Introduction
Pulpitis is one of the most common dental pulp
diseases, which brings a great burden to the life of afflicted
individuals (1). Pulpitis includes
reversible pulpitis and irreversible pulpitis (IP). IP indicates a
more severe degeneration process, which, if left untreated, leads
to pulp necrosis followed by apical periodontitis (2). Dental pulp cells (DPCs), the main
components of the dental pulp, serve a deterministic role in innate
immune response and inflammatory reactions (3), which can secrete inflammatory factors
and chemokines, thus initiating and regulating the inflammatory
response (4). As potential
critical targets, DPCs have therefore acted as the
anti-inflammation target in pulpitis treatment.
Wnts belong to a highly conserved family of secreted
growth factors, which couple to various receptors, thereby
activating different downstream pathways which classify as either
canonical or noncanonical signaling pathways (5). The Wnt pathway controls cellular
processes, such as proliferation, differentiation and migration
(5). Wnt4 is known to regulate
noncanonical Wnt signaling (6). It
has been proved to serve a role in pulpitis. Wnt4 promotes the
recovery of differentiation of dental pulp stem cells into dentin
cells in pulpitis through the JNK signaling pathway (7). However, the role of Wnt4 in pulpitis
remains to be elucidated.
The NF-κB pathway mediates inflammation, immunity
and cell survival (8,9). Extracellular stimulation is
recognized by the receptor and transmitted to initiate a cascade
and IKK is activated by a cascade signal. Activated IKK
phosphorylates IκB at specific N-terminal serine residue and the
phosphorylated IκB is ubiquitinated and degraded. Then, the NF-κB
is released from the inhibitory complex and translocated into the
nucleus, regulating the expression of multiple genes (10,11).
Evidence has shown that NF-κB controls the development of pulpitis
(12,13). Meanwhile, Wnt4 regulates the
expression of NF-κB. For example, Wnt4 alleviates bone loss and
inflammation by suppressing NF-κB in vivo (14). Melatonin inhibits NF-κB in a
dependent manner with Wnt4(15).
Based on the aforementioned studies, it was
hypothesized that Wnt4 serves an anti-inflammatory and
anti-apoptotic role in pulpitis through the NF-κB signaling
pathway. The present study investigated the effect and underlying
mechanisms of Wnt4 on the inflammatory levels and apoptosis of
lipopolysaccharide (LPS)-induced human dental pulp cells
(LPS-HDPCs), which might provide a novel therapy target for
pulpitis. In addition, exploring effective treatment target
therapeutic targets combined with advanced materials such as
polyvinylidene fluoride/barium titanate composites (16) and antibacterial scaffold (17) applications could advance the
treatment of pulpitis.
Materials and methods
Isolation, culture and treatment of
cells
Normal human impacted third molars free of carious
lesions and oral infection were obtained from patients in Anhui
Medical College after informed consent was collected from each
patient. HDPCs were isolated from the dental pulp tissues of
non-decayed third molars. In brief, human dental pulp tissue
obtained from sectioned teeth was removed aseptically and minced
into small pieces. The pulp was treated with 3 mg/ml collagenase
type I solution for 0.5 h at 37˚C. The digested tissues were
cultured in DMEM (Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Thermo Fisher Scientific, Inc.), 300 mg/ml L-glutamine
(Thermo Fisher Scientific, Inc.) and 100 U/ml antibiotics (Thermo
Fisher Scientific, Inc.) in an incubator at 37˚C with 5%
CO2; 3 to 5 passages were used for the experiments. For
LPS stimulation, LPS (10 µg/ml) was administrated for 24 h as
reported previously (18). NF-κB
inhibitor (BAY11-7082; 10 Mm; Sigma-Aldrich; Merck KGaA) was used
to treat HDPCs at 37˚C for 6 h and cells were acquired for
follow-up experiments.
Immunofluorescence
HDPCs were cultured in an 8-well slide for 24 h.
Then cells were fixed with 4% paraformaldehyde at room temperature
for 15 min, permeabilized with 0.5% Triton X-100 (Thermo Fisher
Scientific, Inc.) and blocked with goat serum (cat. no. SL038;
Beijing Solarbio Science & Technology Co., Ltd.) at room
temperature for 20 and 30 min, respectively. Then primary
anti-keratin (cat. no. 13063; 1:200; CST), anti-vimentin (cat. no.
5741; 1:600; CST) antibodies and anti-p-p65 (cat. no. 3033; 1:800l;
CST) were applied to incubate HDPCs at 4˚C overnight. Next, the
cells were cultured in secondary antibody goat against rabbit IgG
(cat. no. A32731; 1:200; Thermo Fisher Scientific, Inc.) at room
temperature for 1 h. DAPI (1:1,000) was applied for nuclei
staining. The images were obtained using a confocal microscope
(Leica Microsystems GmbH) (19).
Western blotting
Cells were harvested and lysed a lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd.). Protein
concentrations were measured using a BCA kit (Thermo Fisher
Scientific, Inc.). Proteins (25 µg per lane) were separated using
10% SDS-PAGE and transferred onto a PVDF membrane (BD Biosciences).
The membrane was blocked with 5% skimmed milk at room temperature
for 60 min and incubated with following primary antibodies: IL-8
(cat. no. ab289967; 1:1,200; Abcam), IL-6 (cat. no. ab233706;
1:1,000; Abcam), TNF-α (cat. no. ab183218; 1:1,000; Abcam), IL-1β
(cat. no. ab254360; 1:1,000; Abcam), cleaved-caspase-3 (cat. no.
ab32042; 1:800; Abcam), caspase-3 (cat. no. ab32351; 1:3,000;
Abcam), Bax (cat. no. 2774S; 1:1,000; CST), Bcl-2 (cat. no. 15071;
1:1,000; CST), Wnt4 (cat. no. ab277798; 1:800, Abcam), p-IKK2 (cat.
no. 2694; 1:1,000; CST), IKK (cat. no. ab124957; 1:1,000; Abcam),
p-p65 (cat. no. 3033; 1:1,000; CST), p65 (cat. no. 8242; 1:1,000;
CST), p-IκBα (cat. no. 2859; 1:1,000; CST), IκBα (cat. no. 9242;
1:1,000; CST), β-actin (cat. no. 4970; 1:5,000; CST), GAPDH (cat.
no. 5174; 1:5,000; CST) at 4˚C overnight. Then, the HRP-conjugated
secondary antibody (goat against rabbit IgG (cat. no. 7074;
1:2,000; CST) and goat against mouse IgG (cat. no. 96714; 1:3,000;
CST) was administrated to the cells at room temperature for 1 h.
