Introduction
Pulmonary embolism (PE) is a common thromboembolic
condition that is associated with significant morbidity and
mortality. Data from the USA suggest that the incidence of the
condition has increased from 65/100,000 population in 1999 to
137/100,000 population in 2014 with a similar increase in the
incidence of high-risk PE. An alarming finding was the excessive
mortality rates of high-risk PE patients which were ~52.2%
(1). Indeed, patients with PE
present with a wide spectrum of severity and with several
predictors of survival. It may manifest as an acute cardiac failure
with sudden cardiac arrest and mortality or may be completely
asymptomatic with only mild dyspnea (2). It is important to carefully stratify
such patients as studies have reported a significant difference in
the mortality rates amongst low-risk (3.4%) and high-risk patients
(31.8%) (3). Identification of
factors and comorbid conditions which influence the severity and
outcomes of PE can help in risk stratification and health-resource
utilization for such patients (4).
One such comorbid condition of interest is
obstructive sleep apnea (OSA). OSA is a sleep-related breathing
disorder that presents with repeated upper airway obstructions
during sleep causing intermittent hypoxia and sleep fragmentation
(5). The hypoxic episodes
experienced during nighttime due to OSA leads to several
cardiovascular changes including a surge in catecholamine levels,
heightened total peripheral resistance, increased heart rate and
venous return resulting in increased cardiac output, hypertension,
tachyarrhythmias, left ventricular hypertrophy and heart failure
(6). Furthermore, research
suggests that PE and OSA share a bidirectional relationship with
OSA being a risk factor for PE and conversely, patients with PE
having an increased risk of moderate-severe OSA (7). Given such associations, it would be
worth knowing how OSA influences the severity of PE and if patients
with comorbid OSA have worse outcomes. Over the last decade,
several studies have evaluated the influence of OSA on patients
with PE but with variable results (8-10).
Xu et al (11) in a
systematic review published in 2020 attempted to collate evidence
but could include only a limited number of studies and were unable
to assess the influence of OSA on patient survival. Therefore, to
overcome these limitations, the current review was designed to
provide the most current and up-to-date evidence on the effect of
OSA on the severity and outcomes of PE.
Materials and methods
The review conforms with the Preferred Reporting
Items for Systematic Reviews and Meta-analyses (PRISMA) statement
(12). The present study protocol
was also pre-registered on PROSPERO (CRD42022311475; https://www.crd.york.ac.uk/prospero/).
Database search
Relevant articles were searched by two reviewers
separately on the electronic databases of PubMed, CENTRAL, Embase,
ScienceDirect and Google Scholar from inception to 1st March 2022.
A combination of MeSH and free-text keywords consisting of ‘Sleep
apnea’, ‘OSA’, ‘Sleep breathing’, ‘PE’, ‘venous thrombosis’,
‘thromboembolism’ and ‘VTE’ were used during the search process
(Table SI). First all the search
results were compiled, duplicates removed and screened by titles
and abstracts. Only appropriate studies were selected for complete
text analysis, to be conducted by the two reviewers. Disagreements
were solved by consensus. The reviewers also went through the
reference list of included studies for any missed articles.
Eligibility criteria
Inclusion criteria were framed according to
Population, Exposure, Comparison, Outcomes and Study Design (PECOS)
(12). Included were i) all types
of studies carried out on patients with PE (Population); ii)
the Exposure group was patients with a history of OSA; iii)
The comparison group was patients without any comorbid OSA;
iv) Outcomes to be reported were (any one): Severity of PE
using simplified PE severity index (sPESI) or pulmonary artery
obstruction index (PAOI), the occurrence of deep vein thrombosis
(DVT), right ventricle to left ventricle short-axis diameter (RV/LV
ratio), use of non-invasive ventilation (NIV), mechanical
ventilation, mortality, PE recurrence and length of hospital stay
(LOS).
Exclusion criteria were: i) Non-comparative studies;
ii) studies not reporting relevant outcomes; iii) Editorials,
review articles; and iv) studies reporting duplicate data. In case
of overlapping data, the study with greater number of patients was
included.
Data extraction and quality
assessment
The following data were extracted from the studies:
Author details, publication year, study type, study location,
sample size, demographic details, smokers, comorbidities, such as
diabetes mellitus and hypertension and body mass index, diagnostic
criteria for PE and OSA and study outcomes.
The Newcastle-Ottawa scale (NOS) was used for
assessing risk of bias (13). All
studies were judged for selection of study population,
comparability and outcomes assessment. These were then given a
maximum of four, two, or three points respectively.
Statistical analysis
The meta-analysis was carried out using Review
Manager [RevMan, version 5.3; Nordic Cochrane Centre (Cochrane
Collaboration), 2014; https://revman.cochrane.org/#/myReviews]. For
quantitative analysis a minimum of two studies reporting similar
data for the same outcome was required.
Dichotomous outcomes were pooled in a random effect
model and odds ratios (OR) with 95% confidence intervals (CI) were
calculated. Continuous outcomes were also combined in a
random-effects model to obtain mean difference (MD) and 95% CI.
When available, multivariable-adjusted hazard ratios (HR) of
mortality were combined using the generic inverse variance function
of RevMan. Heterogeneity was assessed using the I2
statistic. The present study did not assess for publication bias
using funnel plots as <10 studies were included in each
meta-analysis.
