Introduction
Non-Hodgkin lymphoma (NHL) usually originates from
lymphoid tissues and may involve other organs, such as the stomach,
intestines, lungs, breasts and kidneys. Autopsy findings have
indicated that ~30-50% of NHL patients have renal involvement,
which occurs in the late stage of the disease, is usually
asymptomatic and is rarely diagnosed before mortality. Overall,
0.9-23% of such cases progress to renal failure (1), but renal damage as an initial symptom
of lymphoma is very rare (2).
Renal involvement may manifest as a renal mass or invasive kidney
disease. Pathologically, renal involvement is characterized by
diffuse large B-cell lymphoma (3)
and, very rarely, B-LBL. The present study reported the clinical
data for a patient with lymphoma-associated renal damage whose
initial symptoms included acute renal failure and bilateral renal
enlargement and the pathological results confirmed B-LBL.
Case presentation
A 30-year-old male patient presented with
unexplained leg pain, weight loss and intermittent fever for more
than one month. He was then admitted to the Renmin Hospital of
Wuhan University due to the discovery of abnormal kidney function
on October 11, 2019. His history of past illness was as follows.
Other than intellectual disability, the patient was healthy, as
were his parents.
Physical examination upon admission revealed
elevated blood pressure (BP146/96 mm Hg) as the only positive
result. From the laboratory examination, the following results were
obtained. Blood routine (automatic blood cell analyzer; XN-9000;
Sysmex Europe SE) showed a white blood cell count (WBC) of
8.96x109/l, neutrophil count (Neu#) of
6.42x109/l, lymphocyte count (LYM#) of
1.55x109/l, and hemoglobin (Hb) of 93 g/l (Table I). Blood chemistry (biochemistry
analyzer; ADVIA 2400/1800/Chemistry XPT; Siemens AG) indicated a
urea level of 12.48 mmol/l, creatinine level of 312 µmol/l, and an
estimated glomerular filtration rate of 21.89 ml/min. Urine
analysis (full-auto urine sediment analyzer; UF-5000; Sysmex Europe
SE) showed blood 1+ and a 24-hour urinary total protein level of
0.19 g. The erythrocyte sedimentation rate (Ves-matic cube; TEST1;
Diesse Diagnostica Senese S.p.A.) was 120 mm/h. Humoral immunity
(Immune analyzer; IMMAGE800; Beckman Coulter, Inc.) indicated an
immunoglobulin E level of 884 IU/ml, a C4 level of 0.445 g/l, an
IgG4 level of 4.26 g/l. Moreover, antinuclear antibodies and
extractable nuclear antigens (fluorescence microscope; EUROStar),
antineutrophil cytoplasmic antibodies (fluorescence microscope;
EUROStar), anti-glomerular basement membrane (automatic enzyme
immunoassay analyzer; Alegria; ORGENTEC Diagnostika),
cytomegalovirus and Epstein-Barr virus (PCR; AGS4800; Daan Gene
Co., Ltd.) were negative. Urinary Doppler ultrasound showed
bilateral renal enlargement (left kidney 15.7x8.9 cm, parenchymal
thickness 2.5 cm; right kidney 14.4x8.3 cm, parenchymal thickness
2.6 cm). No swollen lymph nodes were detected with ultrasound or
computed tomography (CT).
 | Table ILaboratory findings of the
patient. |
Table I
Laboratory findings of the
patient.
