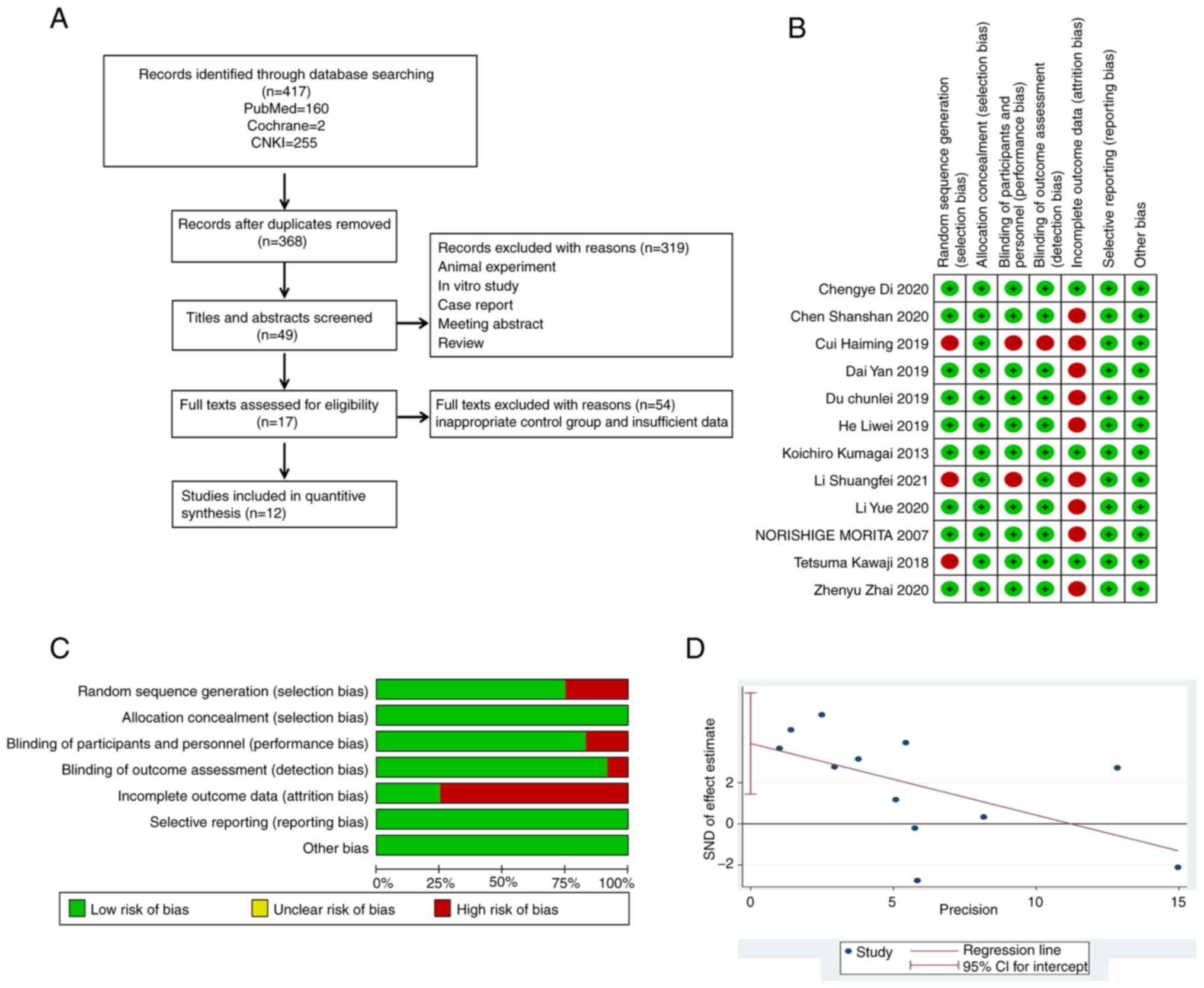
A flow chart of the study selection process is shown in Fig. 1A. A total of 12 studies including
1,162 patients were considered by the present meta-analysis. All
included studies were published between 1999 and 2022. Table I summarizes the information on the
main baseline characteristics of patients and the doses used for
nifekalant and the controls.
 | Table ICharacteristics of patients. |
Table I
Characteristics of patients.
