1. Introduction
Esketamine is the only pharmacological agent with
glutamatergic neuromodulatory properties approved by the US Food
and Drug Administration (FDA) and European Medicines Agency (EMA)
in 2019 to enhance the effects of serotonin selective or serotonin
and norepinephrine reuptake inhibitors (1). In the context of high rates of
partial responsivity or non-response to currently available
antidepressants, multiple mechanisms of action for novel
pharmacological agents are being explored besides the stimulation
of monoaminergic neurotransmission (2,3).
However, although numerous molecules have been studied in phases II
and III of clinical research, it is difficult to predict which will
reach the market in the following decades. Until now, only
esketamine and brexanolone, the latter being a γ-aminobutyric acid
(GABA)-A receptor positive allosteric modulator, are
antidepressants with non-monoaminergic activity, that have been
approved by FDA for use under supervision in patients with
treatment-resistant depression (TRD) and post-partum depression,
respectively (4). Furthermore,
tolerability issues associated with antidepressants already in
clinical use indicate the need to find novel pharmacological agents
for treating major depression (5).
Esketamine nasal spray is recommended for adults
diagnosed with major depressive disorder (MDD) who did not respond
to at least two antidepressants, and who currently have a major
depressive episode of moderate or severe intensity (1,6).
Intensive monitoring is mentioned in the EMA approval and specified
in the summary of the characteristics of the product, with an
algorithm of pre- and post-administration assessment (6). Similar recommendations have been
formulated by the FDA (1,6).
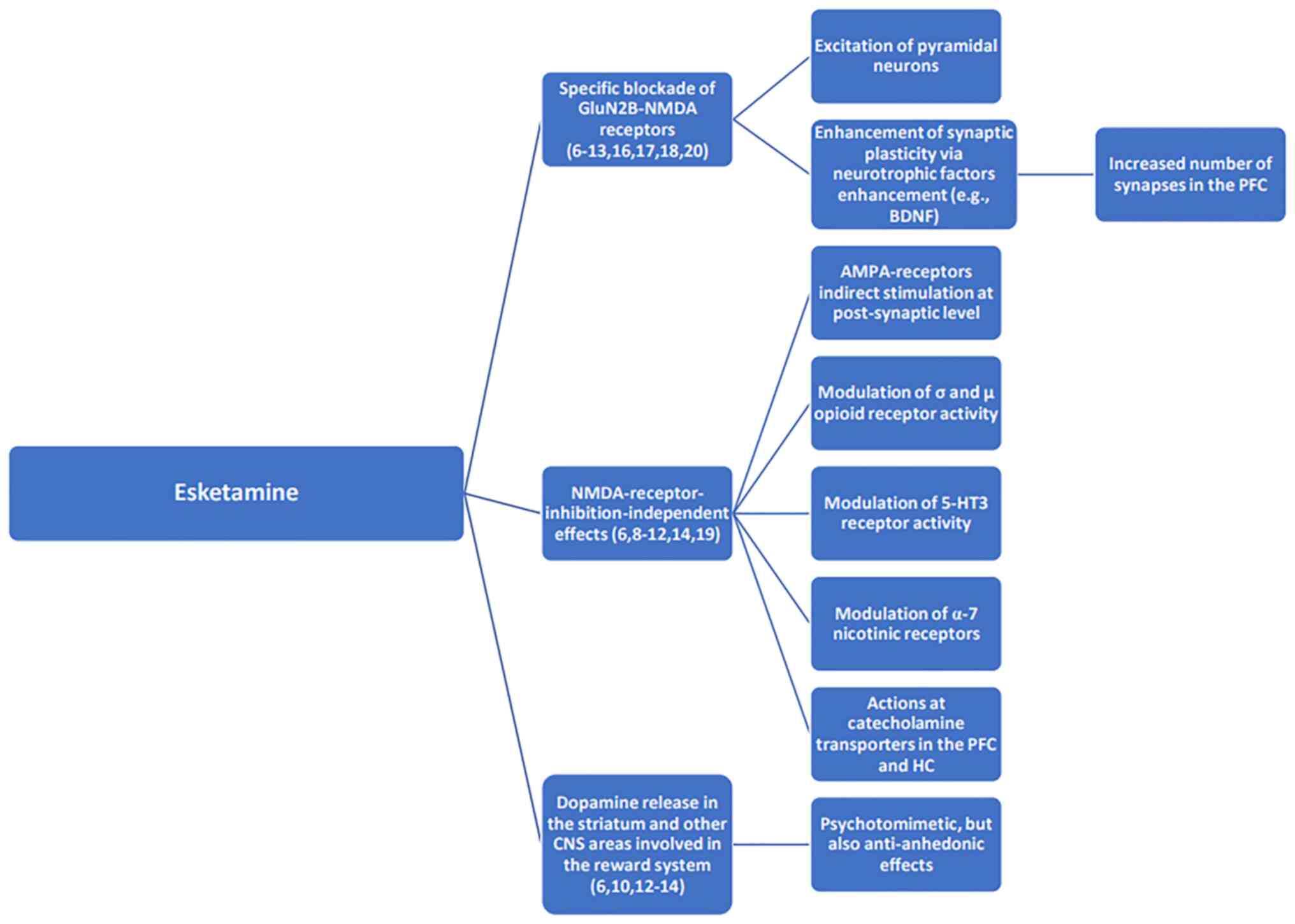
The pharmacodynamic profile of esketamine is
characterized by non-selective, non-competitive antagonism of
N-methyl-D-aspartate (NMDA) receptors, which are ionotropic
glutamatergic receptors (Table I)
(6). Activation of NMDA receptors
causes a transient increase in glutamate release, leading to
stimulation of the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic
acid receptors (6). Furthermore,
signaling via neurotrophic factors is enhanced and synaptogenesis
is improved in brain regions involved in regulating mood and
emotional behavior (6). Restoring
dopaminergic neurotransmission from areas responsible for
regulating motivation and reward contributes to a rapid clinical
response (for example, reduction of anhedonia), but the release of
dopamine in the striatum may explain the psychotomimetic effects of
this agent (Fig. 1) (6,7). The
fast onset of esketamine may be associated with direct stimulation
of the mammalian target of rapamycin complex 1 (mTORC1), a
signaling pathway involved in the regulation of protein synthesis,
which stimulates synaptogenesis and brain-derived neurotrophic
factor production (8).