| Parameter | 2019.10.11 | 2019.12.01 | 2019.12.16 |
|---|
| White blood cell
count |
8.96x109/l |
26.26x109/l |
62.6x109/l |
| Neutrophil count |
6.42x109/l |
7.88x109/l |
1.83x109/l |
| Lymphocyte count |
1.55x109/l |
9.32x109/l |
12.36x109/l |
| Hemoglobin | 93 g/l | 96 g/l | 70 g/l |
| Platelet |
145x109/l |
33x109/l |
13x109/l |
| Urea | 12.48 mmol/l | 18.9 mmol/l | - |
| Creatinine | 312 µmol/l | 289 µmol/l | - |
| Uric acid | 616.00 µmol/l | 1,260 µmol/l | - |
| Lactate
dehydrogenase | 774 U/l | 4,459 U/l | - |
| 24-hour urinary total
protein | 0.19 g | - | - |
First, it was considered that the patient might have
had acute interstitial nephritis. To avoid treatment delays,
methylprednisolone (40 mg/d, intravenous drip) was added
empirically on October 14. Then, renal biopsy was performed for the
patient. The results indicated severe acute renal interstitial
lesions and a large number of interstitial lymphocytes with a
uniform morphology. Renal cortices were minced into 1
mm3 pieces and fixed with glutaraldehyde followed by
osmic acid. Ultrathin sections (40-60 nm) were stained with lead
citrate and alcoholic uranyl acetate for 10 minutes and electron
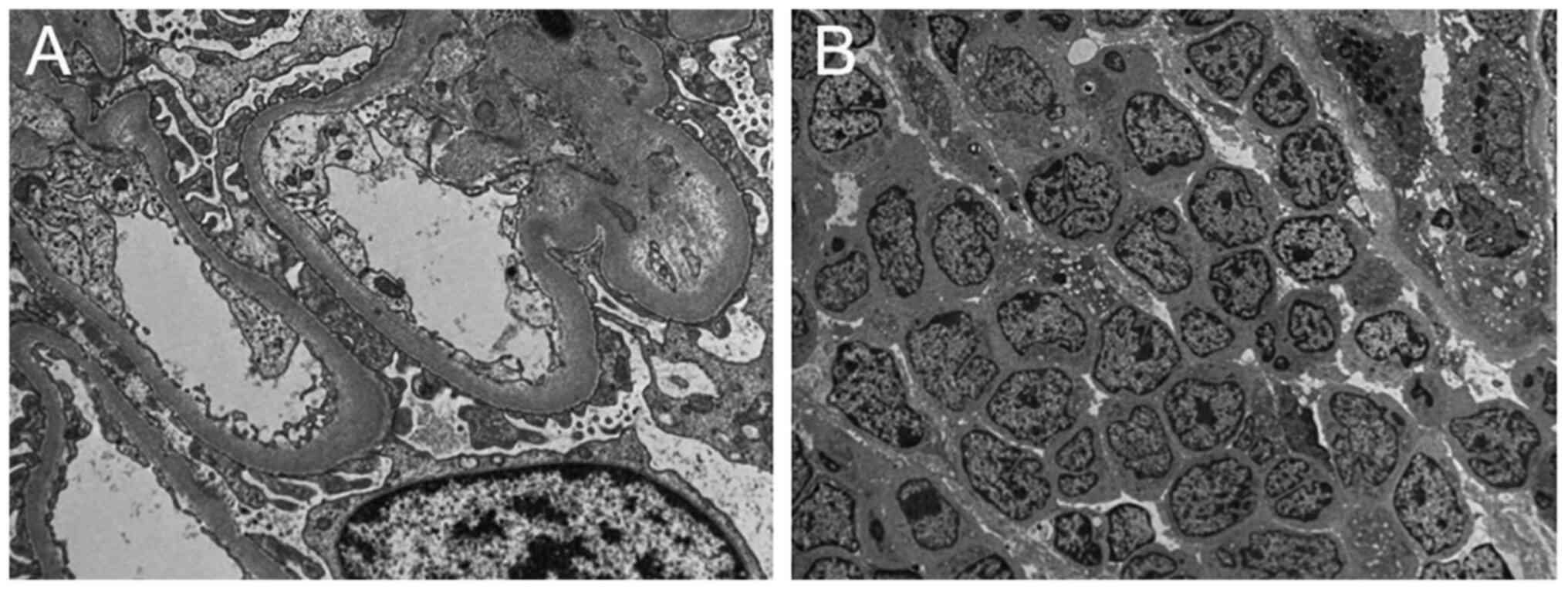
microscopy (TEM) imaging (Hitachi, Japan). The result also revealed
a small amount of renal tissue and a large number of lymphocytes
that were morphologically uniform and immature (Fig. 1). The kidney tissues were fixed
overnight at 4˚C in 4% paraformaldehyde, embedded in paraffin and
sectioned. The sections were dewaxed and hydrated in sequence, and
then antigen retrieval was performed using sodium citrate buffer at
~100˚C for 30 min. Subsequently, the tissue sections were incubated
with 5% normal goat serum for 20 min and incubated overnight at 4˚C
with corresponding primary antibody (Maixin Biotechnology; 1:100).
