1. Metabolic dysfunctions in patients
undergoing antipsychotic treatment: General considerations
Fasting hyperglycaemia, impaired glucose tolerance,
prediabetes, diabetes, obesity and metabolic syndrome are
frequently diagnosed in patients with psychotic or mood disorders
undergoing treatment with atypical antipsychotics (1). The prevalence of diabetes in
individuals diagnosed with severe psychiatric disorders is 2- to
3-fold higher compared with that in the general population
(2). Data in the literature
suggest that the prevalence of diabetes increases shortly after
antipsychotics are initiated and this phenomenon seems mediated by
the negative influences of these medications on weight gain,
insulin sensitivity and secretion alteration (2). However, the causality between
schizophrenia spectrum disorders, antipsychotic use and impaired
glucose metabolism is difficult to determine. Case-control studies
reported alterations in fasting plasma glucose level, plasma
glucose level after an oral glucose tolerance test, fasting plasma
insulin level and insulin resistance in antipsychotic-naïve
patients with a first episode of psychosis compared with those in
the control (3). A high level of
plasma triglycerides was found in patients with the first episode
of psychosis, which may be another piece of evidence to support
glucose dysregulation in this population, as concluded by a
meta-analysis (20 case-control studies; 1,167 patients and 1,184
controls) (4). Excessive caloric
intake and especially the consumption of refined grain foods and
discretionary foods have been considered major contributors to
weight gain in patients with the first episode of psychosis soon
after the initiation of antipsychotic medication (8 months)
(5). In these patients, a study
found an excess of 26% in the energy balance, with a median of
1,837 kJ daily (5).
Not all antipsychotics have the same negative impact
on the metabolic profile. According to a meta-analysis (48
head-to-head studies that compared second-generation
antipsychotics), olanzapine and clozapine were associated most
frequently with weight gain, olanzapine led to a greater
cholesterol increase than aripiprazole, risperidone or ziprasidone,
and a greater increase in the glucose blood level vs. amisulpride,
aripiprazole, quetiapine, risperidone and ziprasidone (6). Antipsychotics have multiple
mechanisms through which they can induce metabolic disturbances,
including increased appetite, sedation with secondary low energy
expenditure and changes in dietary patterns, among others (Fig. 1) (6,7).
Antipsychotics may significantly increase body weight and waist
circumference, which leads to insulin resistance and type 2
diabetes (1). However, diabetes
has been reported in patients treated with second-generation
antipsychotics who did not develop obesity, raising the hypothesis
of a direct effect of these psychotropics on insulin secretion
(1,8,9).
Antipsychotics increase appetite and caloric intake by blocking
serotonergic 5-hydroxytryptamine receptor 2C (5HT2C), dopamine D2
and histaminergic H1 receptors (10). Also, the sedation related to
adrenergic α1 and histaminergic H1 receptor antagonism may enhance
the propensity toward weight gain by decreasing the patients'
involvement in physical activity (10). An association between the affinity
for muscarinic M3 receptors and the ability of antipsychotics to
induce diabetes has been demonstrated (11,12).
A systematic review and meta-analysis showed that several
pharmacogenetic studies found that single nucleotide polymorphisms
in adrenergic α2, dopaminergic D2, serotonergic 5HT2C and
melanocortin-4 receptor genes were significantly associated with
antipsychotic-related weight gain (13). Dysregulation of insulin, cortisol,
glucagon, cholecystokinin, adiponectin, ghrelin, leptin, orexin,
prolactin and oxytocin has also been reported during treatment with
antipsychotic agents (14,15). The possible impairment of the
mitochondrial dynamics in the pathogenesis of metabolic syndrome in
patients undergoing antipsychotic treatment has been suggested,
although data to support this hypothesis are still insufficient
(16).
The existence of several third-generation
antipsychotics, which possess partial D2/D3 receptor agonistic
properties and lower affinity for other receptors, may be useful
for reducing the risk of weight gain or dyslipidaemia (17,18).
However, there is an intense need for more research in this field,
as newer antipsychotic agents (e.g., brexanolone, cariprazine and
blonanserin), with the exception of aripiprazole, have not been
extensively explored for their long-term effects on metabolism.
The vast majority of the existing antipsychotics are
associated with adverse metabolic effects, and switching from the
currently administered agent to a metabolically more favourable one
is not always possible or even recommended (19). In addition, monotherapy is rarely
used in clinical practice in patients with severe mental disorders,
and other pharmacological agents (mood stabilizers, antidepressants
and sedatives) used as add-ons may further complicate the adverse
events profile (20).
