Introduction
Gastric cancer is a major cancer worldwide, with the
fifth highest incidence and fourth highest mortality (1). Although endoscopy is the most
sensitive diagnostic screening method, the detection of gastric
cancer in the early stages is challenging (2,3). The
effectiveness of gastric cancer treatment in developing countries
is very low, and certain countries are unable to treat gastric
cancer. However, the success rate of gastric cancer treatment in
Japan is high (4,5). Japan mainly performs tumor resection
for patients where gastric cancer has been detected early; the
detection of cancer at an early stage is important for obtaining a
good outcome. Early gastric cancer only shows mild morphological
changes and color differences in relation to the surrounding mucosa
(2,6). Due to these subtle manifestations, it
can be challenging for junior endoscopists to diagnose, and
numerous experts use white light imaging (WLI) to screen and
diagnose patients with gastric cancer.
Several image-enhanced endoscopic (IEE) systems
(7), including narrow-band imaging
(NBI) (8,9), flexible spectral imaging color
enhancement, blue laser imaging (BLI) and linked color imaging
(LCI), have recently emerged as simple and convenient imaging
methods for the detection of gastric tumours (10). Early gastric cancers may be missed
by conventional endoscopy as they may not be easy to recognize
(11). NBI and LCI are more
intuitive than WLI regarding the visualization of epidermal vessels
(12). LCI enhances the surface
color and the boundary between the malignant lesion and the
surrounding mucosa (12), with
high diagnostic performance for gastrointestinal endoscopy
(13). However, data on this
subject are insufficient, with few papers reporting the diagnostic
performance of LCI for high-grade gastric intraepithelial
neoplasia. Therefore, the aim of the present study was to
investigate whether LCI had improved diagnostic accuracy for
high-grade gastric intraepithelial neoplasia compared with WLI,
particularly when performed by junior endoscopists.
Materials and methods
Patients
From January 2017 to December 2017, 84 lesions from
81 patients diagnosed using LCI and WLI, and confirmed to have
high-grade gastric intraepithelial neoplasia by the pathological
examination of biopsy tissue, were enrolled in the present study.
High-grade gastric intraepithelial neoplasia includes severe
dysplasia and carcinoma in situ. Carcinoma in situ is
distinguished from intramucosal carcinoma by the destruction of the
basement membrane of the gland which occurs in the latter. Three
patients who had two lesions were excluded and the remaining 78
patients who had only one lesion were included in the study. The
lesions of macroscopic type are classified using the Paris
classification (14-15): 0-IIa, slightly elevated; 0-IIb,
flat; and 0-IIc, slightly depressed. The mean age of the patients
was 46.44±11.99 years, and their sex ratio was 0.73:1 (male:
female). The patients were enrolled at Shanxi Cancer Hospital
(Taiyuan, China), and the study design was approved by the Ethics
Committee of Shanxi Cancer Hospital (ref. no. 201992). Written
informed consent was obtained from patients or their guardians for
participation in the study. The inclusion criteria were as follows:
Patients undergoing gastroscopy for the first time; diagnosed with
high-grade gastric intraepithelial neoplasia by pathology after
biopsy; and agreed to undergo detailed examination using the
linkage imaging mode of the gastroscope (LASEREO EG L590ZW;
Fujifilm Corporation). The exclusion criteria were as follows:
Patients with incomplete clinical, endoscopic and pathological
data; multiple gastric cancer lesions; and underwent radiotherapy
or chemotherapy before the examination.
Endoscopic image acquisition and
selection
The HD EG-L590WR endoscope (Fujifilm Corporation)
was used for all examinations. Images of high-grade gastric
intraepithelial neoplasia were captured carefully from the same
place and angle as the unmagnified photographs with LCI and
conventional WLI. Only endoscopic images of high-grade
intraepithelial neoplasia were included; benign erosions, ulcers or
polyps, which are also distinctive on WLI, were not examined.