Then, the sections were incubated with HRP-conjugated secondary
antibody (Maixin Biotechnology; 1:200) at room temperature for 30
min. Finally, images were captured under a light microscope
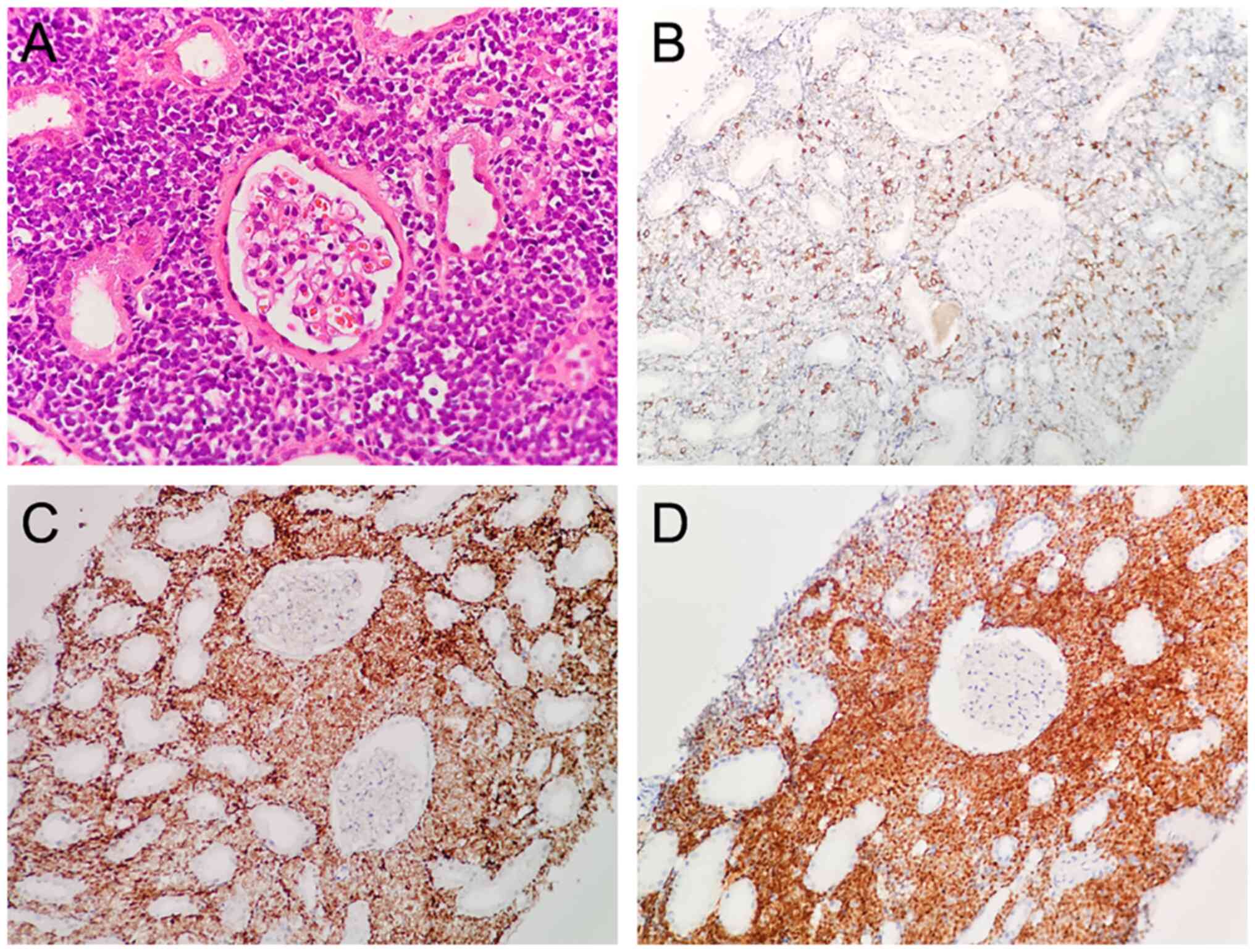
(Olympus, Japan). The result showed lymphoid cells positive for
cluster of differentiation (CD)3 (scattered), CD20 (focal), Ki67
(60%), Pax 5, CD10, and TdT but negative for CD23, Bcl-6, Mum1,
Bcl-2, CD5, P53, and CD30 (Fig.
