Introduction
The inflammasome is formed by cytosolic
multi-protein complexes that consist of pattern recognition
receptors (PRRs), including nucleotide-binding oligomerization
domain-like receptors (NLRs) or absent in melanoma 2 (AIM2),
adaptor protein apoptosis-associated speck-like protein containing
a caspase-recruitment domain (CARD; ASC) and effector protein
procaspase-1(1). Activation of the
inflammasome by specific stimuli, such as pathogen-associated
molecular patterns (PAMPs) or danger-associated molecular patterns
(DAMPs), induces ASC oligomerization and caspase-1 cleavage. Active
caspase-1 proteolytically processes pro-IL-1β and pro-IL-18 into
IL-1β and IL-18, respectively. Inflammasome-mediated caspase-1
activity also induces a specific form of inflammatory cell death
known as pyroptosis (2). These
activations serve important roles in the immune defense against
pathogens, whereas excessive production of IL-1β causes
inflammatory diseases (3).
The NLR family pyrin domain containing 3 (NLRP3)
inflammasome has been extensively studied and is known to mediate
the inflammatory response (4-9).
This inflammasome is activated by pathogen-derived ligands, such as
components of bacterial cell walls, pore-forming toxins and DAMPs,
including uric acid crystals, ATP and β-amyloid (4,10).
Two steps, namely signal 1 and signal 2, control NLRP3 inflammasome
activation. Signal 1 is a priming step that leads to gene
expression of NLRP3 and pro-IL-1β. Molecular sensing by PRRs
activates signaling cascades, such as the NF-κB signaling pathway,
which is responsible for priming (2). Signal 2 is an activation step
triggered by PAMPs and DAMPs, which results in formation of the
NLRP3 inflammasome assembly, caspase-1-mediated IL-1β cleavage and
pyroptosis (2). Excessive and
persistent activation of the NLRP3 inflammasome results in
inflammatory and metabolic disease, as well as neurological
disorders, including inflammatory bowel disease, rheumatoid
arthritis, type 2 diabetes and Alzheimer's disease (4-9).
Therefore, regulation of the NLRP3 inflammasome may be a new
therapeutic strategy for the treatment of metabolic disorders, and
novel compounds to regulate the activity of the NLRP3 inflammasome
are being actively explored at present (11,12).
Chrysanthemum zawadskii (C.
zawadskii), also known as ‘Gu-jeol-cho’ in Korea, is a
perennial plant belonging to the genus Chrysanthemum in the
family Asteraceae. This plant has been used in traditional Chinese
medicine for the treatment of various diseases, such as bone
disease (13,14) and lung injury (15). Previous studies have suggested that
an ethanol extract of C. zawadskii (CZE) has pharmacological
properties, including antioxidant, anti-inflammatory and anticancer
effects (16-18).
Although CZE has potential pharmacological effects, the mechanism
of action underlying its biological effects remains unclear.
Therefore, the present study investigated the effect of CZE on
activation of the NLRP3 inflammasome in macrophages and the
underlying mechanism.
Materials and methods
Plant extraction
The dried aerial parts of C. zawadskii were
purchased from Canaanherb and used for extractions. The water
extract (CZW) was prepared by boiling the dried plant (150 g) in 1
liter of sterilized water at 90˚C for 4 h, while CZE was prepared
by refluxing C. zawadskii (50 g) with 1 liter ethanol at
room temperature for 24 h. These extracts were filtered, evaporated
in a rotary vacuum evaporator and lyophilized with a freeze-dryer.
The powder extracts were dissolved in PBS or DMSO and diluted to
30-300 µg/ml (CZW) or 25-100 µg/ml (CZE) using Iscove's Modified
Dulbecco's medium (IMDM; Gibco; Thermo Fisher Scientific,
Inc.).
Animals
A total of 20 8-week-old male wild-type C57BL/6 mice
weighing 20-25 g were purchased from Jackson Laboratory. Mice were
housed in standard plastic cages in controlled conditions at 23±2˚C
with a humidity of 55±10% under a 12/12-h light/dark cycle, with
ad libitum access to food and water. For tissue collection,
mice were anesthetized with 3% isoflurane (for induction) and
euthanized by cervical dislocation. Death was pronounced by
ascertaining cardiac and respiratory arrest. All animal studies
were performed using protocols approved by the Institutional Animal
Care and Use Committee of Chonnam National University (Gwangju,
Korea; approval no. CNU IACUC-YB-2018-02).
Reagents and bacteria culture
Lipopolysaccharide (LPS) from Escherichia
coli O111:B4 was purchased from InvivoGen (cat. no.
tlrl-eblps). ATP (cat. no. A2383), nigericin (cat. no. N7143) and
poly(dA:dT; cat. no. P0883) were purchased from Sigma-Aldrich
(Merck KGaA). Monosodium urate (MSU) crystals (cat. no. tlrl-msu)
were purchased from InvivoGen. Salmonella enterica serovar
typhimurium (S. typhimurium) was cultured on
Luria-Bertani (LB) agar or broth (both BD Biosciences) at 37˚C. To
prime the inflammasome, LPS was used to treat bone marrow-derived
macrophages (BMDMs) in a 5% CO2 incubator at 37˚C for 6
h. To activate the NLRP3 inflammasome, LPS-primed BMDMs were
treated with ATP (2 mM), or nigericin (10 µM) for 40 min or MSU
(200 µg/ml) for 4 h in a 5% CO2 incubator at 37˚C. For
AIM2 inflammasome activation, LPS-primed BMDMs were treated with
poly(dA:dT) (2 µg/ml) in a 5% CO2 incubator at 37˚C for
4 h. To activate the NLR family CARD domain-containing protein 4
(NLRC4) inflammasome, S. typhimurium was grown on LB agar at
37˚C for 24 h to obtain single colonies, which were inoculated into
LB broth and cultured at 37˚C with shaking for 16 h. A 1:10
dilution of culture suspension was grown in fresh medium at 37˚C
with shaking for an additional 2 h. The bacteria were concentrated
to 1x109 colony-forming units/ml in PBS and diluted to
1x107 colony-forming units/ml in cell culture media
(IMDM, Gibco; Thermo Fisher Scientific, Inc.). LPS-primed BMDMs
were infected with S. typhimurium (multiplicity of
infection=10) in the presence or absence of CZE in a 5%
CO2 incubator at 37˚C for 1 h, and the medium was
replaced with media containing gentamicin and incubated at 37˚C for
3 h. As major components of CZE, linarin (Merck KGaA; cat. no.
PHL80822) and chlorogenic acid (Merck KGaA; cat. no. PHR2202) were
purchased from Sigma-Aldrich, while 3,5-di-caffeoylquinic acid
(3,5-di-CQA; cat. no. ALX-350-320-M001) was purchased from Enzo
Life Sciences, Inc.
Cell culture
BMDMs were isolated from mice and differentiated as
previously described (19).
Briefly, BMDMs were incubated in IMDM (Gibco; Thermo Fisher
Scientific, Inc.) containing 30% L929 cell culture supernatant, 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.), 1%
MEM non-essential amino acids (Gibco; Thermo Fisher Scientific,
Inc.), 1% sodium pyruvate (Gibco; Thermo Fisher Scientific, Inc.)
and 10% fetal bovine serum (Costar; Corning, Inc.) in a 5%
CO2 incubator at 37˚C for 6 days. Fresh medium was added
3 days later, and the cells were cultured for 3 days. Next, cells
were seeded into 48- or 6-well plates at a density of
2x106 cells/ml and incubated in a 5% CO2
incubator at 37˚C.
Measurement of cytokines
The concentrations of IL-1β (Mouse IL-1β/IL-1F2
DuoSet ELISA kit; cat. no. DY401), IL-6 (Mouse IL-6 DuoSet ELISA
kit; cat. no. DY406) and TNF-α (Mouse TNF-α DuoSet ELISA kit; cat.
no. DY410) in the culture supernatants were determined using
commercial ELISA kits (all R&D Systems, Inc.) according to the
manufacturer's instructions.
Measurement of lactate dehydrogenase
(LDH)
The level of LDH in culture supernatants was
determined using a commercial kit (CytoTox 96®
Non-Radioactive Cytotoxicity Assay; cat. no. G1780; Promega
Corporation) according to the manufacturer's instructions.
Western blotting
For detection of the pro- and cleaved forms of IL-1β
and caspase-1, LPS-primed BMDMs were treated with ATP (2 mM) in the
presence or absence of CZE in a 5% CO2 incubator at 37˚C
for 40 min. The attached cells and culture supernatant were lysed
with 1% Triton-X 100 solution (Sigma-Aldrich; Merck KGaA)
containing cOmplete™ Protease Inhibitor Cocktail (Roche
Applied Science). The total protein concentration of cell lysates
was measured using the Bradford protein assay kit II (cat no.
5000002; Bio-Rad Laboratories, Inc.). The supernatant was mixed
with sample loading buffer (5X), separated by 15% SDS-PAGE with 20
µg protein loaded per lane and transferred to nitrocellulose (NC)
membranes.