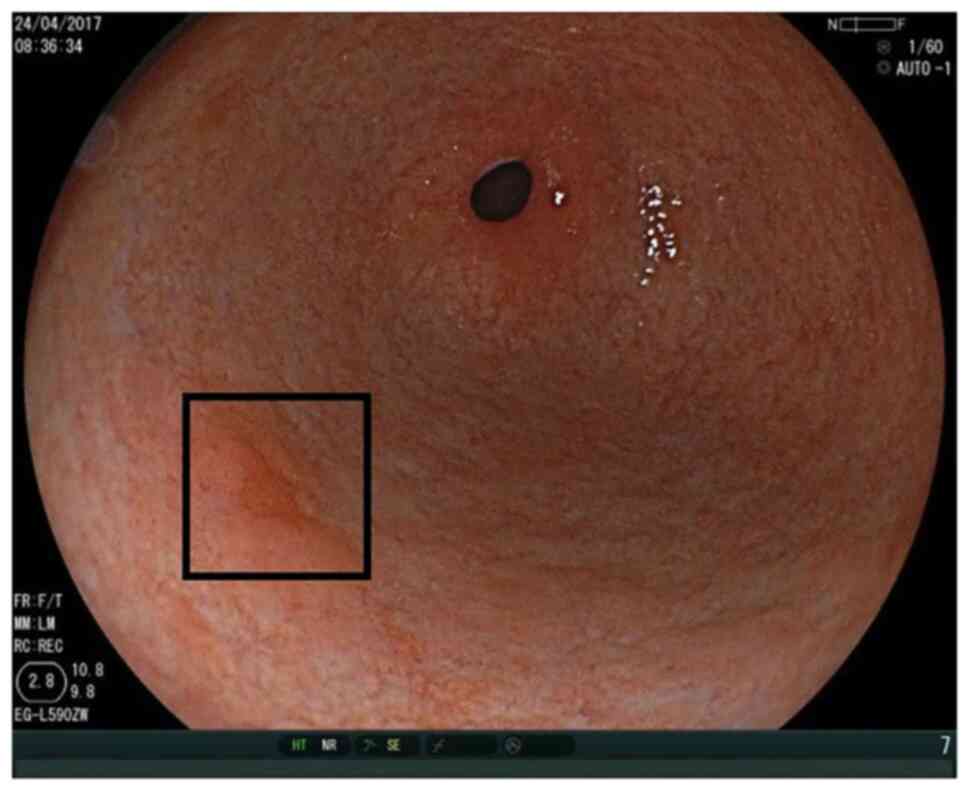
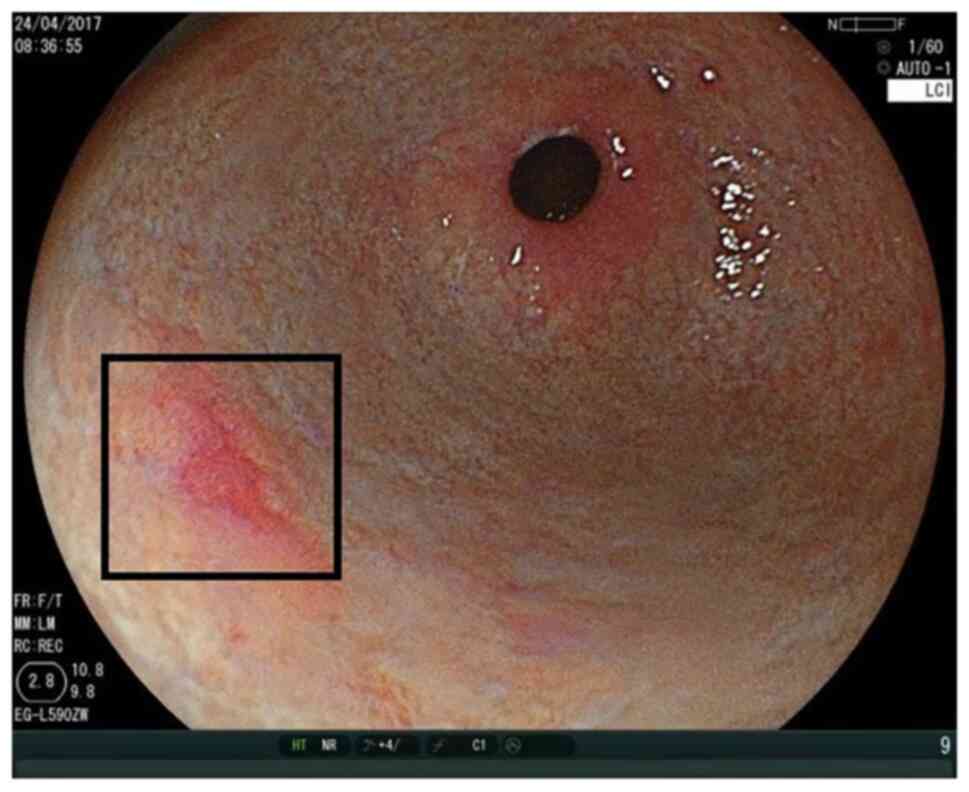
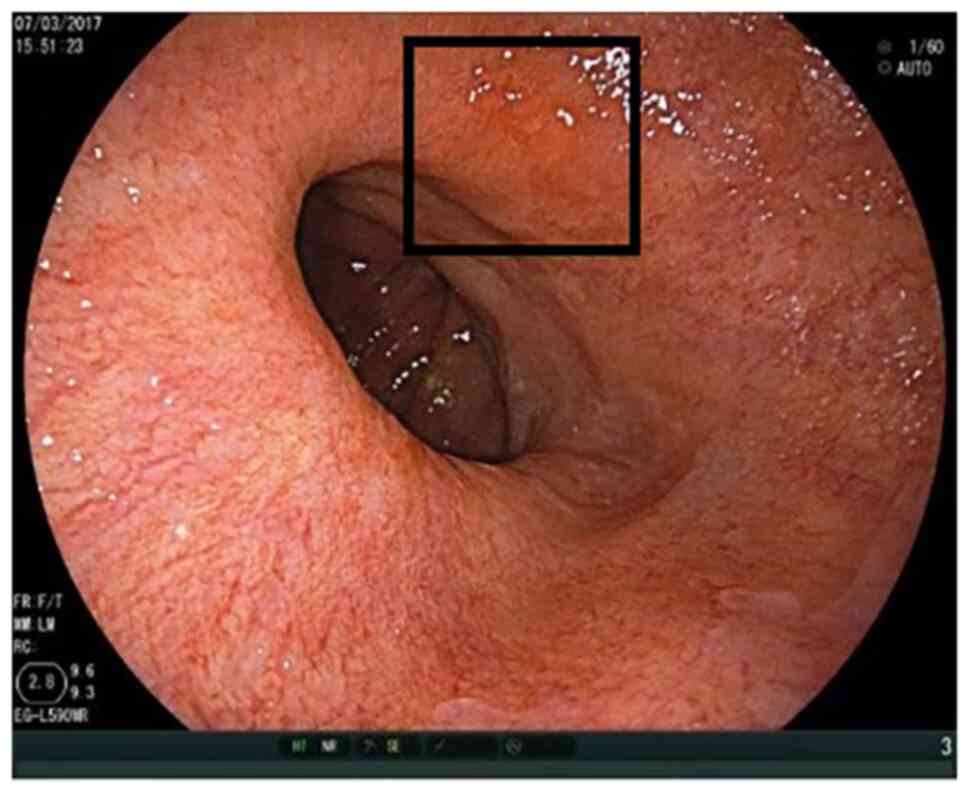
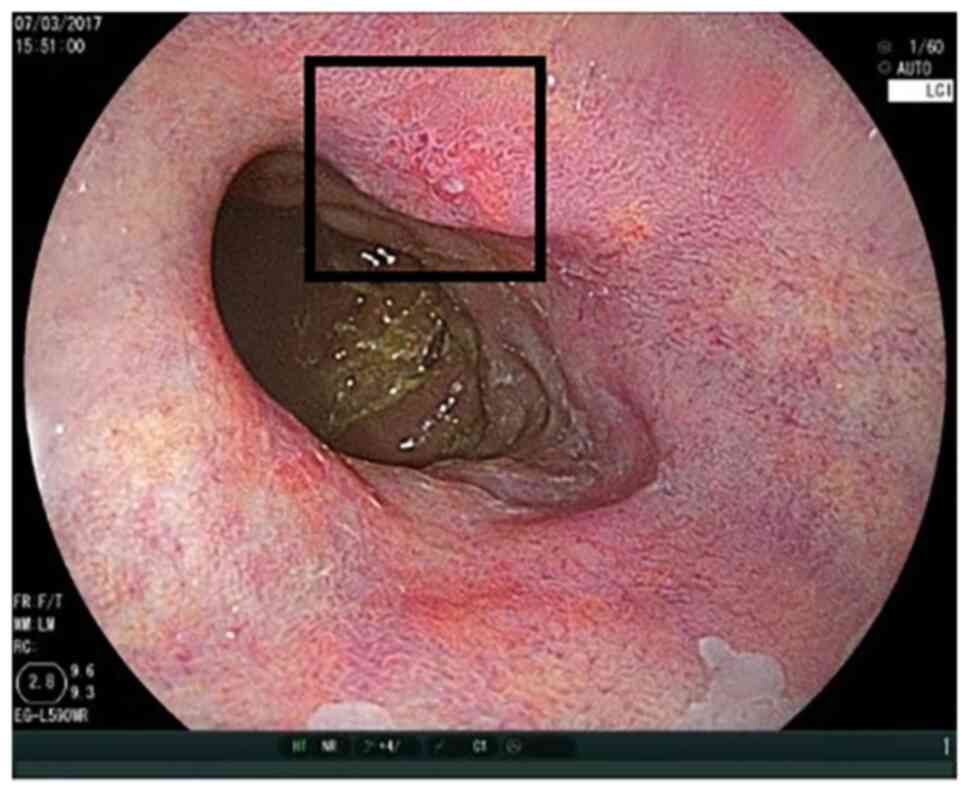
Figs. 1 and 2 show the ease of recognition of
high-grade intraepithelial neoplasia by WLI as well as LCI. By
contrast, Figs. 3 and 4 show that it can be challenging to
recognise high-grade intraepithelial neoplasia by WLI, while
recognition is easier using LCI. Two sets of images were collected
for each patient, one acquired by WLI and the other by LCI. Next,
both sets of images were numbered randomly. The images were
captured by two expert endoscopists who had previously performed
>5,000 esophagogastroduodenoscopies and did not participate in
the subsequent study.