 | Table IPharmacodynamic properties of
esketamine. |
Table I
Pharmacodynamic properties of
esketamine.
| First author/s,
year |
Receptor/neurotransmitters | Description | (Refs.) |
|---|
| Janssen-Cilag,
2019; Salahudeen et al, 2020; De Berardis et al,
2018 | NMDA receptors | Non-selective,
non-competitive, activity-dependent antagonism | (6,8,18) |
| Janssen-Cilag,
2019; Salahudeen et al, 2020 | AMPA receptors | Indirect
stimulation at the post-synaptic level | (6,8) |
| Janssen-Cilag,
2019; Salahudeen et al, 2020 | Neurotrophic
factors | Downstream
activation | (6,8) |
| Janssen-Cilag,
2019; Salahudeen et al, 2020 | Dopamine | Release of
endogenous dopamine from the presynaptic terminal in the striatum
(in monkey trials) | (6,8) |
Glutamatergic mechanisms of depression are currently
the focus of attention after preclinical studies supported the
importance of this neurotransmission system in the pathogenesis of
mood disorders (9,10). Abnormal levels of glutamate are
found in cerebrospinal fluid, plasma and brain tissue during
autopsy studies in individuals with mood disorders (10). Magnetic resonance spectroscopy
detects abnormal levels and ratios of glutamate/GABA in brain areas
associated with MDD (10). Glial
cell functionality may be decreased after exposure to prolonged
stress, leading to synaptic loss and activation of the apoptotic
pathway via glutamatergic mediation (10). Ketamine is associated with
rapid-acting antidepressant effects and NMDA receptor-mediated
signaling, inhibition of extrasynaptic NMDA receptors and blockade
of NMDA receptors in the synapse (9,10).
Ketamine blocks excitatory glutamate transmission and increases
overall activity of the prefrontal cortex by inhibiting NMDA
receptors expressed in GABA neurons preferentially (10,11).
The disinhibition of cortical GABA interneurons by NMDA receptor
inhibition explains the excitatory effect of ketamine on the firing
of pyramidal neurons (10,11). Also, ketamine triggers an
antidepressant effect through direct inhibition of extrasynaptic
NMDA receptors by specific blocking of extrasynaptic GluN2B-NMDA
receptors (via mTOR signaling), which leads to the excitation of
pyramidal neurons (10).
Furthermore, the blockade of NMDA receptors enhances synaptic
plasticity based on mTORC1 signaling via Akt and ERK activation;
this mechanism explains the increasing number and function of
synapses in the prefrontal cortex (10). A less explored mechanism of action
of ketamine assumes the existence of NMDA receptor
inhibition-independent effects because other pharmacological
agents, such as memantine or lanicemine, are not efficient in
treating mood disorders (10,12,13).
Intravenous administration of esketamine has been
reported to induce rapid-acting and sustained activity in
refractory patients with MDD, but it is also associated with
favorable results in treatment-resistant patients with imminent
risk of suicide in phase II studies (14,15).
Additionally, the effects of intranasal esketamine have been
explored in patients with depression and suicidal intent because of
the rapid onset of antidepressant effects reported after
single-dose administration (16,17).
The anti-suicidal effect of esketamine represents a
key reason to explore its properties since managing suicidal
behavior in patients with MDD is difficult with the use of previous
generations of antidepressants. The identification of risk markers
for suicidal behaviors by combining genomic assessment and clinical
evaluation has led to an increased interest in drugs that may
modulate mechanisms such as neural connectivity and activity,
immune and inflammatory response (18). Ketamine and esketamine have been
explored in this type of pathology because the dysregulation of
glutamate neurotransmission has been suggested to serve a central
role in the onset of suicidal behavior (18-20).
Besides its ability to non-selectively antagonize NMDA receptors,
ketamine may modulate the activity of σ and µ opioid, serotonin
5HT3, muscarinic and α7 nicotinic receptors, as well as
catecholamine transporters in the prefrontal cortex and
hippocampus; these pharmacodynamical properties may be involved to
a certain degree in anti-suicidal properties of ketamine (18-20).
However, one study has suggested that ‘the
excitement over a new treatment for depressed patients with
suicidal intentions should be re-evaluated after real-world
experience’ (16). Therefore, a
carefully structured assessment of suicide risk should be combined
with an empathic approach focused on the subjective experiences of
patients with MDD (16). Suicide
is not ingrained in the experience of depression; therefore,
suicidal risk should be regarded as a complex dimension that only
partially overlaps with the depressive phenomenology (16). This is why suicide may be perceived
as a way to escape extreme negative emotions or acute anguish, not
simply as another MDD symptom (16,21).
The pharmacokinetic properties of esketamine are
summarized in Table II. An active
metabolite, (S)-norketamine, has been identified, which results
from cytochrome P450 metabolization of its parent compound and
possesses a notable affinity for NMDA receptors, higher than that
of (R, S)-ketamine and (S)-ketamine [inhibitor constant (Ki)=1.7 µM
vs. 0.53 µM and 0.3 µM, respectively] (7,8,22).