To confirm the involvement of CZE in the priming
step of the NLRP3 inflammasome, BMDMs were stimulated with LPS (100
ng/ml) for 4 or 24 h in the presence or absence of CZE (100 µg/ml)
in a 5% CO2 incubator at 37˚C. BMDMs were lysed in lysis
buffer containing 1% NP-40, 50 mM Tris (pH 7.4), 5 mM EDTA, 250 mM
NaCl, 50 mM NaF, 0.02% NaN3 and 1 mM
Na3VO4 supplemented with phosphatase
inhibitors (Phosphatase Inhibitor Cocktail 2; Sigma-Aldrich; Merck
KGaA), protease inhibitors (Complete Mini EDTA-free Protease
Inhibitor; Roche Applied Science) and 2 mM dithiothreitol. The
total protein concentration of cell lysate was measured using the
Bio-Rad Protein Assay kit II (cat no. 5000002; Bio-Rad
Laboratories, Inc.). A standard curve was generated using bovine
serum albumin (BSA, cat no. 10735086001; Sigma-Aldrich; Merck KGaA)
in the 1-10 µg/µl range. The cell lysates were separated by 10%
SDS-PAGE with 20 µg protein/lane and transferred to NC membranes.
Following blocking for 2 h at room temperature in 5% skimmed milk,
all membranes were probed with primary antibodies against IL-1β
(cat. no. AF-401-NA; R&D Systems, Inc.), caspase-1 (cat no.
AG-20B-0042; Adipogen Life Sciences), IκB-α (cat no. 9242; Cell
Signaling Technology, Inc.), phosphorylated p65 (cat. no. 3031;
Cell Signaling Technology, Inc.), p65 (cat. no. 8242; Cell
Signaling Technology, Inc.), NLRP3 (cat. no. 15101; Cell Signaling
Technology, Inc.) and β-actin (cat. no. sc-47778; Santa Cruz
Biotechnology, Inc.) at 4˚C overnight. The primary antibodies were
diluted 1:1,000 in TBST (TBS containing 0.05% Tween-20). After
immunoblotting with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit (1:10,000 in 5% skim milk; cat. no. 31460; Invitrogen;
Thermo Fisher Scientific, Inc.) or anti-mouse IgG (H+L) secondary
antibody (1:10,000 in 5% skim milk; cat. no. 31430; Invitrogen;
Thermo Fisher Scientific, Inc.) at room temperature for 2 h, the
proteins were detected with Clarity Western ECL Substrate (Bio-Rad
Laboratories, Inc.). β-actin was used as the loading control.
Quantification of protein bands was performed using ImageJ Software
version 1.53t (National Institutes of Health).
ASC oligomerization assay
Following inflammasome activation in the absence or
presence of CZE, the cells were harvested, resuspended in cold
lysis buffer containing 0.2% Triton X-100 (cat no. T9284;
Sigma-Aldrich; Merck KGaA) and Complete Protease Inhibitor Cocktail
(Roche Applied Science) and passed 10 times through a 26-gauge
syringe. Following centrifugation at 330 x g for 10 min at 4˚C, the
supernatants (Triton-X-100 soluble fraction) were mixed with sample
loading buffer (5X). The remaining cell pellets were resuspended in
PBS containing 2 mM disuccinimidyl suberate cross-linker (cat. no.
S1885; Sigma-Aldrich; Merck KGaA) and incubated at room temperature
for 30 min, followed by centrifugation at 330 x g for 10 min at
4˚C. The total protein concentration of cell lysates was measured
using the Bradford protein assay kit II (cat no. 5000002; Bio-Rad
Laboratories, Inc.). A standard curve was generated using bovine
serum albumin (BSA, cat no. 10735086001; Sigma-Aldrich; Merck KGaA)
in the 1-10 µg/µl range. To detect ASC oligomerization,
cross-linked pellets (Triton-X-100-insoluble fraction) were
separated by 12% SDS-PAGE with 20 µg protein/lane and transferred
to NC membranes. The membranes were blocked with 5% skimmed milk at
room temperature for 2 h. Following this, membranes were incubated
with ASC antibody (cat. no. 67824S; Cell Signaling Technology,
Inc.) diluted 1:1,000 in TBST (TBS containing 0.05% Tween-20)
overnight at 4˚C. The Triton-X-100-soluble fraction was used to
detect the total form of ASC (1:1,000 in TBST; cat. no. 67824S;
Cell Signaling Technology, Inc.) and β-actin (1:1,000 in TBST; cat.
no. sc-47778; Santa Cruz Biotechnology, Inc.). After immunoblotting
with HRP-conjugated goat anti-rabbit (1:10,000 in 5% skimmed milk;
cat. no. 31460; Invitrogen; Thermo Fisher Scientific, Inc.) or
anti-mouse IgG (H+L) secondary antibodies (1:10,000 in 5% skim
milk; cat. no. 31430; Invitrogen; Thermo Fisher Scientific, Inc.)
at room temperature for 2 h, the proteins were detected using
Clarity Western ECL Substrate (Bio-Rad Laboratories, Inc.). β-actin
was used as the loading control.
ASC speck assay
For ASC speck assay, BMDMs were cultured in IMDM
(Gibco; Thermo Fisher Scientific, Inc.) containing 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) and
10% fetal bovine serum (Costar; Corning, Inc.) overnight in round
glass dishes in a 5% CO2 incubator at 37˚C. The cells
were stimulated with LPS (100 ng/ml) for 6 h and subsequently
treated with CZE (100 µg/ml) for 30 min. The cells were incubated
with ATP (2 mM) for 40 min, nigericin (10 µM) for 40 min and MSU
(200 µg/ml) for 4 h at 37˚C, then fixed with 2% formaldehyde
solution for 5 min at room temperature and permeabilized with 0.2%
Triton X-100, followed by staining with rabbit ASC antibody (1:100
in 2% BSA; cat. no. 67824S; Cell Signaling Technology, Inc.)
overnight at 4˚C. The cells were incubated with FITC-conjugated
anti-rabbit IgG (1:100 in 2% BSA; cat. no. F0382; Sigma-Aldrich;
Merck KGaA) at room temperature for 2 h and mounted on slides.
Nuclei were stained with DAPI (ProLong™ Gold Antifade
Mountant; Invitrogen; Thermo Fisher Scientific, Inc.) at 4˚C for 16
h. ASC speck images were acquired using a Leica TCS SP5/AOBS/Tandem
laser confocal scanning microscope (x200 magnification, Leica
Microsystems) at Gwangju Center of the Korea Basic Science
Institute.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA was extracted from LPS-treated BMDMs with or
without CZE pretreatment using the Easy-BLUE™ Total RNA
Extraction kit (Intron Biotechnology, Inc.) and cDNA was
synthesized using ReverTra Ace™ qPCR RT Master Mix
(Toyobo Life Science) according to the manufacturer's instructions.
qPCR was performed to detect gene expression of NLRP3 and pro-IL-1β
using the CFX Connect™ Real-time PCR Detection System
(Bio-Rad Laboratories, Inc.) and QGreen™ 2X SybrGreen
qPCR Master Mix (cat. no. QGHR-05; CellSafe). Thermocycling was
performed using a two-step protocol of 95˚C for 10 sec followed by
40 cycles at 58˚C for 45 sec. The primers used for qPCR were as
follows: Mouse NLRP3 forward, 5'-ATGGTATGCCAGGAGGACAG-3' and
reverse, 5'-ATGCTCCTTGACCAGTTGGA-3'; mouse IL-1β forward,
5'-GATCCACACTCTCCAGCTGCA-3' and reverse,
5'-CAACCAACAAGTGATATTCTCCATG-3' and mouse GAPDH forward,
5'-CGACTTCAACAGCAACTCCCACTCTTCC-3' and reverse,
5'-TGGGTGGTCCAGGGTTTCTTACTCCTT-3'. GAPDH was used as an internal
control. The method used to analyze the relative quantification of
mRNA expression was 2-∆∆Cq, where ∆∆Cq=(Cqtarget
gene-CqGAPDH)target
sample-(Cqtarget
gene-CqGAPDH)reference sample (20).
Statistical analysis
Data are presented as the mean ± SD and of three
independent experiments. The statistical significance of
differences between groups was evaluated using one-way ANOVA
followed by Tukey's post-hoc test or two-way ANOVA followed by
Bonferroni's post-hoc test. Statistical analysis was performed
using GraphPad Prism version 5.01 (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
CZE has a stronger inhibitory activity
than CZW on ATP-induced NLRP3 inflammasome activation in LPS-primed
macrophages
To investigate whether C. zawadskii extract
inhibits activation of the NLRP3 inflammasome, LPS-primed BMDMs
were treated with CZW or CZE for 30 min and stimulated with ATP to
activate the NLRP3 inflammasome. Treatment with ATP led to large
secretion of IL-1β from the LPS-primed BMDMs (Fig. 1A and D). Both CZW and CZE suppressed
ATP-induced IL-1β secretion in a dose-dependent manner (Fig. 1A and D) but did not affect production of IL-6
or TNF-α (Fig. 1B, C, E and
F). Since CZE was more effective
at lower concentrations than CZW, only CZE was selected for further
experiments. Next, the effect of CZE on cleavage of IL-1β and
caspase-1 to a mature form was examined. Treatment with ATP led to
notable cleavage of IL-1β and caspase-1 but this was suppressed by
CZE in a dose-dependent manner in LPS-primed BMDMs (Fig. 1G).