Pathological image acquisition
The fresh specimen was preserved by prompt immersion
in a 10% neutral buffered formalin solution for fixation at room
temperature for 24-48 h. The specimen was then dehydrated by
immersion in 75% ethanol for 1 h, 85% ethanol for 1 h, 95% ethanol
for 1 h twice and absolute ethanol for 1 h three times, then washed
with xylene for 40 min twice and immersed in paraffin for 1 h four
times. Wax embedding was subsequently performed by pouring molten
wax into an embedding box, placing the specimens at the center of
the bottom surface of the embedding box and injecting paraffin. The
prepared embedding box was frozen at -20˚C for 1 h. Wax blocks were
decanted from the embedding frame and sliced into 4-µm sections
using a microtome.
To prepare the sections for hematoxylin and eosin
staining, each specimen was promptly immersed in xylene for 5 min
three times, then in 100, 95, 85 and 75% ethanol for 20 sec each,
and finally in tap water for 20 sec. Subsequently, the specimen was
immersed in hematoxylin solution for 5 min at room temperature and
washed with water for 30 sec. It was then differentiated in 1%
hydrochloric ethanol for 2 sec, rinsed with water for 30 sec and
treated with lithium carbonate reverse blue solution for 1 min at
room temperature. After this, it was immersed in 0.5% eosin ethanol
solution for 1 min at room temperature, followed by 100% ethanol
for 20 sec, 75, 85 and 95% ethanol for 10 sec each, 100% ethanol
for 20 sec twice and xylene for 1 min three times. The stained
specimens were finally sealed using a neutral gum sealing
piece.
Pathological evaluation was performed by an
experienced clinical pathologist using plain light microscopy.
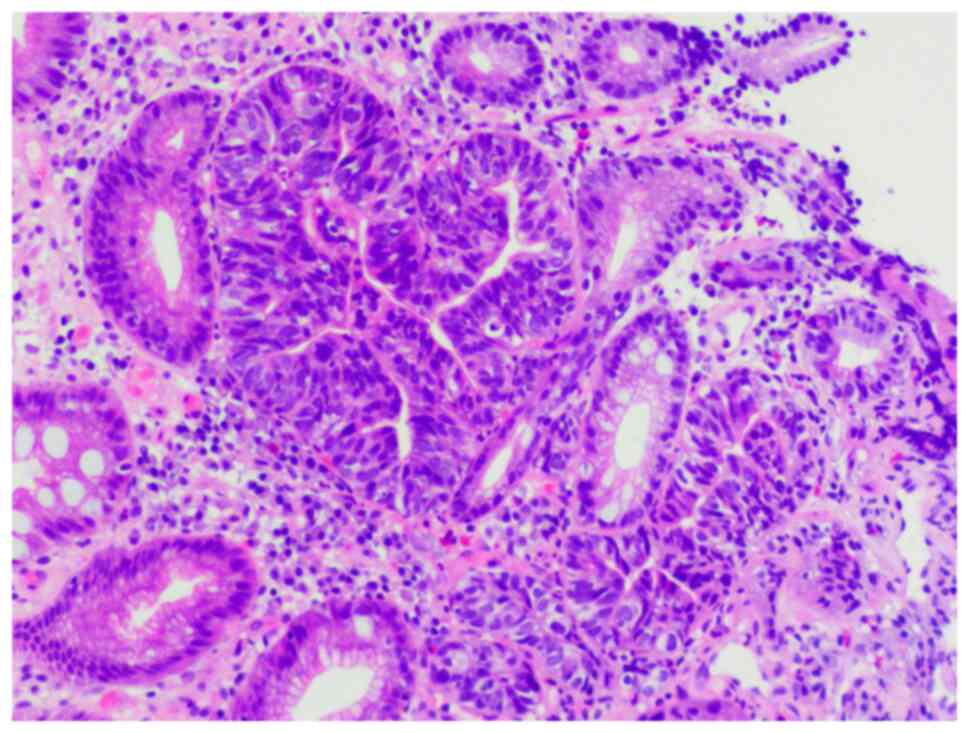
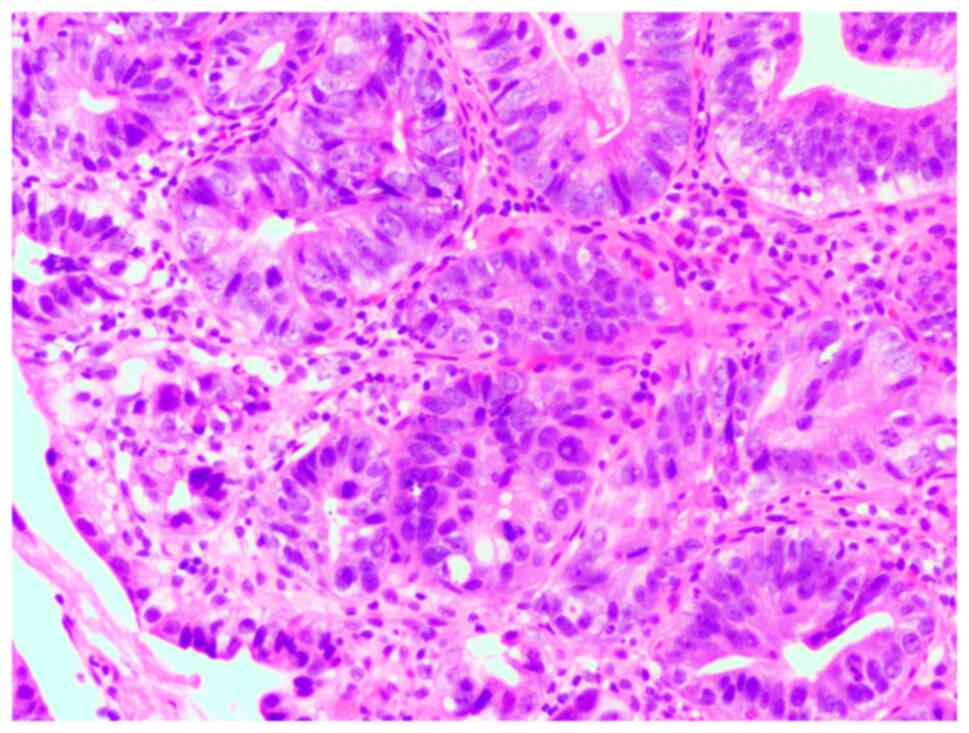
Figs. 5 and 6 show pathological images of high-grade
gastric intraepithelial neoplasms that were easy and challenging,
respectively, to recognize by imaging.
Methods of image analysis
Four senior endoscopists, each of whom had performed
>5,000 esophagogastroduodenoscopies, and four junior
endoscopists, each of whom had performed ~1,000
esophagogastroduodenoscopies, evaluated the images retrospectively.
The patients were examined using conventional endoscopies with WLI
and LCI under the same conditions. The sets of endoscopic images,
divided into 156 groups, were presented to each endoscopist
randomly. The study was designed only to evaluate the diagnosis of