Results
Search and study details
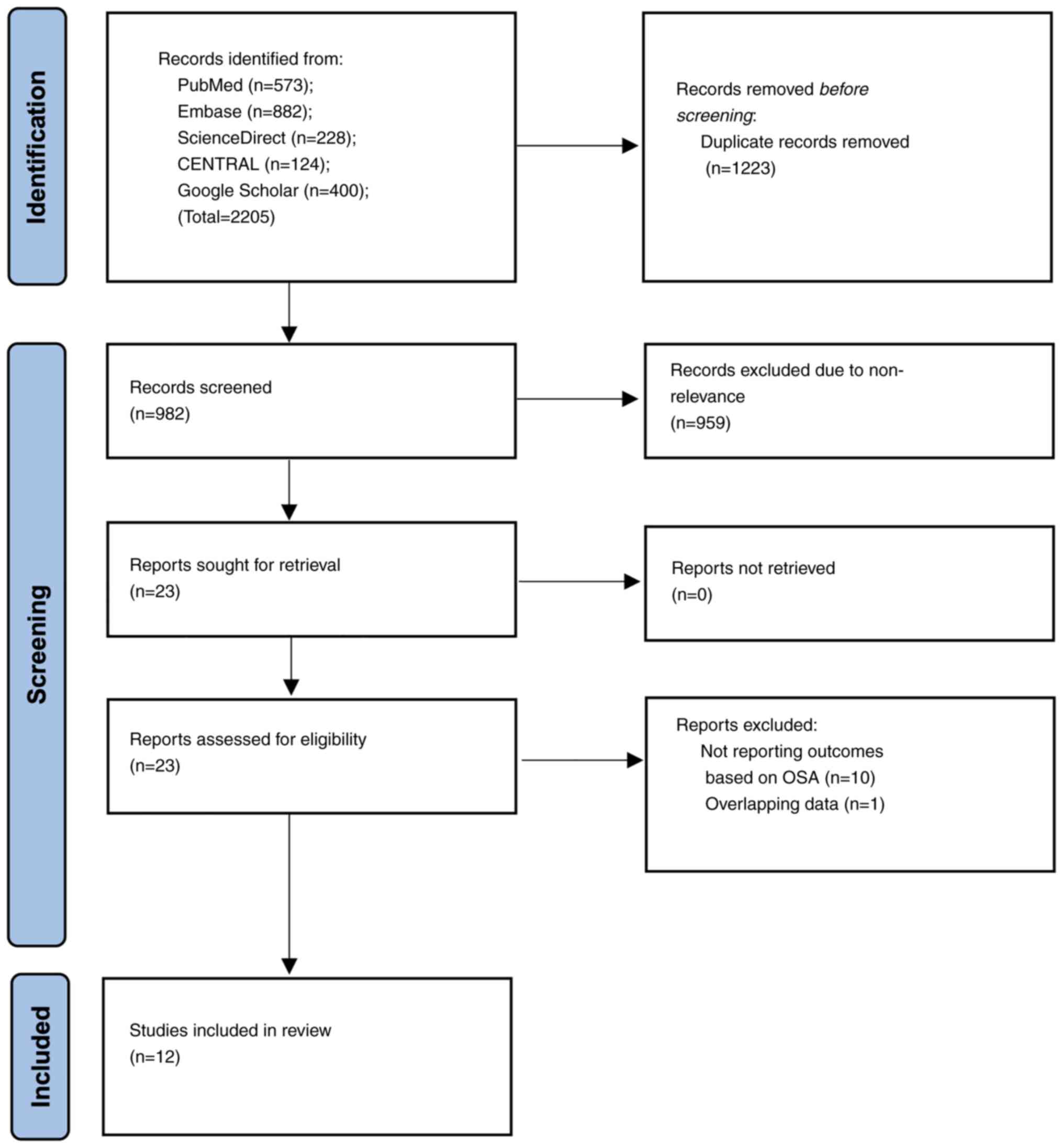
Details of the search results at every stage are
presented in Fig. 1. A total of
982 unique articles were retrieved with the search strategy. These
were then screened to extract 23 articles relevant to the review
topic. After evaluation of full-texts 11 were excluded as they did
not satisfy the inclusion criteria. Finally, 12 studies were
included in the present review (8-10,14-22).
Study details are shown in Table I. The included studies were
published between 2012 and 2021. Of the 12, three studies each were
from Germany, Spain and China, two were from the USA and one was a
multinational study. The four studies from Germany were from the
same institute and with an overlapping database. However, the
studies reported different outcomes in each article. For this
review, overlapping outcomes were not included from these studies.
Except for three cross-sectional studies, all others were cohort in
nature. The diagnostic criteria of PE and PSA were not uniform
across the included studies; two studies used only the
International classification of disease codes for retrieving PE and
OSA cases from their database. The sample size of the OSA group
ranged from 28-61,050 patients while that of the control group
ranged from 26-694,482 patients. All outcomes were not uniformly
reported by the included studies. The NOS score ranged from 6 to
8.
 | Table IDetails of included studies. |
Table I
Details of included studies.