| First author/s,
year | Description
(nifekalant/control) | Follow-up
duration | Atrial fibrillation
duration (nifekalant/control) | Number of patients
(nifekalant/control) | Sex
(male/female) | Age
(nifekalant/control), years | (Refs.) |
|---|
| Di et al,
2020 | 0.3 mg/kg
intravenous injection/amiodarone was infused at 150 mg
intravenously, and then continuously pumped in at 1 | 12 months |
0.40±9.25/0.40±10.70 months | 60/42 | 65/37 |
68.6±9.6/67.1±9.1 | (18) |
| Kumagai and Toyama,
2013 | 0.3 mg/kg
intravenous injection/the control was unknown | 12 months |
310±34.00/30.00±32.00 months | 50/50 | 89/11 |
56.0±10.0/57.0±11.0 | (19) |
| Morita et
al, 2007 | 0.3 mg/kg
intravenous injection/the control was unknown | NA |
17.00±41.50/21.40±44.50 h | 15/16 | 25/6 |
67.9±12.5/67.9±9.7 | (20) |
| Kawaji et
al, 2018 | 0.3 mg/kg
intravenous injection/the control was unknown | 12 months | NA | 79/78 | 121/36 |
65.9±10.2/65.4±7.6 | (10) |
| Zhai et al,
2021 | 0.3 mg/kg
intravenous injection/the method of placebo used was unknown | NA | Data not
available | 110/110 | 142/78 |
55.0-68.0/58.0-67.0 | (21) |
| Chen et al,
2020 | 0.3 mg/kg
intravenous injection/amiodarone was infused at 150 mg
intravenously, and then continuously pumped in at 1 mg/min | NA | 6.60±0.50/6.10±0.80
months | 45/37 | 43/39 |
61.0±11.0/63.0±9.0 | (22) |
| He et al,
2019 | 0.3 mg/kg
intravenous injection/electrical cardioversion | NA | NA | 41/60 | 56/45 |
60.7±12.1/58.2±17.6 | (23) |
| Cui et al,
2019 | 0.3 mg/kg
intravenous injection/electrical cardioversion | NA |
25.10±14.40/22.00±14 months | 23/15 | 27/11 |
66.7±8.6/66.2±7.1 | (24) |
| Li et al,
2021 | 0.3 mg/kg
intravenous injection/amiodarone was infused at 150 mg
intravenously, and then continuously pumped in at 1 mg/min | NA |
22.30±2.30/21.50±2.20 months | 37/34 | 35/36 |
57.9±2.0/59.6±2.1 | (25) |
| Du et al,
2019 | 0.3 mg/kg
intravenous injection/amiodarone was infused at 150 mg
intravenously, and then continuously pumped in at 1 mg/min | NA | <48.00 h | 52/54 | 88/18 |
68.0±10.0/67.0±10.0 | (26) |
| Li et al,
2020 | 0.3 mg/kg
intravenous injection/amiodarone was infused at 150 mg
intravenously, and then continuously pumped in at 1 mg/min | NA | NA | 47/47 | 51/43 |
61.64±2.35/62.03±3.21 | (27) |
| Dai et al,
2019 | 0.3 mg/kg
intravenous injection/amiodarone was infused at 150 mg
intravenously, and then continuously pumped in at 1 mg/min | NA | <48.00 h | 30/30 | 40/20 |
61.33±11.76/62.10±10.64 | (28) |
Methodological quality assessment of
the included studies
All included studies were of medium to high quality
(Fig. 1B and C). Bias analysis was performed with
P=0.864, demonstrating the lack of significant publication bias in
the included studies (Fig. 1D and
Table II).
 | Table IIEgger test for 12 studies. |
Table II
Egger test for 12 studies.