Weight loss in patients with type 2 diabetes may
have important consequences to glycaemic control and potential
cardiovascular complications, and it may even lead to diabetes
remission in certain individuals (21). First-generation antidiabetic agents
have been associated with weight gain, while newer agents, such as
sodium-glucose co-transporter 2 inhibitors (SGLT2Is) and
glucagon-like peptide receptor agonists (GLP-1RAs), treat obesity
and diabetes simultaneously (21).
SGLT2Is are approved for treating diabetes mellitus
due to their properties of reducing plasma glucose levels by
inhibiting glucose and sodium reabsorption in the proximal tubules
of the nephrons, an effect that leads to glucosuria (22). Body weight and adiposity may
decrease as a consequence of the administration of these
pharmacological agents, and the HbA1c, blood glucose and blood
pressure values may also improve during this treatment due to
natriuresis, glucosuria and negative caloric imbalance in patients
with diabetes (1,22). In clinical trials (randomized,
controlled and real-world studies), patients treated with SGLT2Is
reported weight loss of 1-3 kg (23). Also, SGLT2I administration was
associated with benefits in cardiovascular functioning within 6
months of therapy initiation, and also with improvements in renal
function in patients with type 2 diabetes (23). SGLT2Is have few adverse effects and
a lower degree of pharmacodynamic/pharmacokinetic interactions, but
they must be cautiously administered in patients with severe renal
or hepatic impairment (24).
SGLT2Is have been combined with other agents
involved in decreasing weight gain (e.g. GLP-1RAs), which possess
different mechanisms of action, to increase the probability of
weight loss (22). These
associations are needed, as SGLT2I administration may trigger
compensatory mechanisms, which negatively impact weight loss (e.g.
hyperphagia) (23). The clinical
and pharmacological properties of SGLT2Is are presented in Table I.
 | Table ICharacteristics of the SGLT2Is. |
Table I
Characteristics of the SGLT2Is.
| Pharmacological
agent | Pharmacological
properties | Clinical
particularities | Observations | (Refs.) |
|---|
| Empagliflozin | ↑Potency,
↑selectivity for SGLT2, ↑bioavailability (>86%), Tmax=1.5 h | Low risk of
hypoglycaemia, modest reductions of BW and BP, cardioprotective and
renoprotective properties | Monotherapy or
add-on to other ADM. Administered orally once daily | (25) |
| Canagliflozin | ↓Selectivity for
SGLT2 compared to other SGLT2Is, ↓bioavailability (65%), Tmax=1-2
h | Low risk of
hypoglycaemia. ↓Glycaemic levels in adults and older patients
+/-higher cardiovascular risk. Reduces cardiovascular risk and may
lead to renal benefits in patients with type 2 diabetes mellitus.
Adverse events: Genital/urinary tract infections, ↑urination | Administered orally
once daily. Monotherapy or add-on to other ADM | (26) |
| Dapagliflozin | ↑Potency,
reversible, selective agent, ↑bioavailability (78%), Tmax=1-1.5
h | Effective glycaemic
control, ↓BW, ↓BP, ↓the rate of cardiovascular death,
↓hospitalization due to heart failure. Low risk of
hypoglycaemia/diabetic ketoacidosis. ↑Risk of genital
infections | Administered orally
once daily. Monotherapy or add-on to other ADM | (27) |
| Ertugliflozin | ↑selectivity for
SGLT2, ↑bioavailability(~100%), Tmax=1 h | ↓HbA1c, ↓fasting
plasma glucose, ↓BW, ↓BP. Good tolerability in clinical trials | Administered orally
once daily. Monotherapy or add-on to other ADM | (28) |
Empagliflozin is a potent, highly selective SGLT2I
that is administered orally once daily, with a low risk of
hypoglycaemia, and can be administered either as monotherapy or as
an add-on to other antidiabetic agents (25). Empagliflozin induces modest
reductions in body weight and blood pressure, and it has
cardioprotective and renoprotective properties independent of its
glycaemic control effects (25).
