Introduction
Myocardial ischemia/reperfusion injury (I/R) is the
restoration of blood perfusion following ischemia, which causes
metabolic dysfunction and aggravates the structural damage of
myocardial cells, leading to cell death and infarction enlargement
(1). The pathogenesis of
myocardial I/R is complicated, but studies have shown that it is
related to oxygen-free radicals, calcium overload and inflammatory
mediators (2-4).
Currently, there is no effective treatment for myocardial I/R and
it is of great clinical significance to explore the molecular
mechanism of the myocardial I/R pathological process.
Long non-coding RNAs (LncRNAs) are a class of
non-coding RNAs with a length of more than 200 nucleotide molecules
(5). LncRNAs can regulate gene
expression at multiple levels, including epigenetic regulation,
transcription and post-transcriptional regulation mediated by
regulatory factors (6,7). Many studies have shown that lncRNA is
closely related to myocardial I/R injury (8,9). A
recent study found that CHRF exacerbated myocardial I/R injury by
enhancing autophagy via modulation of the miR-182-5p/ATG7 pathway
(10). Studies have shown that
SNHG16 is highly expressed in a variety of tumor tissues and plays
the role of an oncogenic gene (11,12).
Silencing SNHG16 can inhibit tumor cell growth, invasion and
metastasis and induce cell apoptosis (13). However, the mechanism of SNHG16 in
myocardial I/R injury remains to be elucidated.
LncRNA can regulate target gene expression through
competitively binding microRNA (miRNA/miR) (14). miRNAs play essential roles in
anti-apoptotic cardiovascular diseases and myocardial I/R injury
(2,15). miR-183 has been suggested to act as
a regulator in cell apoptosis of hypoxia-induced H2c9 cells
(16). The present study explored
SNHG16 functional role and mechanism in myocardial I/R injury by
establishing an animal model of myocardial I/R in rats and an H9C2
cell model of H/R injury.
Materials and methods
Animals and groups
A total of 26 male SPF grade Sprague-Dawley (SD)
rats (male rats are more resilient and their hormone levels change
more steadily than female ones) weighing 200-250 g (7-8 week-old)
were obtained from Beijing Experimental Animal Center and kept at
25±3˚C and 50-60% humidity in a room with a 12-h light/dark cycle.
Experiments were conducted according to the Declaration of
Helsinki. The Animal Care and Use Committee of Cangzhou Central
Hospital (approval no. 2022-013-01z) approved all rat protocols and
procedures. Rats were randomly divided into four groups, including
sham (Sham group, n=6) and the reperfusion 24 h group, including
I/R group, n=7; I/R + shNC, n=7 and I/R + shSNHG16, n=6).
Establishment of myocardial I/R injury
model
SD rats were fasted for 12 h before surgery,
anesthetized by intraperitoneal injection of pentobarbital sodium
(50 mg/kg body weight) and fixed in an inverted position.
Tracheotomy and intubation were performed and an artificial
ventilator (frequency: 70 times/min; tidal volume: 20 ml;
respiration ratio: 1:1) was connected. A needle electrode was
inserted subcutaneously into the extremities to record the ECG and
connect the BL420S biological function experimental system. The
left anterior descending coronary artery was found between the
pulmonary artery conus and the left atrial appendage. The anterior
descending coronary artery was ligated with a 5/0 thread between
the pulmonary artery conus 2-4 mm below the root of the left atrial
appendage to block the coronary blood flow. Following ligation for
30 min, the ligation line was loosened and reperfusion for 120 min.
The rats were allowed to regain consciousness during reperfusion
period. At the end of experiment, the rats were anesthetized with
3% pentobarbital sodium (50 mg/kg) for approximately 10 min (until
the rat was immobile) followed by sacrifice via cervical
dislocation.
Determination of LDH activity
After 120 min of reperfusion, blood samples from
each group were collected. The levels of lactate dehydrogenase
(LDH) in the serum were detected by ELISA according to the
manufacturer's instructions (cat. no. A020-2-2; Nanjing Jiancheng
Bioengineering Institute).
Hematoxylin and eosin (HE)
staining
At the end of reperfusion, the myocardium tissue was
fixed with 10% neutral buffer formalin for 48 h. After processed in
a series of graded ethanol and dimethyl benzene, the tissues were
embedded in paraffin, and 4-5 µm sections were stained with stained
with hematoxylin for 5 min and eosin for 3 min at room temperature,
sealed and observed under light microscope (magnification, x200)
and the results from 3 fields of each group were obtained.
Cell culture and transfection
Rat cardiomyocytes H9C2 were obtained from the cell
bank of Shanghai Institute of Biology, Chinese Academy of Sciences.
The H9C2 cells were cultured in high glucose DMEM (Gibco; Thermo
Fisher Scientific, Inc.) medium containing 10% FBS and 1%
penicillin-streptomycin at 37˚C in an atmosphere of 95% air and 5%
CO2. The culture medium was changed every 3 days and the
logarithmic growth cells were digested with 0.25% trypsin. For cell
transfection, cells were seeded into 12-well plates at a density of
1.5x105 cells per well and transfected with the short
hairpin (sh)SNHG16 and negative control (NC) shRNA-NC plasmid,
miR-183 specific inhibitor (miR-183 antisense
oligodeoxyribonucleotide, miR-183-ASO) and miR-NC, miR-183-mimics
and mimics control, pcDNA3.1-forkhead box O1 (FoxO1) and pcDNA3.1
vectors were synthesized by Hanbio Biotechnology Co., Ltd. and
infection was performed according to the manufacturer's manual at
37˚C. For each transfection, 1 µg of each construct was added to
each well. At 24 h after transfection, the cells were collected and
the depletion efficiency was validated through reverse
transcription-quantitative (RT-q) PCR analysis. miRNA sequences
were as follows: miR-183 mimics, 5'-UAUGGCACUGGUAGAAUUCACU-'3;
mimics control, 5'-ACUACUGAGUGACAGUAGA-3'.