| Type of
measurement | Coefficient | Standard error | t-value | P-value
>|t| | 95% CI |
|---|
| Standardized
effect | 1.252067 | 1.427434 | 0.88 | 0.401 |
-1.92845-4.43258 |
| Slope bias | 0.3900094 | 2.221697 | 0.18 | 0.864 |
-4.56024-5.34026 |
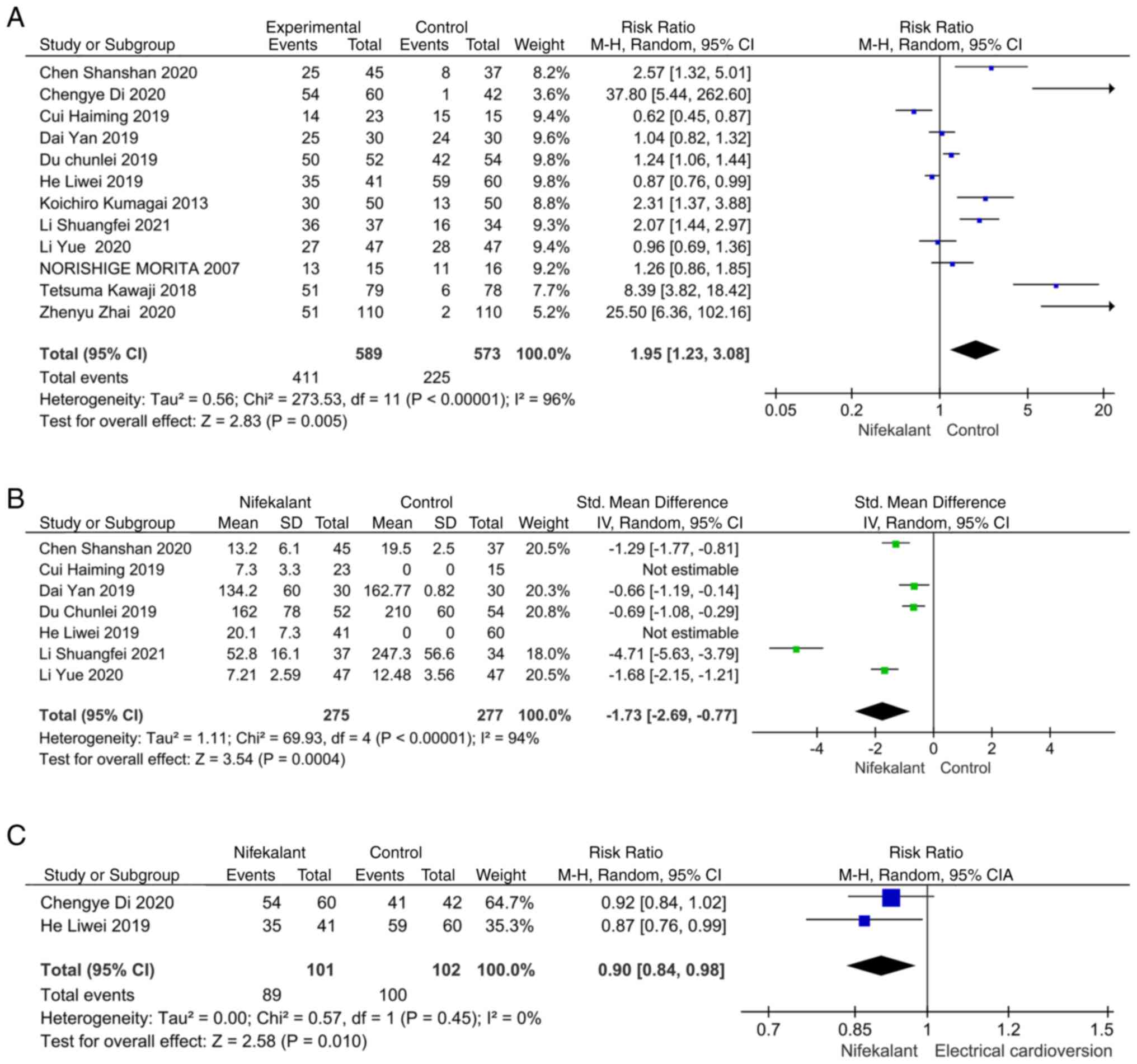
Effect of nifekalant on diversion
success rates
A total of 12 studies were screened for analysis,
nine of which reported the success rate of nifekalant on AF
reversal (Fig. 2A). Some degree of
heterogeneity was present as ascertained through the heterogeneity
test with I2>50. A random effects model was selected
for the analysis and the RR value was 1.95 (95% CI, 1.23-3.08;
P=0.005), which was statistically significant and therefore
demonstrated a success rate of conversion of AF to SR with
nifekalant higher than that achieved with the control treatment
modalities (amiodarone or lidocaine).
Effect of nifekalant on time to
reversal
Out of the 12 screened studies, seven reported
indicators related to time to conversion. Some heterogeneity was
present as indicated by the heterogeneity test with
I2>50. A random effects model was selected for
analysis, and the MD was -1.73 [95% CI, -2.69-(-0.77), P=0.0004],
which was statistically significant, thus demonstrating that
nifekalant had less time to conversion than the control group
(Fig. 2B). The RR value was 0.090
(95% CI, 0.84-0.98; P=0.01) compared with the electro-recovery
group, which was statistically different and demonstrated that the
success rate of electro-recovery was higher than that of nifekalant
(Fig. 2C).