| Author, year | Location | Study type | Diagnostic criteria
for PE | Diagnostic criteria
for OSA | Groups | Sample size | Mean age (years) | Male sex (%) | DM (%) | HT (%) | BMI
(kg/m2) | NOS score | (Refs) |
|---|
| Zhang, 2012 | China | Cross- sectional | Chinese Medical
Association guidelines 2001 | PSG (AHI >5
events/h) | With OSA | 28 | NA | NA | NA | NA | NA | 6 | (14) |
| Without OSA | 30 | | | | | | | |
| Xie, 2015 | China | Cross-
sectional | 2014 ESC
guideline | Portable monitoring
(AHI >5 events/h) | With OSA | 32 | 59.9±12.9 | 62.5 | 43.7 | 78.1 | 30.7±5.4 | 8 | (22) |
| Without OSA | 65 | 62.9±12.7 | 38.5 | 13.8 | 55.3 | 26.1±4.1 | | |
| Alonso- Fernandez,
2016 | Spain | Cohort | CTPA | Portable monitoring
(AHI >10 events/h) | With OSA | 71 | NR | NR | NR | NR | NR | 7 | (21) |
| Without OSA | 59 | | | | | | | |
| Konnerth, 2018 | Germany | Cohort | CTPA or V/Q
scan | PSG (AHI >15
events/h) | With OSA | 89 | 60±NR | 51.7 | NR | NR | NR | 7 | (20) |
| Without OSA | 164 | 56±NR | 49.4 | | | | | |
| Toledo-Pons,
2020 | Spain | Cohort | CTPA | Respiratory
polygraphy (AHI >15 events/h) | With OSA | 55 | 61±12.5 | 80 | NR | NR | 27.9±4.4 | 6 | (18) |
| Without OSA | 65 | 54.1±16.2 | 47.7 | | | 28.3±6.1 | | |
| Xiao, 2019 | China | Cross-
sectional | 2014 ECS
guideline | Portable monitoring
(AHI >5 events/h) | With OSA | 49 | NA | NA | NA | NA | NA | 6 | (19) |
| Without OSA | 26 | | | | | | | |
| Berghaus, 2020 | Germany | Cohort | CTPA | PSG (AHI >15
events/h) | With OSA | 66 | 71±11 | 53 | NR | NR | NR | 7 | (9) |
| Without OSA | 131 | 55±18 | 44.3 | | | | | |
| Geissenberger,
2020 | Germany | Cohort | CTPA | PSG (AHI >15
events/h) | With OSA | 45 | 70.8±11.1 | 53.7 | 20 | NR | 29±NR | 7 | (8) |
| Without OSA | 56 | 53.2±18.1 | 49.3 | 3.6 | | 29.4±NR | | |
| Le Mao, 2020 | Multicentric | Cohort | CTPA or V/Q scan or
signs and symptoms for PE, along with objectively confirmed lower
limb deep vein thrombosis | NR | With OSA | 241 | 66.8±12.2 | 69 | NR | NR | NR | 7 | (16) |
| Without OSA | 3912 | 66.2±17 | 48 | | | | | |
| Seckin, 2020 | USA | Cohort | CTPA or V/Q scan or
signs and symptoms for PE, along with objectively confirmed lower
limb deep vein thrombosis | ICD codes | With OSA | 3184 | 66 (54-76) | 58.3 | 34 | 68.1 | 34.1
(29-40)a | 7 | (17) |
| Without OSA | 21854 | 52 (32-71) | 37.5 | 11.8 | 34.7 | 27.4 (24-32) | | |
| De-Miguel-Diez,
2021 | Spain | Cohort | ICD codes | ICD codes | With OSA | 2561 | 68.4±12.2 | 64.4 | 23.2 | NR | NR | 7 | (15) |
| Without OSA | 44233 | 70.8±15.9 | 45.5 | 14.3 | | | | |
| Joshi, 2021 | USA | Cohort | ICD codes | ICD codes | With OSA | 61050 | 61 (51-71) | 58.9 | NR | 70 | NR | 7 | (10) |
| Without OSA | 694,482 | 65 (52-77) | 46.3 | | 52.1 | | | |
Meta-analysis
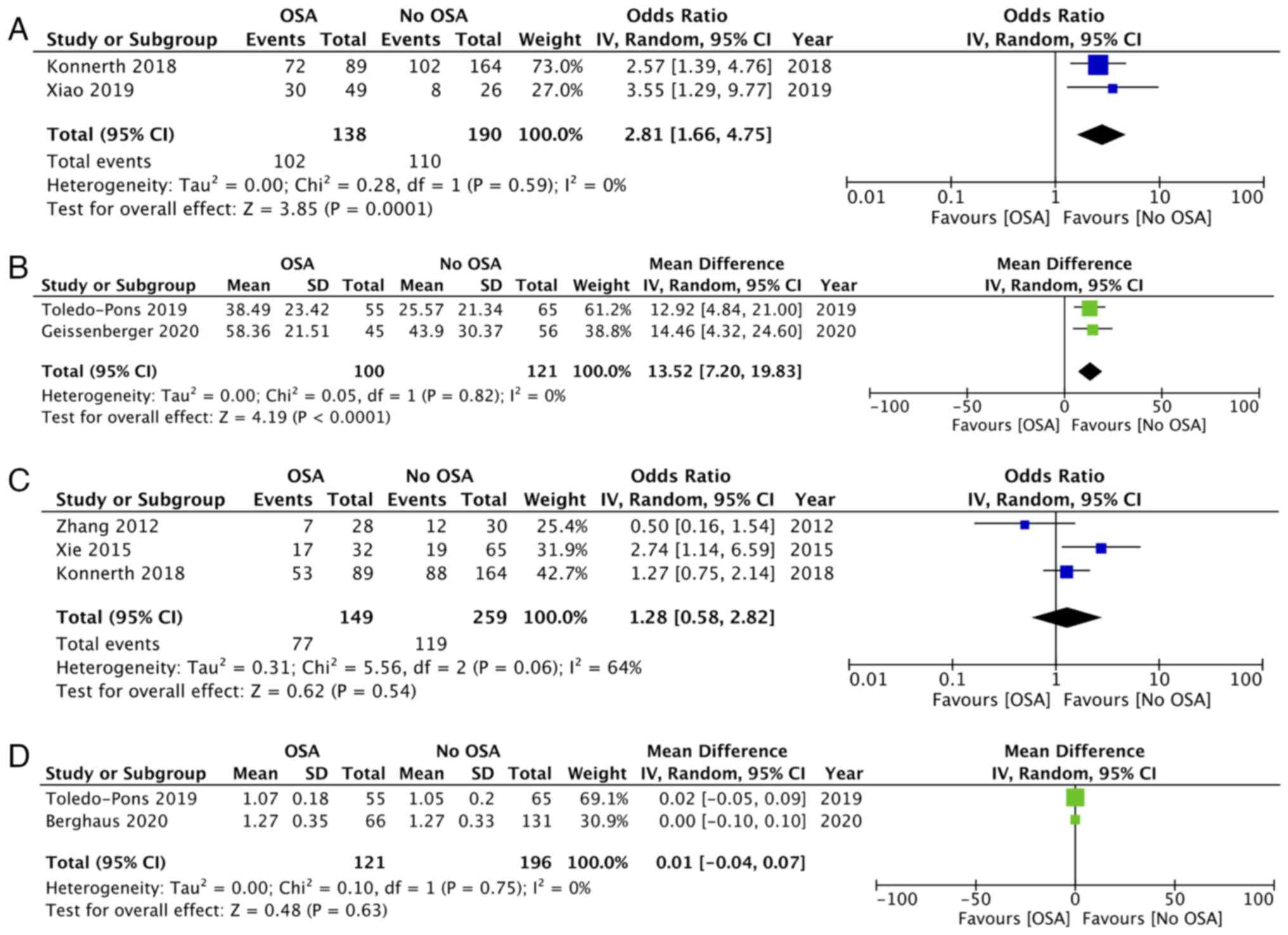
The severity of PE measured by sPESI and PAOI was
reported by only two studies each. Meta-analysis revealed that an
sPESI score of >1 was significantly higher in patients with OSA
as compared with the control group (OR: 2.81; 95% CI: 1.66, 4.85;
I2=0%; P=0.0001; Fig.