Cell counting kit (CCK8) assay
Cells were seeded into 96-well plates at a density
of 0.25x104/well to assess cell proliferation. After 24
h of cell adherent growth, each well was replaced with 100 µl DMEM
complete culture medium, 10 µl CCK-8 reagent was added and then
placed in an incubator for dark incubation for 2 h. The D value of
each well at 450 nm wavelength was measured with an enzyme-linked
immunodetection and the cell growth curve was plotted.
Flow cytometry to detect the apoptosis
of cardiomyocytes
H9C2 cells suspension of each group was collected
and centrifuged at 1,000 x g for 5 min at room temperature, washed
with PBS, stained in Annexin V-FTTC in the presence of 50 µg/ml
RNase A and then the cells were incubated at room temperature for
10-15 min in the dark. A FACScan (Becton Dickinson) was used and
the apoptotic rate (early + late apoptosis) was detected and
analyzed using CytExpert version 2.0 software (Beckman Coulter,
Inc.).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
Apoptosis of H9C2 cells was detected by TUNEL
staining for 60 min at 37˚C and then stained with DAPI for 5 min at
37˚C. The percentage of apoptotic cells was calculated by dividing
TUNEL-positive cells by DAPI-positive cells.
Dual-luciferase reporter assay
The putative interacting sites between SNHG16 and
miR-183 and between miR-183 and FOXO1 were predicted using StarBase
version 2.0 (http://starbase.sysu.edu.cn/), miRDB (http://mirdb.org/miRDB/index.html), miRWalk
(http://mirwalk.umm.uni-heidelberg.de), DIANA
(http://diana.imis.athena-innovation.gr/DianaTools/)
and TargetScan7 (http://www.targetscan.org/vert_71/) tools. H9C2 cells,
following transfection with trypsin digestion for 48 h, were
inoculated in 24-well plates with 1x104 cells/well, then
cultured for 24 h. If the cells fused into one layer, the
transfection was carried out. The wild-type (WT-SNHG16) and
mutant-type (MUT-SNHG16) dual-luciferase reporter vectors of SNHG16
were constructed (Promega Corporation) and then co-transfected with
miR-NC or miR-183 using Lipofectamine® 3000 reagent
(Invitrogen), respectively. After 48 h of transfection, the cells
were collected and lysed in lysate buffer at room temperature for
20 min. The Dual Luciferase Reporter Assay kit (Promega
Corporation) was used to examine the Renilla and firefly
luciferase activity following the manufacturer's protocol.
RT-qPCR Reverse
transcription-quantitative (RT-q) PCR
Tissue and cells (seeded into a 6-well plate at a
density of 4x104 cells/well and cultured for 24 h) were
collected to extract total RNA using TRIzol® reagent
(Thermo Fisher Scientific, Inc.). RNA was reversely transcribed
into cDNA using the cDNA Reverse Transcription kit (Thermo Fisher
Scientific, Inc.) or MicroRNA Reverse Transcription kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocols.
The expression levels were determined using the SYBR PremixEx Taq
II kit (Takara Biotechnology Co., Ltd.) with GAPDH as an internal
control on ABI 7500 RT-PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The qPCR thermocycling conditions were:
Initial denaturation at 95˚C for 10 min; followed by 40 cycles of
95˚C for 15 sec and 64˚C for 30 sec. The relative gene expression
was analyzed by using the 2-ΔΔCq method (11). The experiment was repeated three
times. The primer sequences were: SNHG16: forward:
5'-GCAGAATGCCATGGTTTCCC-3'; SNHG16: reverse:
5'-GGACAGCTGGCAAGAGACTT-3'; miR-183: forward:
5'-CGCGGTATGGCACTGGTAGA-3'; miR-183: reverse:
5'-AGTGCAGGGTCCGAGGTATTC-3'; FOXO1: forward:
5'-GGATGGCATGTTCATTGAGCG-3'; FOXO1: reverse:
5'-ACTGCTTCTCTCAGTTCCTGC-3'; GAPDH: forward:
5'-CATGAGAAGTATGACAACAGCCT-3'; GAPDH: reverse:
5'-AGTCCTTCCACGATACCAAAGT-3'; U6: forward: 5'-CTCGCTTCGGCAGCACA-3';
U6: reverse: 5'-AACGCTTCACGAATTTGCGT-3'.
Western blot assay
Total protein was isolated from spinal cord samples
and cells using a protein extraction kit (Bio-Rad Laboratories,
Inc.). The total protein concentration was determined by the BCA
method, the proteins (50 µg per lane) were separated via 8%
SDS-PAGE. Proteins were then transferred onto PVDF membranes. The
membranes were blocked using 5% skimmed milk for 2 h at room
temperature, the primary antibody was incubated overnight and the
HRP-conjugated secondary antibody was incubated at 1:5,000 for 2 h.
Protein bands were developed with BeyoECL Plus (Beyotime Institute
of Biotechnology). ImageJ software (National Institutes of Health,
v1.8) was used to analyze the gray value for a gel imaging system
for imaging. Western blotting was performed using the following
antibodies: Bax (cat. no. ab32503; 1:1,000), Bcl-2 (cat. no.
ab32124; 1:1,000), cleaved Caspase 3 (cat. no. ab32042; 1:1,000)
and β-actin (cat. no. ab8227; Abcam) antibodies were purchased from
Abcam, FOXO1 (cat. no. 2880; 1:1,000) was purchased from Cell
Signaling Technology.
Statistical analysis
Statistical analysis software SPSS 21.0 (IBM Corp.)