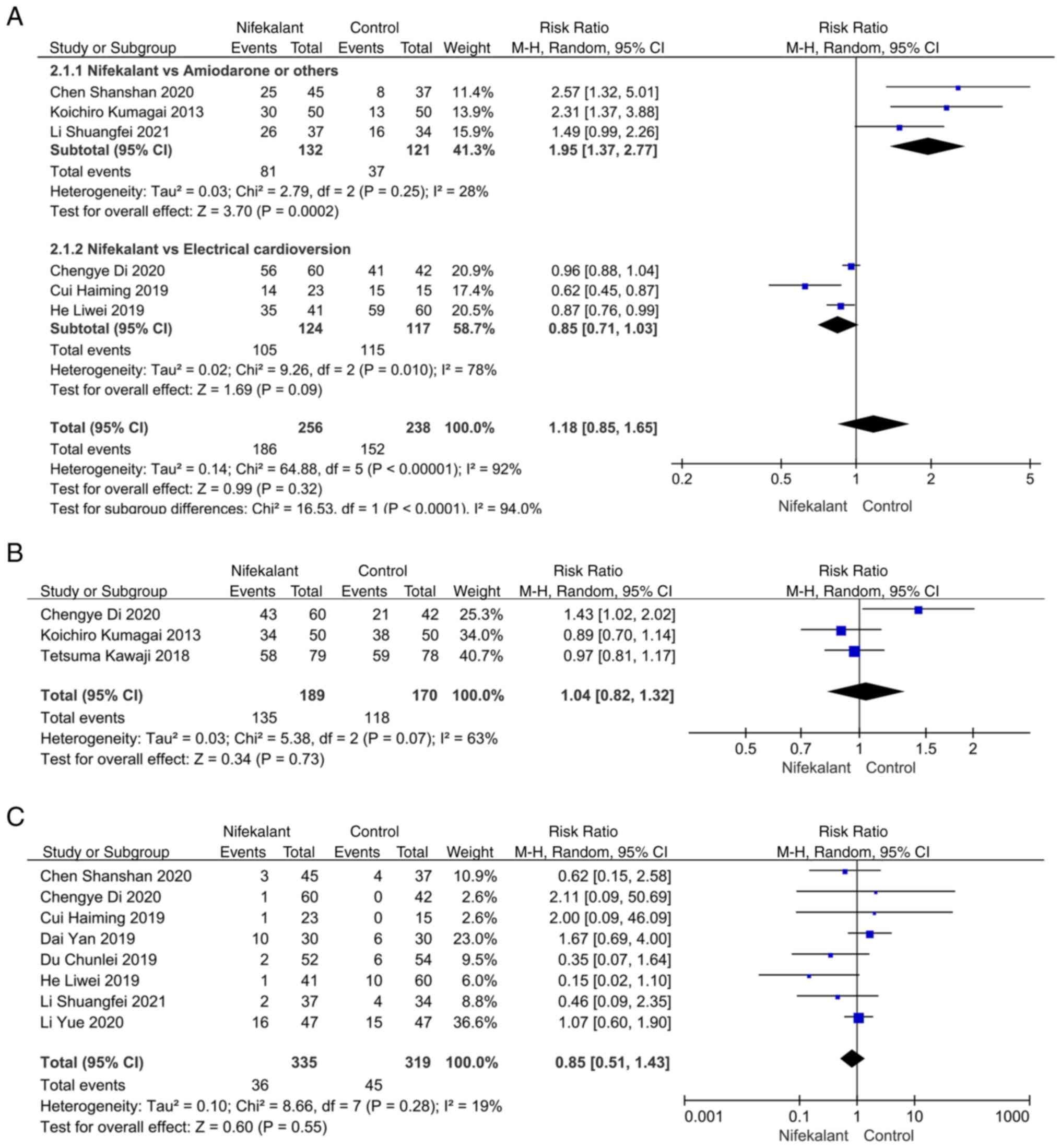
Effect of nifekalant on the success
rate of AF conversion after catheter ablation
A total of six studies reported the success rate of
AF conversion following catheter ablation (18,19,22,23,25,27).