2). The patient was diagnosed with B-cell lymphoblastic
lymphoma (B-LBL)-associated renal damage. To further identify the
primary lymphoma lesion, the patient underwent bone marrow
aspiration and positron emission tomography (PET)-CT. Reverse
transcription quantitative PCR technology was used to screen
leukemia fusion genes and the result showed the E2A-PBX1 fusion
gene (+). The immunophenotyping of leukemia as detected by flow
cytometry (BD FACSCanto II; BD Biosciences) revealed elevated
abnormal cells (immature B cells), suggesting the possibility of
precursor B-cell acute lymphoblastic leukemia (B-ALL). Bone marrow
was taken for smearing and Wright-giemsa staining and then observed
under a microscope. The results indicated elevated proliferation of
nucleated cells in the bone marrow. Specifically, the
leukocyte-to-erythrocyte ratio was 1.58:1, granulocytes accounted
for 28.5% of cells, and promyelocytes (and later-stage cells) were
visible; most granulocytes were neutrophils with rod-shaped nuclei
and a largely normal morphology. Erythrocytes accounted for 18% of
cells; proerythroblasts (and later-stage cells) were visible and
were mostly metarubricytes; and mature erythrocytes varied in size
and morphology. Lymphocytes accounted for 52% of cells; of these,
lymphoblasts accounted for 29.5% of cells, and prolymphocytes
accounted for 9.5% of cells. These data suggested the possibility
of acute lymphoblastic leukemia and lymphoma. Bone marrow
aspiration revealed an extremely high level of myeloproliferation
(90%), suppression of three lineages of hemopoietic cells
(granulocytes, erythrocytes and megakaryocytes) and diffuse, patchy
type I cell proliferation. Combined with the patient's
immunohistochemistry, these results suggested B-cell-derived
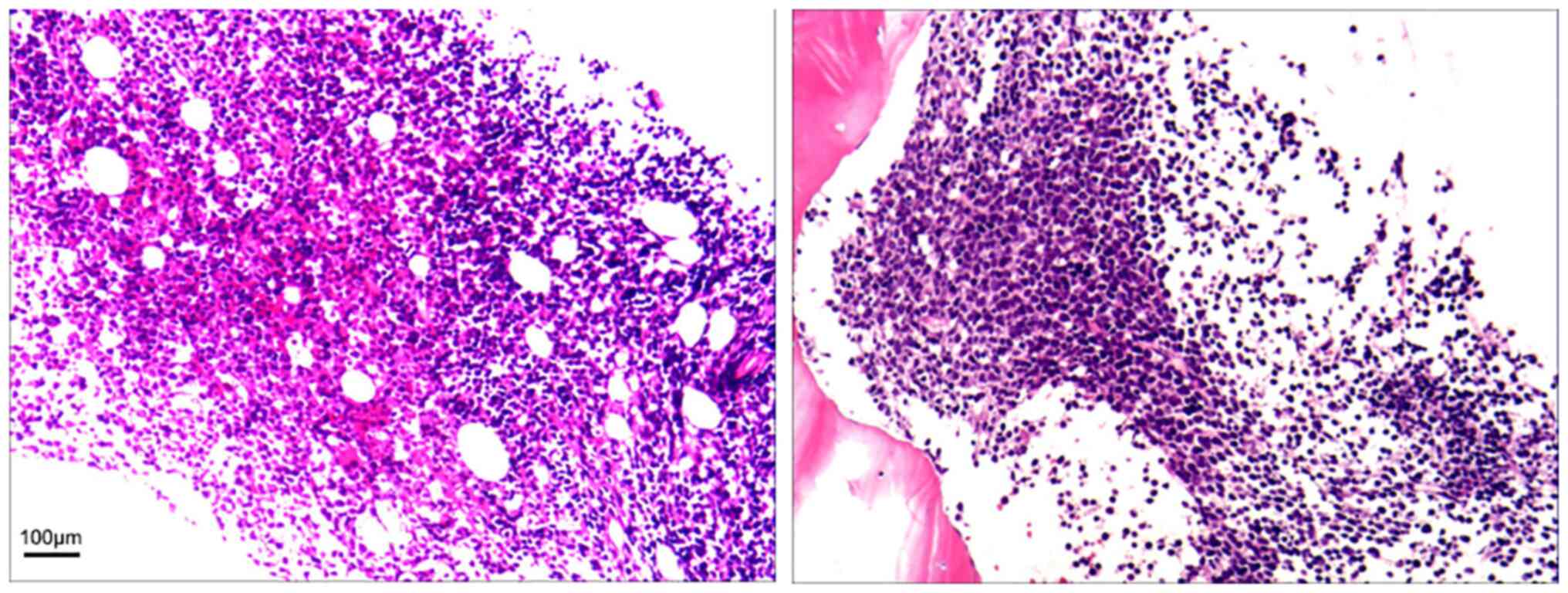
leukemia/lymphoma. However, the immunohistochemistry results were