This metabolite is associated with a rapid and potent
antidepressant effect in rodent models of depression (22). While preclinical studies reported
the abuse potential of esketamine, its metabolite was reported as
being safer (i.e., lower risk of psychotomimetic and addictive
potential) (7,8,22).
 | Table IIPharmacokinetics of esketamine. |
Table II
Pharmacokinetics of esketamine.
| Parameter | Description |
|---|
| Absorption | A total of ~48% of
intranasally administered drug is absorbed |
| |
Tmax=20-40 min |
| Distribution | Vd at
steady state=709 l |
| | Plasma protein
binding=43-45% |
| | Esketamine is not
an inhibitor of the P-glycoprotein transport system |
|
Biotransformation | There is extensive
hepatic metabolism, primarily by CYP2B6 and 3A4 isoenzymes; the
contribution of CYP2C19 and 2C9 is lower compared with that of
CYP2B6 |
| Special
populations | Elderly (≥65
years): Cmax is 18% higher compared with young
individuals at a dose of 28 mg and 67% higher at a dose of 84 mg
esketamine. T1/2 is similar in the
elderly and young individuals |
| | Cmax in
individuals with kidney failure is 20-26% higher in mild, moderate
or severe renal insufficiency compared with individuals without
kidney failure. There are no data on the pharmacokinetics of
esketamine in patients undergoing dialysis |
| | There are no
differences in pharmacokinetics between individuals with mild
hepatic failure and healthy individuals; Cmax is 8%
higher compared with healthy individuals in the case of moderate
insufficiency; in the severe cases, no pharmacokinetic studies have
been conducted |
| Elimination |
T1/2=7-12
h |
| | Elimination occurs
via the urine and feces, with only 1% of the administered dose
being excreted unchanged in the urine |
| Pharmacokinetic
interactions | Administration of
rifampicin, a potent CYP 3A4/2B6 enzyme inhibitor, decreases the
concentration of esketamine by 17-28% |
| | Rifampicin
decreases plasma concentration of midazolam, a CYP3A4 substrate, by
~16%, but does not affect the concentration of bupropion, a CYP2B6
substrate |
2. Objectives
The primary objective of the present review was to
evaluate the existing evidence in the literature on the efficacy
and tolerability of esketamine in the management of TRD. Secondary
objectives were: i) To explore other potential benefits and risks
of esketamine in short- and long-term administration; ii) to
formulate clinical recommendations based on the analysis of these
data and iii) to establish future research directions that may
enrich knowledge on the effects of esketamine.
3. Subjects and methods
The efficacy and safety profile of esketamine was
analyzed by reviewing the results of studies found in electronic
databases (PubMed-https://pubmed.ncbi.nlm.nih.gov/, Cochrane-https://www.cochrane.org/, EMBASE-https://www.embase.com and Clarivate Web of
Science-https://www.webofscience.com/). All
studies published between January 2010 and May 2022 were included
in the primary analysis. Prospective clinical trials were included
regardless of the methodology used (randomized/non-randomized,
open/single-blind/double-blind and controlled/uncontrolled) and
retrospective trials were also included. Studies including nasal or
intravenous (i.v.) infusion of esketamine were allowed in the
reviewing stage. Patients enrolled in the trials within the current
review were diagnosed with TRD, MDD, psychotic depression or any
other type of depressive disorder. Studies exploring esketamine
effects in healthy volunteers were allowed if these effects were
evaluated using validated instruments. There were no limitations
related to the age or sex of the enrolled patients in the clinical
trials. Exclusion criteria were as follows: Case reports, case
series, systematic and narrative reviews, and meta-analyses;
sources written in languages other than English; studies with
unspecified outcomes, duration or outcome measures.
4. Results overview
A total of nine randomized controlled trials
exploring the efficacy and tolerability of esketamine nasal spray
were included in the review. Additionally, one esketamine vs.
ketamine i.v. study, one single dose of esketamine i.v. study, one
retrospective analysis, one post-hoc analysis and one esketamine
vs. ketamine and R-ketamine i.v. study were also reviewed (Table III).
 | Table IIIEsketamine efficacy and
tolerability. |
Table III
Esketamine efficacy and
tolerability.
| First author/s,
year | Methodology | Key results | Trial registration
code | (Refs.) |
|---|
| Fedgchin et
al, 2019 | Phase III, DBL,
RCT, 346 participants with moderate/severe MDD non- responsive to
≥2 AD; ESK 56/84 mg vs. placebo + AD, 4 weeks | MADRS scores did
not support any significant difference between ESK and placebo at
the endpoint | NCT02417064 | (23) |
| Popova et
al, 2019 | Phase III, DBL,
RCT, 227 participants with moderate/severe MDD non-responsive to ≥2
AD; ESK 56/84 mg vs. placebo + AD, 4 weeks | ESK differentiated
itself from placebo on MADRS and PHQ-9 scales | NCT02418585 | (25,26) |
| Floden et
al, 2022 |
| Ochs-Ross et
al, 2020 | Phase III, DBL,
RCT, 138 participants with TRD; ESK 28/56/84 mg vs. placebo + AD, 4
weeks | No significant
difference in decrease of MADRS scores between groups. Patients
aged 65-74 years responded better; first MDE <55 years of age
was a favorable prognostic factor | NCT02422186 | (28) |
| Correia-Melo et
al, 2020 | DBL, RCT, 63
participants with TRD; ESK vs. KET i.v., single dose | The remission rate
was higher under ESK vs. KET treatment at 24 h post-administration
based on MADRS scores | UMIN000032355 | (29) |
| Singh et al,
2016 | DBL, RCT, 30
participants with TRD; ESK vs. placebo i.v., single dose; second
phase: Re-randomization of non-responsive patients on ESK vs.