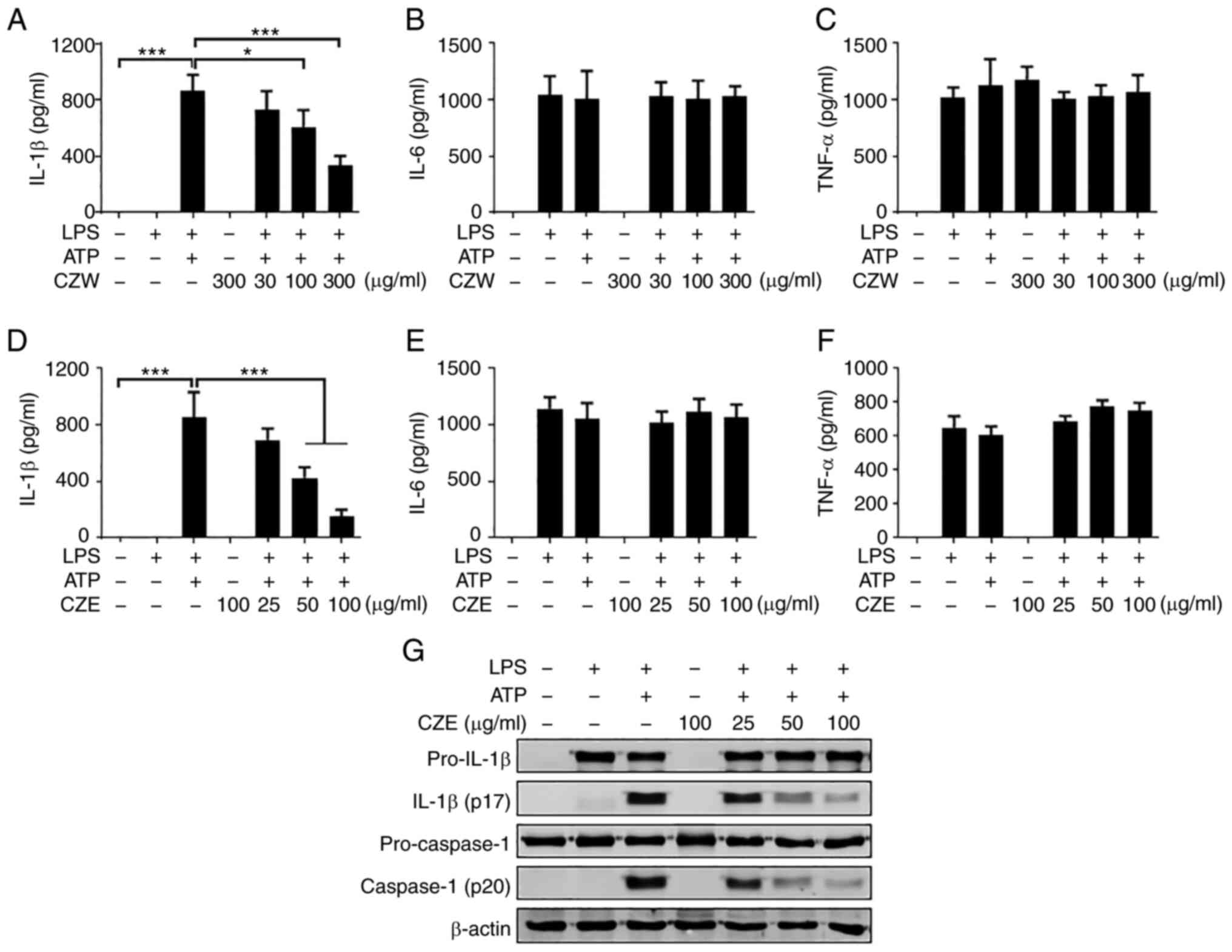 | Figure 1CZE inhibits ATP-induced IL-1β
secretion in LPS-primed BMDMs. BMDMs were primed with LPS (100
ng/ml) for 6 h and subsequently treated with ATP (2 mM) for 40 min
in the presence or absence of CZW for 30 min. Levels of (A) IL-1β,
(B) IL-6 and (C) TNF-α in culture supernatants were measured by
ELISA. Next, LPS-primed BMDMs were treated with ATP (2 mM) for 40
min, with or without CZE. The levels of (D) IL-1β, (E) IL-6 and (F)
TNF-α in culture supernatants were measured by ELISA. The results
are presented as the mean ± SD. *P<0.05,
***P<0.001. (G) Culture supernatant and cell lysates
were used to detect the immature and cleaved forms of IL-1β and
caspase-1 by western blotting. β-actin was used as a loading
control. LPS, lipopolysaccharide; BMDM, bone marrow-derived
macrophage; CZE, ethanol extract of Chrysanthemum zawadskii;
CZW, water extract of Chrysanthemum zawadskii; CASP1,
caspase-1. |
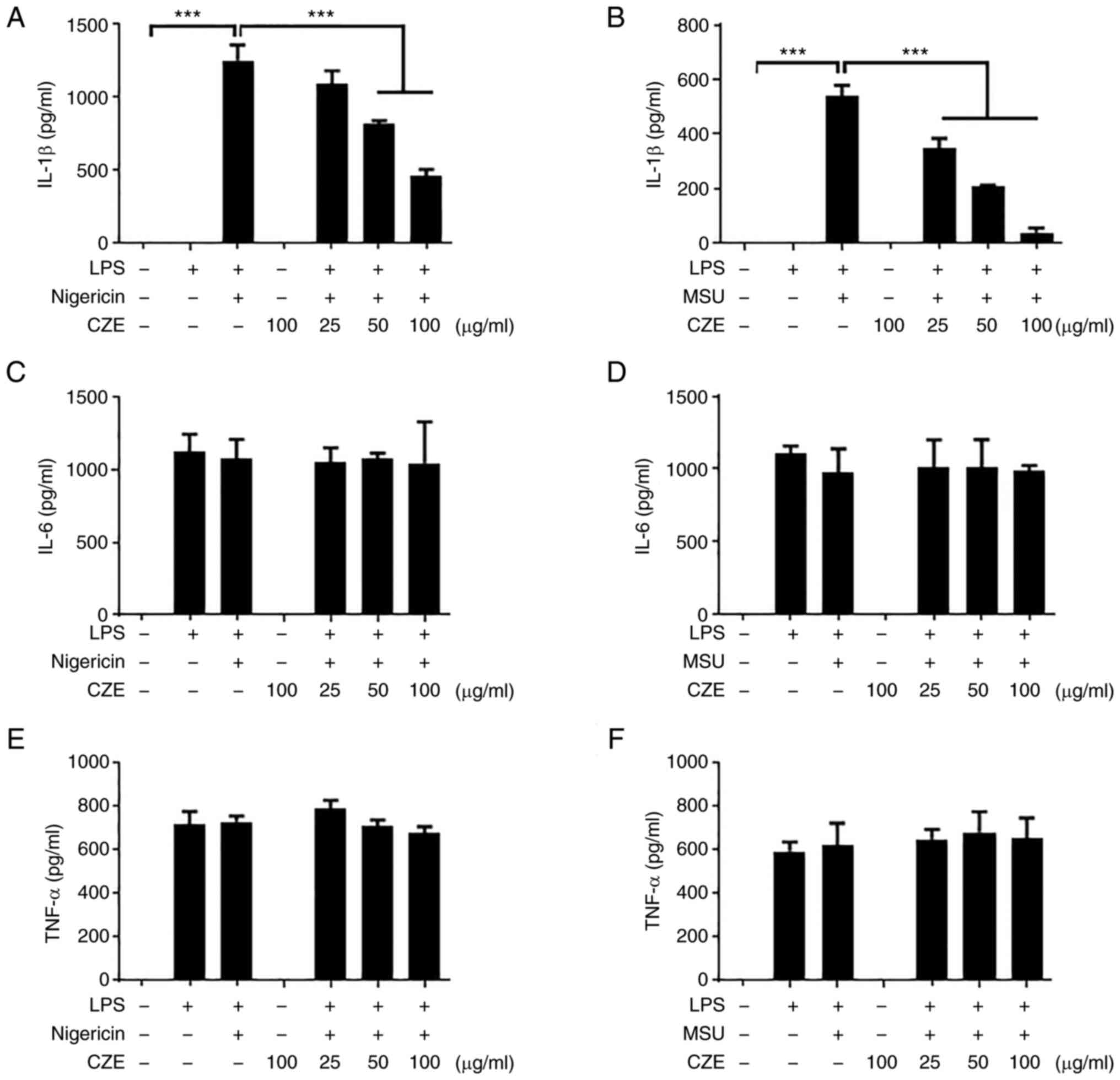
CZE inhibits IL-1β secretion in
response to nigericin and MSU
Various molecules, including nigericin, MSU crystals
and ATP, induce the activation of the NLRP3 inflammasome by
different mechanisms (21). The
present study investigated whether CZE suppressed IL-1β production
in macrophages in response to nigericin and MSU treatment. Both
nigericin and MSU induced IL-1β secretion in LPS-primed BMDMs
(Fig. 2A and B). The level of IL-1β was decreased by
CZE treatment in a dose-dependent manner (Fig. 2A and B). CZE did not affect the production of
IL-6 or TNF-α (Fig. 2C-F). Taken
together, CZE exerted a notable inhibitory effect on the activation
of the NLRP3 inflammasome.