The results were detected using an ECL kit (Beyotime Institute of
Biotechnology) and quantitated using ImageJ (National Institutes of
Health) (18).
Flow cytometry
Treated HDPCs were collected, washed and resuspended
with binding buffer (PBS with 5% FBS). Subsequently, HDPCs were
incubated with FITC annexin V at room temperature for 0.5 h and
washed using PBS. Propidium iodide (PI; 50 mg/ml) was used to
culture HDPCs at room temperature for 5 min in the dark. Analysis
was performed using flow cytometry by a Cytomics FC500 flow
cytometer (Beckman Coulter, Inc.) and Cytomics CXP software version
2.2 (Beckman Coulter, Inc.). Untreated HDPCs were used as the
control (20). Apoptosis rate=the
percentage of early + late apoptotic cells.
Reverse transcription-quantitative
(RT-q) PCR
HDPCs were seeded into six-well plate
(1x106 cells/well) and total RNA was extracted using
TRIzol® according to the manufacturer's protocol
(Invitrogen; Thermo Fisher Scientific, Inc.) after cells were
treated with LPS or transfected for 48 h. Reverse transcription was
executed with PrimeScript RT reagent kit (Takara Bio, Inc.) at 42˚C
for 40 min and 85˚C for 5 min and 4˚C for 60 min according to the
manufacturer's protocol. RT-qPCR was conducted with SYBR-Green I
Master Mix (Roche Diagnostics) via LightCycler 480 (Roche
Diagnostics) according to the manufacturer's protocols. Three
parallel spaces were conducted for each sample and experiment was
performed three times. PCR amplification conditions were: 95˚C for
10 min, followed by 40 cycles of denaturation at 95˚C for 15 sec
and annealing 60˚C for 1 min and extension 72˚C for 20 sec. The
relative changes were calculated using the 2-ΔΔCq method
(18). The primer sequences were:
Wnt4 forward 5'-ACCTGGAAGTCATGGACTCG-3', reverse
5'-TCAGAGCATCCTGACCACTG-3'. GAPDH Forward:
5'-CTGCCCAGAACATCATCC-3', Reverse: 5'-CTCAGATGCCTGCTTCAC-3'.
Cell transfection
The Wnt4 overexpression plasmids (pcDNA-Wnt4) were
constructed using pcDNA3.1 vector by Shanghai GenePharma Co., Ltd.
and empty plasmids were used as normal control (NC). The
constitutive activator of IKK2 (pCMV-IKK2EE) constructed using
pCMV-MCS vector (21) and empty
plasmids (pCMV-NC) were obtained from OriGene Technologies, Inc.
IKK2EE primer: Forward: 5'-GCTCTAGAGCCACCATGAGCTGGTCACCTT-3', and
reverse:
5'-TATGCGGCCGCATCAAGCGTAGTCTGGGACGTCGTATGGGTATGAGGCCTGCTCCAGGCAGCTGTGCTCTTCTT-3'.
HDPCs were inoculated into six-well plates (2x105
cells/well) and when confluence reached 70-80%, the cells were
transfected with 2.5 µg pcDNA-NC/pcDNA-Wnt4 and pCMV-IKK2EE/pCMV-NC
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37˚C for 48 h. Subsequent experiments were
performed 48 h after transfection (21).
Statistical analysis
All experiments were conducted three times and the
data were presented as mean ± standard deviation. Analysis used
GraphPad Prism 8.0 via one-way ANOVA followed by a Bonferroni test
or an unpaired Student's t-test. P<0.05 was considered
statistically significant.
Results
LPS induces inflammatory response and
apoptosis in HDPCs
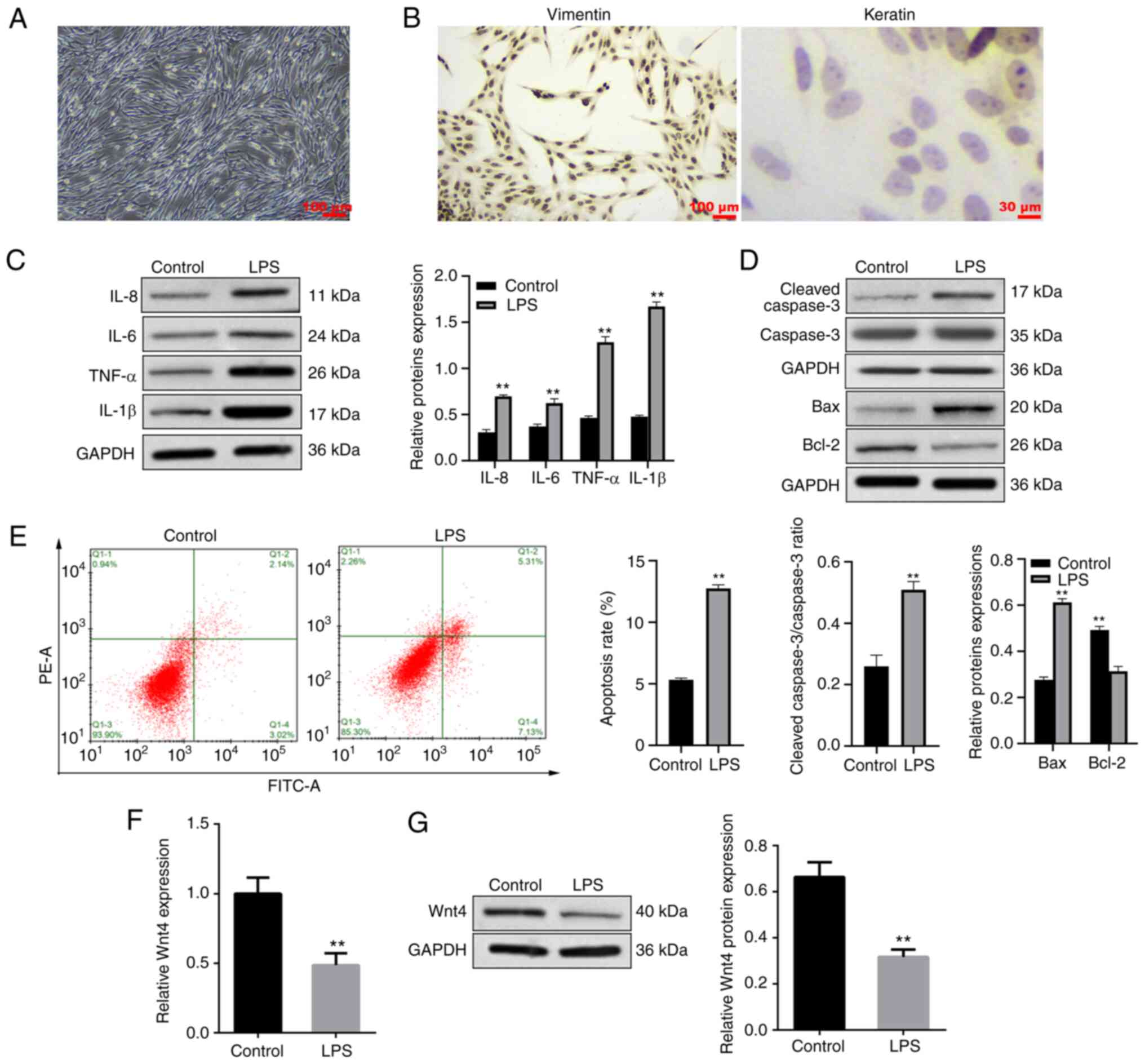
Morphological observation identified that HDPCs of
the third generation were fusiform or polygon (Fig. 1A). Immunofluorescence demonstrated
that the HDPCs were positive for vimentin and negative for keratin
(Fig. 1B), indicating that HDPCs
were derived from mesenchymal tissue and conformed to the
histological features of the pulp. HDPCs were treated with 10 µg/ml
LPS for 24 h to construct a pulpitis cell model. Western blotting
was applied to measure the expressions of proinflammatory cytokines
and apoptosis-related proteins. It was found that the expressions
of proinflammatory cytokines (IL-8, IL-6, TNF-α and IL-1β) were
significantly increased in the LPS group (Fig. 1C). The expressions of Bax and
cleaved-caspase 3 were upregulated while Bcl-2 was downregulated in
the LPS group (Fig. 1D).