was used to complete the data sorting and analysis. Values are
expressed as the mean ± standard deviation from at least three
independent experiments. The differences between the two groups
were compared using a Student's t-test, whereas the differences
among several groups were compared using a one-way ANOVA with a
post-hoc Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Inhibition of SNHG16 relieved
myocardial I/R injury in vivo
SNHG16 has been reported to be a significant
regulator in multiple cancers (12). However, its function in myocardial
I/R injury remains to be elucidated. The present study tested the
expression of the SNHG16 in vivo model of myocardial I/R
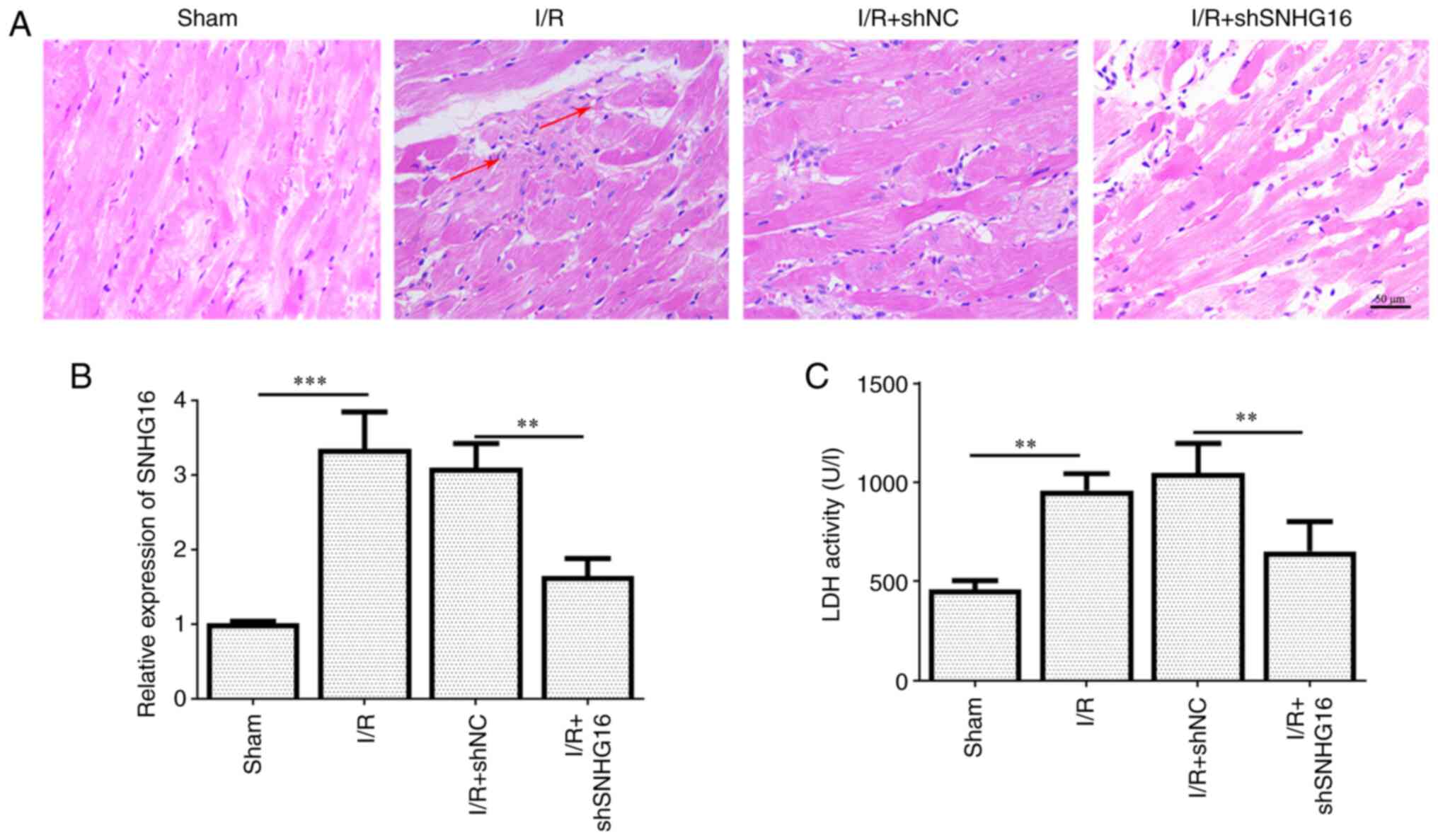
injury. As shown in Fig. 1A,
SNHG16 exhibited a higher expression in the I/R model. Compared
with the Sham group, HE staining also demonstrated that the
myocardium was severely injured in the I/R group, manifested by
myocardial edema, intense eosinophilic change and contraction band
anomaly. This effect was attenuated by the silencing of SNHG16
(Fig. 1B). The activity of LDH was
markedly enhanced in the I/R group, but knockdown of SNHG16 could
reverse this effect (Fig. 1C).