Canagliflozin is administered orally once daily in
the treatment of type 2 diabetes, and it can reduce glycaemic
levels in adults, including those of older age and/or presenting
with higher cardiovascular risk (26). Canagliflozin reduces cardiovascular
risk and may be associated with renal benefits in patients with
type 2 diabetes (26). The overall
tolerability of this agent was good in clinical trials, with a low
risk of hypoglycaemia and the most frequently reported adverse
effects being genital/urinary tract infections and increased
urination (26).
Dapagliflozin is a highly potent, reversible and
selective SGLT2I administered orally once daily, either as
monotherapy or add-on to other antihyperglycemic medications for
patients with type 2 diabetes (27). This agent produced effective
glycaemic control and reduced body weight and blood pressure, while
also decreasing the rate of cardiovascular death or hospitalization
for heart failure (27). The
tolerability was good in clinical trials, with a low risk of
hypoglycaemia, diabetic ketoacidosis and genital infections, which
were more common with dapagliflozin than with the placebo (27).
Ertugliflozin significantly reduced HbA1c, fasting
plasma glucose, body weight and blood pressure levels compared with
a placebo and other hypoglycaemic agents (28). No significant difference in the
rate of adverse events, serious adverse events, deaths or
discontinuations due to adverse events was recorded between the
active drug and placebo (28).
SGLT2Is reduced weight and waist circumference in
overweight or obese patients without diabetes compared with those
in the control group (other drugs or placebo), according to a
systematic review (13 studies) (29). The mean body weight loss due to
SGLT2Is in this population was -1.62 kg compared with that in the
placebo group, and the BMI decreased by -0.47 kg/m2,
which was superior to the placebo, according to a meta-analysis (5
clinical trials) (30). The mean
reduction of the waist circumference due to SGLT2Is vs. placebo was
1.29 cm, which was not statistically significant (30).
2. Objectives and methods
The main objective of this review was to investigate
the evidence that may support SGLT2Is as a potentially useful
intervention in patients diagnosed with severe mental illnesses who
are undergoing therapy with atypical antipsychotics and present
with metabolic dysfunctions.
A secondary objective was to find the most important
aspects that need to be addressed by future research in the field
of therapeutic management in patients treated with atypical
antipsychotics and comorbid metabolic dysfunctions.
A narrative literature review was initiated to
comply with the two aforementioned objectives. The major electronic
databases (PubMed, https://pubmed.ncbi.nlm.nih.gov/; Cochrane, https://www.cochrane.org/; CINAHL, https://www.ebsco.com/; EMBASE, https://www.embase.com/; and Clarivate/Web of Science,
https://www.webofscience.com/) were
searched using the words ‘sodium-glucose co-transporter 2
inhibitors’ AND ‘atypical antipsychotics’ OR ‘second-generation
antipsychotics’ AND ‘obesity’ OR ‘diabetes mellitus’ OR ‘metabolic
syndrome’. All studies published between January 2000 and June 2022
were included in the primary analysis. Clinical trials,
irrespective of their methodology (open-label, randomized,
controlled, single-blind and double-blind), case reports and case
series, preclinical studies, systematic reviews and meta-analyses,
clinical guidelines, good practices and expert consensus
recommendations were included. Studies with imprecise methodology
(e.g., unspecified duration of the intervention, undefined setting
and unstructured methods of outcome measurement) were excluded from
the analysis. In addition, sources that did not explore the
efficacy and/or tolerability of the SGLT2Is (either as monotherapy
or add-on) were excluded. Reports on patients without psychiatric
disorders or on subjects without metabolic syndrome possibly
related to the administration of antipsychotic agents were not
included in the final stage of the review.