2A). Similarly, PAOI scores were significantly higher in
patients with OSA compared with controls (MD: 13.52; 95% CI: 7.20,
19.83; I2=0%; P<0.0001; Fig. 2B). Incidence of DVT was reported by
three studies. Pooled analysis indicated no difference in the
incidence of DVT between the two groups (OR: 1.28; 95% CI: 0.58,
2.82; I2=64%; P=0.54) (Fig.
2C). Data on RV/LV ratio diameter was reported by two studies.
Meta-analysis indicated no difference between the two groups (MD:
0.01; 95% CI: -0.04, 0.07; I2=0%; P=0.63; Fig. 2D).
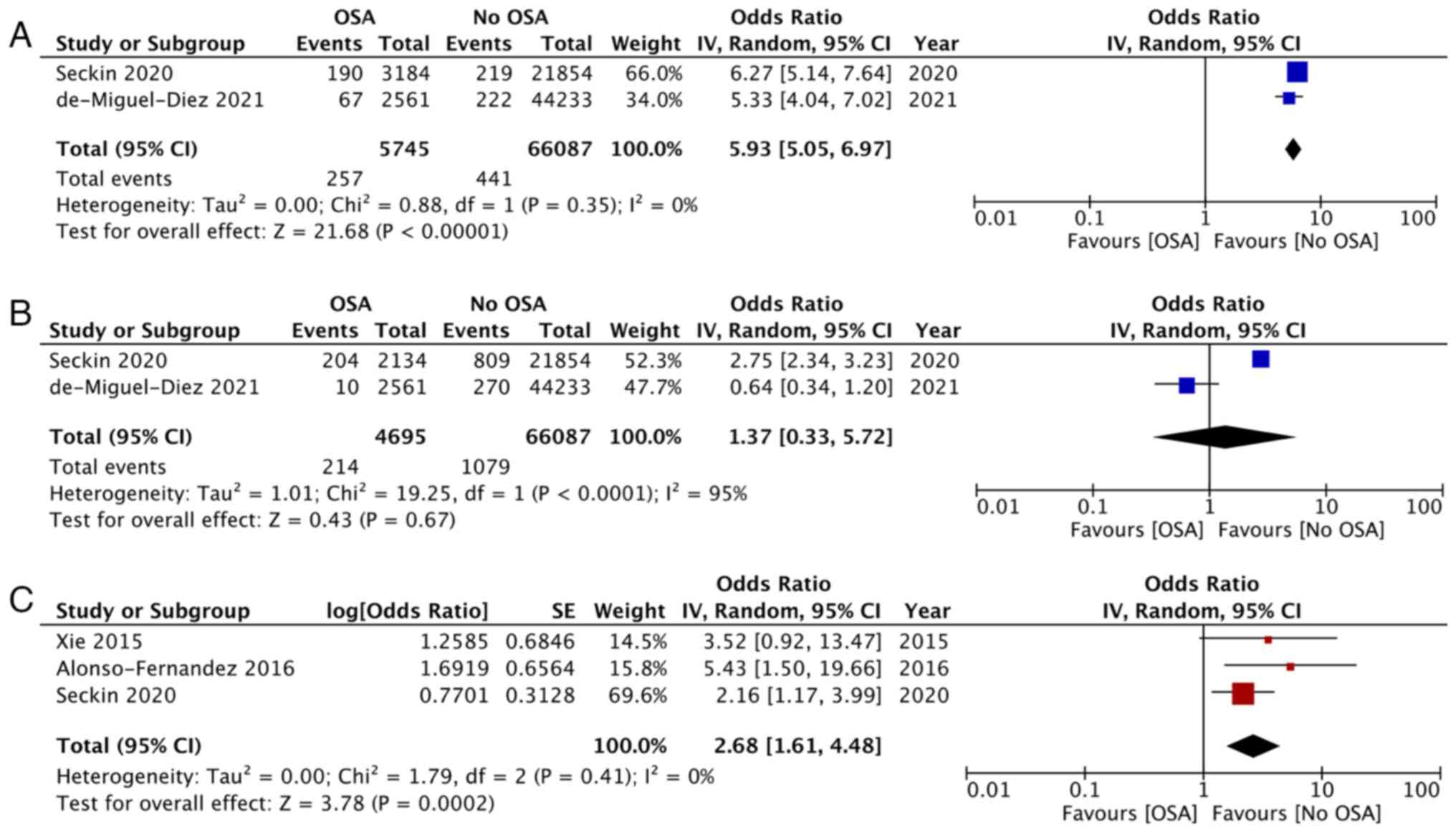
The need for NIV and mechanical ventilation was
reported by two studies each. Pooled analysis indicated that the
need for NIV was significantly higher in patients with OSA (OR:
5.93; 95% CI: 5.05, 6.97; I2=0%; P<0.00001; Fig. 3A) but there was no difference in
the need for mechanical ventilation (OR: 1.37; 95% CI: 0.33, 5.72;
I2=95%; P=0.67; Fig.