These results indicated that the inhibition of SNHG16 relieved
myocardial I/R injury in vivo.
Knockdown of SNHG16 alleviates
H/R-induced cardiomyocyte apoptosis
To verify the efficiency of knockdown SNHG16 in
H/R-induced injury cardiomyocytes, the present study transfected
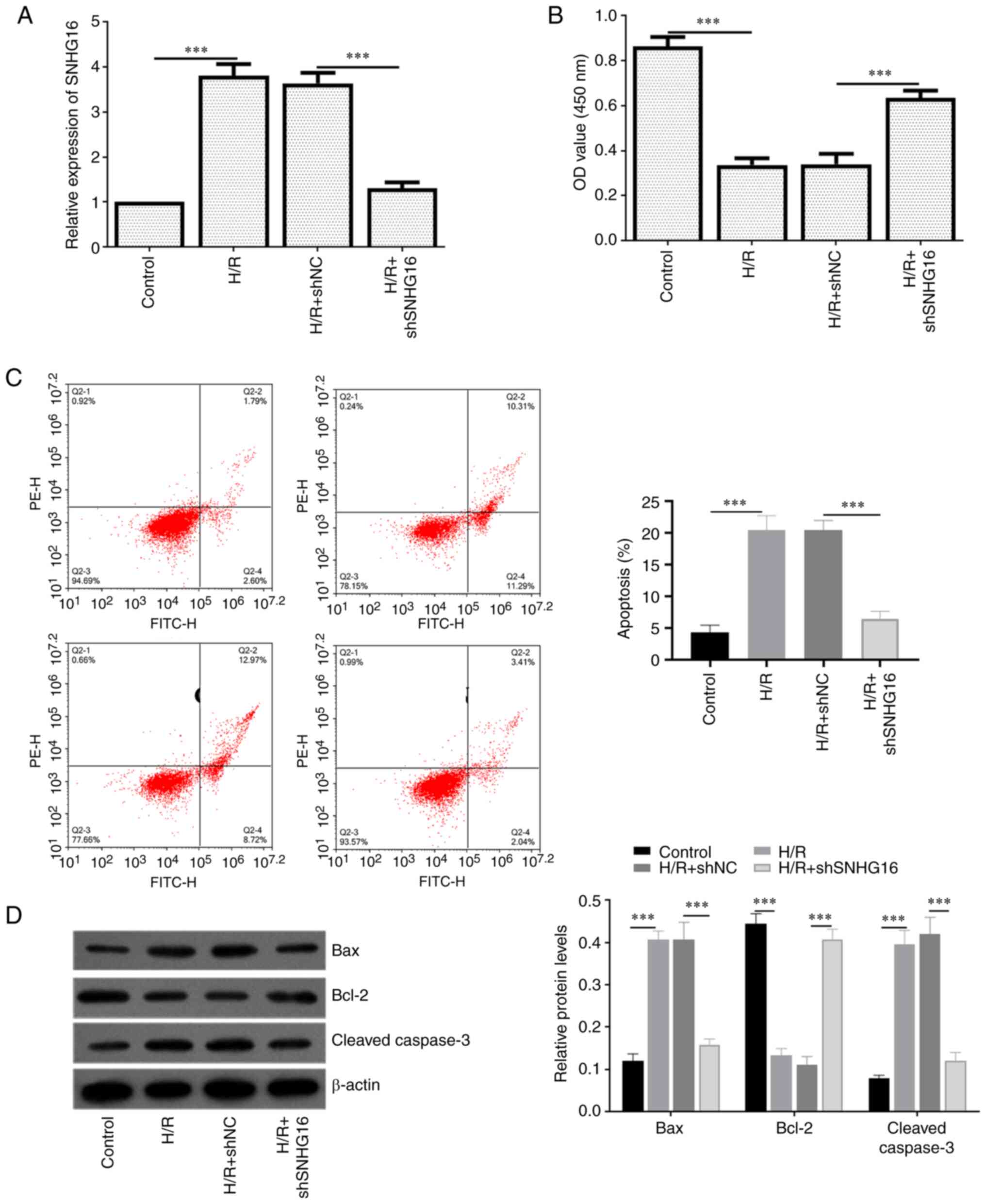
shSNHG6 into H9C2 cells before treatment with H/R. As shown in
Fig. 2A, SNHG16 in the H/R +
shSNHG16 group was significantly lower compared with the H/R group.
CCK8 assay indicated that the cell viability of H9C2 cells was
decreased by H/R treatment, while shSNHG16 reduced this effect
(Fig. 2B). Results of flow
cytometry (Fig. 2C) showed that
inhibiting SNHG16 could partially reverse the H/R-induced apoptosis
in H9C2 cells. The present study detected the apoptosis-related
protein level. Fig. 2D showed that
transfection of shSNHG16 significantly reduced the high expression
of apoptotic proteins Caspase 3 and Bax induced by H/R injury. The
expression trend of anti-apoptotic protein Bcl-2 was the opposite
to that of Caspase 3 and Bax.
LncRNA SNHG16 targeted miR-183 in H9C2
cells
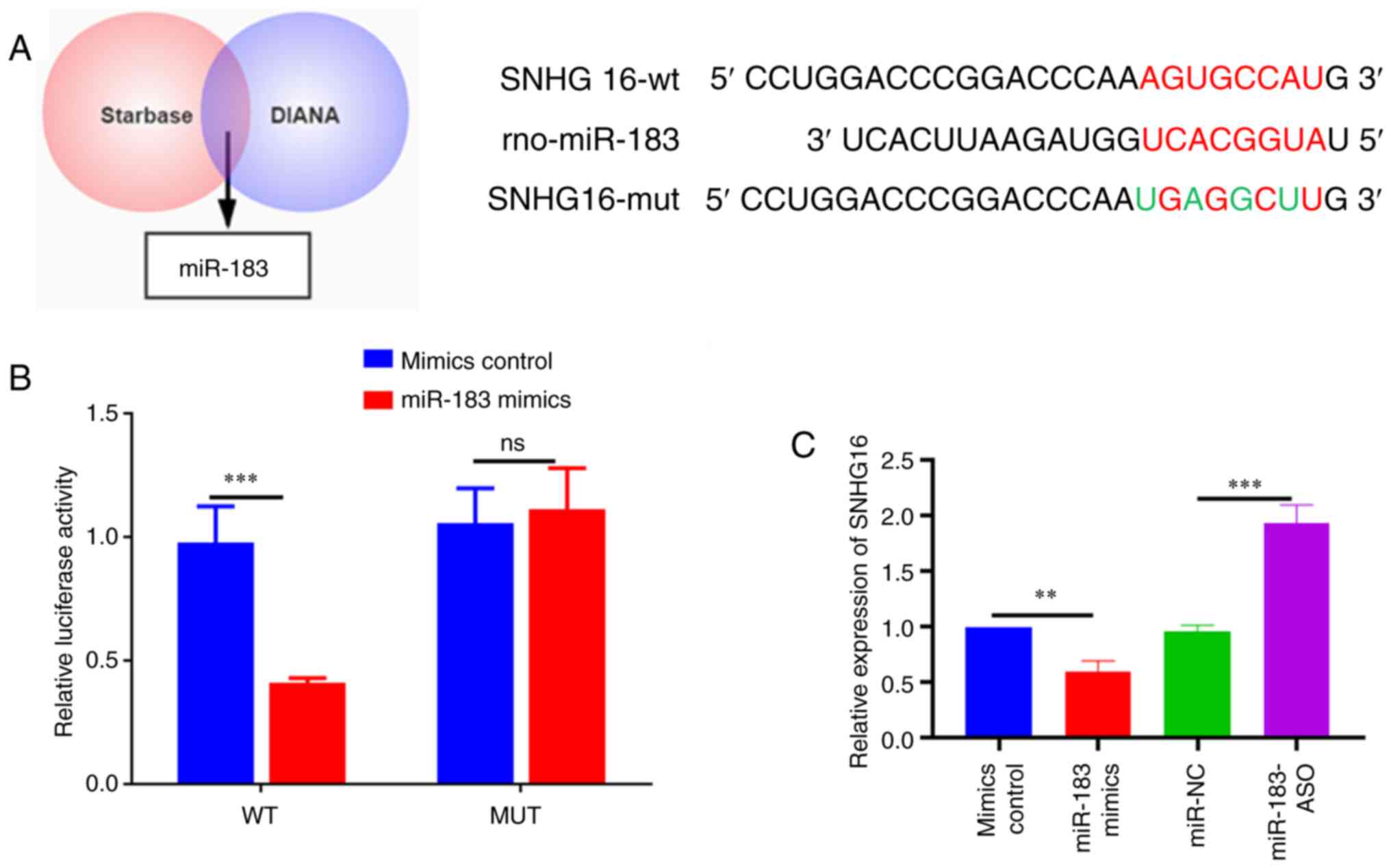
StarBase version 2.0 (http://starbase.sysu.edu.cn/) predicted that SNHG16
had a binding site with miR-183 (Fig.