3. Results
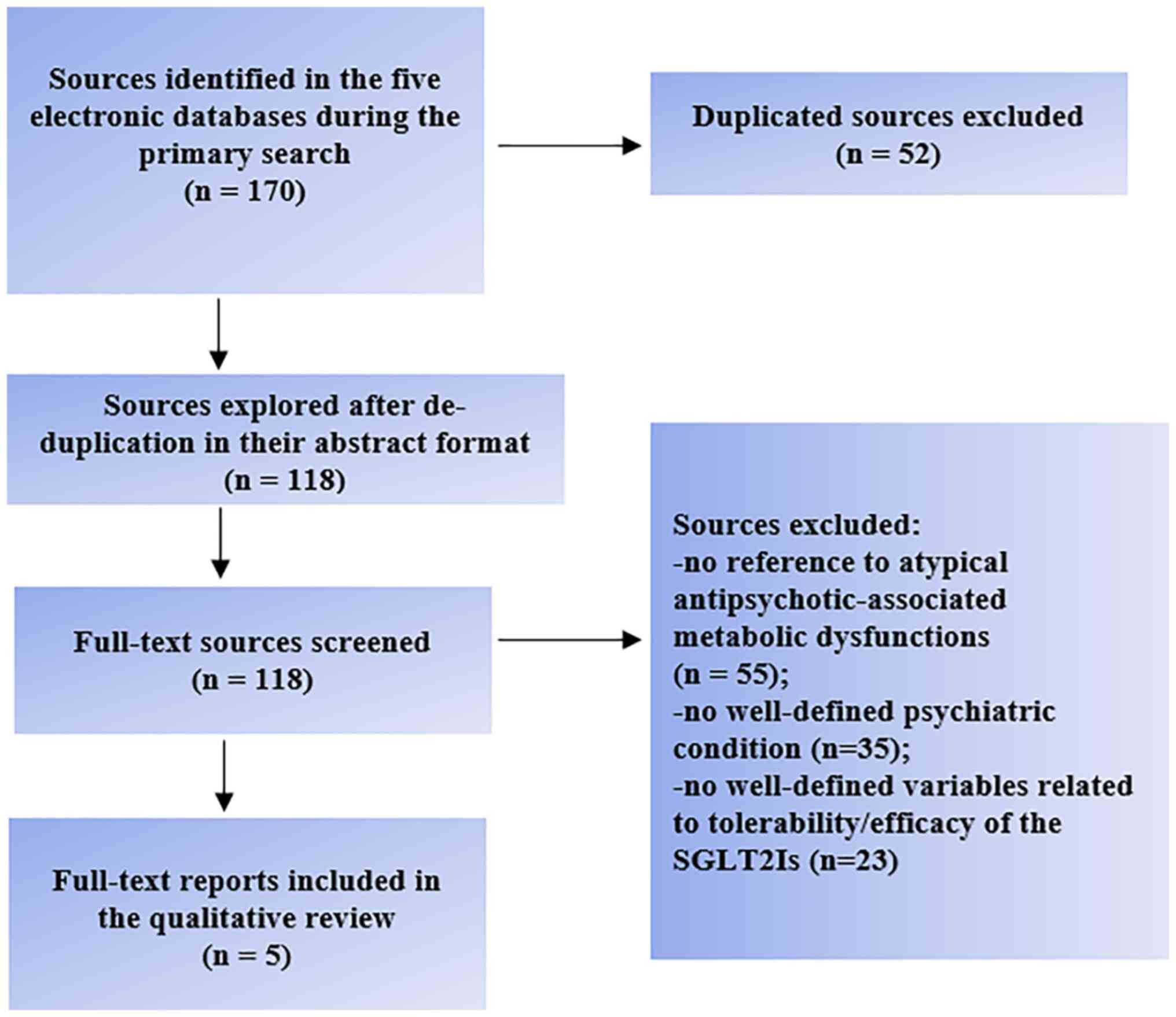
Out of the 170 references initially found, only five
corresponded to the main objective of the present review (Fig. 2). A total of one preclinical trial,
two guideline-format clinical recommendations, one systematic
review and one case report were found, and their conclusions are
reported in this section (Table
II). The other 165 papers did not refer to psychiatric
conditions or atypical antipsychotic-associated metabolic
dysfunctions (n=90), were duplicates (n=52) or did not report on
well-defined variables regarding the tolerability and/or efficacy
of the SGLT2Is (n=23).
 | Table IIMain results of the reviewed
studies. |
Table II
Main results of the reviewed
studies.