placebo, on day 4 | ESK was superior to
placebo (based on MADRS scores) on day 2 after therapy. The effect
of ESK was fast (2 h post-infusion) | NCT01640080 | (15) |
| Souza-Marques et
al, 2022 | Retrospective
analysis, 15 patients with PMD; ESK single dose i.v. | ESK improved MADRS
scores 24 h post-administration. No difference was observed between
patients with MDD and PMD in relation to ESK treatment | N/A | (30) |
| Fu et al,
2020 | Phase III, DBL,
RCT, 226 participants with MDD + active suicidal ideation and
intent; ESK 84 mg vs. placebo + SOC (AD included), 4 weeks | ESK was associated
with significantly greater improvement after 24 h post- first dose
administration. No difference in severity of suicide risk was
reported. The favorable effect was detected earlier in patients who
received ESK (4 h post-administration) | NCT03039192 | (31) |
| Ionescu et
al, 2021 | Phase III, DBL,
RCT, 230 patients with MDD + suicidal ideation with intent, ESK 84
mg vs. placebo, 4 weeks + SOC (AD included) | ESK led to
significantly greater improvement in MADRS scores 24 h from the
first dose. The CGI-S scores were improved in both groups, without
differences between them. The positive effect was detected earlier
in patients with ESK (4 h) | NCT03097133 | (32) |
| Caruso et
al, 2021 | Post hoc analysis,
ESK vs. placebo + SOC, 24 h post-treatment administration, two
trials | Patients with a
history of suicide attempts had a significantly greater decrease in
suicidal behavior and/or ideation (CGI-SS-R) post-treatment | N/A | (34) |
| Takahashi et
al, 2021 | Phase IIb, DBL,
RCT, 202 patients with MDD non-responsive to 1-4 Ads; 4 weeks | No differences
between active and placebo groups were reported, based on the
primary outcome of MADRS scores | NCT02918318 | (36) |
| Araújo-de-Freitas
et al, 2021 | DBL, RCT, 54
patients with TRD; ESK vs. KET i.v. single dose | No difference
between groups was observed in cognitive functioning following
treatment but both drugs improved cognitive performance in patients
with TRD | UMIN000032355 | (37) |
| Pfenninger et
al, 2002 | RCT, DBL, healthy
subjects, cross- over design; KET vs. ESK vs. R-KET i.v. | Multiple cognitive
parameters improved significantly after 5 min post-isomer
administration vs. racemic KET | N/A | (38) |
| Dijkstra et
al, 2022 | 27 participants
with mild/moderate MDD; ESK 84 mg vs. placebo, 6±0.5 and 18±2 h
post- administration, 3 weeks | ESK did not
negatively affect driving performance vs. placebo | NCT02919579 | (39) |
| Daly et al,
2019 | Phase III, 297
patients, ESK vs. placebo + AD, 16 weeks | The risk of relapse
was decreased by 51-70% in patients treated with ESK + AD vs.
placebo + AD | NCT02493868 | (40) |
| Wajs et al,
2020 | Phase III, DBL,
RCT, 802 patients with TRD; ESK 28/56/84 mg + AD, 4 weeks (first
phase) + OL second phase (48 weeks) | MADRS scores
improved compared with baseline up to the end of the second
phase | NCT02497287 | (41) |
5. Short-term efficacy results
TRANSFORM-1 was a phase III, double-blind,
randomized, multicenter trial, which enrolled 346 patients with
moderate/severe MDD that did not respond to ≥2 antidepressants
(23). The effects of esketamine
nasal spray (56 or 84 mg, twice weekly) were compared with placebo
as an add-on to a newly initiated oral antidepressant (such as
duloxetine, escitalopram, sertraline or venlafaxine extended
release), which was administered in an open-label manner for 4
weeks (23). The primary objective
of this trial was to observe the decrease in the Montgomery-Asberg
Depression Rating Scale (MADRS) (24) from baseline to the end of the
study. After 28 days, there were no significant differences between
the active and placebo groups regarding the MADRS score (23).
Another phase III study, TRANSFORM-2, had a
double-blind, randomized, multicenter, active comparator design,
and 227 patients with moderate/severe MDD who did not respond to ≥2
antidepressants were enrolled (25,26).
The intervention consisted of esketamine (56 or 84 mg, twice
weekly) vs. placebo nasal spray as an add-on to antidepressants,
and the study duration was 4 weeks. The change in MADRS score in
patients receiving esketamine + antidepressant was significantly
greater than in those treated with an antidepressant + placebo
spray (25,26). Patient Health
Questionnaire-9(27) scores also
improved significantly in the active group vs. placebo and the
improvement in depressive symptoms was observed earlier in patients
who received the active intervention (25,26).
The most improved items on MADRS in the esketamine vs. placebo
group were ‘apparent sadness’ and ‘inability to feel’ (26).
In TRANSFORM-3, another phase III, double-blind
trial, 138 patients ≥65 years of age with TRD were randomized on
flexible doses of esketamine (28, 56 or 84 mg, administered twice
weekly) or placebo nasal spray in addition to a newly initiated
oral antidepressant (28). The
duration of the study was 4 weeks, and the primary outcome was
change in MADRS total score from baseline to endpoint (28). No significant difference was
reported in the improvement of depressive symptoms in patients
receiving esketamine + antidepressant vs. placebo + antidepressant,
according to the MADRS score (28). However, there were more significant
differences between groups that favored patients aged 65 to 74
years vs. those >75 years of age and patients with an earlier
onset of depression (<55 years of age) (28).
A randomized, double-blind, non-inferiority clinical
trial compared the effects of esketamine and ketamine, both
administered as i.v. infusion, in a single dose of 0.25 and 0.5
mg/kg, respectively, in 63 patients with TRD (29). The results collected 24 h
post-infusion showed that 24.1% of patients who received ketamine
and 29.4% of those treated with esketamine obtained remission,
according to MADRS scores (29).
Therefore, the non-inferiority hypothesis was confirmed in this
trial at 24 h post-treatment (29).
Another randomized, multicenter, double-blind,
placebo-controlled study included 30 patients with TRD who received
0.2 or 0.4 mg/kg i.v. infusion of esketamine or placebo for 40 min
(15). In the second phase,
non-responsive patients who received a placebo in the first stage
were randomized again to i.v. esketamine or placebo on day
4(15). Patients treated with
esketamine (both dosing regimens) achieved clinical improvements
(according to MADRS scores) vs. placebo on the second day after
therapy (15). The effect of
esketamine was fast, its onset being detected 2 h post-treatment
administration (15).
A retrospective analysis of medical records included
15 patients with MDD with psychotic features and evaluated the
effects of a single dose of 0.5 mg/kg esketamine (30). A significant difference was
observed in MADRS scores 24 h after administration, but no
differences were reported between patients with MDD who exhibited
psychotic features vs. those who do not (30).
6. Efficacy of esketamine in lowering
suicide risk
In a double-blind, multicenter, phase III study,
ASPIRE I, 226 patients with MDD and active suicidal ideation who
also presented suicidal intent were randomized to 84 mg esketamine
or placebo nasal spray twice/week for 4 weeks as an add-on to
standard-of-care therapy (hospitalization and newly initiated or
enhanced oral antidepressant) (31). MADRS scores indicated a
significantly greater improvement in patients treated with
esketamine 24 h after the first dose (31). The favorable effect was observed
earlier in the esketamine group vs. placebo (4 h after
administration of the drug) (31).