3A), which is associated with the degree of myocardial ischemic
injury (17). To validate this
bioinformatics prediction, luciferase reporter further verified the
direct interaction of SNHG16 and miR-183 (Fig. 3B). It was subsequently discovered
that SNHG16 overexpression in H9c2 cells significantly inhibited
miR-183 expression while knockdown of SNHG16 increased it (Fig. 3C). Overall, these findings
suggested that SNHG16 interacted with miR-183.
miR-183 inhibitor rescues the effect
of shSNHG16 on H/R-induced cardiomyocyte apoptosis
To investigate whether SNHG6 aggravated H/R-induced
myocardial apoptosis through regulating miR-183, rescue experiments
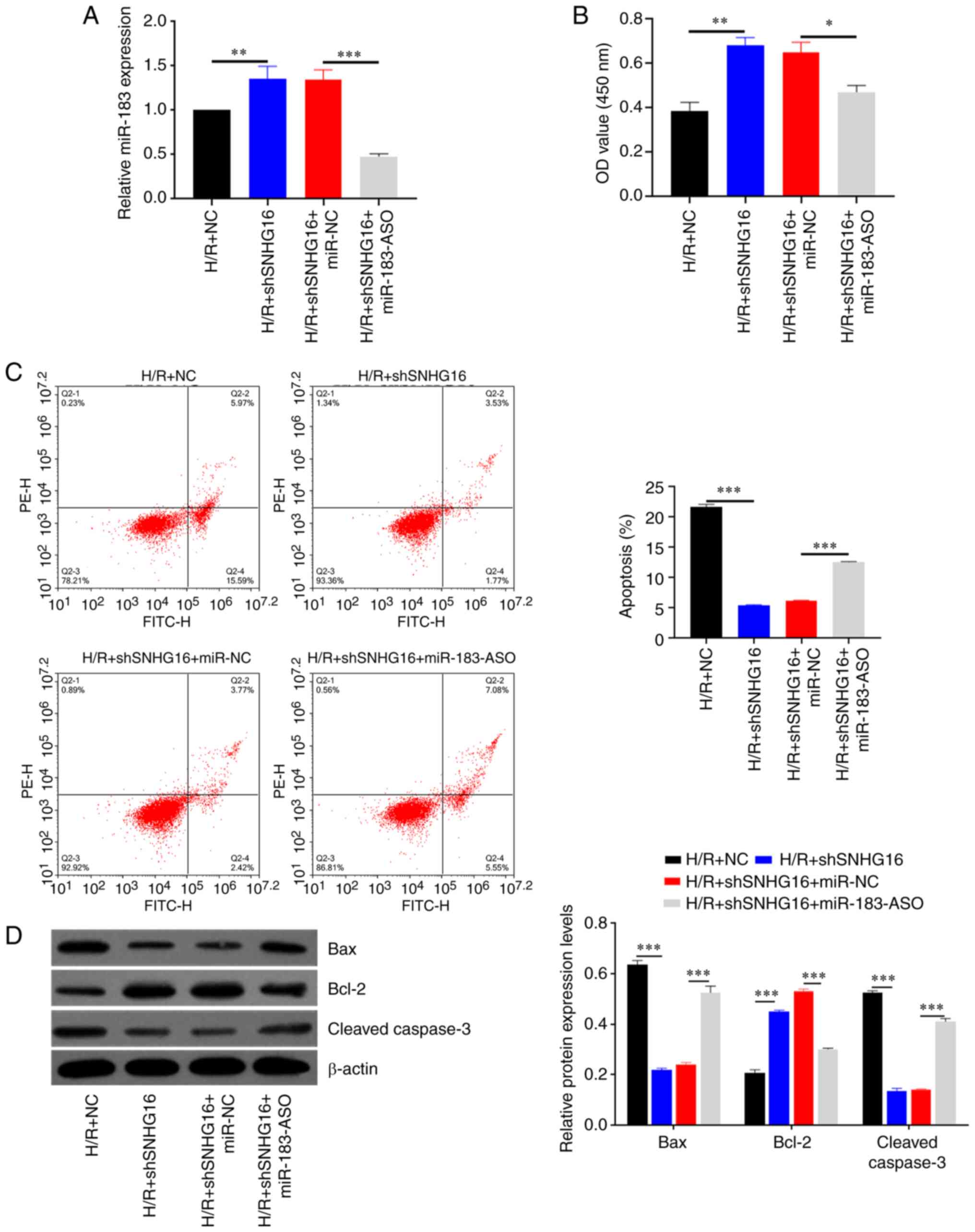
were conducted in H9C2 cells. As shown in Fig. 4A, miR-183 expression was increased
in the H/R + shSNHG16 group, whereas cotransfection of shSNHG6 and
miR-183-ASO partly restored the expression of miR-183 (Fig. 4A). Inhibition of SNHG6 increased
cell viability compared with H/R + NC, whereas inhibition of
miR-183 reduced cell viability following co-transfected with the
shSNHG6 on H/R-induced H9C2 myocardial cell injury (Fig. 4B). Flow cytometry results showed
that apoptosis was decreased in the H/R + shSNHG16 group compared
with the H/R group, whereas cotransfection of shSNHG16 and
miR-183-ASO dramatically increased apoptotic cells compared with
the H/R + shSNHG16 + miR-NC group (Fig. 4C). Similar apoptosis results were
also shown by western blotting (Fig.