| First author,
year | Methodology | Results | Observations | (Refs.) |
|---|
| Ashraf et
al, 2021 | Wistar rats, the
empagliflozin effect on olanzapine-induced body weight increase was
observed | SGLT2I reduced the
weight gain determined by olanzapine | This preclinical
study supports the SGLT2I efficacy in mitigating the effects of
olanzapine on weight gain | (31) |
| Cooper et
al, 2016 | Therapeutic
guidelines for the management of metabolic disturbances | Metformin + an
SGLT-2 inhibitor may be appropriate as an intensification approach
if HbA1c remains ≥6.5% after 3-6 months | SGLT2Is are
recommended as an add-on to metformin in selected cases of type 2
diabetes mellitus | (32) |
| Lally et al,
2018 | Expert consensus
for the treatment of patients with severe mental disorders and type
2 diabetes | SGLT2Is may be used
in combination with metformin if HbA1c target values cannot be
obtained with metformin monotherapy after 3 months | SGLT2Is, GLP1RAs
and dipeptidyl-peptidase-4 inhibitors are second-line therapy for
these dually diagnosed patients | (33) |
| Cernea et
al, 2020 | A systematic review
(14 randomized controlled trials) | SGLT2Is and GLP1RAs
are second-line therapy, and they may be used as add-ons in
patients with diabetes and comorbid conditions | The support for
SGLT2Is is still not very solid | (1) |
| Barbosa and
Fernandes, 2021 | Case report,
45-year-old overweight patient, treated with clozapine | Metformin was
initiated, but insulin, exenatide and empagliflozin were also
added. Clozapine was discontinued | SGLT2Is may be
added for the control of glycaemic dysfunction induced by
clozapine | (37) |
Preclinical studies
In the reported preclinical study, researchers
evaluated the effects of empagliflozin on body weight gain induced
by olanzapine administration in female and male Wistar rats
(31). Olanzapine induced a
sustained increase in body weight in this population, while the
subsequent treatment with empagliflozin attenuated the
antipsychotic-induced weight gain only in female rats (31).
Clinical guidelines and expert
consensus
British Association of Psychopharmacology guidelines
on the management of weight gain, metabolic disturbances and
cardiovascular risk associated with psychosis and antipsychotic
drug treatment, published in 2016, formulates the following
therapeutic algorithm for patients with type 2 diabetes: i)
First-stage intervention includes educational, lifestyle and
dietary measures; ii) if HbA1c remains >6.5% after 3-6 months
drug therapy should be offered; iii) metformin should be the
first-line medication, but if it is not tolerated or it is
contraindicated, then a dipeptidyl-peptidase-4 (DPP-4) inhibitor,
or pioglitazone, or a sulfonylurea treatment will be initiated; and
iv) the first intensification of drug treatment includes
combinations of DPP-4 inhibitor + metformin, metformin +
pioglitazone or metformin + sulfonylurea, but metformin + an
SGLT-2I may be appropriate (32).
It may be observed that SGLT-2Is are recommended in selected cases
of type 2 diabetes in the context of antipsychotic treatment, but
only as an add-on to metformin (32).
Based on an expert consensus, in the management of
hyperglycaemia in patients diagnosed with severe mental disorders
and type 2 diabetes, SGLT2Is, as well as GLP-1RAs,
dipeptidyl-peptidase-4 inhibitors (DPP-4Is), pioglitazone, insulin
and sulfonylureas, may be used in combination with metformin if the
HbA1c target value cannot be reached after 3 months of monotherapy
with metformin, administered at the maximum tolerated doses
(33). Agents with a low risk of
hypoglycaemia and with weight loss properties, such as SGLT2Is,
GLP-1RAs and DPP-4Is, are the preferred options for second-line
therapy in patients with severe mental disorders (33).
Data from systematic reviews
A systematic review (14 randomized controlled trials
with patients treated with second-generation antipsychotics) found
metformin to be the most efficient first-line therapy for
individuals with diabetes mellitus and co-morbid conditions, while
the GLP-1RAs and SGLT-2 inhibitors were supported by evidence as
add-on therapies (1). Both
GLP-1RAs and SGLT2Is favourably influenced glucose metabolism and
BMI in general in these patients, but the support for the use of
SGLT2Is is not yet very solid (1).
Using these new-generation antidiabetics offers additional benefits
such as weight control, cardiovascular and renal protection, and a
low risk of hypoglycaemia (1).
Metformin is also an option for patients with
prediabetes, especially if additional conditions, such as obesity,
exist (1). The mechanisms by which
metformin improves antipsychotic-induced metabolic dysfunctions
remain unclear, but preclinical studies support the hypothesis of
attenuation of hepatic insulin resistance during olanzapine
administration (1,34). Moreover, the anorectic effects of
metformin could mitigate the increased appetite induced by
antipsychotics (1,35). Kidney function monitoring is
indicated during metformin administration, as it can be safely used
only if the glomerular filtration rate is >30 ml/min/1.73
m2 (1,36).
The same systematic review showed that the prognosis
of lifestyle-based strategies for treating diabetes or prediabetes
in this population was modest (1).