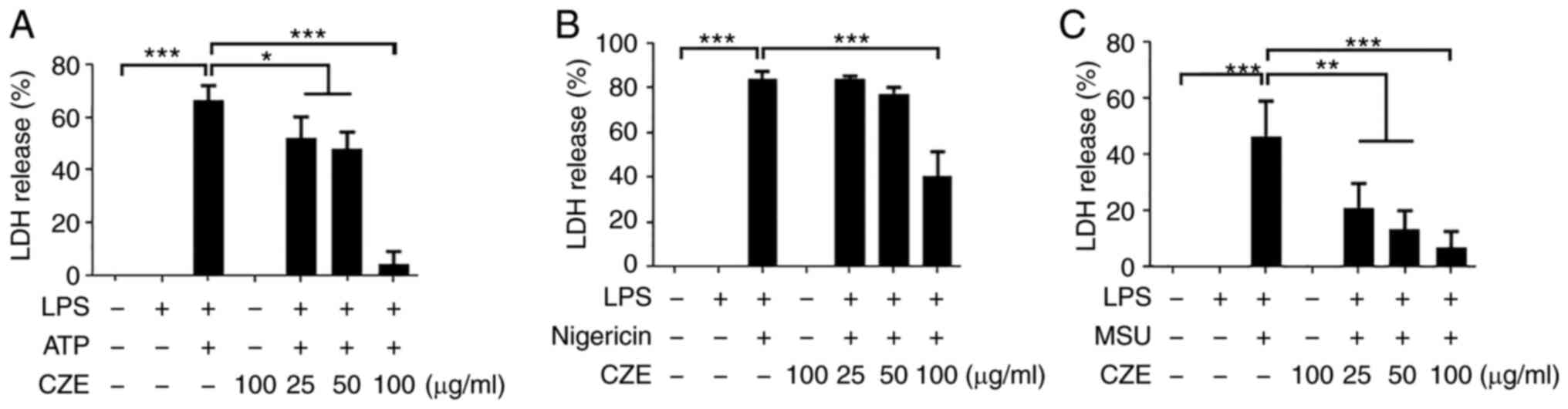
CZE inhibits pyroptosis in BMDMs
following activation of the NLRP3 inflammasome
Since activation of the NLRP3 inflammasome induces
caspase-1-dependent programmed cell death (pyroptosis) (2), the current study investigated whether
CZE influenced NLRP3 inflammasome-mediated pyroptosis in LPS-primed
BMDMs. Pyroptosis was quantified by measuring the quantity of LDH
released into the cell culture supernatant. The levels of LDH
released by LPS-primed cells in response to ATP, nigericin and MSU
were ~66, 84 and 46%, respectively (Fig. 3A-C). ATP-induced LDH release was
slightly decreased by CZE at concentrations of 25 and 50 µg/ml; CZE
at 100 µg/ml inhibited LDH release by >90% (Fig. 3A). In addition, nigericin-induced
LDH release was decreased by CZE at 100 µg/ml (Fig. 3B). CZE treatment also inhibited LDH
release in response to MSU in a dose-dependent manner (Fig. 3C). These findings indicate that CZE
inhibited NLRP3 inflammasome-induced pyroptotic cell death.
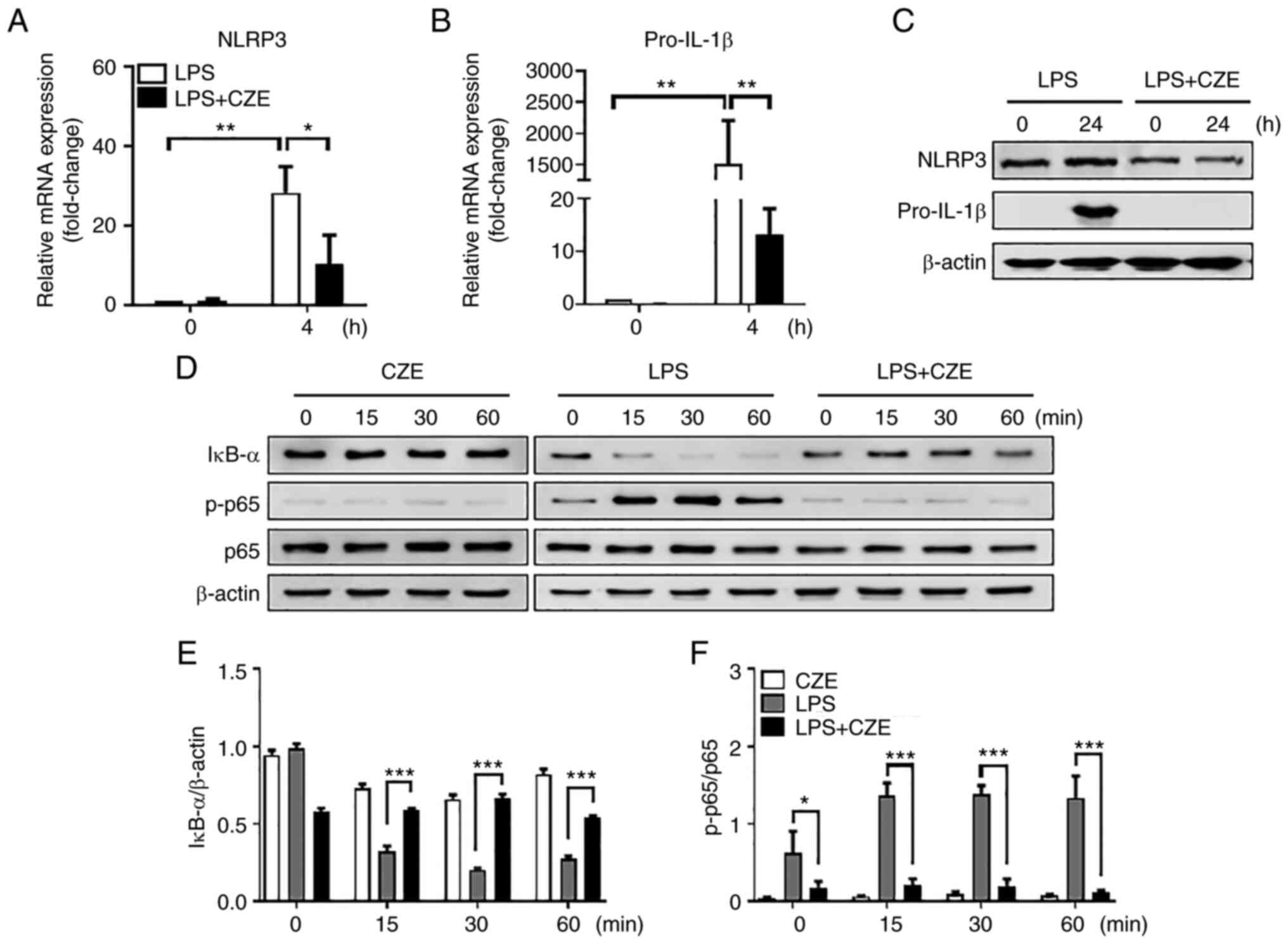
CZE decreases gene expression of NLRP3
and pro-IL-1β and the activation of NF-κB in BMDMs in response to
LPS
To examine whether CZE inhibits the priming step of
the NLRP3 inflammasome, BMDMs were treated with LPS for 4 and 24 h
in the presence or absence of CZE (Fig. 4A-C). LPS-induced gene expression of
NLRP3 and pro-IL-1β was decreased in CZE-treated cells compared
with LPS-stimulated cells (Fig. 4A
and B). Protein expression of
NLRP3 and pro-IL-1β in response to LPS was decreased by CZE
(Fig. 4C). Western blot analysis
showed that IκB-α degradation was prevented by CZE treatment in
BMDMs in response to LPS; p65 phosphorylation was also decreased by
CZE (Fig. 4D-F). CZE alone did not
affect IκB-α degradation or p65 phosphorylation in BMDMs (Fig. 4D-F). Accordingly, it is likely that
CZE regulated NLRP3 inflammasome activation by inhibiting both the
priming and activation steps.