4D).
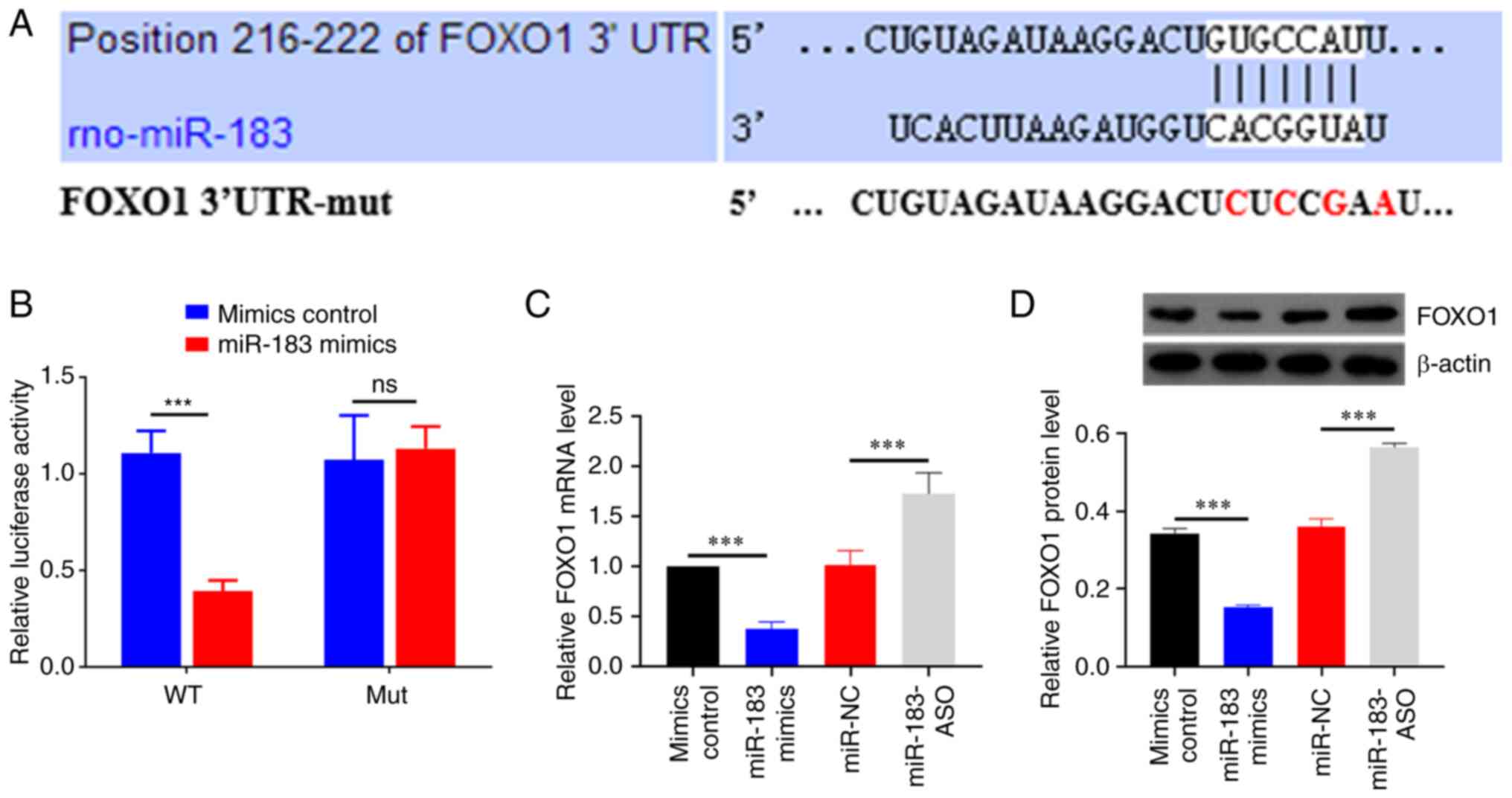
FOXO1 3'-UTR is the direct target of
miR-183 in cardiomyocyte
The downstream target genes of miR-183 were
predicted using online analysis tools, including TargetScan
(http://www.targetscan.org), miRWalk
(http://mirwalk.umm.uni-heidelberg.de)
and miRDB (http://www.mirdb.org). FOXO1 was one of
the 118 genes predicted by all three tools and there is evidence
that it is closely related to myocardial I/R injury (Fig. 5A). To further confirm this
hypothesis, a dual-luciferase reporter assay was used to verify the
regulation between miR-183 and FOXO1. As shown in Fig. 5B, the luciferase activity was
significantly decreased in H9C2 cells co-transfected with miR-183
mimics and FOXO1-WT. Furthermore, overexpression of miR-183
significantly suppressed the mRNA level of FOXO1 (Fig. 5C). Consistently, the same results
were confirmed by western blotting (Fig. 5D). Collectively, these results
indicated that miR-183 targeted FOXO1.