high-grade intraepithelial neoplasia; the location or size of the
lesions was not considered. After the diagnoses were completed, the
endoscopists immediately recorded the results as either positive or
negative. The method of verification was through two sets of LCI
and WLI image evaluation experiments conducted by different
doctors.
Study outcomes
The primary outcome of this study was the rate of
diagnosis between modalities and endoscopists. The secondary
outcome was the interobserver agreement (16), which was calculated at 5 levels:
0.0-0.20=slight; 0.21-0.40=fair; 0.41-0.60=moderate;
0.61-0.80=substantial; and 0.81-1.00=almost perfect.
Statistical analysis
Quantitative data are expressed as the mean and
standard deviation. The rates of diagnosis between modalities and
endoscopists were compared using the χ2 test. P<0.05
was considered to indicate a statistically significant result. The
interobserver agreement was evaluated using Kappa test. All
statistical analysis was performed using SPSS 19.0 statistical
software (IBM Corp.).
Results
Baseline characteristics
From January 2017 to December 2017, 78 patients were
enrolled in the study. The patients were confirmed to have
high-grade gastric intraepithelial neoplasia by pathological
examination of the biopsy tissues. The mean age of the patients was
46.44±11.99 years and their sex ratio was 0.73:1 (male:female).
Table I displays characteristics
of the patients and their tumors.
 | Table IPatient and tumor characteristics. |
Table I
Patient and tumor characteristics.
| Factors | Value |
|---|
| Total patients,
n | 78 |
| Patient
characteristics | |
|
Sex, n | |
|
Male | 33 |
|
Female | 45 |
|
Age, years,
mean ± SD | 46.44±11.99 |
| Tumour
characteristics | |
|
Location,
n | |
|
Upper | 40 |
|
Middle | 18 |
|
Lower | 20 |
|
Macroscopic
typea, n | |
|
0-IIa | 34 |
|
0-IIc | 44 |
|
Size, mm,
mean ± SD | 13.99±4.61 |
Evaluation of the WLI images
Table II reports
the results of the evaluation of the WLI images by the eight
endoscopists. The lesion detection rates by the four junior
endoscopists were 30/78 (38.46%), 32/78 (41.03%), 35/78 (44.87) and
31/78 (39.74). There were no significant differences among the four
junior endoscopists in their evaluation of the WLI images. The
lesion detection rates by the four senior endoscopists were 47/78
(60.26%), 45/78 (57.69%), 45/78 (57.69%) and 50/78 (64.10%). There
were also no significant differences in detection rates among the
four senior endoscopists.