3B). Data on recurrence of PE and LOS was reported by three
studies each. Meta-analysis indicated that the incidence of
recurrence of PE was significantly higher in patients with OSA as
compared with controls (OR: 2.68; 95% CI: 1.61, 4.97;
I2=0%; P<0.00001; Fig.
3C). However, the present study noted no difference in the LOS
between the two groups (MD: 0.40; 95% CI: -1.21, 2.01;
I2=78%; P=0.62; Fig.
3D).
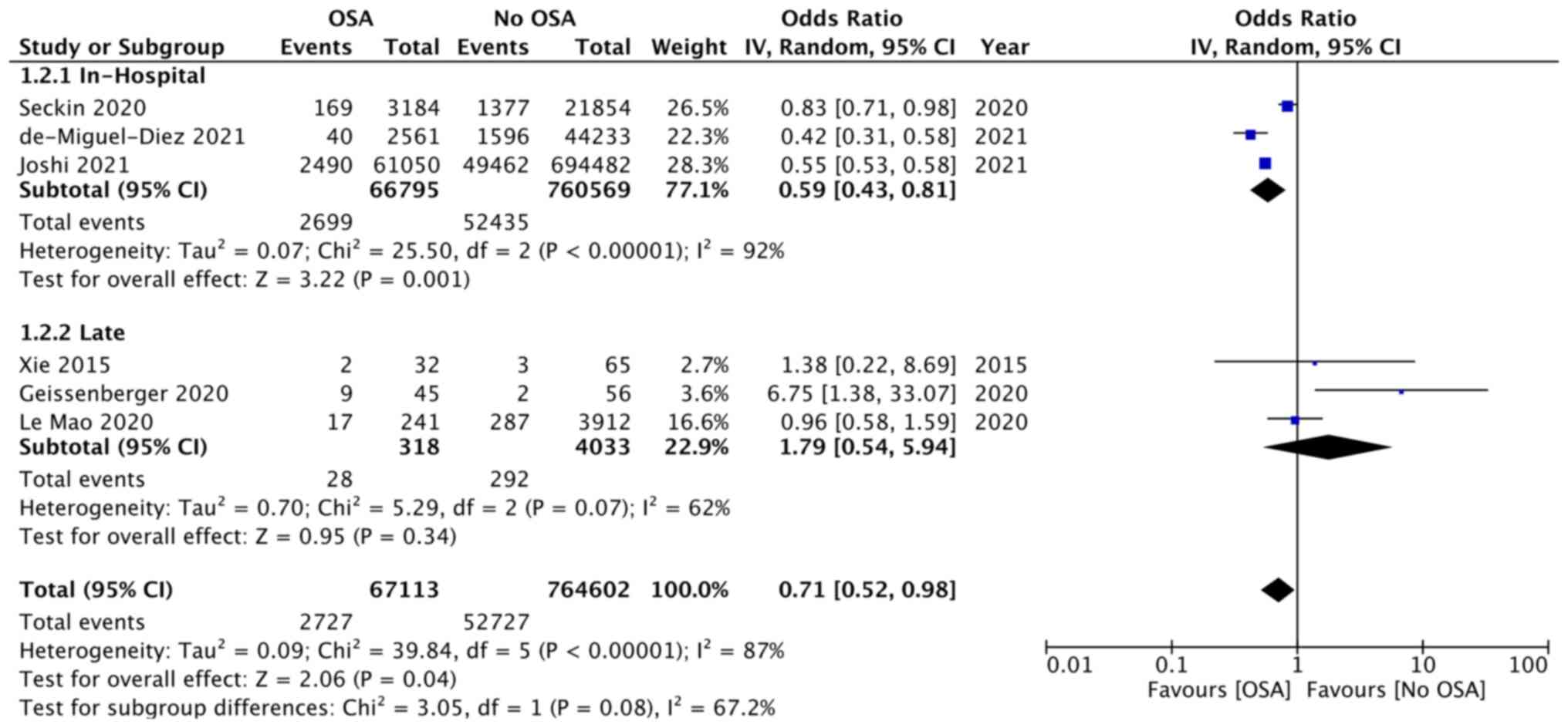
Data on mortality was reported by six studies. Three
reported data on in-hospital mortality while another three reported
on a follow-up of 3-53 months. Meta-analysis revealed statistically
significant lower risk of in-hospital mortality in patients with
OSA as compared with controls (OR: 0.59; 95% CI: 0.43, 0.81;
I2=92%; P=0.01), but there was no difference in the risk
of late mortality (>1 year) (OR: 1.79 95%; CI: 0.54, 5.94;
I2=62%; P=0.34; Fig.
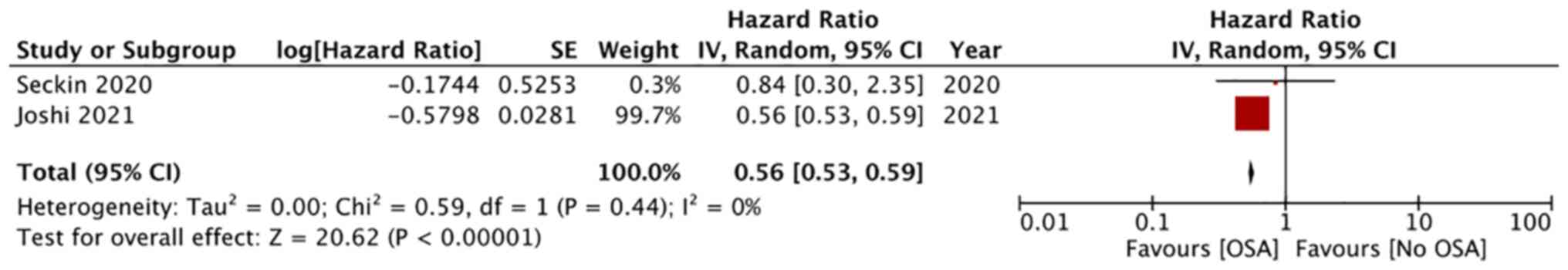
4). Adjusted data on mortality was available from two studies.