A subgroup analysis was performed due to a heterogeneity test score
of I2>50, and the control group was divided into
amiodarone and electrical resuscitation groups. Compared with the
amiodarone group, I2 was <50 and the RR value was
1.95 (95% CI, 1.37-2.77; P=0.0002), which was statistically
significant, indicating that the success rate of conversion was
higher in the nifekalant compared with the control group. There was
no statistical difference with I2>50 and an RR value
of 0.85 (95% CI, 0.71-1.03; P=0.09), in the nifekalant compared
with the electrical resuscitation group (Fig. 3A). AF recurrence rates after 12
months of follow-up were reported in three studies, with an RR
value of 1.04 (95% CI, 0.82-1.32; P=0.73) and no statistically
significant difference, demonstrating that nifekalant did not
affect AF recurrence differently from the control group (Fig. 3B).
Incidence of adverse events with
nifekalant
A total of eight studies reported the incidence of
adverse events with nifekalant with a heterogeneity of
I2<50 and an RR value of 0.85 (95% CI, 0.51-1.43;
P>0.05) with no statistical difference, demonstrating no
difference in the incidence of adverse events between nifekalant
and the control group (Fig.
3C).
Discussion
AF is one of the most common types of
tachyarrhythmia, whose incidence increases significantly with age,
causing high morbidity, disability and mortality rates (8,29).
In addition, cardiac emergencies, such as acute myocardial
infarction, can induce new-onset AF, which in severe cases can lead
to heart failure and induce severe hemodynamic dysfunction
(30). Therefore, early paroxysmal
AF should be reversed as early as possible. Patients with
persistent AF should receive regular anticoagulation treatment and,
if necessary, interventional therapy may be provided (31,32).
Pharmacological conversion is the most traditional
and classical treatment for patients with AF for restoring the SR
of the heart (33). However, each
of the traditional antiarrhythmic drugs have their advantages and
disadvantages. Propafenone, although recommended as a class I drug,
is <50% effective and is contraindicated in patients with left
ventricular systolic dysfunction and ischemic heart disease due to
its negative ionic nature (34,35).
Amiodarone is recommended as a class III drug, but studies have
shown that it is ineffective in AF reversal and is prone to causing
adverse events (36). Ibutilide,
while it exhibits improved efficacy over the first two, is prone to
QT prolongation and may induce torsional angles and even
ventricular arrhythmias (37).
Catheter radiofrequency ablation is an alternative
and an important treatment for AF and is more effective than
antiarrhythmic drugs. However, due to the specific
pathophysiological mechanism of AF, atrial arrhythmias still recur
in 25-50% of patients following ablation, of which 21-38% are early
recurrences (38). Some patients
still have persistent AF following radiofrequency ablation, and
although the traditional drug amiodarone and electrical
resuscitation can be used to revert sinus rhythm, the time required
for reversion and the need for complex electric shock operations
are increasingly unable to meet the treatment needs of patients
(3,39).
The current high recurrence rate is the main reason
catheter radiofrequency ablation is not widely available (40,41).
The primary treatment options are electrical cardioversion,
intravenous pharmacological cardioversion and oral pharmacological
rhythm control. Although electrical resuscitation can be rapid and
effective, it requires intravenous anaesthesia and may cause skin
burns and myocardial damage, and in some cases may even induce
acute pulmonary oedema (42).
There is also no consensus on the choice of antiarrhythmic drugs
(18).
Nifekalant is a new class III antiarrhythmic drug
that was approved in 1999 for the treatment of ventricular
tachyarrhythmias. Nifekalant is a single-channel blocker that does
not block sodium and calcium channels and has no significant effect
on myocardial cell conduction velocity or myocardial contractility.
Therefore, the incidence of adverse events, such as bradycardia and
hypotension, is low and the efficiency of conversion is high;
however, the effectiveness of nifekalant in the treatment of
postoperative AF recurrence has not been established (43).
In the present study, patient information from 12
studies was comprehensively evaluated to demonstrate the
superiority of nifekalant in AF conversion. The present
meta-analysis showed that nifekalant had a higher success rate of
conversion compared with conventional drugs in the control group,
particularly during radiofrequency ablation and in the treatment of
postoperative recurrence. In the 12-month follow-up study, no
difference was identified between the nifekalant group and the
control group. However, further studies are required, since only
three studies were included, and the sample was small. During
conversion, the incidence of adverse events in the nifekalant group
did not differ from the control group, and although it has been
reported that nifekalant may cause prolongation of the QT interval
or tip-twisting ventricular tachycardia, no significant tendency
was found in the present study (44).