 | Table IILesion detection rate using white
light imaging. |
Table II
Lesion detection rate using white
light imaging.
| Endoscopist | Positive, n (%) | Negative, n (%) | χ2 | P-value |
|---|
| Junior | | | 0.74 | 0.86 |
|
A | 30 (38.46) | 48 (61.54) | | |
|
B | 32 (41.03) | 46 (58.97) | | |
|
C | 35 (44.87) | 43 (55.13) | | |
|
D | 31 (39.74) | 47 (60.26) | | |
| Senior | | | 0.89 | 0.83 |
|
E | 47 (60.26) | 31 (39.74) | | |
|
F | 45 (57.69) | 33 (42.31) | | |
|
G | 45 (57.69) | 33 (42.31) | | |
|
H | 50 (64.10) | 28 (35.90) | | |
Evaluation of the LCI images
Table III reports
the results of the evaluation of the LCI images by the eight
endoscopists. The lesion detection rates by the four junior
endoscopists were 64/78 (82.05%), 66/78 (84.62%), 69/78 (88.46%)
and 67/78 (85.90%), respectively. No significant differences were
identified among the four junior endoscopists in the evaluation of
the images. The lesion detection rates by the four senior
endoscopists were 70/78 (89.74%), 70/78 (89.74%), 68/78 (87.18%)
and 71/78 (91.03%), respectively. No significant differences in
detection rates were detected among the four senior
endoscopists.
 | Table IIILesion detection rate using linked
color imaging. |
Table III
Lesion detection rate using linked
color imaging.
| Endoscopist | Positive, n (%) | Negative, n (%) | χ2 | P-value |
|---|
| Junior | | | 1.33 | 0.72 |
|
A | 64 (82.05) | 14 (17.95) | | |
|
B | 66 (84.62) | 12 (15.38) | | |
|
C | 69 (88.46) | 9 (11.54) | | |
|
D | 67 (85.90) | 11 (14.1) | | |
| Senior | | | 0.64 | 0.89 |
|
E | 70 (89.74) | 8 (10.26) | | |
|
F | 70 (89.74) | 8 (10.26) | | |
|
G | 68 (87.18) | 10 (12.82) | | |
|
H | 71 (91.03) | 7 (8.97) | | |
Detection rates
Table IV reports
the detection rates obtained using WLI and LCI for the junior and
senior endoscopists. The lesion detection rates by the junior and
senior endoscopists based on images obtained by WLI were 128/312
(41.03%) and 187/312 (59.94%), respectively. The lesion detection
rates of the junior and senior endoscopists were significantly
different. The lesion detection rates by the junior and senior
endoscopists based on images obtained by LCI were 266/312 (85.26%)
and 279/312 (89.42%), respectively. The lesion detection rates of
the junior and senior endoscopists were not significantly
different.
 | Table IVLesion detection rates by WLI and LCI
for junior and senior endoscopists. |
Table IV
Lesion detection rates by WLI and LCI
for junior and senior endoscopists.
| Imaging type | Junior
endoscopists, n (%) | Senior
endoscopists, n (%) | χ2 | P-value |
|---|
| WLI | 128 (41.03) | 187 (59.94) | 22.32 | <0.01 |
| LCI | 266 (85.26) | 279 (89.42) | 2.45 | 0.12 |
Interobserver agreement values
Table V reports
that the interobserver agreement values for WLI and LCI were 0.54
and 0.59 for junior endoscopists and 0.63 and 0.65 for senior
endoscopists, respectively. These values indicate that the
interobserver agreement was good to satisfactory.
 | Table VInterobserver agreement of evaluation
by junior and senior endoscopists. |
Table V
Interobserver agreement of evaluation
by junior and senior endoscopists.
| Imaging type | Junior
endoscopists | Senior
endoscopists |
|---|
| WLI | 0.54 | 0.63 |
| LCI | 0.59 | 0.65 |
Discussion
Gastric cancer is a malignant disease that is
challenging to treat (17).
Following years of research, a consensus on the utility of
endoscopy for gastric cancer diagnosis has been reached, along with
recommendations to reduce Helicobacter pylori infections,
take sufficient exercise and follow a healthy lifestyle (18). The early detection of gastric
cancer increases the likelihood of treatments being effective
(19-21).
For the early diagnosis of gastric cancer, the
timely detection of early lesions is very important.
Characteristics of lesions revealed by IEE techniques are
enhancement and a change in color. Observation using image-enhanced
endoscopy enables the early signs of gastric cancer to be detected
effectively. LCI aids visualization of the difference between
gastric lesions and normal stomach tissue and enables junior
endoscopists to observe the difference between the two types of
tissue (22).
The present study is a comparative analysis of the
diagnostic utility of LCI for high-grade gastric intraepithelial
neoplasia. Gastric lesions and normal stomach tissue may be
distinguished when observed using LCI due to a difference in color.