On pooled analysis, a significantly lower risk of mortality was
noted in PE patients with comorbid OSA (HR: 0.56; 95% CI: 0.53,
0.59; I2=0%; P<0.0001; Fig. 5).
Discussion
The present study with limited data demonstrated
that the presence of OSA resulted in higher severity of PE.
However, OSA was not found to influence the RV/LV ratio of the
right heart function, incidence of DVT and LOS. The need for NIV
was significantly higher in patients with OSA along with the
increased risk of recurrent PE; but by contrast, in-hospital
mortality was significantly lower in patients with OSA.
Between the spectrum of mild dyspnoeic symptoms and
acute cardiac arrest followed by sudden mortality, PE as a
condition can present with a wide range of signs and symptoms with
different grades of dyspnea, chest pain, hypoperfusion, respiratory
failure and hemodynamic instability (2). The vital signs of patients, presence
of right ventricle dysfunction and evidence of myocardial injury
are important predictors of early survival (23). Several clinical models based on the
medical history and clinical condition of patients have been
developed for risk stratification of PE, of which, the sPESI has
been one of the most validated tools. In a meta-analysis, Zhou
et al (24) showed that the
sPESI has good discrimination power to predict early survival and
adverse events in patients with PE. The sPESI encompasses six
clinical variables and patients showing none of them (0 points) are
classified as low-risk while those with any of the six variables
(1-6 points) are classified as high risk (25). Similarly, PAOI is another common
tool used for risk stratification of PE based on computed
tomography pulmonary angiography (CTPA) findings (26). The present meta-analysis noted that
sPESI >1 and mean PAOI scores were significantly higher in
patients with OSA as compared with controls indicating high-risk PE
in such patients.
Results should be interpreted with caution as such
data was not universally reported and each meta-analysis could
include just two studies. The increased severity of PE in OSA can
be due to several reasons. Notably, a difference in age and
comorbidities between OSA and control groups was noted in some
studies (20). It is known that
elderly patients have a more complicated course of venous
thromboembolism (20).
Furthermore, the majority of OSA patients have several
comorbidities such as hypertension, dyslipidemia, obesity,
depression, obstructive lung disease, diabetes and other
cardiovascular diseases which can significantly affect the clinical
course of PE (27,28). However, Konnerth et al
(20) in their study noted that
OSA was independently associated with a worse clinical course of PE
irrespective of other confounding variables. The authors
hypothesized that reduced oxygen saturation in patients with OSA
could worsen the myocardial injury and lead to high-risk PE.
Additionally, apneic events and desaturation episodes in OSA
patients lead to a prothrombotic state with increased levels of
blood coagulability markers. This in turn could result in higher
pulmonary thrombus load and increased right ventricular strain
leading to high-risk PE (29).
Research has also noted that D-dimer levels in PE patients with
comorbid OSA are higher as compared with controls. Elevated levels
of D-dimer suggest a hypercoagulable state and are associated with
high rates of PE recurrence (30).
Thus, it was not surprising to note a higher risk of recurrent PE
in patients with OSA in our meta-analysis.
It is known that desaturation episodes in OSA cause
changes in intrathoracic pressure and pulmonary hypertension which
can affect right heart performance. Furthermore, increased severity
of OSA is known to worsen right ventricular dysfunction (31). CTPA-based assessment of right
ventricular dilation measured by RV/LV ratio is a proxy for right
ventricular dysfunction and can accurately predict early outcomes
in PE patients (32). However, the
present meta-analysis noted no difference in RV/LV ratio in PE
patients based on the presence or absence of OSA. One reason for
such lack of difference could be due to the hypothesis that
heightened pulmonary arterial pressure is mild in OSA and severe
right ventricular dysfunction is noted only with other comorbid
conditions such as chronic heart failure (33). Second, right ventricular strain is
not seen in all patients with a high thrombus burden. The RV/LV
ratio may be unchanged even with increasing thrombus load up to a
critical point, after which right ventricular strain may be evident
(34). Also, long-term OSA
patients may develop right ventricular compensatory mechanisms
which may counterbalance right ventricular dysfunction (18). The present meta-analysis
demonstrated increased use of NIV in OSA patients but no difference
in the use of invasive ventilation. The higher use of NIV in OSA
patients could be due to the inability of the retrospective studies
to differentiate between NIV used for respiratory failure compared
with nighttime airway support for OSA (17).
An intriguing and paradoxical finding of the present
study was lower in-hospital mortality in PE patients with comorbid
OSA. Such findings are inconsistent with the fact that OSA and PE
patients are known to have a higher incidence of comorbidities as
compared with those with only PE (27,28).
Furthermore, the present meta-analysis also revealed that OSA
patients had higher severity of PE which should, in turn, lead to
worse outcomes in these patients. However, all three studies in the
meta-analysis of in-hospital mortality reported improved survival
in patients with OSA as compared with controls. The consistent
results and the large sample size of all the three studies in the
analysis further add support to the results. There are several
hypotheses put forward to support these results. First, it has been
suggested that right ventricle dysfunction due to PE may be more
tolerated in patients with OSA as they are adapted to recurring
right heart pressure overloads provoked by poor pulmonary perfusion
due to repeated oxygen desaturation during sleep. Thus, the right
ventricles in OSA patients may be more resistant to injury and
hemodynamic collapse due to acute PE which may, in turn, reduce the
risk of early mortality (9,15).