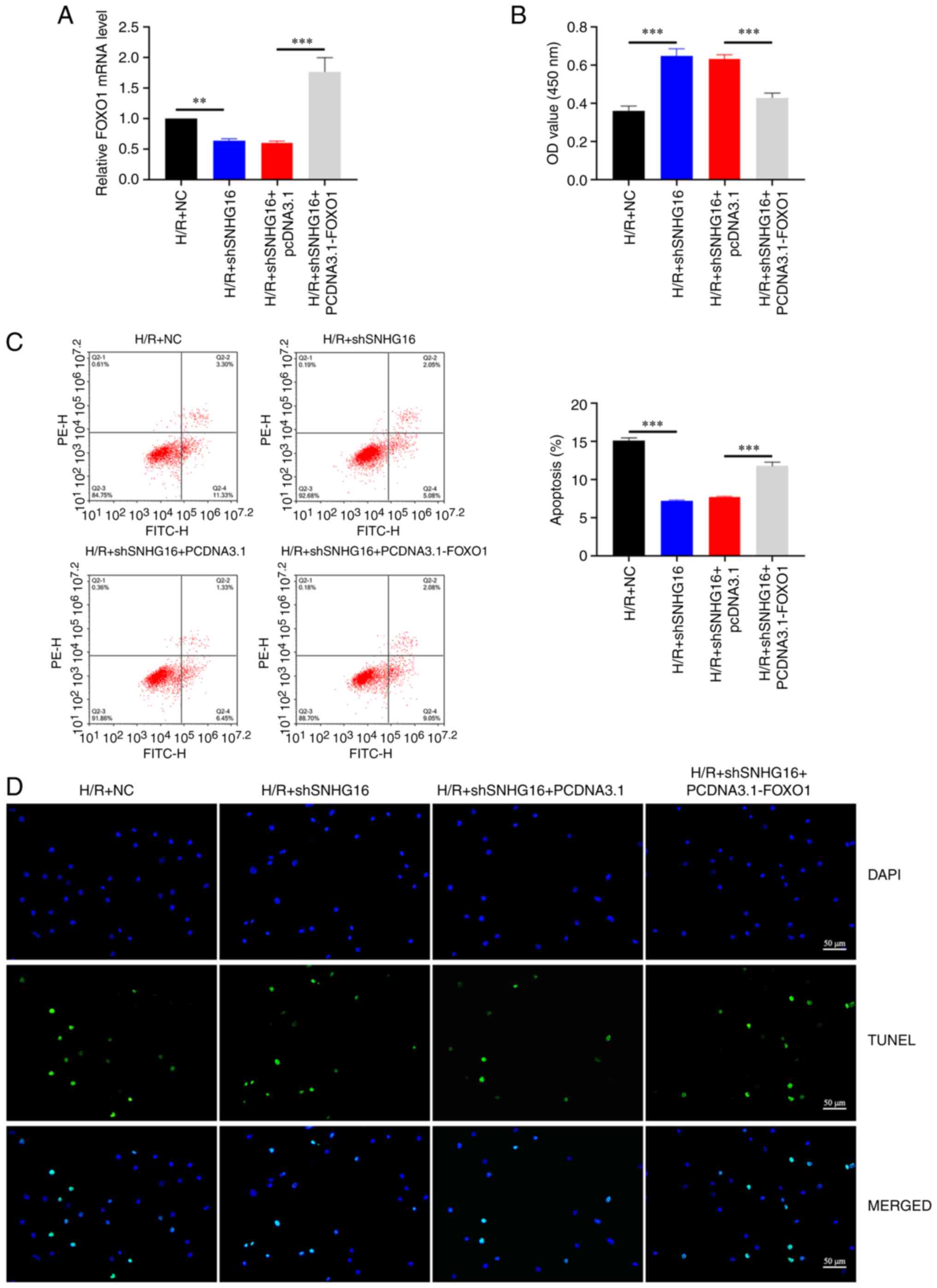
Upregulation of FOXO1 can rescue the
effect of shSNHG16 on H/R induced cardiomyocyte apoptosis
To further explore the effect of FOXO1 on SNHG16,
H9C2 cells were transfected with pcDNA3.1-FOXO1 and shSNHG16. The
RT-qPCR results showed that inhibition of SNHG16 could reduce the
expression of FOXO1 and FOXO1 expression is significantly increased
following overexpression of FOXO1 (Fig. 6A). As shown in Fig. 6B, the high cell viability caused by
shSNHG16 following H/R treatment was considerably weakened by
pcDNA3.1-FOXO1 transfection (Fig.
6B). Furthermore, flow cytometry (Fig. 6C) and TUNEL (Fig. 6D) showed the same trend. These
findings emphasized the significance of FOXO1 for SNHG16-induced
myocardial injury.
Discussion
Cardiomyocyte H/R injury is a classic model to
simulate the pathological and physiological processes of myocardial
I/R. Short-time ischemia and reperfusion of myocardial cells can
cause dysfunction of tissue cell function metabolism, aggravation
of structural and functional damage and even irreversible damage
(1). The current study found that
lncRNA SNHG16 knockdown may reduce the myocardial I/R injury in
rats and the H/R injury in H9C2 cells. The SNHG16/miR-183/FOXO1
axis modulated apoptosis in I/R injury.
A previous study indicates that silenced SNHG16
represses Ang II-imposed cardiac hypertrophy (18). In the present study, SNHG16 was
upregulated in myocardial I/R injury and inhibition of SNHG16 could
significantly improve I/R damage. A number of lncRNAs have been
demonstrated to serve as competing endogenous (ce)RNAs of miRNAs in
myocardial I/R injury progression. Guo et al (19) reported that lncRNA PART1 protects
mitochondrial function via miR-503-5p/BIRC5 in myocardial I/R
injury. A report from Li et al (20) noted that lncRNA XIST acts as a
ceRNA of miR-133a to improve myocardial I/R injury by regulating of
SOCS2 and inhibiting autophagy. In the current study, miR-183 was
identified as a functional target gene of SNHG16 through
bioinformatics and dual-luciferase reporter analysis. Evidence has
demonstrated that miR-183 plays an essential role in cardiovascular
disease. For example, Lin et al (21) found that overexpression of
miR-183-5p by agomiR transfection alleviates cardiac dysfunction
and significantly reduces the infarct size in rats with myocardial
I/R. In addition, miR-183 acts as a cardioprotective regulator for
the development of cardiomyocyte hypertrophy via direct regulation
of TIAM1(22). Furthermore, FOXO1
was confirmed to be a target of miR-183 and can be regulated by
both SNHG16 and miR-183 to affect the process of myocardial I/R
injury. FOXO1, a member of the FOX family, mainly plays a reactive
oxygen species scavenging role by regulating the oxidative stress
response through transcriptional modifications such as
phosphorylation and acetylation (23,24).
It has been reported that overexpression of FOXO1 in H9C2
cardiomyocytes can regulate PDK4 transcription and inhibit the
oxidative stress response of cardiomyocytes (25).