Color differences observed by LCI are more obvious than those
observed by WLI. Improved detection rates were observed for junior
and senior endoscopists using LCI compared with WLI, and the
difference in detection rates was statistically significant. These
results indicate that in the early diagnosis of gastric cancer, LCI
is more effective than WLI.
With WLI, the lesion detection rate for junior
endoscopists ranged from 38.46 to 44.87%, and for senior
endoscopists, it ranged from 57.69 to 64.10%. These results suggest
that senior endoscopists had a much higher detection rate than
junior endoscopists, and the lesion detection rate using WLI was
significantly different between junior and senior endoscopists. By
contrast, the detection rate using LCI improved to >80% for both
senior and junior endoscopists, and no significant differences were
detected between them. The results of the present study indicate
that LCI allows the easy recognition and early detection of gastric
cancer even by junior endoscopists, which may improve the rate of
diagnosis and reduce that of misdiagnosis, regardless of the level
of experience of the endoscopist. LCI may enable the gap in the
detection rate of high-grade gastric intraepithelial neoplasia
between junior and senior endoscopists to be narrowed.
Yoshifuku et al (23) evaluated the visibility of early
gastric cancer using LCI and BLI. The study found that LCI improved
the visibility of early gastric cancer, regardless of the
endoscopist's experience or Helicobacter pylori eradication
in patients, compared with BLI; the visibility obtained with LCI
was significantly higher with than that obtained with BLI. In
another study, Suzuki et al (24) reported the efficacy of LCI in
improving the visibility of flat colorectal lesions compared with
BLI. When compared with WLI and BLI, LCI presents greater color
differences between gastric lesions and the mucosa around the
stomach, thereby improving the detection of gastric cancer, as
verified by the current study and previous research.
The present study has some limitations. First, a
relatively small patient sample was examined rather than a
large-scale cohort and type 0-IIb lesions were not included.
Therefore, the effectiveness of LCI for the detection of 0-IIb
lesions remains unclear. To address this, random evaluations and
controlled multicenter clinical trials are required. Secondly, only
endoscopic images of high-grade intraepithelial neoplasia were
examined, as they were the focus of the study. Benign erosions,
ulcers and polyps, which are also distinctive when viewed using
WLI, were not examined. Therefore, other reddish lesions require
further examination in future studies.
In conclusion, the present study shows that junior
endoscopists can detect gastric cancer early using LCI, which may
improve the rate of diagnosis and reduce misdiagnosis, regardless
of the level of experience of the endoscopist. Additionally, the
use of LCI may narrow the gap in detection rates between junior and
senior endoscopists for high-grade gastric intraepithelial
neoplasia.
Acknowledgements
The authors would like to thank Dr Qi Zhao (Shanxi
Cancer Hospital, Department of Pathology) for their theoretical
guidance on pathology.
Funding
Funding: The study was funded by Key Research and Development
Projects of Shanxi Province (grant no. 201903D321140).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC conceived and supervised the study. JHL and HHC
designed the study. HHC, JHL and HZZ performed data collection and
processing. HZZ, WBZ and JF analyzed and interpreted the data. JHL
and WBZ conducted the literature search. JHL and HHC wrote the
manuscript. XC critically reviewed the manuscript. All authors read
and approved the final version of the manuscript. XC and JHL
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Ethics committee approval was provided for this
study by the Ethics Committee of Shanxi Cancer Hospital (approval.
no. 201992). Written informed consent was obtained from patients or
their guardians for participation in the study.
Patient consent for publication
Not applicable.
Authors' information
Junhui Lu is a PhD student, ORCID no.
0000-0003-2387-5945; Haihua Chen is a Bachelor of Medicine, ORCID
no. 0000-0002-1581-0490; Xing Chen is a Doctor of Medicine, ORCID
no. 0000-0003-3806-5494; Hezhao Zhang is a PhD student, ORCID no.
0000-0002-9565-4298; Jing Fan is a Master of Medicine, ORCID no.
0000-0002-7080-0666; and Wenbin Zhang is a Master of Medicine,
ORCID no. 0000-0003-1995-6750.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yao K, Uedo N, Kamada T, Hirasawa T,
Nagahama T, Yoshinaga S, Oka M, Inoue K, Mabe K, Yao T, et al:
Guidelines for endoscopic diagnosis of early gastric cancer. Dig
Endosc. 32:663–698. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tanabe S, Hirabayashi S, Oda I, Ono H,
Nashimoto A, Isobe Y, Miyashiro I, Tsujitani S, Seto Y, Fukagawa T,
et al: Gastric cancer treated by endoscopic submucosal dissection
or endoscopic mucosal resection in Japan from 2004 through 2006:
JGCA nationwide registry conducted in 2013. Gastric Cancer.