Meanwhile, the results of flow cytometry indicated that LPS
dramatically induced the apoptosis of HDPCs (Fig. 1E).
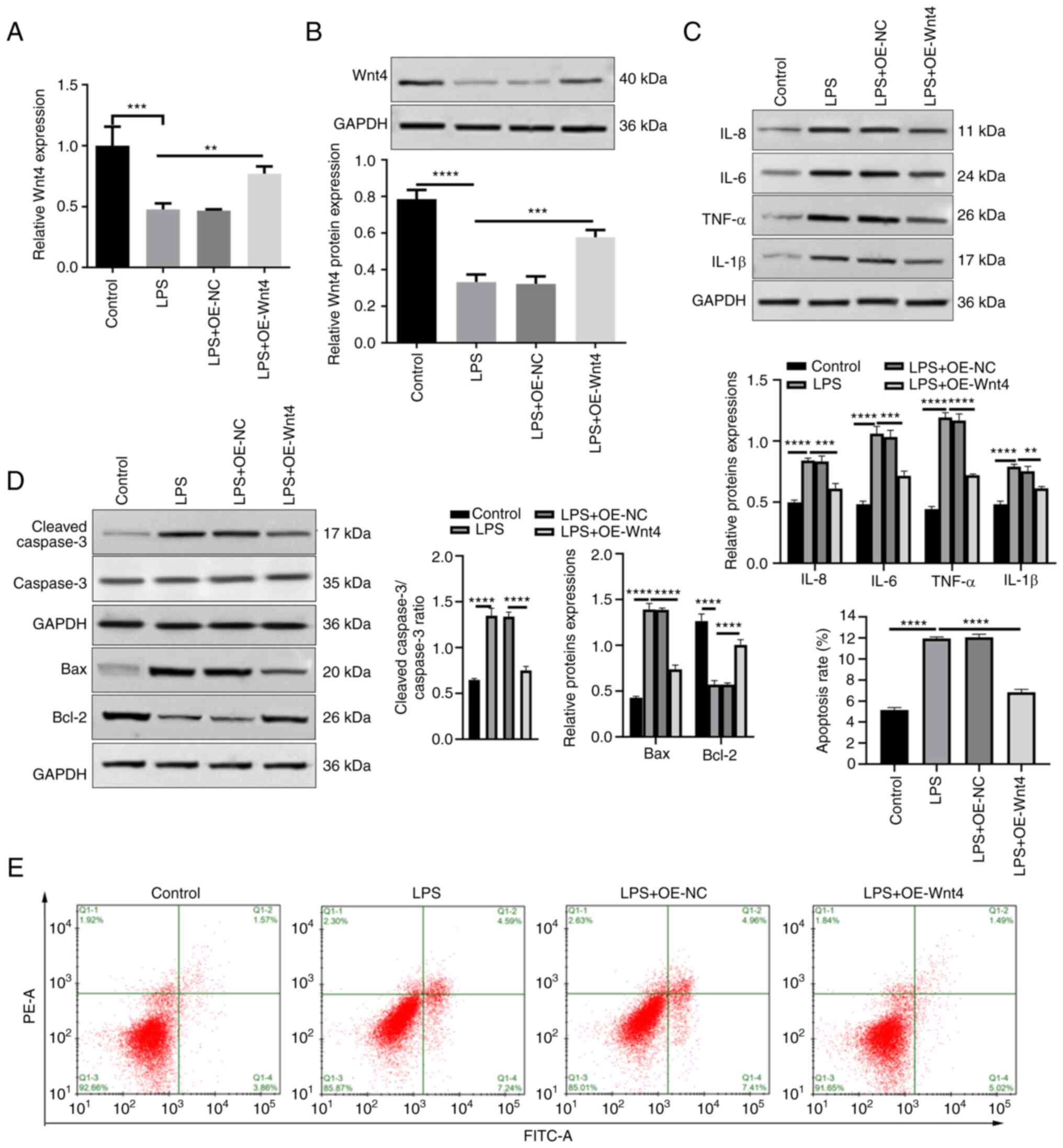
Overexpression of Wnt4 inhibits
inflammation and apoptosis in HDPCs induced by LPS
The expression of Wnt4 in HDPCs was detected. In
Fig. 1F and G, LPS significantly inhibited the
expression of Wnt4 in HDPCs. To explore the effect of Wnt4 on
LPS-induced inflammation and apoptosis, Wnt4 was overexpressed with
plasmids in LPS-HDPCs. The results showed that the mRNA and protein
levels of Wnt4 were significantly increased in the LPS + OE-Wnt4
group compared with LPS + OE-NC and LPS groups (Fig. 2A and B, P<0.01). Overexpression of Wnt4
significantly inhibited the upregulation of inflammatory factors
induced by LPS in HDPCs (Fig. 2C).