unsatisfactory despite multiple attempts (Fig. 3). Immunohistochemistry showed CD10
(+), CD19 (weak +), CD20 (scattered +), CD23 (-), CD3 (-), CD34
(-), CD5 (-), CD79α (-), TdT (-), Pax-5 (weak+), lymphoid enhancer
factor (LEF)1 (scattered+), Mum-1 (-), Bcl-6 (-), myeloperoxidase
(MPO) (-), Cyclin-D1 (-), Kappa (-), Lambda (-), and Ki67 (labeling
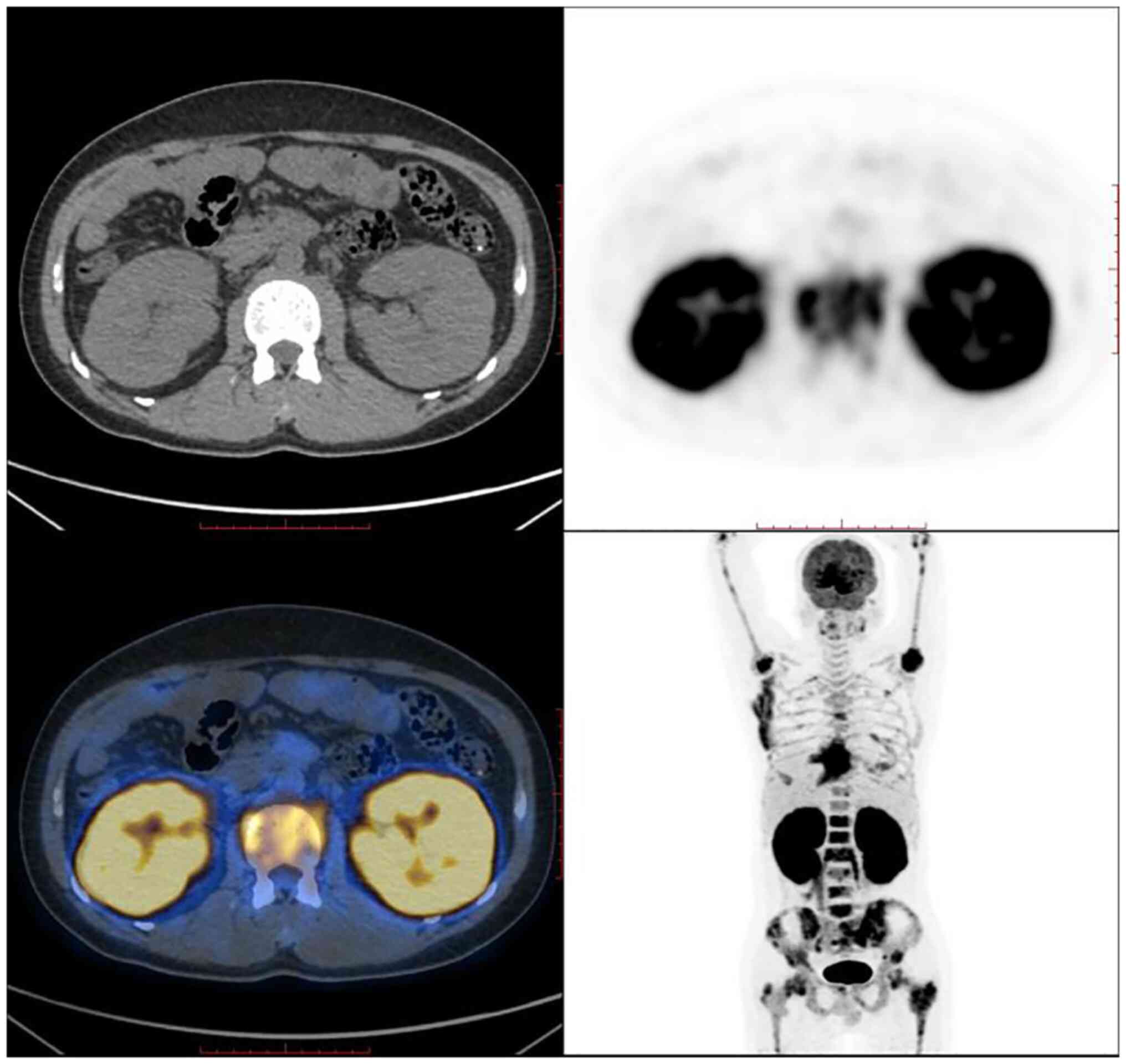
index indicating low proliferation). PET-CT revealed bilateral
diffuse renal enlargement with increased metabolism, increased
metabolism of the left and right psoas major muscles, increased
metabolism of the lymph nodes next to the left iliac vessels, and
extensive localized bone metabolism throughout the body (Fig. 4). The final diagnosis was B-LBL,
stage IV, with associated renal damage (renal interstitial
infiltration). Regular tests indicated gradual improvement in renal
function. On October 28, a laboratory test showed a serum
creatinine (Scr) of 107 µmol/l.
Once diagnosed, the patient was transferred to the
Department of Hematology for further treatment; however, the
patient requested discharge due to financial reasons and continued
to take steroids with tapering after discharge. Once the steroids
were withdrawn, he developed generalized pain and fever. On
December 1, 2019, he was transferred to the Department of
Hematology of Renmin Hospital of Wuhan University again due to
uncontrolled conditions. Blood tests showed a WBC of
26.26x109/l, a Neu% of 3.6%, an LYM% of 6.9%, a Neu# of
7.88x109/l, an LYM# of 9.32x109/l, an Hb
level of 96 g/l, and a platelet (PLT) count of 33x109/l.
Blood chemistry indicated a urea level of 18.9 mmol/l, an Scr level
of 289 µmol/l, a uric acid (UA) level of 1260 µmol/l, a lactate
dehydrogenase (LDH) level of 4459 U/l, and a procalcitonin (PCT)
level of 9.8 ng/ml. Throat swabs showed gram-positive cocci and
bacilli. In consideration of concomitant infection, the patient was