However, no differences were reported between groups in the
severity of suicide risk during monitoring (31).
In ASPIRE II, a phase III, double-blind, randomized
trial, 230 patients with MDD and active suicidal ideation with
intent received treatment with 84 mg esketamine or placebo nasal
spray twice/week with a monitoring period of 4 weeks, together with
comprehensive standard care (antidepressant being included)
(32). MADRS scores indicated a
significantly greater improvement in patients treated with
esketamine after 24 h from the first dose of treatment (32). The favorable effect was observed
earlier in the active group vs. placebo, at 4 h after esketamine
administration (32). The Clinical
Global Impression-Severity (33)
scores also improved in both groups, but without significant
differences between groups (32).
A post hoc analysis of data collected from the
ASPIRE I and II studies on 24 h post-treatment outcomes of patients
at risk of suicide showed that the active group improved
significantly (indicated by MADRS scores) vs. placebo + standard
care (34). Patients who had ≥1
suicide attempt in their history showed a more significant
reduction in suicidal behavior and/or ideation, based on the
between-group difference in the Clinical Global Impression-Severity
of Suicidality-Revised (35)
scores 24 h post-treatment (34).
A placebo-controlled phase IIb study involved the
enrollment of 202 patients with MDD who were non-responsive to ≥1
but <5 different antidepressants, randomized to esketamine (28,
56 or 84 mg) or placebo nasal spray as an add-on to a new
antidepressant, with an active monitoring period of 4 weeks
(36). In the double-blind phase,
a similar improvement in depressive symptoms was observed in all
groups, with no difference between the active substance and
placebo, according to the primary outcome (MADRS score) (36).
7. Efficacy of esketamine on cognitive
functioning
A randomized, double-blind study that evaluated the
comparative efficacy of esketamine and ketamine i.v. infusion on
cognition in 54 patients with treatment-resistant MDD at 24 h and 7
days post-treatment found no significant differences between the
two substances on cognitive function (37). Both drugs improved short-term
visuospatial memory, executive functioning, processing speed and
episodic verbal memory, evaluated via neuropsychological tests
(37). These results were
different from those of a previous study, which compared i.v.
racemic ketamine (0.5 mg/kg) with esketamine (0.25 mg/kg) and
R-ketamine (1 mg/kg), in a prospective, randomized, double-blind,
crossover design on healthy subjects (38). Objective concentration capacity and
retention in primary memory were less affected by esketamine
compared with R-ketamine and racemic ketamine at 1 min
post-administration (38). After 5
min, immediate recall, anterograde amnesia, retention in primary
memory, short-term storage memory and intelligence quotient were
less decreased after administration of isomers vs. racemic ketamine
(38).
A single-blind, randomized, cross-titration study
compared the effects of 84 mg intranasal esketamine vs. placebo on
driving performance at 18±2 h post-administration (38). Another phase of the same study
evaluated the impact of the same drug on driving ability at 6.0±0.5
h post-administration, in a regimen of esketamine delivered
twice/week for three weeks (39).
All patients had a diagnosis of mild-to-moderate MDD without
psychotic features (n=27) (39).
In both the first and second phase, esketamine did not
significantly alter driving performance compared with placebo
(39).
8. Efficacy of esketamine in the prevention
of depressive recurrence
SUSTAIN-1 was a phase III study that monitored the
effects of controlled withdrawal of active medication in patients
(n=297) who achieved a stable level of remission/response under
esketamine + antidepressant and who were randomized to continue
this drug or discontinue it and switch to placebo nasal spray
(40). The duration of monitoring
was 16 weeks. The risk of relapse was lower by 51-70% under
continued treatment with esketamine + antidepressant vs. placebo +
antidepressant (40).
9. Long-term results of esketamine
administration
In another phase III trial, SUSTAIN-2, which had an
open, multicenter design, 802 patients with treatment-resistant MDD
were randomized to 28, 56 or 84 mg esketamine nasal spray added to
a new oral antidepressant (41).
Esketamine was administered twice/week during a 4-week induction
phase, then weekly or every 2 weeks in patients who obtained a
response in the first phase of the study. The duration of
monitoring was 48 weeks; MADRS scores decreased during the
induction phase (41). This
improvement was maintained in the continuation, open-label phase,
with decreased MADRS scores from baseline at the endpoint (41).
10. Safety profile of esketamine in clinical
trials
The overall tolerability of intranasally
administered esketamine in clinical trials is good, with no mood
switches or emergent psychotic manifestations being observed
(23). Adverse effects, such as
transient dissociative phenomena as well as a potential addictive
risk (unconfirmed in short-term clinical trials and ≤2 weeks after
completion of adjuvant therapy), require caution in the
administration of esketamine (23,25,26,28).
In the TRANSFORM-1 study, the most commonly reported side effects
were nausea, dissociation, dizziness, and headache (23). In the TRANSFORM-2 study, patients
primarily reported nausea, dizziness, dissociation, dysgeusia and
dizziness (25,26). In the TRANSFORM-3 study, the
incidence of the reported adverse effects in the esketamine +
antidepressant therapy group was 70.8% vs. 60% in the esketamine +
placebo group and were primarily dizziness, nausea, transient
increase in blood pressure, fatigue, headache and dissociation
(28).
Adverse events in the SUSTAIN-1 and -2 trials in
patients receiving esketamine were dysgeusia, vertigo,
dissociation, drowsiness, nausea, headache and dizziness (40,41).
These side effects were of moderate or mild intensity, being
detected especially following administration of the medication and
typically remitted on the same day (40,41).
Discontinuation due to side effects was reported in 7% of patients
in the SUSTAIN-2 trial (41).
In the ASPIRE I and II trials, the most commonly
reported side effects (>20%) were dizziness, dissociation,
nausea, drowsiness and headache (31,32,34).