The results of western blotting showed that Bax and cleaved-caspase
3 were reduced, while Bcl-2 was increased in LPS + OE-Wnt4 group
compared with the LPS + OE-NC group and LPS groups (Fig. 2D). Meanwhile, the result of flow
cytometry showed that overexpression of Wnt4 significantly reduced
LPS-induced apoptosis (Fig.
2E).
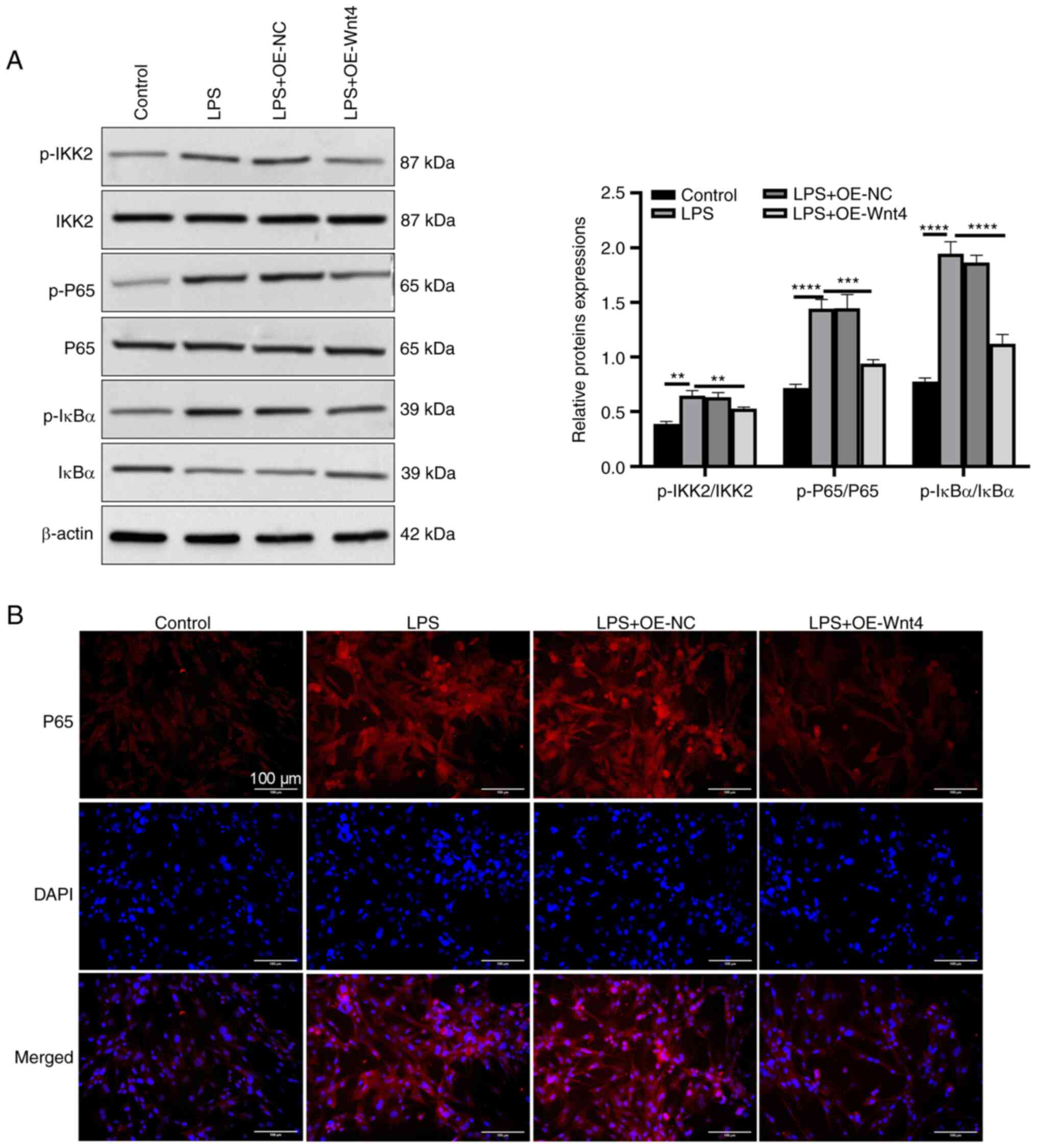
Wnt4 suppresses the activation of the
IKK/NF-κB pathway by LPS
It has been found that Wnt4 inhibits the NF-kB
pathway in the bone disease mouse model (14). To explore whether Wnt4 regulated
the activation of the NF-kB pathway in LPS-HDPCs, the present study
detected the expressions of NF-κB pathway-related proteins. The
results of western blotting presented that LPS upregulated the
phosphorylation levels of IKK2, IκBα and p65, while overexpression
of Wnt4 downregulated the phosphorylation levels of IKK2, IκBα and
p65 (Fig. 3A). In addition, the
result of immunofluorescent assay demonstrated that p65 nuclear
translocation was promoted in LPS-HDPCs compared with the control
group, overexpression of Wnt4 effectively inhibited the nuclear
translocation of p65 (Fig.
3B).