In summary, the experimental data from the present
study indicated the function and mechanism of lncRNA SNHG16 in
regulating myocardial I/R injury in rats and the H/R injury in H9C2
cells. The present study also showed that inhibition of SNHG16
could improve myocardial I/R injury by regulating the miR-183/FOXO1
axis, which could be a promising therapeutic agent for myocardial
I/R injury.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TG and ZX initiated and designed the present study,
analyzed and interpreted the results and wrote the manuscript. JX,
YY and JL performed various experiments. TG and ZX confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Experiments were conducted according to the
Declaration of Helsinki. The Animal Care and Use Committee of
Cangzhou Central Hospital (approval no. 2022-013-01z) approved all
rat protocols and procedures.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pagliaro BR, Cannata F, Stefanini GG and
Bolognese L: Myocardial ischemia and coronary disease in heart
failure. Heart Fail Rev. 25:53–65. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Makkos A, Ágg B, Petrovich B, Varga ZV,
Görbe A and Ferdinandy P: Systematic review and network analysis of
microRNAs involved in cardioprotection against myocardial
ischemia/reperfusion injury and infarction: Involvement of redox
signalling. Free Radic Biol Med. 172:237–251. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wang S, Luo Q, Chen H, Huang J, Li X, Wu
L, Li B, Wang Z, Zhao D and Jiang H: Light emitting diode therapy
protects against myocardial ischemia/reperfusion injury through
mitigating neuroinflammation. Oxid Med Cell Longev.
2020(9343160)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wu SZ, Tao LY, Wang JN, Xu ZQ, Wang J, Xue
YJ, Huang KY, Lin JF, Li L and Ji KT: Amifostine pretreatment
attenuates myocardial ischemia/reperfusion injury by inhibiting
apoptosis and oxidative stress. Oxid Med Cell Longev.
2017(4130824)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ulitsky I and Bartel DP: lincRNAs:
Genomics, evolution and mechanisms. Cell. 154:26–46.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kopp F and Mendell JT: Functional
classification and experimental dissection of long noncoding RNAs.
Cell. 172:393–407. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang K, Liu F, Liu CY, An T, Zhang J, Zhou
LY, Wang M, Dong YH, Li N, Gao JN, et al: The long noncoding RNA
NRF regulates programmed necrosis and myocardial injury during
ischemia and reperfusion by targeting miR-873. Cell Death Differ.
23:1394–1405. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Meng K, Jiao J, Zhu RR, Wang BY, Mao XB,
Zhong YC, Zhu ZF, Yu KW, Ding Y, Xu WB, et al: The long noncoding
RNA hotair regulates oxidative stress and cardiac myocyte apoptosis
during ischemia-reperfusion injury. Oxid Med Cell Longev.
2020(1645249)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mo Y, Wu H, Zheng X, Xu L, Liu L and Liu
Z: LncRNA CHRF aggravates myocardial ischemia/reperfusion injury by
enhancing autophagy via modulation of the miR-182-5p/ATG7 pathway.
J Biochem Mol Toxicol. 35(e22709)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cheng T, Shuang W, Ye D, Zhang W, Yang Z,
Fang W, Xu H, Gu M, Xu W and Guan C: SNHG16 promotes cell
proliferation and inhibits cell apoptosis via regulation of the
miR-1303-p/STARD9 axis in clear cell renal cell carcinoma. Cell
Signal. 84(110013)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang M and Wei W: SNHG16: A novel long-non
coding RNA in human cancers. Onco Targets Ther. 12:11679–11690.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Xiao Y, Xiao T, Ou W, Wu Z, Wu J, Tang J,
Tian B, Zhou Y, Su M and Wang W: LncRNA SNHG16 as a potential
biomarker and therapeutic target in human cancers. Biomark Res.
8(41)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Thomson DW and Dinger ME: Endogenous
microRNA sponges: Evidence and controversy. Nat Rev Genet.
17:272–283. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao Y, Ponnusamy M, Dong Y, Zhang L, Wang
K and Li P: Effects of miRNAs on myocardial apoptosis by modulating
mitochondria related proteins. Clin Exp Pharmacol Physiol.
44:431–440. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gong L, Xu H, Chang H, Tong Y, Zhang T and
Guo G: Knockdown of long non-coding RNA MEG3 protects H9c2 cells
from hypoxia-induced injury by targeting microRNA-183. J Cell
Biochem. 119:1429–1440. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao X, Jia Y, Chen H, Yao H and Guo W:
Plasma-derived exosomal miR-183 associates with protein kinase
activity and may serve as a novel predictive biomarker of
myocardial ischemic injury. Exp Ther Med. 18:179–187.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang D, Lin B, Zhang W and Wang X:
Up-regulation of SNHG16 induced by CTCF accelerates cardiac
hypertrophy by targeting miR-182-5p/IGF1 axis. Cell Biol Int.
44:1426–1435. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guo Z, Zhao M, Jia G, Ma R and Li M:
LncRNA PART1 alleviated myocardial ischemia/reperfusion injury via
suppressing miR-503-5p/BIRC5 mediated mitochondrial apoptosis. Int
J Cardiol. 338:176–184. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li Z, Zhang Y, Ding N, Zhao Y, Ye Z, Shen
L, Yi H and Zhu Y: Inhibition of lncRNA XIST improves myocardial
I/R injury by targeting miR-133a through inhibition of autophagy
and regulation of SOCS2. Mol Ther Nucleic Acids. 18:764–773.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lin D, Cui B, Ma J and Ren J: MiR-183-5p
protects rat hearts against myocardial ischemia/reperfusion injury
through targeting VDAC1. Biofactors. 46:83–93. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gong FH, Chen XL, Zhang Q, Xiao XQ, Yang
YS, Song BJ, Chao SP and Cheng WL: MicroRNA-183 as a novel
regulator protects against cardiomyocytes hypertrophy via targeting
TIAM1. Am J Hypertens. 35:87–95. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
van der Horst A and Burgering BM:
Stressing the role of FoxO proteins in lifespan and disease. Nat
Rev Mol Cell Biol. 8:440–450. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Storz P: Forkhead homeobox type O
transcription factors in the responses to oxidative stress.
Antioxid Redox Signal. 14:593–605. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chien HC, Greenhaff PL and
Constantin-Teodosiu D: PPARδ and FOXO1 mediate palmitate-induced
inhibition of muscle pyruvate dehydrogenase complex and CHO
oxidation, events reversed by electrical pulse stimulation. Int J
Mol Sci. 21(5942)2020.PubMed/NCBI View Article : Google Scholar
|