11. Synthetic parameters
Based on results from four phase III studies, the
number needed to treat (NNT) value (the number of patients that
would need to be treated to obtain a favorable outcome) was 8 for
patients treated with esketamine + antidepressant in the acute
phase trials (38). Regarding the
relapse prevention study, the NNT value was <10(42). These values suggest a potential
benefit of combining esketamine with an antidepressant for both
acute and maintenance phases.
Another NNT for esketamine was 6 in the short-term,
based on the results of 297 adults treated with esketamine as an
add-on to antidepressants in a double-blind study (40). In the long term, the NNT was 4 for
preventing relapse during esketamine treatment (40).
Based on analysis of the results from the same four
phase III studies, the number needed to harm (NNH) value (the
number of patients that would need to receive treatment to report
an adverse effect) was <10 for patients treated with esketamine
and antidepressants (34). NNH
reached 17 for discontinuation due to adverse effects in the acute
phase studies and 178 (insignificant) in the maintenance study
(38).
12. Limitations of the review
The present study is not a systematic review, and
therefore, relevant studies on this topic may be missing. Also,
several trials included in the present review are short-term, which
limits the possibility of analyzing the efficacy and tolerability
of esketamine in the long term.
13. Conclusions
Esketamine has been shown to be effective in
decreasing the severity of short-term depressive symptoms, but
questions about its medium- and long-term action, as well as its
tolerability profile, remain to be elucidated as novel clinical
studies explore its pharmaco-clinical properties. The advantages
and disadvantages of esketamine treatment are presented in Table IV.
 | Table IVAdvantages and disadvantages of
esketamine use in clinical practice. |
Table IV
Advantages and disadvantages of
esketamine use in clinical practice.
| Advantages | Disadvantages |
|---|
| Short and
medium-term effectiveness | Cost |
| Add-on to SSRI/SNRI
treatment | Need to monitor the
tolerability of the drug |
| Superior
tolerability to racemic ketamine | Accessibility
(special prescription regimen) |
| Intranasal
administration once weekly | Limited experience
of psychiatrists with this medication |
| Emergency
treatment, which allows rapid reduction of the symptoms of acute
depression | The risk of abuse
cannot be ruled out on the basis of existing evidence |
Regarding the secondary objectives, based on the
reviewed data, esketamine nasal spray may be recommended in
patients with TRD (defined by ≥2 periods of treatment with
different antidepressants that did not result in remission of
symptoms) as an adjunct to antidepressant treatment. No significant
results have been shown in patients >75 years of age, but this
conclusion is based on limited data (28). There are also studies exploring
esketamine which reported a lack of significant efficacy on
depressive severity symptoms in patients with TRD, but there is
insufficient data about potential factors that may adversely
influence the progression of these patients (23,28,36,37).
Active suicidal ideation with intent was not significantly
decreased by esketamine, but prior suicidal behavior may indicate a
favorable response to this drug (31,34).
Regarding suicidal risk in MDD and the potential benefits of
esketamine, several models show the complexity of the suicide
dimension and its partial overlap with MDD (16,17,21).
This makes the interpretation of pharmacological trials difficult
as these are based on limited descriptions of suicidal scenarios
(43).
The presence of psychotic features in MDD was not
apparently associated with a different progression compared with
non-psychotic depression during esketamine treatment (30). The impact of esketamine on
cognitive functioning may be positive or neutral (37-39).
As reported by previous reviews and expert opinions
(44,45), the data about the effects of
esketamine in TRD might position it above racemic ketamine in terms
of safety (although it must be noted that no direct comparison
between S-ketamine, R-ketamine and racemic ketamine exists).
Several aspects in this domain require further investigation,
including methodological variables, such as duration, frequency of
administration and continuing with safety data (such as abuse
potential), as well as logistical parameters, such as cost and
availability (44,45). Unlike the aforementioned reviews,
in the present paper, studies regarding the cognitive effects of
esketamine were included in healthy individuals and patients with
MDD (37-39).
Also, studies comparing ketamine and esketamine were included, as
well as trials exploring both nasal spray and i.v. infusion forms
of administration. The present review supports conclusions similar
to the meta-analysis of Papakostas et al (46), which found that esketamine is
significantly more effective than placebo as an adjuvant to ongoing
antidepressant treatment, based on MADRS scores. These results
remained significant regardless of whether esketamine was added to
newly initiated antidepressants or to already ongoing treatment
(46). The safety of intranasal
esketamine was also supported by a meta-analysis conducted by Jawad
et al (47), which explored
the tolerability of this agent in long-term studies (>4 weeks
and ≤1 year).
Research directions for future studies are the
efficacy of esketamine for bipolar and geriatric depression,
depression with addictive comorbidities and MDD in adolescents, as
well as the detection of favorable/unfavorable response factors of
this treatment. The pharmacogenetics of adjuvant therapy with
esketamine is another potential domain that should be explored from
the perspective of individualized medicine.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
OV was responsible for collecting, analyzing and
presenting data within the current review. Data authentication is
not applicable. OV has read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that they have no competing
interests.
References
|
1
|
Gastaldon C, Papola D, Ostuzzi G and
Barbui C: Esketamine for treatment resistant depression: A trick of
smoke and mirrors? Epidemiol Psychiatr Sci. 29(e79)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vasiliu O: Investigational drugs for the
treatment of depression (Part 1): Monoaminergic, orexinergic,
GABA-ergic, and anti-inflammatory agents. Front Pharmacol.
13(884143)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vasiliu O: Investigational drugs for the
treatment of depression (Part 2): Glutamatergic, cholinergic,
sestrin modulators, and other agents. Front Pharmacol.
13(884155)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cristea IA and Naudat F: US Food and Drug
Administration approval of esketamine and brexanolone. Lancet
Psychiatry. 6:975–977. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vasiliu O: Effects of the selective
serotonin reuptake inhibitors over coagulation in patients with
depressive disorders-a systematic review and retrospective
analysis. Rom J Mil Med CXXII. 2:7–11. 2019.
|
|
6
|
Janssen-Cilag Intl. Spravato, Summary of
Product Characteristics. 2019. Retrieved online at https://www.ema.europa.eu/en/documents/product-information/spravato-epar-product-information_en.pdf.