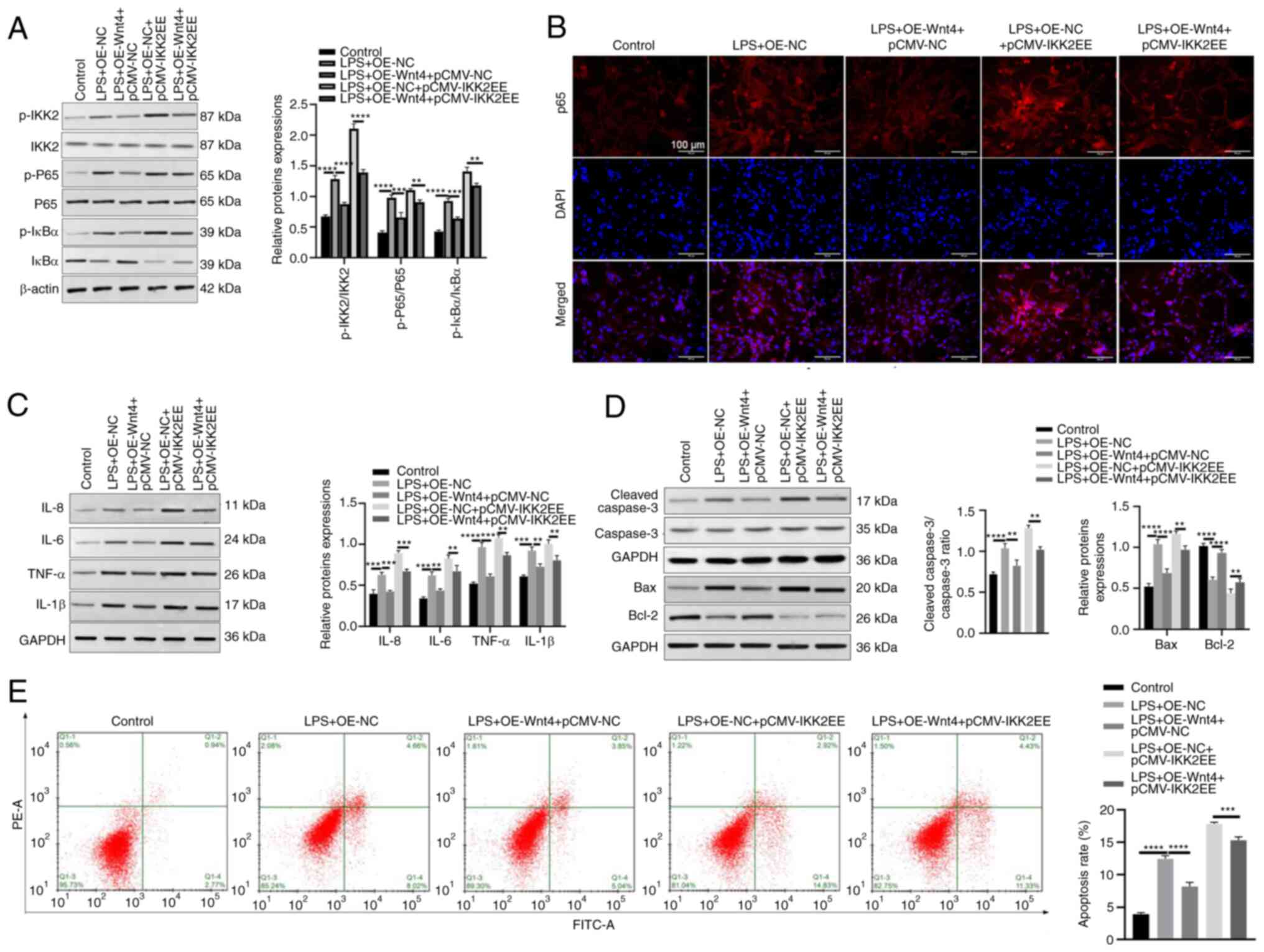
Wnt4 inhibits inflammation and
apoptosis through IKK/NF-κB pathway in LPS-HDPCs
To investigate whether IKK/NF-κB pathway was
involved in the regulation of Wnt4 on inflammation and apoptosis in
LPS-HDPCs, pcDNA-Wnt4 or negative control and pCMV-IKK2EE which
could mimic the IKK2 loop to activate the downstream NF-κB
signaling pathway (21), or
negative control were transfected into LPS-HDPCs. The results of
western blotting revealed that overexpression of Wnt4 reduced the
upregulated phosphorylation levels of IKK2, IκBα and p65 induced by
LPS while overexpression of IKK2EE reversed the effect of Wnt4
(Fig. 4A). The result of
immunofluorescence demonstrated that Wnt4 significantly suppressed
p65 nuclear translocation induced by LPS, while overexpression of
IKK2 significantly promoted p65 nuclear translocation in the LPS +
OE-Wnt4 + OE-IKK2EE group (Fig.
4B). Moreover, the detection results of inflammatory factors
showed that overexpression of IKK2EE increased the expressions of
inflammatory factors (IL-8, IL-6, TNF-α and IL-1β) and reversed the
inhibitory effect of Wnt4 on LPS induced upregulation of
inflammatory factors IL-8, IL-6, TNF-α and IL-1β (Fig. 4C). Moreover, the results of western
blotting showed that the expressions of Bax and cleaved-caspase 3
were significantly downregulated, Bcl-2 was upregulated in the LPS
+ OE-Wnt4 + pCMV-NC group as compared with the LPS + OE-NC +
pCMV-NC group, while the expressions of Bax and cleaved-caspase 3
were strikingly increased, Bcl-2 was decreased in the LPS + OE-Wnt4
+ pCMV-IKK2EE group as compared with LPS + OE-Wnt4 + pCMV-NC group
(Fig. 4D). Meanwhile, the result
of flow cytometry indicated that the apoptosis rate of HDPCs was