given low-dose intravenous dexamethasone and anti-infective
treatment instead of standard chemotherapy. On December 16, blood
tests showed a WBC of 62.6x109/l, a Neu# of
1.83x109/l, an LYM# of 12.36x109/l, an Hb
level of 70 g/l, and a PLT count of 13x109/l, suggesting
disease progression and the need for chemotherapy. Chemotherapy
with anti-infective support was suggested to the patient since the
patient's infection was serious, but the patient and his family
members eventually discontinued treatment. The diagnosis at
discharge included lymphoma cell leukemia and B-LBL with associated
renal damage (renal interstitial infiltration).
The present study was carried out in accordance with
the code of Ethics of the World Medical Association (Declaration of
Helsinki) for experiments involving humans. It was reviewed and
approved by the ethics committee of Renmin Hospital of Wuhan
University (Wuhan, China; approval no. WDRY2021-KS015).
Discussion
In cases of lymphoma, renal involvement is known as
secondary renal lymphoma with evidence of extensive lymph node or
extranodal lymphoma or as primary renal lymphoma in the absence of
other organ involvement (i.e., in cases with only renal
involvement) (4). Primary renal
lymphoma is very rare, accounting for <1% of extranodal
lymphomas (5). Some researchers
have speculated that primary renal lymphoma may originate from the
renal capsule, which is rich in lymphoid tissue that can penetrate
the renal parenchyma (6). In the
present study, primary renal lymphoma was excluded because bone
marrow aspiration and PET-CT found infiltration on organs other
than the kidney.
The main acute B-cell lymphoproliferative disease is
B-ALL (80%), followed by B-LBL (10%) and mixed B-ALL/B-LBL (10%)
(7). The World Health Organization
classification groups B-ALL and B-LBL together as B-lymphoblastic
leukemia/lymphoma (B-ALL/LBL) (8).