Several limitations should be noted in the present
meta-analysis. Firstly, there were few post-reversal follow-up
studies and the overall sample size was small, with most of the
included studies being in the Chinese region; further clinical
trial study centres are therefore needed to perform validation of
the effects of nifekalant on reversal. Secondly, some of the
studies included in the present analysis were retrospective or
prospective studies with insufficient research evidence. In
addition, although there was no significant variability in the
basic information of the patients included in the current study,
the underlying disease of the patients included and whether the
patients were using other medications was not detailed in the
literature, and therefore there may be a potential confounding
effect; this may have also been a cause of the large heterogeneity.
In addition, there were differences in the treatment modalities of
the controls included in the present study, and although the
majority of the control group was treated with amiodarone, there
was also a proportion of treatments, such as electrical
cardioversion and lidocaine, which may also have contributed to the
heterogeneity of the analysis. The molecular mechanism of
nifekalant in AF reversal is not fully understood; therefore,
further studies of nifekalant in AF regression are required.
In conclusion, patients with AF in the nifekalant
group had a better success rate of conversion and time consumed for
conversion than the control group. In particular, the success rate
of cardiac SR conversion with the aid of nifekalant was
significantly better than that in the control group during catheter
ablation, and there was no difference in the incidence of adverse
events between the two groups. In addition, the results of the
12-month follow-up showed that the incidence of AF recurrence was
not associated with choice of drug.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL, KL and SW designed the study, searched
databases, extracted and assessed the literature and drafted the
manuscript. PL, LW and ML statistically analyzed the data. KW and
LW confirm the authenticity of all the raw data. PL and GS
conceived and designed the present study, provided general
supervision and finalized the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
No authors listed. Atrial fibrillation.
Nat Rev Dis Primers. 8(20)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Baman JR and Passman RS: Atrial
fibrillation. JAMA. 325(2218)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
John RM, Michaud GF and Stevenson WG:
Atrial fibrillation hospitalization, mortality, and therapy. Eur
Heart J. 39:3958–3960. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yandrapalli S, Malik AH, Namrata F,
Pemmasani G, Bandyopadhyay D, Vallabhajosyula S, Aronow WS,
Frishman WH, Jain D, Cooper HA and Panza JA: Influence of diabetes
mellitus interactions with cardiovascular risk factors on
post-myocardial infarction heart failure hospitalizations. Int J
Cardiol. 348:140–146. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sepehri Shamloo A, Dagres N and Hindricks
G: 2020 ESC guidelines on atrial fibrillation: Summary of the most
relevant recommendations and innovations. Herz. 46:28–37.
2021.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
6
|
Kuck KH, Lebedev DS, Mikhaylov EN, Romanov
A, Gellér L, Kalējs O, Neumann T, Davtyan K, On YK, Popov S, et al:
Catheter ablation or medical therapy to delay progression of atrial
fibrillation: The randomized controlled atrial fibrillation
progression trial (ATTEST). Europace. 23:362–369. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li Z, Liu Q, Liu F, Hidru TH, Yang Y, Wang
S, Bai L, Chen J, Yang X and Xia Y: Atrial cardiomyopathy markers
and new-onset atrial fibrillation risk in patients with acute
myocardial infarction. Eur J Intern Med. 102:72–79. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Savelieva I, Graydon R and Camm AJ:
Pharmacological cardioversion of atrial fibrillation with
vernakalant: Evidence in support of the ESC guidelines. Europace.
16:162–173. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lévy S: Cardioversion of recent-onset
atrial fibrillation using intravenous antiarrhythmics: A European
perspective. J Cardiovasc Electrophysiol. 32:3259–3269.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kawaji T, Shizuta S, Yamagami S, Aizawa T,
Komasa A, Yoshizawa T, Kato M, Yokomatsu T, Miki S, Ono K and
Kimura T: Clinical utility of intravenous nifekalant injection
during radiofrequency catheter ablation for persistent atrial
fibrillation. J Atr Fibrillation. 11(1839)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Harayama N, Nihei SI, Nagata K, Aibara K,
Kamochi M and Sata T: Drug therapy for shock-resistant ventricular
fibrillation: Comparison of nifekalant and amiodarone. J UOEH.
38:35–46. 2016.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
12
|
Kofune T, Watanabe I, Okubo K, Okumura Y,
Masaki R, Shindo A and Saito S: Effect of IKr blocker nifekalant on
atrial action potential duration after successful internal
cardioversion of chronic atrial fibrillation. Pacing Clin
Electrophysiol. 28:391–396. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sekita G, Sawaki D, Otani Y, Kobayakawa N,
Fukushima K, Takeuchi H and Aoyagi T: Pretreatments with a novel
pure potassium channel blocker, nifekalant, were effective in the
electrical atrial defibrillation: A report of two cases. Cardiovasc
Drugs Ther. 16:551–552. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tagami T, Yasunaga H and Yokota H:
Antiarrhythmic drugs for out-of-hospital cardiac arrest with
refractory ventricular fibrillation. Crit Care.