Also, if prediabetes or diabetes appears, switching from the
current antipsychotic to another with an improved metabolic
profile, is a very useful strategy, but the risk of worsening
psychiatric outcomes should be anticipated and monitored carefully
(1).
Case reports
In a 45-year-old overweight female patient diagnosed
with Parkinson's disease who received clozapine for refractory
dyskinesia and a history of gestational diabetes, the acute onset
of a glycaemic dysfunction (blood glucose, 505 mg/dl; reference
range, <99 mg/dl; HbA1c, 12.4%; reference range, <5.7%) was
observed (37). Metformin was
started and clozapine was discontinued, but the glycemia could not
be controlled until insulin was added, together with exenatide and
empagliflozin (37).
4. Conclusions and clinical
observations
Atypical antipsychotic-associated weight gain and
metabolic disorders are clinical challenges that may reduce
therapeutic adherence, health-related quality of life and life
expectancy in patients with severe mental illnesses (38,39).
Early interventions focused on nutritional counselling, an increase
in physical exercise and the use of adequate antipsychotic
treatment have been suggested for patients with schizophrenia
spectrum disorders, starting from the first episode of psychosis to
chronic forms of disorders (4).
Changing the currently administered antipsychotic to a
metabolically less harmful agent has also been suggested as a
measure to improve the prognosis of patients with both severe
mental disorders and diabetes mellitus, obesity or dyslipidaemia
(39,40). Adding metformin has also been
associated with favourable results on the metabolic parameters in
this population, and other add-on therapeutic agents, such as
amantadine, topiramate and orlistat, have been explored, with
various results (39,40). However, an optimum strategy is
still missing, as all the previously listed options are associated
with significant risks, from the possibility of psychotic symptoms
worsening in the case of antipsychotic switching, to adding new
adverse events in the case of using other pharmacological agents
for controlling metabolic dysfunctions.
In this context, newer antidiabetics, such as
SGLT2Is, may represent a therapeutic option in patients undergoing
antipsychotic treatment, and their favourable effects on blood
pressure and serum lipids (41)
may be useful in these patients. These newer agents are not without
adverse effects, and more trials are required to determine their
effect on macrovascular outcomes (41). Based on the current literature
review, it can be concluded that SGLT2Is may be added to metformin
in selected cases of type 2 diabetes mellitus in the context of
antipsychotic treatment, as suggested by a clinical guideline and
an expert consensus; a case report and a systematic review also
support this strategy (1,32,33,37).
Evidence to support the administration of SGLT2Is as second-line
treatment in patients with diabetes mellitus who are also treated
with olanzapine or clozapine is derived from very limited
preclinical (31) and clinical
data (37). The advantages of
SGLTI2s, i.e., the low risk of hypoglycaemia and the associated
weight loss properties, may recommend these agents, along with
GLP-1RAs and DPP-4Is, as preferred options for second-line therapy
in patients with severe mental disorders (33).
Regarding the secondary objective of this review,
further domains of interest for the clinical research that may help
improve the health and related functional outcomes in patients with
severe mental illnesses and metabolic pathology are as follows: i)
The identification of prognosis factors that may predict a
favourable response to SGLT2I add-on in patients with a dual
diagnosis (severe mental disorders and metabolic dysfunctions)
undergoing treatment with second-generation antipsychotics; ii)
randomized clinical trials to help find positive effects and
adverse events during long-term treatment with SGLT2Is; iii)
comparative studies with different new generation antidiabetic
agents (e.g. GLP1-RAs); iv) identification of possible interactions
between specific second-generation antipsychotics and SGLT2Is; and
v) identification of potential pharmacogenetic determinants of
SGLT2Is responsiveness.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
OV is responsible for the collection, processing and
presentation of the data within the review. OV has read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and informed consent
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that they have no competing
interests.
References
|
1
|
Cernea S, Dima L, Correll CU and Manu P:
Pharmacological management of glucose dysregulation in patients
treated with second-generation antipsychotics. Drugs. 80:1763–1781.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Holt RIG: Association between
antipsychotic medication use and diabetes. Curr Diab Rep.
19(96)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pillinger T, Beck K, Gobjila C, Donocik
JG, Jauhar S and Howes OD: Impaired glucose homeostasis in
first-episode schizophrenia: A systematic review and meta-analysis.