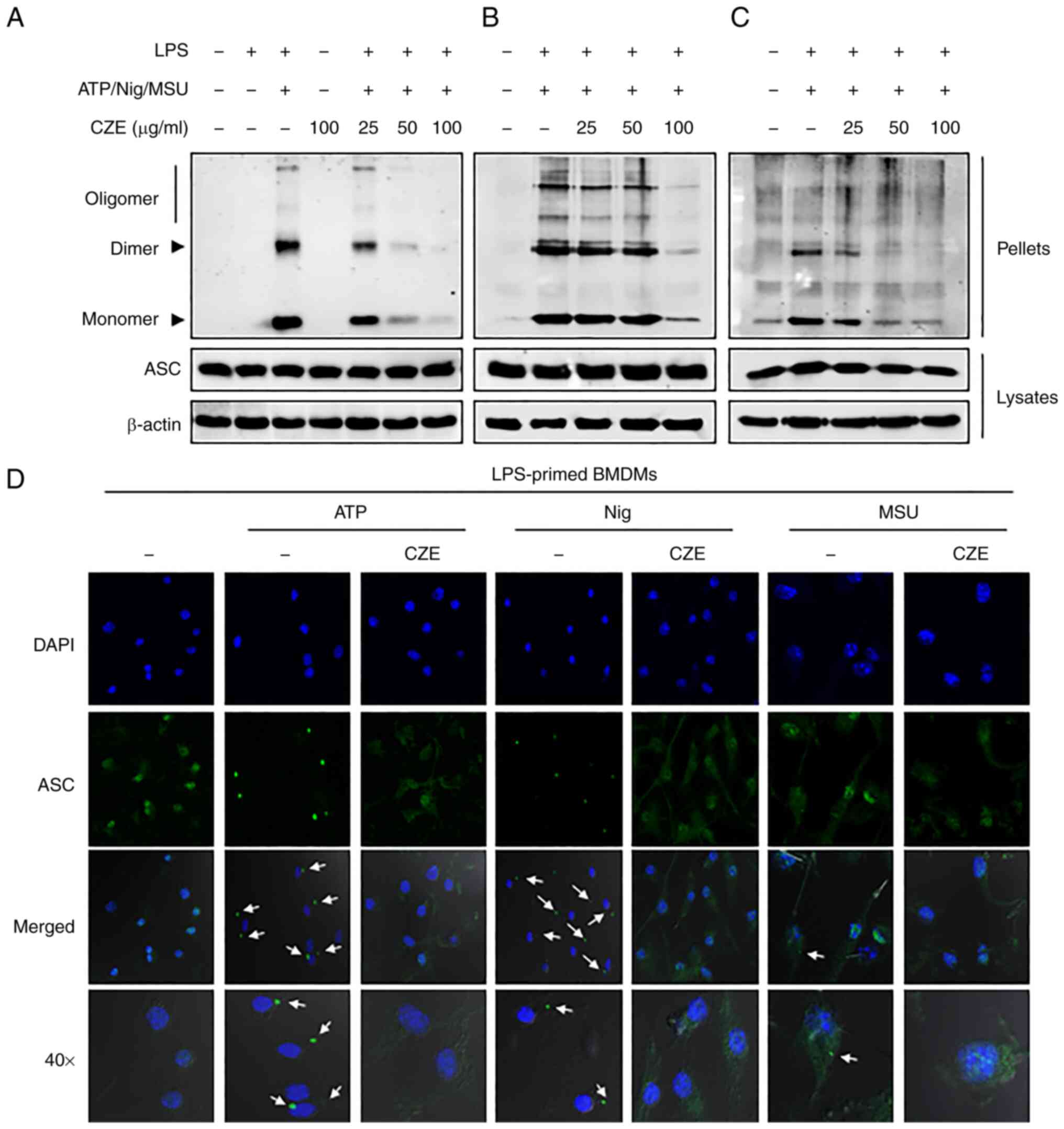
CZE inhibits NLRP3
inflammasome-mediated oligomerization and speck formation of
ASC
ASC oligomerization is a hallmark of inflammasome
activation (2,22). Therefore, the present study sought
to determine whether CZE inhibits ASC oligomerization in response
to ATP, nigericin and MSU in LPS-primed BMDMs. ATP, nigericin and
MSU treatment led to ASC oligomerization in LPS-primed BMDMs, which
was decreased by CZE in a dose-dependent manner (Fig. 5A-C). The present study further
examined the effect of CZE on ASC speck formation, which
contributes to signal amplification (22,23).
ATP, nigericin and MSU treatment induced ASC speck formation in
LPS-primed macrophages, which was prevented by CZE treatment
(Fig. 5D). These results indicated
that CZE may regulate the NLRP3 inflammasome by inhibiting ASC
oligomerization and speck formation.
CZE does not inhibit inflammasome
activation of NLRC4 or AIM2
In addition to NLRP3, NLRC4 and AIM2 are also
involved in the formation of the inflammasome (24). Therefore, the present study
examined whether CZE also regulates NLRC4 or AIM2 inflammasome
activation. LPS-primed BMDMs were infected with S.
typhimurium to induce NLRC4 inflammasome activation and treated
with poly(dA:dT) to activate the AIM2 inflammasome. CZE treatment
slightly reduced the S. typhimurium-induced IL-1β secretion,
whereas the production of IL-6 or TNF-α was not affected (Fig. 6A-C). Western blot analysis revealed
that expression of both pro- and cleaved IL-1β was decreased by CZE
(Fig. 6D). By contrast, the
expression of pro-caspase-1 was not affected by CZE and cleaved
caspase-1 expression was slightly decreased only at high
concentrations (Fig. 6D),
suggesting that the decrease in IL-1β induced by CZE in S.
typhimurium-infected BMDMs may have been due to a decrease in
pro-IL-1β rather than inflammasome activation. In addition, CZE did
not affect the poly(dA:dT)-induced production of IL-1β, IL-6 or
TNF-α (Fig. 6E-G). These findings
suggested that CZE did not inhibit NLRC4 or AIM2 inflammasome
activation, although it reduced the protein expression of pro-IL-1β
in response to S. typhimurium.
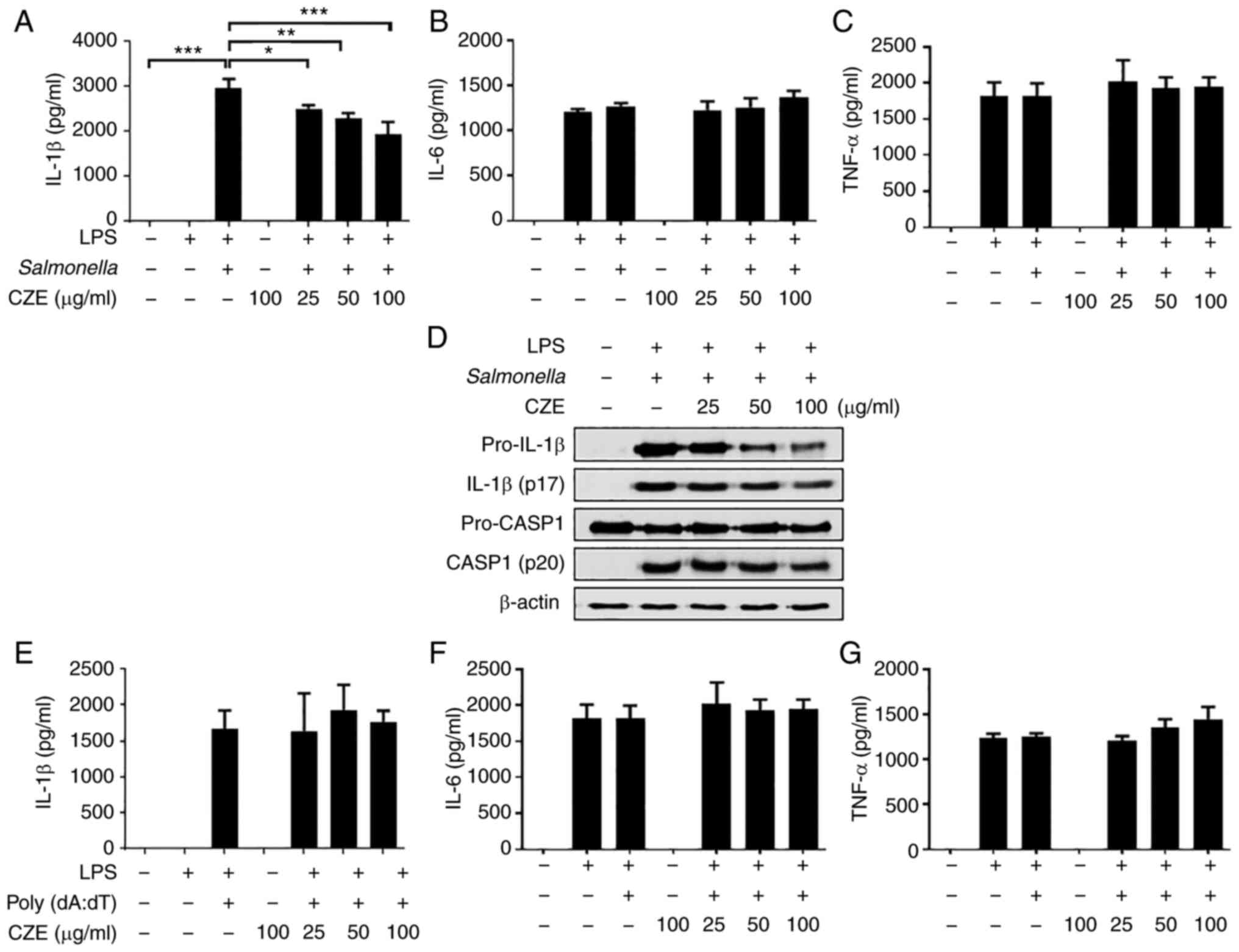 | Figure 6CZE inhibits activation of the NLR
family CARD domain-containing protein 4 inflammasome but not absent
in melanoma 2 inflammasome in BMDMs. BMDMs were primed with LPS
(100 ng/ml) for 6 h and subsequently treated with CZE for 30 min.