21(59)2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dong Y, Zhai Z, Zhu B, Xiao S, Chen Y, Hou
A, Zou P, Xia Z, Yu J and Li J: Development and validation of a
novel prognostic model predicting the atrial fibrillation
recurrence risk for persistent atrial fibrillation patients treated
with nifekalant during the first radiofrequency catheter ablation.
Cardiovasc Drugs Ther: Jun 22, 2022 (Epub ahead of print).
|
|
16
|
Eikelboom R, Sanjanwala R, Le ML,
Yamashita MH and Arora RC: Postoperative atrial fibrillation after
cardiac surgery: A systematic review and meta-analysis. Ann Thorac
Surg. 111:544–554. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cumpston M, Li T, Page MJ, Chandler J,
Welch VA, Higgins JP and Thomas J: Updated guidance for trusted
systematic reviews: A new edition of the cochrane handbook for
systematic reviews of interventions. Cochrane Database Syst Rev.
10(ED000142)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Di C, Gao P, Wang Q, Wu Y and Lin W:
Intraprocedural conversion efficacy of intravenous nifekalant
administration for persistent atrial fibrillation after pulmonary
vein isolation. Int Heart J. 61:1157–1164. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kumagai K and Toyama H: Usefulness of
ablation of complex fractionated atrial electrograms using
nifekalant in persistent atrial fibrillation. J Cardiol. 61:44–48.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Morita N, Tanaka K, Yodogawa K, Hayashi M,
Akutsu K, Yamamoto T, Satoh N, Kobayashi Y, Katoh T and Takano T:
Effect of nifekalant for acute conversion of atrial flutter: The
possible termination mechanism of typical atrial flutter. Pacing
Clin Electrophysiol. 30:1242–1253. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhai Z, Xia Z, Xia Z, Hu J, Hu J, Zhu B,
Xiong Q, Wu Y, Hong K, Chen Q, et al: Comparison of the efficacy
and safety of different doses of nifekalant in the instant
cardioversion of persistent atrial fibrillation during
radiofrequency ablation. Basic Clin Pharmacol Toxicol. 128:430–439.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chen S, Tao S, Yang Z, Wei W and Han M:
Observation on the efficacy of nifedipine in the conversion of
atrial fibrillation patients undergoing radiofrequency ablation.
Chin J Card Pacing Electrophysiol. 342–344.
2020.doi:10.13333/j.cnki.cjcpe.2020.04.006.
|
|
23
|
He LW, Huang XF, Liu SR and Peng J:
Initial use of nifedipine in radiofrequency ablation of atrial
fibrillation. Chin J Card Pacing Electrophysiol. 233–235.
2019.doi:10.13333/j.cnki.cjcpe.2019.03.007.
|
|
24
|
Cui HM, Tang YH, Wan WT, Yang J, Zhang J
and Liao D: Efficacy of Nifikalan in persistent atrial fibrillation
without end-reversal by radiofrequency ablation. Chin J Card Pacing
Electrophysiol. 28–31.
2019.doi:10.13333/j.cnki.cjcpe.2019.01.007.
|
|
25
|
Li S, Li D, Wang Q, Di Y, Zhang H, Zhao Y,
Xu M and Wenping ZD: Efficacy of nifikalan on early recurrence
after radiofrequency ablation of atrial fibrillation. Chin J Card
Pacing Electrophysiol. 439–442.
2021.doi:10.13333/j.cnki.cjcpe.2021.05.009.
|
|
26
|
Du C, Guo M, Zhang Y, Liang H, Tian S,
Wang Z and Song Y: Comparative study on the efficacy and safety of
nifikalan and amiodarone in the treatment of new-onset atrial
fibrillation in acute myocardial infarction. J Tianjin Med Univ.