JAMA Psychiatry. 74:261–269. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pillinger T, Beck K, Stubbs B and Howes
OD: Cholesterol and triglyceride levels in first-episode psychosis:
Systematic review and meta-analysis. Br J Psychiatry. 211:339–349.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Teasdale SB, Ward PB, Jarman R, Wade T,
Rossimel E, Curtis J, Lappin J, Watkins A and Samaras K: Is obesity
in young people with psychosis a foregone conclusion? Markedly
excessive energy intake is evident soon after antipsychotic
initiation. Front Psychiatry. 9(725)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rummel-Kluge C, Komossa K, Schwarz S,
Hunger H, Schmid F, Lobos CA, Kissling W, Davis JM and Leucht S:
Head-to-head comparison of metabolic side effects of second
generation antipsychotics in the treatment of schizophrenia: A
systematic review and meta-analysis. Schizophr Res. 123:225–233.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dipasquale S, Pariante CM, Dazzan P,
Aguglia E, McGuire P and Mondelli V: The dietary pattern of
patients with schizophrenia: A systematic review. J Psychiatr Res.
47:197–207. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Polcwiartek C, Kragholm K, Rohde C,
Hashemi N, Vang T and Nielsen J: Diabetic ketoacidosis and diabetes
associated with antipsychotic exposure among a previously
diabetes-naive population with schizophrenia: A nationwide nested
case-control study. Diabetologia. 60:1678–1690. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Leslie DL and Rosenheck RA: Incidence of
newly diagnosed diabetes attributable to atypical antipsychotic
medications. Am J Psychiatry. 161:1709–1711. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Miron IC, Baroana VC, Popescu F and Ionica
F: Pharmacological mechanisms underlying the association of
antipsychotics with metabolic disorders. Curr Health Sci J.
40:12–17. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Silvestre JS and Prous J: Research on
adverse drug events. I. Muscarinic M3 receptor binding affinity
could predict the risk of antipsychotics to induce type 2 diabetes.
Methods Find Exp Clin Pharmacol. 27:289–304. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Weston-Green K, Huang XF and Deng C:
Second generation antipsychotic-induced type 2 diabetes: A role for
the muscarinic M3 receptor. CNS Drugs. 27:1069–1080.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang JP, Lencz T, Zhang RX, Nitta M,
Maayan L, John M, Robinson DG, Fleischhacker WW, Kahn RS, Ophoff
RA, et al: Pharmacogenetic associations of antipsychotic
drug-related weight gain: A systematic review and meta-analysis.
Schizophr Bull. 42:1418–1437. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Goh KK, Chen CY, Wu TH, Chen CH and Lu ML:
Crosstalk between schizophrenia and metabolic syndrome: The role of
oxytocinergic dysfunction. Int J Mol Sci. 23(7092)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cheng SC, Goh KK and Lu ML: Metabolic
disturbances associated with antipsychotic drug treatment in
patients with schizophrenia: State-of-the-art and future
perspectives. World J Psychiatry. 11:696–710. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
del Campo A, Bustos C, Mascayano C,
Acuña-Castillo C, Troncoso R and Rojo LE: Metabolic syndrome and
antipsychotics: The role of mitochondrial fission/fusion imbalance.
Front Endocrinol (Lausanne). 9(144)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Vasiliu O: Case report: Cariprazine
efficacy in young patients diagnosed with schizophrenia with
predominantly negative symptoms. Front Psychiatry.
12(786171)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mailman RB and Murthy V: Third generation
antipsychotic drugs: Partial agonism or receptor functional
selectivity? Curr Pharm Des. 16:488–501. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vasiliu O, Vasile D and Voicu V: Efficacy
and tolerability of antibiotic augmentation in schizophrenia
spectrum disorders-A systematic literature review. Rom J Military
Med. CXXIII:3–20. 2020.
|
|
20
|
Vasiliu O and Vasile D: Risk factors and
quality of life in late-life depressive disorders. Rom J Military
Med. CXIX:24–28. 2016.
|
|
21
|
Brown E, Wilding JPH, Barber TM, Alam U
and Cuthbertson DJ: Weight loss variability with SGLT2 inhibitors
and GLP-1 receptor agonists in type 2 diabetes mellitus and
obesity: Mechanistic possibilities. Obes Rev. 20:816–828.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pereira MJ and Eriksson JW: Emerging role
of SGLT-2 inhibitors for the treatment of obesity. Drugs.