significantly downregulated in the LPS + OE-Wnt4 + pCMV-NC group as
compared with the LPS + OE-NC + pCMV-NC group, while the apoptosis
rate was remarkably increased in the LPS + OE-Wnt4 + pCMV-IKK2EE
group as compared with LPS + OE-Wnt4 + pCMV-NC group (Fig. 4E)
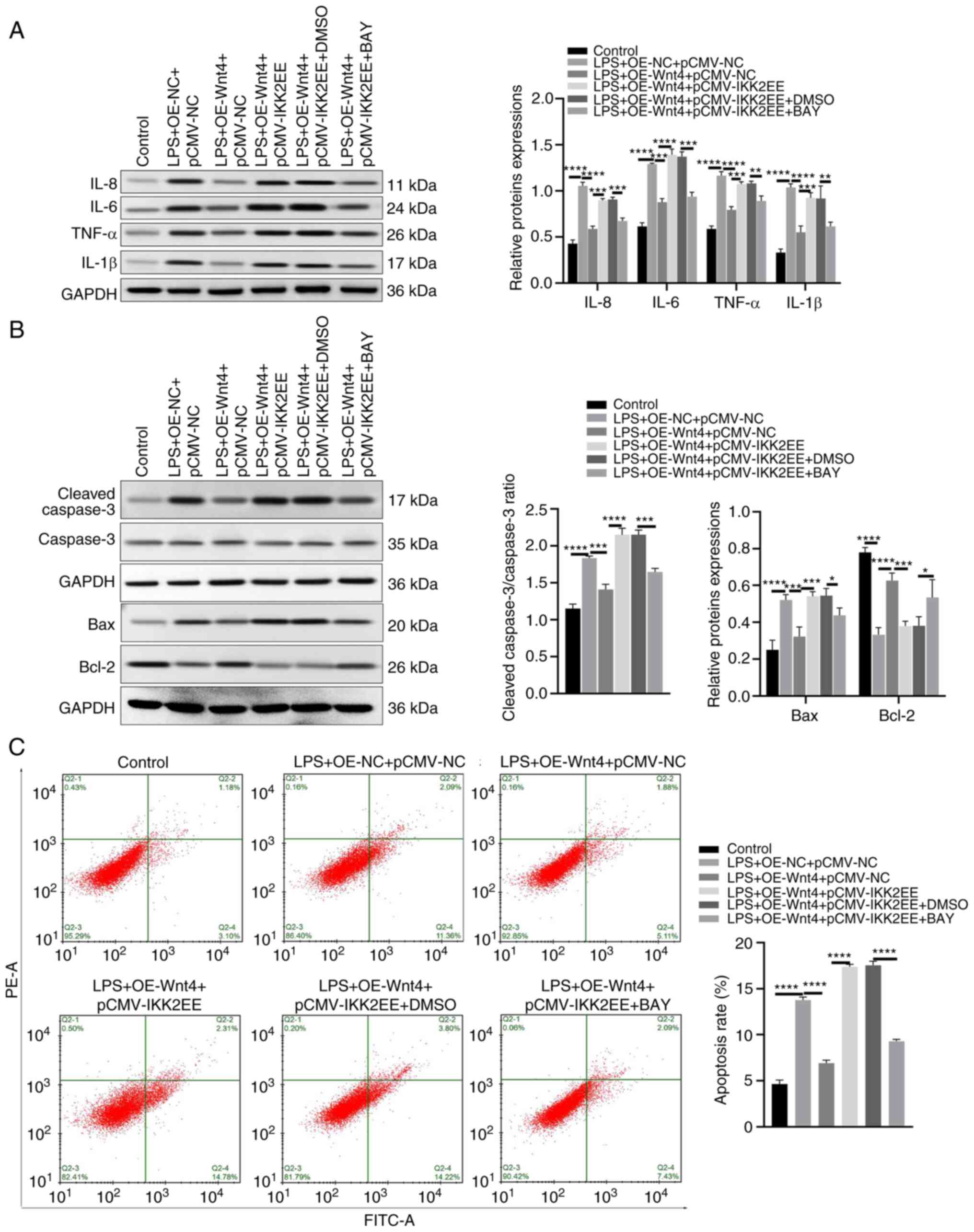
To further explore the effect of the IKK/NF-κB
pathway on the inflammatory and apoptosis of HDPCs overexpressing
of Wnt4. NF-κB inhibitor (BAY11-7082) was used to treat HDPCs
co-transfected with OE-Wnt4 and pCMV-IKK2EE. The results showed
that BAY11-7082 decreased the expression levels of IL-8, IL-6,
TNF-α and IL-1β in the LPS + OE-Wnt4 + pCMV-IKK2EE + BAY group
compared with the LPS + OE-Wnt4 + pCMV-IKK2EE + DMSO group
(Fig. 5A). Similarly, The
expression levels of Bax and cleaved-caspase 3 were markedly
weakened and Bcl-2 expression was raised by in the LPS + OE-Wnt4 +
pCMV-IKK2EE + BAY group compared with the LPS + OE-Wnt4 +
pCMV-IKK2EE + DMSO group (Fig.
5B). Adding BAY11-7082 reduced the apoptosis of OE-Wnt4 and
pCMV-IKK2EE co-transfected HDPCs (Fig.
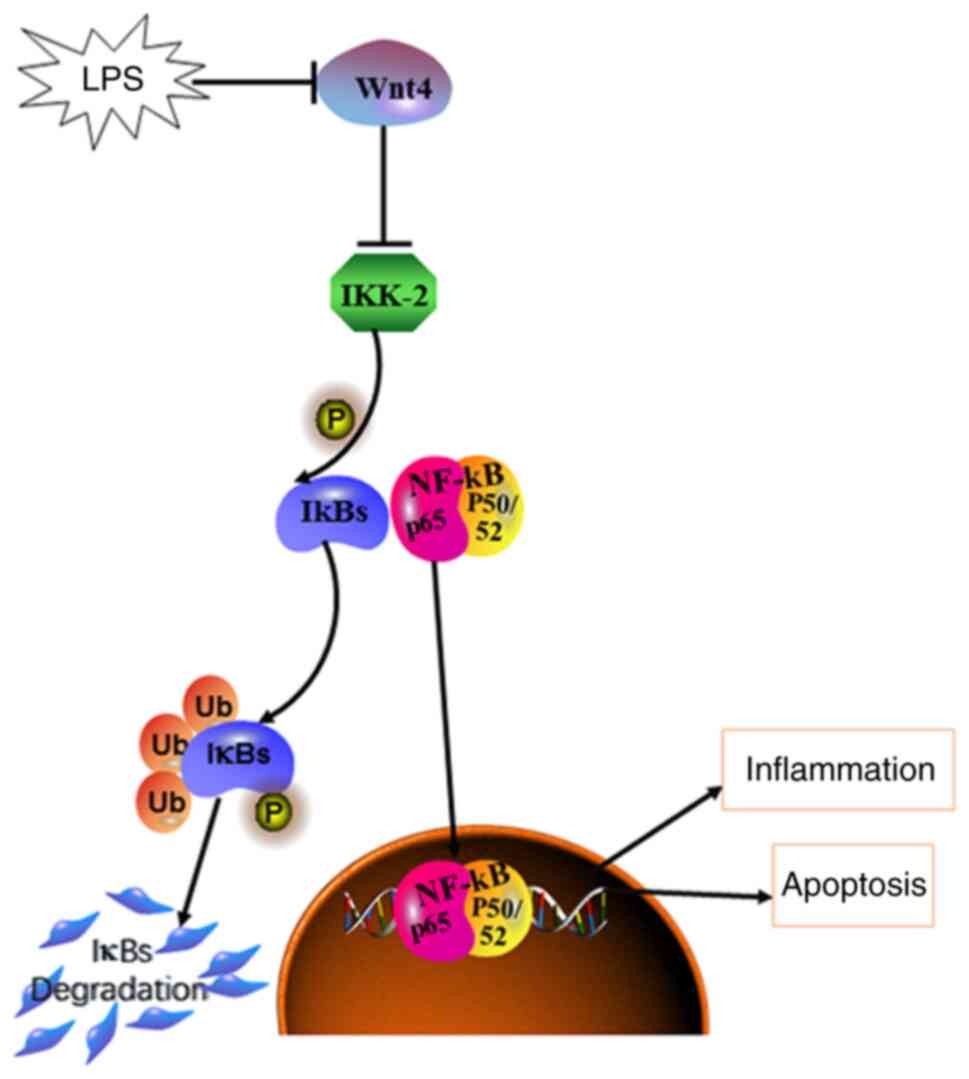
5C). Collectively, these results suggested that Wnt4 alleviated
inflammation and apoptosis via IKK/NF-κB pathway in LPS-HDPCs
(Fig. 6).
Discussion
Pulpitis is a physiological response to bacterial
infections, physical and chemical injuries (22). Bacterial invasion triggers immune
reactions to initiate pulpitis (23). Pulpitis may continuously develop
into pulp necrosis, periapical periodontitis and severe infections
(24). Exploring the abnormal
molecular changes in the pathological process of pulpitis can
provide new ideas for the prevention and treatment of pulpitis.