471–474+480. 2019.
|
|
27
|
Li Y, Yang R and Lin W: Analysis of the
effects of nifikalan on cardiac function indices and adverse
effects in patients with atrial fibrillation. Chin Prescription
Drugs. 89–90. 2020.
|
|
28
|
Dai Y, Xie H, Kan J and Zhou P: Comparison
of the efficacy of intravenous application of nifikalan and
amiodarone in patients with paroxysmal atrial fibrillation. Lingnan
J Cardiovasc Dis. 192–194. 2019.
|
|
29
|
Vora P, Morgan Stewart H, Russell B,
Asiimwe A and Brobert G: Time trends and treatment pathways in
prescribing individual oral anticoagulants in patients with
nonvalvular atrial fibrillation: An observational study of more
than three million patients from Europe and the United States. Int
J Clin Pract. 2022(6707985)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Carlisle MA, Fudim M, DeVore AD and
Piccini JP: Heart failure and atrial fibrillation, like fire and
fury. JACC Heart Fail. 7:447–456. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Obayashi Y, Shiomi H, Morimoto T, Tamaki
Y, Inoko M, Yamamoto K, Takeji Y, Tada T, Nagao K, Yamaji K, et al:
Newly diagnosed atrial fibrillation in acute myocardial infarction.
J Am Heart Assoc. 10(e021417)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li M, Gao Y, Guo K, Wu Z, Lao Y, Li J,
Huang X, Feng L, Dong J and Yuan Y: Association between fasting
hyperglycemia and new-onset atrial fibrillation in patients with
acute myocardial infarction and the impact on short- and long-term
prognosis. Front Cardiovasc Med. 8(667527)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Heldal M and Atar D: Pharmacological
conversion of recent-onset atrial fibrillation: A systematic
review. Scand Cardiovasc J Suppl. 47:2–10. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Boriani G, Martignani C, Biffi M, Capucci
A and Branzi A: Oral loading with propafenone for conversion of
recent-onset atrial fibrillation: A review on in-hospital
treatment. Drugs. 62:415–423. 2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Botto GL, Bonini W, Broffoni T, Espureo M,
Cappelletti G, Lombardi R, Molteni S, Pedraglio E and Ferrari G:
Randomized, crossover, controlled comparison of oral loading versus
intravenous infusion of propafenone in recent-onset atrial
fibrillation. Pacing Clin Electrophysiol. 21:2480–2484.
1998.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Freemantle N, Lafuente-Lafuente C,
Mitchell S, Eckert L and Reynolds M: Mixed treatment comparison of
dronedarone, amiodarone, sotalol, flecainide, and propafenone, for
the management of atrial fibrillation. Europace. 13:329–345.
2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hanley CM, Robinson VM and Kowey PR:
Status of antiarrhythmic drug development for atrial fibrillation:
New drugs and new molecular mechanisms. irc Arrhythm
Electrophysiol. 9(e002479)2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zink MD, Chua W, Zeemering S, di Biase L,
Antoni BL, David C, Hindricks G, Haeusler KG, Al-Khalidi HR,
Piccini JP, et al: Predictors of recurrence of atrial fibrillation
within the first 3 months after ablation. Europace. 22:1337–1344.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chew DS, Li Y, Cowper PA, Anstrom KJ,
Piccini JP, Poole JE, Daniels MR, Monahan KH, Davidson-Ray L,
Bahnson TD, et al: Cost-effectiveness of catheter ablation versus
antiarrhythmic drug therapy in atrial fibrillation: The CABANA
randomized clinical trial. Circulation. 146:535–547.
2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Grimaldi M, Di Monaco A, Gomez T, Berman
D, Datta K, Sharma T, Govari A, Altmann A and Di Biase L: Time
course of irreversible electroporation lesion development through
short- and long-term follow-up in pulsed-field ablation-treated
hearts. Circ Arrhythm Electrophysiol. 15(e010661)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Han J, Li G, Zhang D, Wang X and Guo X:
Predicting late recurrence of atrial fibrillation after
radiofrequency ablation in patients with atrial fibrillation:
Comparison of C2HEST and HATCH scores. Front Cardiovasc Med.
9(907817)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Van Gelder IC, Hagens VE, Bosker HA,
Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ,
Tijssen JG, et al: A comparison of rate control and rhythm control
in patients with recurrent persistent atrial fibrillation. N Engl J
Med. 347:1834–1840. 2002.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yang L, Yu L, Chen Z and Zhang M:
Nifekalant: A new option for pre-excited atrial fibrillation with a
high-risk accessory pathway. JACC Case Rep. 2:235–239.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wang J, Hua W, Zhu J, Yang YM, Wang FZ, Pu
JL, Chen KP and Zhang S: Nifekalant hydrochloride terminating
sustained ventricular tachycardia accompanied with QT dispersion
prolongation. Chin Med J (Engl). 123:2028–2033. 2010.PubMed/NCBI
|