Accessed 19 July 2022.
|
|
7
|
Hashimoto K, Kakiuchi T, Ohba H, Nishiyama
S and Tsukada H: Reduction of dopamine
D2/3 receptor binding in the
striatum after a single administration of esketamine, but not
R-ketamine: A PET study in conscious monkeys. Eur Arch Psychiatry
Clin Neurosci. 267:173–176. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Salahudeen MS, Wright CM and Peterson GM:
Esketamine: New hope for the treatment of treatment-resistant
depression? A narrative review. Ther Adv Drug Saf.
11(2042098620937899)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tomasetti C, Montemitro C, Fiengo ALC,
Santone C, Orsolini L, Valchera A, Carano A, Pompili M, Serafini G,
Perna G, et al: Novel pathways in the treatment of major
depression: Focus on the glutamatergic system. Curr Pharm Des.
25:381–387. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shin C and Kim YK: Ketamine in major
depressive disorder: Mechanisms and future perspectives. Psychiatry
Investig. 17:182–192. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Homayoun H and Moghaddam B: NMDA receptor
hypofunction produces opposite effects on prefrontal cortex
interneurons and pyramidal neurons. J Neurosci. 27:11496–11500.
2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zarate CA Jr, Singh JB, Quiroz JA, De
Jesus G, Denicoff KK, Luckenbaugh DA, Manji HK and Charney DS: A
double-blind, placebo-controlled study of memantine in the
treatment of major depression. Am J Psychiatry. 163:153–155.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zarate CA Jr, Mathews D, Ibrahim L, Chaves
JF, Marquardt C, Ukoh I, Jolkovsky L, Brutsche NE, Smith MA and
Luckenbaugh DA: A randomized trial of a low-trapping nonselective
N-methyl-D-aspartate channel blocker in major depression. Biol
Psychiatry. 74:257–264. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
De Berardis D, Tomasetti C, Pompili M,
Serafini G, Vellante F, Fornaro M, Valchera A, Perna G, Volpe U,
Martinotti G, et al: An update on glutamatergic system in suicidal
depression and on the role of esketamine. Curr Top Med Chem.
20:554–584. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Singh JB, Fedgchin M, Daly E, Xi L, Melman
C, De Bruecker G, Tadic A, Sienaert P, Wiegand F, Manji H, et al:
Intravenous esketamine in adult treatment-resistant depression: A
double-blind, double-randomization, placebo-controlled study. Biol
Psychiatry. 80:424–431. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pompili M: Intranasal esketamine and
current suicidal ideation with intent in major depression disorder:
Beat the clock, save a life, start a strategy. Front Psychiatry.
11(325)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Daly EJ, Singh JB, Fedgchin M, Cooper K,
Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H and
Drevets WC: Efficacy and safety of intranasal esketamine adjunctive
to oral antidepressant therapy in treatment-resistant depression: A
randomized clinical trial. JAMA Psychiatry. 75:139–148.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
De Berardis D, Fornaro M, Valchera A,
Cavuto M, Perna G, Di Nicola M, Serafini G, Carano A, Pompili M,
Vellante F, et al: Eradicating suicide at its roots: Preclinical
bases and clinical evidence of the efficacy of ketamine in the
treatment of suicidal behaviors. Int J Mol Sci.
19(2888)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bernstein HG, Tausch A, Wagner R, Steiner
J, Seeleke P, Walter M, Dobrowolny H and Bogerts B: Disruption of
glutamate-glutamine-GABA cycle significantly impacts on suicidal
behaviour: Survey of the literature and own findings on glutamine
synthetase. CNS Neurol Disord Drug Targets. 12:900–913.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tomasetti C, Iasevoli F, Buonaguro EF, De
Berardis D, Fornaro M, Fiengo AL, Martinotti G, Orsolini L,
Valchera A, Di Giannantonio M and de Bartolomeis A: Treating the
synapse in major psychiatric disorders: The role of postsynaptic
density network in dopamine-glutamate interplay and
psychopharmacologic drugs molecular actions. Int J Mol Sci.
18(135)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schneidman ES: Suicide as psychache. J
Nerv Ment Dis. 181:145–147. 1993.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hashimoto K and Yang C: Is (S)-norketamine
an alternative antidepressant for esketamine? Eur Arch Psychiatry
Clin Neurosci. 269:867–868. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fedgchin M, Trivedi M, Daly EJ, Melkote R,
Lane R, Lim P, Vitagliano D, Blier P, Fava M, Liebowitz M, et al:
Efficacy and safety of fixed-dose esketamine nasal spray combined
with a new oral antidepressant in treatment-resistant depression:
Results of a randomized, double-blind, active-controlled study
(TRANSFORM-1). Int J Neuropsychopharmacol. 22:616–630.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Montgomery SA and Asberg M: A new
depression scale designed to be sensitive to change. Br J
Psychiatry. 134:382–389. 1979.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Popova V, Daly EJ, Trivedi M, Cooper K,
Lane R, Lim P, Mazzucco C, Hough D, Thase ME, Shelton RC, et al:
Efficacy and safety of flexibly dosed esketamine nasal spray
combined with a newly initiated oral antidepressant in
treatment-resistant depression: A randomized double-blind
active-controlled study. Am J Psychiatry. 176:428–438.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Floden L, Hudgens S, Jamieson C, Popova V,
Drevets WC, Cooper K and Singh J: Evaluation of individual items of
the Patient Health Questionnaire (PHQ-9) and Montgomery-Asberg
Depression Rating Scale (MADRS) in adults with treatment-resistant
depression treated with esketamine nasal spray combined with a new
oral antidepressant. CNS Drugs. 36:649–658. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Spitzer RL, Kroenke K and Williams JB:
Validation and utility of a self-report version of PRIME-MD: The
PHQ primary care study. Primary care evaluation of mental
disorders. Patient health questionnaire. JAMA. 282:1737–1744.