The cells were infected with Salmonella typhimurium
(multiplicity of infection=10) for 1 h, and the medium was replaced
with medium containing gentamicin and incubated for 3 h. Levels of
(A) IL-1β, (B) IL-6 and (C) TNF-α in the culture supernatants were
measured by ELISA. (D) Culture supernatant and cell lysates were
used to detect the immature and cleaved forms of IL-1β and CASP1 by
western blotting. β-actin was used as a loading control. LPS-primed
BMDMs were treated with CZE. The cells were additionally incubated
with poly(dA:dT) (2 µg/ml) for 4 h. Levels of (E) IL-1β, (F) IL-6,
(G) TNF-α in the culture supernatants were measured by ELISA. The
results are presented as the mean ± SD. *P<0.05,
**P<0.01, ***P<0.001. LPS,
lipopolysaccharide; BMDM, bone marrow-derived macrophage; CZE,
ethanol extract of Chrysanthemum zawadskii; CASP1,
caspase-1. |
Key components of CZE inhibit IL-1β
production in response to NLRP3 inflammasome activators in
LPS-primed BMDMs
CZE is known to contain three key components:
Linarin, 3,5-di-CQA and CGA (13).
These components have been reported to have anti-inflammatory
effects (25-27).
Therefore, the present study investigated whether these components
regulate NLRP3 inflammasome-induced IL-1β production in LPS-primed
BMDMs. LPS-primed BMDMs were treated with 100 µM linarin, 3,5-di-CQ
and CGA and stimulated with ATP, nigericin and MSU. All components
inhibited the ATP-induced secretion of IL-1β, but not IL-6 or
TNF-α, in LPS-primed BMDMs (Fig.
7A-C). Linarin, but not 3,5-di-CQA or CGA, decreased
nigericin-induced IL-1b secretion (Fig. 7D). By contrast, IL-1β induced by
MSU was decreased by 3,5-di-CQA and CGA, but not by linarin
(Fig. 7G). IL-6 secretion in
response to nigericin (Fig. 7E and
F) and MSU (Fig. 7H and I) was not affected by any of the
components. These results indicated that the three major components
of CZE may inhibit the NLRP3 inflammasome via different
mechanisms.
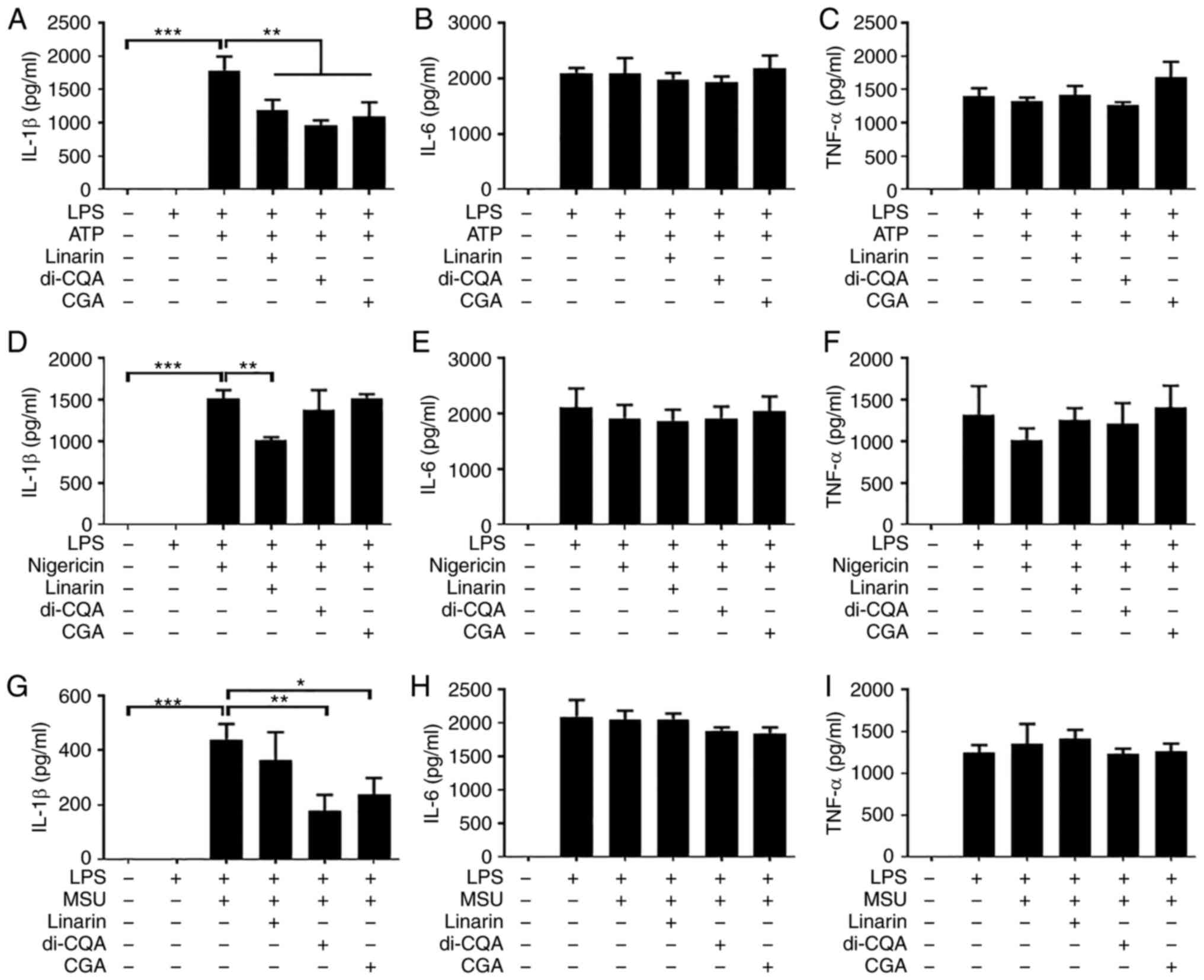 | Figure 7Linarin, di-CQA and CGA decrease
nucleotide-binding oligomerization domain-like receptor family
pyrin domain containing 3 inflammasome-induced IL-1β secretion from
LPS-primed BMDMs. BMDMs were primed with LPS (100 ng/ml) for 6 h
and subsequently treated with 100 µM linarin, di-CQA or CGA for 30
min. Cells were incubated with ATP (2 mM) or nigericin (10 µM) for
40 min or MSU (200 µg/ml) for 4 h. The levels of (A) IL-1β, (B)
IL-6 and (C) TNF-α in culture supernatant from BMDMs treated with
or without LPS and ATP and linarin, di-CQA and CGA were measured by
ELISA. LPS-primed BMDMs were treated with nigericin (10 µM) for 40
min in the presence or absence of linarin, di-CQA and CGA. The
levels of (D) IL-1β, (E) IL-6 and (F) TNF-α in culture supernatants
were measured by ELISA. LPS-primed BMDMs were treated with MSU (200
µg/ml) for 4 h with or without linarin, di-CQA and CGA. Levels of
(G) IL-1β, (H) IL-6 and (I) TNF-α in culture supernatant were
measured by ELISA. The results are presented as the mean ± SD.
*P<0.05, **P<0.01,
***P<0.001. di-CQA, di-caffeoylquinic acid; CGA,
chlorogenic acid; LPS, lipopolysaccharide; BMDM, bone
marrow-derived macrophage; MSU, monosodium urate. |
Discussion
The uncontrolled activation of the NLRP3
inflammasome is associated with various metabolic and inflammatory
diseases (4-9).
Therefore, efforts have been made to find new compounds that
inhibit the NLRP3 inflammasome. Specifically, numerous compounds in
plants, such as curcumin, epigallocatechin gallate, quercetin and
resveratrol have been shown to inhibit the NLRP3 inflammasome
(28). C. zawadskii var.
latilobum (CZ) is widely used in traditional Chinese
medicine. Its extract has been reported to inhibit nitric oxide
(NO) production via induction of heme oxygenase-1(16). CZ extract also increases
transactivation of peroxisome proliferator-activated
receptors-responsive element, suppresses TNF-α and IL-6-induced
NF-κB activation and NO production and plays a role in the
homeostasis of the skin barrier (29). However, to the best of our
knowledge, whether CZ extract regulates NLRP3 inflammasome
activation has not been reported. The present study found that CZE
efficiently inhibited NLRP3 activator (ATP, nigericin and
MSU)-induced IL-1β secretion, cleavage of caspase-1 and IL-1β and
pyroptotic cell death. CZE also inhibited the LPS-induced gene
expression of NLRP3 and pro-IL-1β and the activation of NF-κB,
suggesting that CZE regulated activation of the NLRP3 inflammasome
at both signals 1 and 2.