LPS is an important inducer of pulpitis.
Administration of LPS to dentinal surfaces can trigger pulpal
inflammatory responses in mice (25) and can cause upregulation of
inflammatory factors and adhesion molecules in HDPCs (26). LPS also induces apoptosis of
odontoblast-like cells (27). The
present study also verified that LPS significantly induced the
increased expressions of inflammatory factors IL-8, IL-6, TNF-α and
IL-1β as well as the increase of apoptosis rate in HDPCs, which
were consistent with previous studies that LPS increased the
expression of IL-1β and IL-6 (26,28).
Wnt4 is reported as a nonclassical Wnt signaling
pathway component to exert crucial roles in physiological and
pathological conditions (29).
Wnt4 mediates the protective effect of mesenchymal stromal cells on
vascular endothelial cell apoptosis (30). Wnt4 contributes to
cisplatin-induced acute kidney injury (31). In addition, Wnt4 can promote bone
repair by improving the osteogenic potential of inflammatory dental
pulp stem cells (32). Wnt4 has
also been shown to have a potential effect on pulpitis (7). The present study found that the
expression level of Wnt4 was decreased in LPS-stimulated HDPCs, and
that the upregulation of inflammatory factors induced by LPS was
significantly decreased after overexpression of Wnt4.
Overexpression of Wnt4 also significantly reduced the levels of
apoptosis-related proteins and the apoptosis of HDPCs.
NF-κB is a transcription factor that controls the
inflammatory response, cell cycle and apoptosis (33). In the canonical NF-κB pathway, IκB
is phosphorylated, ubiquitinated and degraded, which triggers the
nuclear translocation of the NF-κB complex and regulates the
transcription of the downstream gene (34). LPS can significantly induce the
activation of NF-κB (35). The
present study also found that LPS treatment activated the IKK/NF-κB
signaling pathway in HDPCs. Wnt signaling presents a critical
effect in the regulation of cell proliferation and differentiation,
while Yu et al (14), found
that Wnt4 could reduce the inflammatory response by inhibiting
NF-kB in osteoclasts. IKK2 (IKKβ) is the main IKK activated by
proinflammatory stimuli and is the kinase primarily responsible for
regulating NF-κB activation (36).
The present study verified that the phosphorylation levels of IKK2,
IκBα and p65 were decreased in Wnt4-overexpressed HDPCs, which
indicated that Wnt4 inactivated IKK2/NF-κB pathway in HDPCs.
Studies have reported that IKK2/NF-κB signaling mediates
neuroinflammation in the cerebellum (37) and regulates the apoptosis of
pulmonary arterial smooth muscle cells (38). In the present study, to verify the
role of the IKK2/NF-κB pathway on Wnt4 mediation of inflammation
and apoptosis, the IKK2/NF-κB pathway was activated by
overexpressing constitutively active IKK2 and inhibiting the
activation of NF-κB. The results indicated that IKK2/NF-κB pathway
promoted the inflammation and apoptosis of HDPCs and Wnt4 inhibited
LPS-induced activation of IKK2/NF-κB and thereby inhibiting
inflammation and apoptosis induced by LPS in HDPCs.
In conclusion, Wnt4 could inhibit inflammation and
apoptosis by hindering the activation of the IKK2/NF-κB pathway in
LPS-HDPCs, which might provide a potential therapeutic target in
pulpitis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the horizontal
project of Anhui Medical College in 2021 ‘Application of Endogenous
Regeneration Technology in periodontal Tissue reconstruction’
(grant no. 2021HXYF001) and the General project of 2021 Outstanding
Young Talents Support Program of Colleges and Universities (grant
no. gxyq2021260).
Availability of data and materials
The datasets generated and/or used during the
present study are available from the corresponding author upon
reasonable request.
Authors' contributions
All authors contributed to the conception and design
of the study. GW, TM and JX performed the experiments, data
collection and analysis and wrote the manuscript. CN conceived and
designed the experiments and revised the manuscript. CN and GW
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Anhui Medical College (approval no. 2022-LLBG-001) and
obeyed the principles of the Declaration of Helsinki. Informed
consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ricucci D, Siqueira JF Jr, Abdelsayed RA,
Lio SG and Rocas IN: Bacterial invasion of pulp blood vessels in
teeth with symptomatic irreversible pulpitis. J Endod.
47:1854–1864. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Agnihotry A, Thompson W, Fedorowicz Z, van
Zuuren EJ and Sprakel J: Antibiotic use for irreversible pulpitis.
Cochrane Database Syst Rev. 5(CD004969)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Song J, Wu Q, Jiang J, Sun D, Wang F, Xin
B and Cui Q: Berberine reduces inflammation of human dental pulp
fibroblast via miR-21/KBTBD7 axis. Arch Oral Biol.
110(104630)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Song F, Sun H, Wang Y, Yang H, Huang L, Fu
D, Gan J and Huang C: Pannexin3 inhibits TNF-α-induced inflammatory
response by suppressing NF-κB signalling pathway in human dental
pulp cells. J Cell Mol Med. 21:444–455. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang Z, Pan X, Chen M and Bai M: Wnt
signalling in oral and maxillofacial diseases. Cell Biol Int.
46:34–45. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jang S, Cho HH, Park JS and Jeong HS:
Non-canonical Wnt mediated neurogenic differentiation of human bone
marrow-derived mesenchymal stem cells. Neurosci Lett. 660:68–73.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhong T, Zhang Z, Gao Y, Lu Z, Qiao H,
Zhou H and Liu Y: Loss of Wnt4 expression inhibits the odontogenic
potential of dental pulp stem cells through JNK signaling in
pulpitis. Am J Transl Res. 11:1819–1826. 2019.PubMed/NCBI
|
|
8
|
Adlimoghaddam A and Albensi BC: The
nuclear factor kappa B (NF-κB) signaling pathway is involved in
ammonia-induced mitochondrial dysfunction. Mitochondrion. 57:63–75.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hunto ST, Kim HG, Baek KS, Jeong D, Kim E,
Kim JH and Cho JY: Loratadine, an antihistamine drug, exhibits
anti-inflammatory activity through suppression of the NF-kB
pathway. Biochem Pharmacol. 177(113949)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Napetschnig J and Wu H: Molecular basis of
NF-κB signaling. Ann Rev Biophys. 42:443–468. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sasaki Y and Iwai K: Roles of the NF-κB
pathway in B-lymphocyte biology. Curr Top Microbiol Immunol.