Second, Joshi et al (10)
suggested that OSA patients have higher hemoglobin levels due to
repeated hypoxemias which may have a protective role in PE by
preventing worsening of hypoxia. Third, OSA patients have higher
rates of obesity (27). An obesity
paradox has been noted wherein patients with higher body mass index
have improved outcomes in acute diseases possibly due to improved
medical care or due to higher metabolic reserves (35,36).
It is plausible that such associations could have led to improved
survival in OSA patients (15).
However, it should also be noted that the data for in-hospital
mortality was mostly from registry-based studies which are prone to
errors in record-keeping and data entry. Furthermore, several other
confounding factors could influence survival in PE patients and
multivariable-adjusted data represents improved evidence rather
than crude mortality rates. However, such data was reported by just
two studies and the results were skewed by the outcomes of Joshi
et al (10) (weight 99.7%).
Further robust studies considering all major confounding variables
are needed to assess the impact of OSA on mortality rates in PE
patients.
There are several limitations to the present review.
First, despite including several new studies (9,10,15-17)
as compared with a previous review (11), the total number of studies in the
present review was not very high. A significant limitation was the
difference in the reporting of outcomes by the studies which
significantly restricted the number of studies in each
meta-analysis. Second, some of the analyses such as in-hospital
mortality and use of mechanical ventilation had very high
heterogeneity. This was expected as the studies pooled were
registry-based including a very heterogeneous group of PE patients
of different severity, clinical status and treated with varied
treatment protocols. The low number of studies in the meta-analyses
prevented the present review from exploring the source of
heterogeneity by subgroup analysis or a meta-regression. Also, in
the meta-analysis of mortality, the study of Joshi et al
(10) with its large sample size
could have skewed the results. Third, there were variations in the
included studies in relation to the diagnostic criteria of PE and
OSA. Not all studies used CTPA or polysomnography to diagnose PE
and OSA respectively. Fourth, the present review was unable to
segregate outcomes based on the severity of OSA due to the limited
availability of data. Last, the majority of the studies did not
conduct baseline matching of study groups and did not report
adjusted outcome data. Thus, the influence of confounding variables
on the outcomes of the studies and the present review cannot be
ignored.
Data from a limited number of retrospective studies
indicate that comorbid OSA increases the severity of PE but has no
effect on right ventricular function. OSA may increase the risk of
recurrent PE. Paradoxically, the presence of OSA may reduce the
risk of in-hospital mortality. Results must be interpreted with
caution owing to high inter-study heterogeneity and lack of
matching of baseline characteristics. Current evidence needs to be
confirmed by high-quality prospective studies.
Supplementary Material
Search strategy
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no data sets were generated or analyzed during the current
study.
Authors' contributions
WZ conceived and designed the study. WZ and YD
collected the data and performed the literature search. WZ was
involved in the writing of the manuscript. All authors read and
approved the final manuscript. Data sharing is not applicable to
this article.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stein PD, Matta F and Hughes MJ:
Hospitalizations for high-risk pulmonary embolism. Am J Med.
134:621–625. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Becattini C and Agnelli G: Risk
stratification and management of acute pulmonary embolism.
Hematology Am Soc Hematol Educ Program. 2016:404–412.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Casazza F, Becattini C, Bongarzoni A,
Cuccia C, Roncon L, Favretto G, Zonzin P, Pignataro L and Agnelli
G: Clinical features and short term outcomes of patients with acute
pulmonary embolism. The Italian pulmonary embolism registry (IPER).
Thromb Res. 130:847–852. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Elias A, Mallett S, Daoud-Elias M, Poggi
JN and Clarke M: Prognostic models in acute pulmonary embolism: A
systematic review and meta-analysis. BMJ Open.
6(e010324)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Patel SR: Obstructive sleep apnea. Ann
Intern Med. 171:ITC81–ITC96. 2019.PubMed/NCBI
|
|
6
|
Diamond JA and Ismail H: Obstructive sleep
apnea and cardiovascular disease. Clin Geriatr Med. 37:445–456.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Alonso-Fernández A, Toledo-Pons N and
García-Río F: Obstructive sleep apnea and venous thromboembolism:
Overview of an emerging relationship. Sleep Med Rev.
50(101233)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Geissenberger F, Schwarz F, Probst M,
Haberl S, Parkhe A, Faul C, von Lewinski D, Kroencke T,
Schwaiblmair M, von Scheidt W and Berghaus TM: Obstructive sleep
apnea is associated with pulmonary artery thrombus load, disease
severity, and survival in acute pulmonary embolism. Clin Res
Cardiol. 109:13–21. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Berghaus TM, Geissenberger F, Konnerth D,
Probst M, Kröncke T and Schwarz F: Right-to-left ventricular
diameter ratio at computed tomographic pulmonary angiography in
patients with acute pulmonary embolism and obstructive sleep apnea.
Clin Med Insights Circ Respir Pulm Med.
14(1179548420976430)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Joshi AA, Hajjali RH, Gokhale AV, Smith T,
Dey AK, Dahiya G, Lerman JB, Sajja AP, Kanwar M and Raina A:
Outcomes of patients hospitalized for acute pulmonary embolism by
obstructive sleep apnea status. Pulm Circ.
11(2045894021996224)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xu J, Wang X, Meng F, Zhao T, Tang T, Wu W
and Wang W: The role of obstructive sleep apnea on the prognosis of
pulmonary embolism: A systemic review and meta-analysis. Sleep
Breath. 25:1419–1426. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. Int J Surg. 88(105906)2021.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Wells G, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of nonrandomised studies in
meta-analyses, 2013.
|
|
14
|
Zhang XF, Gao YM, Zhu GF, Yang JH, Ding SF
and Liu S: Obstructive sleep apnea-hypopnea syndrome in patients
with pulmonary thromboembolism: Clinical features and management.