B-LBL is a type of rare and highly invasive NHL and accounts for
~10% of LBLs, which in turn account for ~2% of NHLs (9). B-LBL often involves lymph nodes and
extranodal locations, such as the skin, bones, and soft tissues.
However, renal involvement is rare in B-LBL (7). A literature search identified <5
such cases in China and elsewhere, including two cases reported in
China, one of which was primary renal B-LBL (8,10).
Many factors are associated with renal failure in
lymphoma patients, including acute tumor lysis syndrome (11) and urinary obstruction (1); of these, urinary obstruction accounts
for ~10% of renal failure. Renal insufficiency is rarely caused by
lymphoma-associated bilateral diffuse renal infiltration, which may
occur in the renal interstitium or glomerular microcirculation.
Lymphoma with glomerular infiltration is a diverse manifestation of
renal malignant intravascular lymphoma (12), whereas lymphoma with interstitial
infiltration is more common in cases of diffuse large B-cell
lymphoma (13). The pathological
mechanism of acute renal failure in lymphoma with interstitial
infiltration is unclear. A study reported that dense
lymphoma-related interstitial infiltration compresses tubules and
interstitial capillaries, resulting in lobular obstruction and
elevated postglomerular vascular resistance (14).
The cyclophosphamide, doxorubicin, vincristine, and
prednisone (CHOP) regimen is the standard chemotherapy scheme for
invasive NHL (15). A 2003
multicenter trial in patients with invasive NHL demonstrated that
in addition to CHOP, the monoclonal anti-CD20 antibody rituximab
was associated with a high survival rate (16). A study also found that early
diagnosis and chemotherapy, including rituximab, cyclophosphamide,
doxorubicin, vincristine, and prednisolone (R-CHOP), may improve
renal function after 2-4 weeks of treatment and may improve 5-year
survival (17). Therefore, renal
involvement is important to detect, and the addition of rituximab
to the standard chemotherapy regimen may improve progression-free
survival and overall survival (18,19).
When the disease progresses to lymphoma cell leukemia, the
treatment scheme for acute lymphocytic leukemia can be adopted, and
Hyper-CVAD is a commonly used regimen for adults with newly
diagnosed acute lymphoblastic leukemia (20).
In the present study, renal damage was the primary
initial symptom. Active prediagnostic use of glucocorticoids
rapidly improved renal function; however, with disease progression,
hematological involvement occurred, with typical leukemia symptoms.
Unfortunately, the patient declined chemotherapy. We wanted to
share this case because this lymphoma patient presented with acute
renal failure with bilateral renal enlargement as initial symptoms
and because this pathologic type is rare, and it is hoped to
provide valuable information about lymphoma-associated renal damage
and help improve the understanding of this disease.
Acknowledgements
The authors thank Dr Jingping Yuan and Dr Yang Guan
from Renmin Hospital of Wuhan University for helping to clarify the
patient's diagnosis.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JD provided relevant medical records. YJ, GD, DY,
and JD participated in the patient's diagnosis and treatment
process. YJ drafted the manuscript. JD revised the manuscript. YJ
and JD confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was carried out in accordance with
the code of Ethics of the World Medical Association (Declaration of
Helsinki) for experiments involving humans. It was reviewed and
approved by the ethics committee of Renmin Hospital of Wuhan
University (Wuhan, China; approval no. WDRY2021-KS015).
Patient consent for publication
The patient and his brother provided written
informed consent for publication of the medical data and images for
this case.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Richmond J, Sherman RS, Diamond HD and
Craver LF: Renal lesions associated with malignant lymphomas. Am J
Med. 32:184–207. 1962.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Obrador GT, Price B, Omeara Y and Salant
DJ: Acute renal failure due to lymphomatous infiltration of the
kidneys. J Am Soc Nephrol. 8:1348–1353. 1997.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Morel P, Dupriez B, Herbrecht R, Bastion
Y, Tilly H, Delannoy A, Haioun C, Nouvel C and Bouabdallah K:
Aggressive lymphomas with renal involvement-a study of 48 patients
treated with the lnh-84 and lnh-87 regimens. Br J Cancer.