393:177–209. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu H, He M, Yang R, Zuo Y and Bian Z:
Astrocyte elevated gene-1 participates in the production of
pro-inflammatory cytokines in dental pulp cells via NF-κB
signalling pathway. Int Endod J. 51:1130–1138. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Du Y, Yu X, Wang W, Zhao X, Zhang L, Sun
Y, Wang X and Yu Q: Elevated expression of importin 8 in inflamed
human dental pulps. Int J Clin Exp Pathol. 10:7699–7706.
2017.PubMed/NCBI
|
|
14
|
Yu B, Chang J, Liu Y, Li J, Kevork K,
Al-Hezaimi K, Graves D, Park N and Wang C: Wnt4 signaling prevents
skeletal aging and inflammation by inhibiting nuclear factor-κB.
Nat Med. 20:1009–1017. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Zhao C, Ling X, Li X, Hou X and Zhao D:
MicroRNA-138-5p inhibits cell migration, invasion and EMT in breast
cancer by directly targeting RHBDD1. Breast Cancer. 26:817–825.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shuai C, Liu G, Yang Y, Qi F and Qian G: A
strawberry-like Ag-decorated barium titanate enhances piezoelectric
and antibacterial activities of polymer scaffold. Nano Energy.
74(104825)2020.
|
|
17
|
Shuai C, Xu Y, Feng P, Wang G, Xiong S and
Peng S: Antibacterial polymer scaffold based on mesoporous
bioactive glass loaded with in situ grown silver. Chem Engineering
J. 374:304–315. 2019.
|
|
18
|
Kim D, Shin M, Kim Y, Bae W, Roh D, Hwang
Y and Kim E: Anti-inflammatory effects of glutamine on
LPS-stimulated human dental pulp cells correlate with activation of
MKP-1 and attenuation of the MAPK and NF-κB pathways. Int Endod J.
48:220–228. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Adibkia K, Ehsani A, Jodaei A, Fathi E,
Farahzadi R and Barzegar-Jalali M: Silver nanoparticles induce the
cardiomyogenic differentiation of bone marrow derived mesenchymal
stem cells via telomere length extension. Beilstein J Nanotechnol.
12:786–797. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fang J, Zhao X, Li S, Xing X, Wang H,
Lazarovici P and Zheng W: Protective mechanism of artemisinin on
rat bone marrow-derived mesenchymal stem cells against apoptosis
induced by hydrogen peroxide via activation of
c-Raf-Erk1/2-p90rsk-CREB pathway. Stem Cell Res Ther.
10(312)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Millen S, Meretuk L, Göttlicher T, Schmitt
S, Fleckenstein B and Thoma-Kress AK: A novel positive
feedback-loop between the HTLV-1 oncoprotein Tax and NF-κB activity
in T-cells. Retrovirology. 17(30)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Larsen T and Fiehn N: Dental biofilm
infections-an update. APMIS. 125:376–384. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Aubeux D, Peters OA, Hosseinpour S,
Tessier S, Geoffroy V, Pérez F and Gaudin A: Specialized
pro-resolving lipid mediators in endodontics: A narrative review.
BMC Oral Health. 21(276)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lei F, Zhang H and Xie X: Comprehensive
analysis of an lncRNA-miRNA-mRNA competing endogenous RNA network
in pulpitis. PeerJ. 7(e7135)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chung M, Lee J, Duraes G and Ro J:
Lipopolysaccharide-induced pulpitis up-regulates TRPV1 in
trigeminal ganglia. J Dent Res. 90:1103–1107. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jung J, Woo S, Kim W, Lee B, Nör J, Min K,
Choi C, Koh J, Lee K and Hwang Y: Simvastatin inhibits the
expression of inflammatory cytokines and cell adhesion molecules
induced by LPS in human dental pulp cells. Int Endod J. 50:377–386.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang H, Yang F, Wang Y, Pei F, Chen Z and
Zhang L: Odontoblastic exosomes attenuate apoptosis in neighboring
cells. J Dent Res. 98:1271–1278. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu X, Cao Y, Zhang Y, Sun B and Liang H:
Teneligliptin inhibits lipopolysaccharide-induced cytotoxicity and
inflammation in dental pulp cells. Int Immunopharmacol. 73:57–63.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Maeda K, Takahashi N and Kobayashi Y:
Roles of Wnt signals in bone resorption during physiological and
pathological states. J Mol Med (Berl). 91:15–23. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang L, Qing L, Liu H, Liu N, Qiao J, Cui
C, He T, Zhao R, Liu F, Yan F, et al: Mesenchymal stromal cells
ameliorate oxidative stress-induced islet endothelium apoptosis and
functional impairment via Wnt4-β-catenin signaling. Stem Cell Res
Ther. 8(188)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
He YX, Diao TT, Song SM, Wang CC, Wang Y,
Zhou CL, Bai YB, Yu SS, Mi X, Yang XY, et al: Wnt4 is significantly
upregulated during the early phases of cisplatin-induced acute
kidney injury. Sci Rep. 8(10555)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhong T, Gao Y, Qiao H, Zhou H and Liu Y:
Elevated osteogenic potential of stem cells from inflammatory
dental pulp tissues by Wnt4 overexpression for treating bone defect
in rats. Ann Palliat Med. 9:2962–2969. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Won M, Byun H, Park K and Hur G:
Post-translational control of NF-κB signaling by ubiquitination.
Arch Pharm Res. 39:1075–1084. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sun SC: The non-canonical NF-κB pathway in
immunity and inflammation. Nat Rev Immunol. 17:545–558.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang L, Wu J, Guo X, Huang X and Huang Q:
RAGE plays a role in LPS-induced NF-κB activation and endothelial
hyperpermeability. Sensors (Basel). 17(722)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Caposio P, Dreano M, Garotta G, Gribaudo G
and Landolfo S: Human cytomegalovirus stimulates cellular IKK2
activity and requires the enzyme for productive replication. J
Virol. 78:3190–3195. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Lattke M, Reichel SN, Magnutzki A, Abaei
A, Rasche V, Walther P, Calado DP, Ferger B, Wirth T and Baumann B:
Transient IKK2 activation in astrocytes initiates selective
non-cell-autonomous neurodegeneration. Mol Neurodegener.
12(16)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Price LC, Shao D, Meng C, Perros F,
Garfield BE, Zhu J, Montani D, Dorfmuller P, Humbert M, Adcock IM
and Wort SJ: Dexamethasone induces apoptosis in pulmonary arterial
smooth muscle cells. Respir Res. 16(114)2015.PubMed/NCBI View Article : Google Scholar
|