Zhonghua Jie He He Hu Xi Za Zhi. 35:180–183. 2012.PubMed/NCBI(In Chinese).
|
|
15
|
de-Miguel-Diez J, Lopez-Herranz M,
Hernandez-Barrera V, Jimenez D, Monreal M, Jiménez-García R and
López-de-Andrés A: Sex-differences in the effect of obstructive
sleep apnea on patients hospitalized with pulmonary embolism and on
in-hospital mortality. Sci Rep. 11(18390)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Le Mao R, Jiménez D, Bikdeli B,
Porres-Aguilar M, García-Ortega A, Rosa V, Schellong S, Mazzolai L,
Rivera-Civico F and Monreal M: RIETE Investigators. Prognostic
impact of obstructive sleep apnea in patients presenting with acute
symptomatic pulmonary embolism. Thromb Haemost. 121:808–815.
2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Seckin ZI, Helmi H, Weister TJ, Lee A and
Festic E: Acute pulmonary embolism in patients with obstructive
sleep apnea: Frequency, hospital outcomes, and recurrence. J Clin
Sleep Med. 16:1029–1036. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Toledo-Pons N, Alonso-Fernández A, de la
Peña M, Pierola J, Barceló A, Fernández-Capitán C, Lorenzo A, Mejía
Núñez JA, Carrera M, Soriano JB, et al: Obstructive sleep apnea is
associated with worse clinical-radiological risk scores of
pulmonary embolism. J Sleep Res. 29(e12871)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xiao Y, Yang LR, Zhu GF, Zhang Y, Wu CT
and Zhang WM: Effect of obstructive sleep apnea on the severity of
acute pulmonary thromboembolism. Zhonghua Yi Xue Za Zhi.
99:739–743. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
20
|
Konnerth D, Schwarz F, Probst M, Seidler
M, Wagner T, Faul C, von Scheidt W, Schwaiblmair M and Berghaus TM:
Is acute pulmonary embolism more severe in the presence of
obstructive sleep apnea? Results from an observational cohort
study. J Thromb Thrombolysis. 46:253–259. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Alonso-Fernández A, Suquia AG, de la Peña
M, Casitas R, Pierola J, Barceló A, Soriano JB, Fernández-Capitán
C, Martinez-Ceron E, Carrera M and García-Río F: OSA Is a risk
factor for recurrent VTE. Chest. 150:1291–1301. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xie J, Wei YX, Liu S, Zhang W, Zhang XF
and Li J: Obstructive sleep apnea hypopnea syndrome as a reason for
active management of pulmonary embolism. Chin Med J (Engl).
128:2147–2153. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Piazza G: Submassive pulmonary embolism.
JAMA. 309:171–180. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhou XY, Ben SQ, Chen HL and Ni SS: The
prognostic value of pulmonary embolism severity index in acute
pulmonary embolism: A meta-analysis. Respir Res.
13(111)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Venetz C, Jiménez D, Méan M and Aujesky D:
A comparison of the original and simplified pulmonary embolism
severity index. Thromb Haemost. 106:423–428. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Guo F, Zhu G, Shen J and Ma Y: Health risk
stratification based on computed tomography pulmonary artery
obstruction index for acute pulmonary embolism. Sci Rep.
8(17897)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Testelmans D, Spruit MA, Vrijsen B, Sastry
M, Belge C, Kalkanis A, Gaffron S, Wouters EFM and Buyse B:
Comorbidity clusters in patients with moderate-to-severe OSA. Sleep
Breath. 26:195–204. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ng ACC, Chow V, Yong ASC, Chung T and
Kritharides L: Prognostic impact of the Charlson comorbidity index
on mortality following acute pulmonary embolism. Respiration.
85:408–416. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hong SN, Yun HC, Yoo JH and Lee SH:
Association between hypercoagulability and severe obstructive sleep
apnea. JAMA Otolaryngol Head Neck Surg. 143:996–1002.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Palareti G: How D-dimer assay can be
useful in deciding the duration of anticoagulation after venous
thromboembolism: A review. Expert Rev Hematol. 8:79–88.
2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Akyol S, Cortuk M, Baykan AO, Kiraz K,
Borekci A, Seker T, Gur M and Cayli M: Biventricular myocardial
performance is impaired in proportion to severity of obstructive
sleep apnea. Texas Hear Inst J. 43:119–125. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ende-Verhaar YM, Kroft LJM, Mos ICM,
Huisman MV and Klok FA: Accuracy and reproducibility of CT
right-to-left ventricular diameter measurement in patients with
acute pulmonary embolism. PLoS One. 12(e0188862)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sajkov D and McEvoy RD: Obstructive sleep
apnea and pulmonary hypertension. Prog Cardiovasc Dis. 51:363–370.
2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wong LF, Akram AR, McGurk S, Van Beek EJR,
Reid JH and Murchison JT: Thrombus load and acute right ventricular
failure in pulmonary embolism: correlation and demonstration of a
‘tipping point’ on CT pulmonary angiography. Br J Radiol.
85:1471–1476. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ho KS, Wu L, Sheehan J and Salonia J:
Obesity paradox? Improved in-hospital mortality in patients
admitted for sepsis vs septic shock: A propensity score match
analysis. Chest. 156 (Suppl 1)(A1141)2019.
|
|
36
|
Niedziela J, Hudzik B, Niedziela N, Gąsior
M, Gierlotka M, Wasilewski J, Myrda K, Lekston A, Poloński L and
Rozentryt P: The obesity paradox in acute coronary syndrome: A
meta-analysis. Eur J Epidemiol. 29:801–812. 2014.PubMed/NCBI View Article : Google Scholar
|