70:154–159. 1994.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kandel LB, Mccullough DL, Harrison LH,
Woodruff RD, Ahl ET Jr and Munitz HA: Primary renal lymphoma-does
it exist. Cancer. 60:386–391. 1987.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Olusanya AA, Huff G, Adeleye O, Faulkner
M, Burnette R, Thompson H, Adeola T and Woods K: Primary renal
non-Hodgkins lymphoma presenting with acute renal failure. J Natl
Med Assoc. 95:220–224. 2003.PubMed/NCBI
|
|
6
|
Betta PG, Bottero G, Cosimi MF and Musante
F: Primary renal lymphoma. Eur Urol. 12:352–354. 1986.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Geethakumari PR, Hoffmann MS, Pemmaraju N,
Hu S, Jorgensen JL, O'Brien S and Daver N: Extramedullary B
lymphoblastic leukemia/lymphoma (B-ALL/B-LBL): A diagnostic
challenge. Clin Lymphoma Myeloma Leuk. 14:e115–e118.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang L, Feng Y, Cheng N, Zou Q, Lai W and
Liu JJ: A case of renal involvement in b lymphoblastic lymphoma
leukemia. Clin Lab. 65:177–180. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Soslow RA, Baergen RN and Warnke RA:
B-lineage lymphoblastic lymphoma is a clinicopathologic entity
distinct from other histologically similar aggressive lymphomas
with blastic morphology. Cancer. 85:2648–2654. 1999.PubMed/NCBI
|
|
10
|
Rajakumar V, Balaraman V, Balasubramaniam
R, Shankar S, Ganesan TS and Kurien AA: Lymphoblastic lymphoma
presenting as bilateral renal enlargement diagnosed by percutaneous
kidney biopsy: Report of three cases. Indian J Nephrol. 26:298–301.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hande KR and Garrow GC: Acute tumor lysis
syndrome in patients with high-grade non-hodgkins-lymphoma. Am J
Med. 94:133–139. 1993.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sheibani K, Battifora H, Winberg CD, Burke
JS, Ben-Ezra J, Ellinger GM, Quigley NJ, Fernandez BB, Morrow D and
Rappaport H: Further evidence that malignant angioendotheliomatosis
is an angiotropic large-cell lymphoma. N Engl J Med. 314:943–948.
1986.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ferry JA, Harris NL, Papanicolaou N and
Young RH: Lymphoma of the kidney-a report of 11 cases. Am J Surg
Pathol. 19:134–144. 1995.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tornroth T, Heiro M, Marcussen N and
Franssila K: Lymphomas diagnosed by percutaneous kidney biopsy. Am
J Kidney Dis. 42:960–971. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Brouland JP, Meeus F, Rossert J, Hernigou
A, Gentric D, Jacquot C, Diebold J and Nochy D: Primary bilateral
b-cell renal lymphoma-a case-report and review of the literature.
Am J Kidney Dis. 24:586–589. 1994.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Boye J, Elter T and Engert A: An overview
of the current clinical use of the anti-CD20 monoclonal antibody
rituximab. Ann Oncol. 14:520–535. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Al-Salam S, Shaaban A, Alketbi M, Haq NU
and Abouchacra S: Acute kidney injury secondary to renal large
B-cell lymphoma: Role of early renal biopsy. Int Urol Nephrol.
43:237–240. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tai WM, Chung J, Tang PL, Koo YX, Hou X,
Tay KW, Quek R, Tao M and Lim ST: Central nervous system (CNS)
relapse in diffuse large B cell lymphoma (DLBCL): Pre- and
post-rituximab. Ann Hematol. 90:809–818. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Villa D, Connors JM, Shenkier TN, Gascoyne
RD, Sehn LH and Savage KJ: Incidence and risk factors for central
nervous system relapse in patients with diffuse large B-cell
lymphoma: The impact of the addition of rituximab to CHOP
chemotherapy. Ann Oncol. 21:1046–1052. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cassaday RD, Stevenson PA, Wood BL, Becker
PS, Hendrie PC, Sandmaier BM, Radich JL and Shustov AR: Description
and prognostic significance of the kinetics of minimal residual
disease status in adults with acute lymphoblastic leukemia treated
with HyperCVAD. Am J Hematol. 93:546–552. 2018.PubMed/NCBI View Article : Google Scholar
|