To the best of our knowledge, little is known about
the role of the Chrysanthemum extract in inflammasome
activation. Methanol extract of Chrysanthemum indicum
suppresses both NLRP3 and AIM2 inflammasome activation by
regulating ASC phosphorylation (30). In high-performance liquid
chromatography fingerprinting analysis of C. indicum, one
major peak corresponding to 1,5-di-CQA and minor peaks
corresponding to luteolin and CGA were observed (30). Kim et al (13) identified that CGA, 3,5-di-CQA and
linarin are three key components of CZE. Linarin, a natural
flavonoid, has been reported to have anti-inflammatory effects by
downregulating phagocytosis and induce pro-inflammatory cytokine
production, such as IL-6, TNF and IL-1β and antigen presentation in
LPS-stimulated macrophages (27).
Kim et al (18) showed that
ethanol extract of Chrysanthemum zawadskii Herbich (ECZ)
induces reactive oxygen species-mediated apoptosis and autophagy in
mouse colon cancer CT-26 cells. In addition, high performance
liquid chromatography analysis was performed to identify and
quantify the components (CGA, 3,5-di-CQA and luteolin) in ECZ.
However, in the aforementioned study, the physiological effects of
the components contained in CZE were not confirmed. In the present
study, it was confirmed that three components (linarin, 3,5-di-CQA
and CGA) of CZE inhibited production of IL-1β by using various
molecules that induce NLRP3 inflammasome activation. CZE and its
components (CGA, 3,5-di-CQA and linarin) contribute to inhibition
of NLRP3 inflammasome activation by regulating both step 1 and 2,
but not NLRC4 and AIM2 inflammasome activation. 3,5-Di-CQA has been
shown to inhibit pro-inflammatory gene expression and NO production
via inducible NO synthase and cyclooxygenase-2(25). CGA exerts an anti-inflammatory
effect in LPS-stimulated macrophages and microglial cells (26) and inhibits fibroblast-like
synoviocyte proliferation by inducing apoptosis (31). In addition, CGA administration
increases expression of nuclear factor E2-related factor-2 (Nrf2)
and antioxidant genes in liver tissue and rescues liver damage
induced by CCl4 in rats (32). The expression of NLRP3
inflammasome-associated proteins, such as caspase-1 and IL-1β (both
full length and cleaved forms), is also reduced in liver tissues of
CCl4-treated rats by CGA administration. CGA inhibits
the activation of the NLRP3 inflammasome by activating
Nrf2(32). Linarin is known to
prevent acute lung injury induced by LPS in mice by suppressing
oxidative stress and inflammation (33). Protein expression of NLRP3, ASC and
caspase-1 are decreased by linarin in LPS-treated lung epithelial
cells. In the present study, linarin, 3,5-di-CQA and CGA
differently regulated NLRP3 inflammasome activation, as only
linarin inhibited nigericin-induced IL-1β production, whereas
3,5-di-CQA and CGA, but not linarin, decreased IL-1β production
induced by MSU. Thus, further studies are required to uncover the
underlying mechanism by which each component controls NLRP3
inflammasome activation.
ASC serves a key role in the formation of the NLRP3
inflammasome complex by recruiting pro-caspase-1 via interaction
with the pyrin domain of the NLRP3 inflammasome (22,23).
Previous studies have demonstrated that NLRP3 activator-mediated
phosphorylation and oligomerization of ASC is affected by plant
extracts (30,34). In the present study, ASC
oligomerization in response to NLRP3 activators was decreased by
CZE in LPS-primed BMDMs. ASC oligomerization is a key step in the
formation of the AIM2 inflammasome complex. Inhibition of ASC speck
formation by plant extracts affects the activation of AIM2, as well
as the NLRP3 inflammasome (30,34).
In the present study, CZE did not affect AIM2 inflammasome-induced
IL-1β secretion. CZE may regulate NLRP3 inflammasome activation
upstream of ASC; alternatively, CZE may be specific for NLRP3
inflammasome complex formation. Further studies are therefore
needed to clarify the precise mechanism. In addition, unlike NLRP3
and AIM2, the CARD of NLRC4 binds pro-caspase-1 without ASC
(23,35). In the present study,
Salmonella-induced IL-1β production was slightly decreased
by CZE treatment, although this was due to decreased production of
pro-IL-1β. The effect of CZE on the cleavage of IL-1β and caspase-1
in response to Salmonella infection was limited. Despite it
being known that activated NLRC4 recruits and interacts with
NLRP3(36), the effect of CZE on
NLRC4 inflammasome activation is not notable. In conclusion, the
present study demonstrated that CZE and its components (CGA,
3,5-di-CQA and linarin) effectively inhibited activation of the
NLRP3 inflammasome at both steps 1 and 2 (Fig. 8). The present results suggested
that CZE and its components may be developed as novel therapeutics
against NLRP3-mediated metabolic or inflammatory disease.
Gnaphalium pensylvanicum extracts containing caffeoylquinic
acid derivatives shows anti-gout activity in mice with MSU-induced
acute gouty arthritis (37).
Therefore, animal experiments are needed to evaluate the potential
treatment effect of NLRP3 inflammasome inhibition using CZE and its
components.
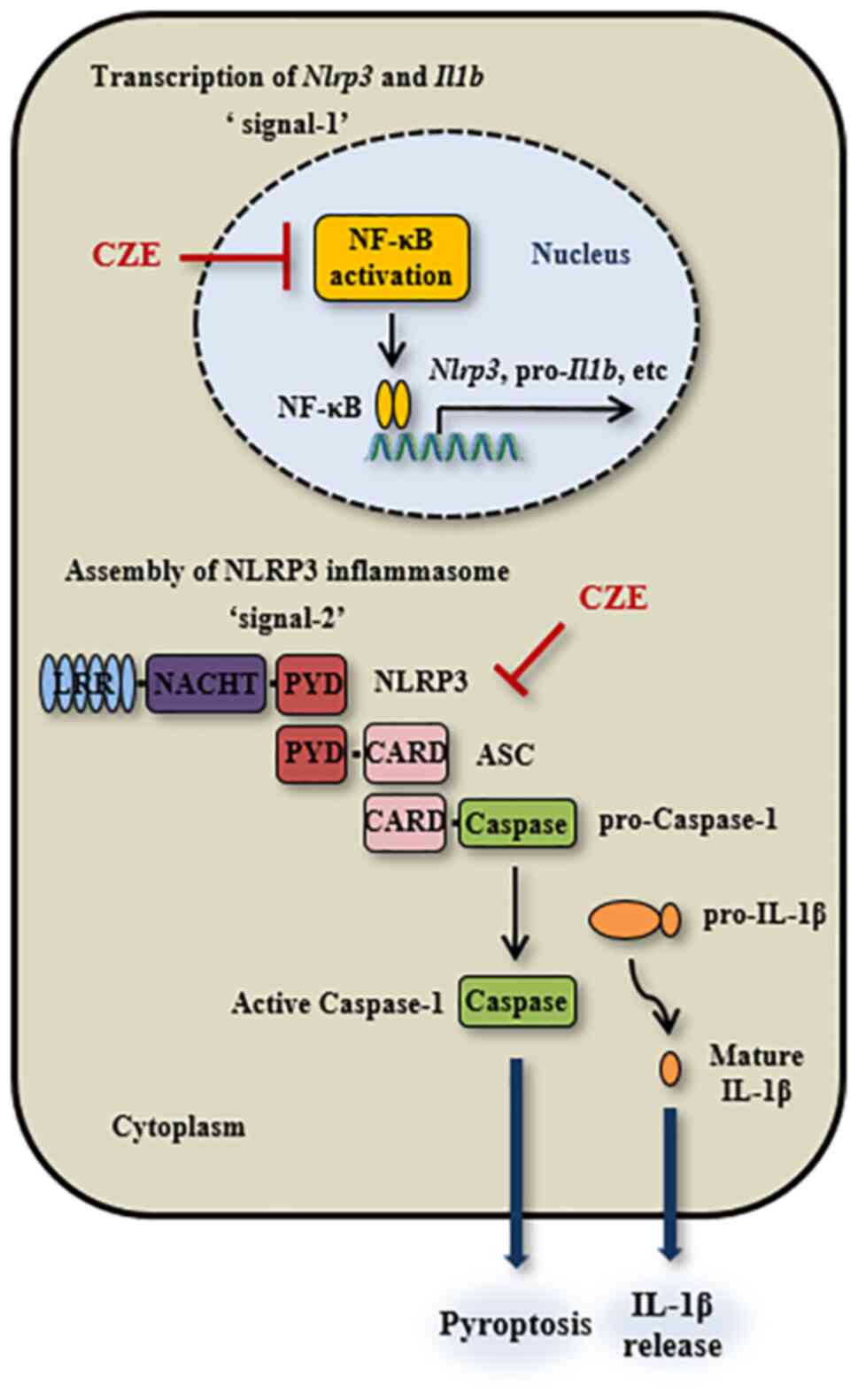 | Figure 8Schematic of the inhibitory effect of
CZE on activation of the NLRP3 inflammasome in macrophages. CZE
downregulates expression of NLRP3 and pro-IL-1β in response to LPS
by inhibiting NF-κB activation in macrophages. It also suppresses
ATP/nigericin/monosodium urate-induced activation of the NLRP3
inflammasome in LPS-primed macrophages via inhibition of ASC
oligomerization and speck formation, resulting in decreased IL-1β
secretion and pyroptosis. NLRP3, nucleotide-binding oligomerization
domain-like receptor family pyrin domain containing 3; LPS,
lipopolysaccharide; CZE, ethanol extract of Chrysanthemum
zawadskii; LRR, leucine-rich repeat; NACHT, NAIP, CIITA, HET-E
and TEP1; PYD, pyrin domain; CARD, caspase-recruitment domain; ASC,
apoptosis-associated speck-like protein containing a CARD. |
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Korea Research
Institute of Bioscience and Biotechnology Research Initiative
Program (grant no. KGM4571922) and the Korean Health Technology
R&D Project funded by the Ministry of Health and Welfare (grant
no. HI17C1238).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JP designed the study. AJ and HL performed
experiments and collected data. AJ, HL, JH, YK and JP analyzed and
interpreted data. AJ and JP wrote, revised and reviewed the
manuscript. All authors have read and approved the final
manuscript. AJ, HL and JP confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
All animal studies were performed using protocols
approved by the Institutional Animal Care and Use Committee of
Chonnam National University (Gwangju, Republic of Korea; approval
no. CNU IACUC-YB-2018-02).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Latz E, Xiao TS and Stutz A: Activation
and regulation of the inflammasomes. Nat Rev Immunol. 13:397–411.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Paik S, Kim JK, Silwal P, Sasakawa C and
Jo EK: An update on the regulatory mechanisms of NLRP3 inflammasome
activation. Cell Mol Immunol. 18:1141–1160. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kaneko N, Kurata M, Yamamoto T, Morikawa S
and Masumoto J: The role of interleukin-1 in general pathology.