79:219–230. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee PC, Ganguly S and Goh SY: Weight loss
associated with sodium-glucose cotransporter-2 inhibition: A review
of evidence and underlying mechanisms. Obes Rev. 19:1630–1641.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ribola FA, Cançado FB, Schouveri JH, De
Toni VF, Medeiros VH and Feder D: Effects of SGLT2 inhibitors on
weight loss in patients with type 2 diabetes mellitus. Eur Rev Med
Pharmacol Sci. 21:199–211. 2017.PubMed/NCBI
|
|
25
|
Frampton JE: Empagliflozin: A review in
type 2 diabetes. Drugs. 78:1037–1048. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Deeks ED and Scheen AJ: Canagliflozin: A
review in type 2 diabetes. Drugs. 77:1577–1592. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dhillon S: Dapagliflozin: A review in type
2 diabetes. Drugs. 79:1135–1146. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu L, Shi FH, Xu H, Wu Y, Gu ZC and Lin
HW: Efficacy and safety or ertugliflozin in type 2 diabetes: A
systematic review and meta-analysis. Front Pharmacol.
12(752440)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Shi Y, Si Y, Fu R, Zhang M, Jiang K, Dai
W, Shen J, Li X and Yuan Y: Efficacy and safety of SGLT-2i in
overweight/obese, non-diabetic individuals: A meta-analysis of
randomized controlled trials. Endokrynol Pol. 73:71–80.
2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cho YK, Kim YJ and Jung CH: Effect of
sodium-glucose cotransporter 2 inhibitors on weight reduction in
overweight and obese populations without diabetes: A systematic
review and a meta-analysis. J Obes Metab Syndr. 30:336–344.
2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ashraf GM, Alghamdi BS, Alshehri FS, Alam
MZ, Tayeb HO and Tarazi FI: Empagliflozin effectively attenuates
olanzapine-induced body weight gain in female Wistar rats. Front
Pharmacol. 12(578716)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cooper SJ and Reynolds GP: With expert
co-authors (in alphabetical order). Barnes T, England E, Haddad PM,
Heald A, Holt R, Lingford-Hughes A, Osborn D, et al: BAP guidelines
on the management of weight gain, metabolic disturbances and
cardiovascular risk associated with psychosis and antipsychotic
drug treatment. J Psychopharmacol. 30:717–748. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lally J, O'Loughlin A, Stubbs B, Guerandal
A, O'Shea D and Gaughran F: Pharmacological management of diabetes
in severe mental illness: A comprehensive clinical review of
efficacy, safety, and tolerability. Expert Rev Clin Pharmacol.
11:411–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Remington GJ, Teo C, Wilson V, Chintoh A,
Guenette M, Ahsan Z, Giacca A and Hahn MK: Metformin attenuates
olanzapine-induced hepatic, but not peripheral insulin resistance.
J Endocrinol. 227:71–81. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Luo C, Wang X, Huang H, Mao X, Zhou H and
Liu Z: Effect of metformin on antipsychotic-induced metabolic
dysfunction: The potential role of gut-brain axis. Front Pharmacol.
10(371)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hsu WH, Hsiao PJ, Lin PC, Chen SC, Lee MY
and Shin SJ: Effect of metformin on kidney function in patients
with type 2 diabetes mellitus and moderate chronic kidney disease.
Oncotarget. 9:5416–5423. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Barbosa M and Fernandes V: Rapid-onset
clozapine-induced hyperglycemia: Pathways of glycaemic
dysregulation. BMJ Case Rep. 14(e243938)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Foldemo A, Wӓrdig R, Bachrach-Lindström M,
Edman G, Holmberg T, Lindström T, Valter L and Osby U:
Health-related quality of life and metabolic risk in patients with
psychosis. Schizophr Res. 152:295–299. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Agarwal SM and Stogios M: Cardiovascular
health in severe mental illness: Potential role for metformin. J
Clin Psychiatry. 83(22ac14419)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lao X, Ye H and Si T: A review of
switching strategies for patients with schizophrenia comorbid with
metabolic syndrome or metabolic abnormalities. Neuropsychiatr Dis
Treat. 17:453–469. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Halimi S and Vergès B: Adverse effects and
safety of SGLT-2 inhibitors. Diabetes Metab. 40 (Suppl.1):S28–S34.
2014.PubMed/NCBI View Article : Google Scholar
|