20:834–842. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Young E, Philpott H and Singh R:
Endoscopic diagnosis and treatment of gastric dysplasia and early
cancer: Current evidence and what the future may hold. World J
Gastroenterol. 27:5126–5151. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nasu J, Doi T, Endo H, Nishina T, Hirasaki
S and Hyodo I: Characteristics of metachronous multiple early
gastric cancers after endoscopic mucosal resection. Endoscopy.
37:990–993. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Loncroft-Wheaton G and Bhandari P: Image
enhanced endoscopy: Optical diagnosis or optical illusion? Saudi J
Gastroenterol. 25:71–72. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Doyama H, Nakanishi H and Yao K:
Image-enhanced endoscopy and its corresponding histopathology in
the stomach. Gut Liver. 15:329–337. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Yoshida N, Doyama H, Yano T, Horimatsu T,
Uedo N, Yamamoto Y, Kakushima N, Kanzaki H, Hori S, Yao K, et al:
Early gastric cancer detection in high-risk patients: A multicentre
randomised controlled trial on the effect of second-generation
narrow band imaging. Gut. 70:67–75. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhenming Y and Lei S: Diagnostic value of
blue laser imaging combined with magnifying endoscopy for
precancerous and early gastric cancer lesions. Turk J
Gastroenterol. 30:549–556. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Onozato Y, Ishihara H, Iizuka H, Sohara N,
Kakizaki S, Okamura S and Mori M: Endoscopic submucosal dissection
for early gastric cancers and large flat adenomas. Endoscopy.
38:980–986. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fujimoto D, Muguruma N, Okamoto K, Fujino
Y, Kagemoto K, Okada Y, Takaoka Y, Mitsui Y, Kitamura S, Kimura T,
et al: Linked color imaging enhances endoscopic detection of
sessile serrated adenoma/polyps. Endosc Int Open. 6:E322–E334.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ono S, Abiko S and Kato M: Linked color
imaging enhances gastric cancer in gastric intestinal metaplasia.
Dig Endosc. 29:230–231. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
The Paris endoscopic classification of
superficial neoplastic lesions: Esophagus, stomach, and colon:
November 30 to December 1, 2002. Gastrointest Endosc. 58 (6
Suppl):S3–S43. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Endoscopic Classification Review Group.
Update on the paris classification of superficial neoplastic
lesions in the digestive tract. Endoscopy. 37:570–578.
2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Landis JR and Koch GG: The measurement of
observer agreement for categorical data. Biometrics. 33:159–174.
1977.PubMed/NCBI
|
|
17
|
Toyokawa T, Inaba T, Omote S, Okamoto A,
Miyasaka R, Watanabe K, Izumikawa K, Fujita I, Horii J, Ishikawa S,
et al: Risk factors for non-curative resection of early gastric
neoplasms with endoscopic submucosal dissection: Analysis of 1,123
lesions. Exp Ther Med. 9:1209–1214. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nakanishi H, Doyama H, Ishikawa H, Uedo N,
Gotoda T, Kato M, Nagao S, Nagami Y, Aoyagi H, Imagawa A, et al:
Evaluation of an e-learning system for diagnosis of gastric lesions
using magnifying narrow-band imaging: A multicenter randomized
controlled study. Endoscopy. 49:957–967. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fujiyoshi MRA, Inoue H, Fujiyoshi Y,
Nishikawa Y, Toshimori A, Shimamura Y, Tanabe M, Ikeda H and
Onimaru M: Endoscopic classifications of early gastric cancer: A
literature review. Cancers (Basel). 14(100)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Park JH, Lee KN, Lee HL, Jun DW, Yoon JH,
Lee OY, Yoon BC and Choi HS: Rates and risk factors for interval
gastric cancers at screening gastroscopy. Turk J Gastroenterol.
32:194–202. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen MJ, Wu W, Pan S, Lin CJ, Dong LM,
Chen ZF, Wu JS and Huang ZM: Sedated gastroscopy improves detection
of gastric polyps. Exp Ther Med. 16:3116–3120. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhu Y, Wang F, Zhou Y, Xia GL, Dong L, He
WH and Xiao B: Blue laser magnifying endoscopy in the diagnosis of
chronic gastritis. Exp Ther Med. 18:1993–2000. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yoshifuku Y, Sanomura Y, Oka S, Kurihara
M, Mizumoto T, Miwata T, Urabe Y, Hiyama T, Tanaka S and Chayama K:
Evaluation of the visibility of early gastric cancer using linked
color imaging and blue laser imaging. BMC Gastroenterol.
17(150)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Suzuki T, Hara T, Kitagawa Y, Takashiro H,
Nankinzan R, Sugita O and Yamaguchi T: Linked-color imaging
improves endoscopic visibility of colorectal nongranular flat
lesions. Gastrointest Endosc. 86:692–697. 2017.PubMed/NCBI View Article : Google Scholar
|