1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ochs-Ross R, Daly EJ, Zhang Y, Lane R, Lim
P, Morrison RL, Hough D, Manji H, Drevets WC, Sanacora G, et al:
Efficacy and safety of esketamine nasal spray plus an oral
antidepressant in elderly patients with treatment-resistant
depression-TRANSFORM 3. Am J Geriatr Psychiatry. 28:121–141.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Correia-Melo FS, Leal GC, Vieira F,
Jesus-Nunes AP, Mello RP, Magnavita G, Caliman-Fontes AT, Echegaray
MVF, Bandeira ID, Silva SS, et al: Efficacy and safety of
adjunctive therapy using esketamine or racemic ketamine for adult
treatment-resistant depression: A randomized, double-blind,
non-inferiority study. J Affect Disord. 264:527–534.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Souza-Marques B, Telles M, Leal GC,
Faria-Guimarães D, Correira-Melo FS, Jesus-Nunes AP, Vieira F,
Souza L, Lins-Silva D, Mello RP, et al: Esketamine for unipolar
major depression with psychotic features: A retrospective chart
review and comparison with nonpsychotic depression. J Clin
Psychopharmacol. 42:408–412. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fu DJ, Ionescu DF, Li X, Lane R, Lim P,
Sanacora G, Hough D, Manji H, Drevets WC and Canuso CM: Esketamine
nasal spray for rapid reduction of major depressive disorder
symptoms in patients who have active suicidal ideation with intent:
Double-blind, randomized study (ASPIRE I). J Clin Psychiatry.
81(19m13191)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ionescu DF, Fu DJ, Qiu X, Lane R, Lim P,
Kasper S, Hough D, Drevets WC, Manji H and Canuso CM: Esketamine
nasal spray for rapid reduction of depressive symptoms in patients
with major depressive disorder who have active suicide ideation
with intent: Results of a phase 3, double-blind, randomized study
(ASPIRE II). Int J Neuropsychopharmacol. 24:22–31. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Busner J and Targum SD: The clinical
global impressions scale: Applying a research tool in clinical
practice. Psychiatry (Edgmont). 4:28–37. 2007.PubMed/NCBI
|
|
34
|
Caruso CM, Ionescu DF, Li X, Qiu X, Lane
R, Turkoz I, Nash AI, Lopena TJ and Fu DJ: Esketamine nasal spray
for the rapid reduction of depressive symptoms in major depressive
disorder with acute suicidal ideation or behavior. J Clin
Psychopharmacol. 41:516–524. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lindenmayer JP, Czobor P, Alphs L, Nathan
AM, Anand R, Islam Z and Chou JC: InterSePT Study Group. The
InterSePT scale for suicidal thinking reliability and validity.
Schizophr Res. 63:161–170. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Takahashi N, Yamada A, Shiraishi A,
Shimizu H, Goto R and Tominaga Y: Esketamine as add-on therapy to
oral antidepressant in Japanese patients with treatment-resistant
depression: A phase 2b randomized clinical study. BMC Psychiatry.
21(526)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Araújo-de-Freitas L, Santos-Lima C,
Mendoça-Filho E, Vieira F, França RJAF, Magnavita G, Cardoso TL,
Correia-Melo FS, Leal GC, Jesus-Nunes AP, et al: Neurocognitive
aspects of ketamine and esketamine on subjects with
treatment-resistant depression: A comparative, randomized and
double-blind study. Psychiatry Res. 303(114058)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Pfenninger EG, Durieux ME and Himmelseher
S: Cognitive impairment after small-dose ketamine isomers in
comparison to equianalgesic racemic ketamine in human volunteers.
Anesthesiology. 96:357–366. 2002.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dijkstra FM, van de Loo AJ, Abdulahad S,
Bosma ER, Hartog M, Huls H, Kuijper DC, de Vries E, Solanki B,
Singh J, et al: The effects of intranasal esketamine on on-road
driving performance in patients with major depressive disorder or
persistent depressive disorder. J Psychopharmacol. 36:614–625.
2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Daly EJ, Trivedi MH, Janik A, Li H, Zhang
Y, Li X, Lane R, Lim P, Duca AR, Hough D, et al: Efficacy of
esketamine nasal spray plus oral antidepressant treatment for
relapse prevention in patients with treatment-resistant depression:
A randomized clinical trial. JAMA Psychiatry. 76:893–903.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wajs E, Aluisio L, Holder R, Daly EJ, Lane
R, Lim R, George JE, Morrison RL, Sanacora G, Young AH, et al:
Esketamine nasal spray plus oral antidepressant in patients with
treatment-resistant depression: Assessment of long-term safety in
phase 3, open-label study (SUSTAIN-2). J Clin Psychiatry.
81(19m12891)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Citrome L, DiBernardo A and Singh J:
Appraising esketamine nasal spray for the management of
treatment-resistant depression in adults: Number needed to treat,
number needed to harm, and likelihood to be helped or harmed. J
Affect Disord. 271:228–238. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Pompili M: Critical appraisal of major
depression with suicidal ideation. Ann Gen Psychiatry.
18(7)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Swainson J, Thomas RK, Archer S, Chrenek
C, MacKay MA, Baker G, Dursun S, Klassen LJ, Chokka P and Demas ML:
Esketamine for treatment resistant depression. Expert Rev
Neurother. 19:899–911. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sapkota A, Khurshid H, Qureshi IA, Jahan
N, Went TR, Sultan W and Alfonso M: Efficacy and safety of
intranasal esketamine in treatment-resistant depression in adults:
A systematic review. Cureus. 13(e17352)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Papakostas GI, Salloum NC, Hock RS, Jha
MK, Murrough JW, Mathew SJ, Iosifescu DV and Fava M: Efficacy of
esketamine augmentation in major depressive disorder: A
meta-analysis. J Clin Psychiatry. 81(19r12889)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jawad MY, Di Vincenzo JD, Ceban F, Jaberi
S, Lui LMW, Gillissie ES, Alnafeesi Y, Rosenblat JD and McIntyre
RS: The efficacy and safety of adjunctive intranasal esketamine
treatment in major depressive disorder: A systematic review and
meta-analysis. Expert Opin Drug Saf. 21:841–852. 2022.PubMed/NCBI View Article : Google Scholar
|