Inflamm Regen. 39(12)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mangan MSJ, Olhava EJ, Roush WR, Seidel
HM, Glick GD and Latz E: Targeting the NLRP3 inflammasome in
inflammatory diseases. Nat Rev Drug Discov. 17:588–606.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Daniels MJD, Rivers-Auty J, Schilling T,
Spencer NG, Watremez W, Fasolino V, Booth SJ, White CS, Baldwin AG,
Freeman S, et al: Fenamate NSAIDs inhibit the NLRP3 inflammasome
and protect against Alzheimer's disease in rodent models. Nat
Commun. 7(12504)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dixit VD: Nlrp3 inflammasome activation in
type 2 diabetes: Is it clinically relevant? Diabetes. 62:22–24.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Fusco R, Siracusa R, Genovese T, Cuzzocrea
S and Di Paola R: Focus on the role of NLRP3 inflammasome in
diseases. Int J Mol Sci. 21(4223)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kanneganti TD: Inflammatory bowel disease
and the NLRP3 inflammasome. N Engl J Med. 377:694–696.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Guo C, Fu R, Wang S, Huang Y, Li X, Zhou
M, Zhao J and Yang N: NLRP3 inflammasome activation contributes to
the pathogenesis of rheumatoid arthritis. Clin Exp Immunol.
194:231–243. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sharma D and Kanneganti TD: The cell
biology of inflammasomes: Mechanisms of inflammasome activation and
regulation. J Cell Biol. 213:617–629. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Coll RC, Robertson AA, Chae JJ, Higgins
SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG,
Stutz A, et al: A small-molecule inhibitor of the NLRP3
inflammasome for the treatment of inflammatory diseases. Nat Med.
21:248–255. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Cragg GM and Newman DJ: Nature products: A
contrinuing source of novel drug leads. Biochim Biophys Acta.
1830:3670–3695. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim AR, Kim HS, Kim DK, Lee JH, Yoo YH,
Kim JY, Park SK, Nam ST, Kim HW, Park YH, et al: The extract of
Chrysanthemum zawadskii var. latilobum ameliorates
collagen-induced arthritis in mice. Evid Based Complement Alternat
Med. 2016(3915013)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gu DR, Hwang JK, Erkhembaatar M, Kwon KB,
Kim MS, Lee YR and Lee SH: Inhibitory effect of Chrysanthemum
zawadskii Herbich var. latilobum kitamura extract on
RANKL-induced osteoclast differentiation. Evid Based Complement
Alternat Med. 2013(509482)2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang WY, Jeong I, Han BK, Kim MJ, Hong J,
Ahn SII, Heo W, Pan JH, Kim JK, Shin EC and Kim YJ:
Chrysanthemum zawadskii Herbich var. latilobum
(Maxim.) Kitamura water extract prevents BALB/c mice lung injury
from particulate matter 10 toxicity. Food Agric Immunol.
33:252–263. 2022.
|
|
16
|
Kim Y, Han J, Sung J, Sung M, Choi Y,
Jeong HS and Lee J: Anti-inflammatory activity of Chrysanthemum
zawadskii var. latilobum leaf extract through haem
oxygenase-1 induction. J Funct Foods. 4:474–479. 2012.
|
|
17
|
Cho BO, Shin JY, Kang HJ, Park JH, Hao S,
Wang F and Jang SI: Anti-inflammatory effect of Chrysanthemum
zawadskii, peppermint, Glycyrrhiza glabra herbal mixture in
lipopolysaccharide-stimulated RAW264.7 macrophages. Mol Med Rep.
24(532)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim KY, Oh TW, Yang HJ, Kim YW, Ma JY and
Park KI: Ethanol extract of Chrysanthemum zawadskii Herbich
induces autophagy and apoptosis in mouse colon cancer cells through
the regulation of reactive oxygen species. BMC Complement Altern
Med. 19(274)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Celada A, Gray PW, Rinderknecht E and
Schreiber RD: Evidence for a gamma-interferon receptor that
regulates macrophage tumoricidal activity. J Exp Med. 160:55–74.
1984.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2ˆ(-delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
21
|
Kelley N, Jeltema D, Duan Y and He Y: The
NLRP3 inflammasome: An overview of mechanisms of activation and
regulation. Int J Mol Sci. 20(3328)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bryan NB, Dorfleutner A, Rojanasakul Y and
Stehlik C: Activation of inflammasomes requires intracellular
redistribution of the apoptotic speck-like protein containing a
caspase recruitment domain. J Immunol. 182:3173–3182.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hara H, Tsuchiya K, Kawamura I, Fang R,
Hernandez-Cuellar E, Shen Y, Mizuguchi J, Schweighoffer E,
Tybulewicz V and Mitsuyama M: Phosphorylation of the adaptor ASC
acts as a molecular switch that controls the formation of
speck-like aggregates and inflammasome activity. Nat Immunol.
14:1247–1255. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zheng D, Liwinski T and Elinav E:
Inflammasome activation and regulation: Toward a better
understanding of complex mechanisms. Cell Discov.
6(36)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hong S, Joo T and Jhoo JW: Antioxidant and
anti-inflammatory activities of 3,5-dicaffeoylquinic acid isolated
from Ligularia fischeri leaves. Food Sci Biotechnol. 24:257–263.
2015.
|
|
26
|
Hwang SJ, Kim YW, Park Y, Lee HJ and Kim
KW: Anti-inflammatory effects of chlorogenic acid in
lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm Res.
63:81–90. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kim B, Lee JH, Seo MJ, Eom SH and Kim W:
Linarin down-regulates phagocytosis, pro-inflammatory cytokine
production, and activation marker expression in RAW264.7
macrophages. Food Sci Biotechnol. 25:1437–1442. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tőzsér J and Benkő S: Natural compounds as
regulators of NLRP3 inflammasome-mediated IL-1β production.
Mediators Inflamm. 2016(5460302)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim B and Kim HS: Chrysanthemum
zawadskii extract activates peroxisome proliferator-activated
receptor-α and has an anti-inflammatory activity: Potential
interest for the skin barrier function. Korean J Chem Eng.
31:1831–1838. 2014.
|
|
30
|
Yu SH, Sun X, Kim MK, Akther M, Han JH,
Kim TY, Jiang J, Kang TB and Lee KH: Chrysanthemum indicum
extract inhibits NLRP3 and AIM2 inflammasome activation via
regulating ASC phosphorylation. J Ethnopharmacol.
239(111917)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lou L, Zhou J, Liu Y, Wei YI, Zhao J, Deng
J, Dong B, Zhu L, Wu A, Yang Y and Chai L: Chlorogenic acid induces
apoptosis to inhibit inflammatory proliferation of IL-6-induced
fibroblast-like synoviocytes through modulating the activation of
JAK/STAT and NF-κB signaling pathways. Exp Ther Med. 11:2054–2060.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shi A, Shi H, Wang Y, Liu X, Cheng Y, Li
H, Zhao H, Wang S and Dong L: Activation of Nrf2 pathway and
inhibition of NLRP3 inflammasome activation contribute to the
protective effect of chlorogenic acid on acute liver injury. Int
Immunopharmacol. 54:125–130. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Han X, Wu YC, Meng M, Sun QS, Gao SM and
Sun H: Linarin prevents LPS-induced acute lung injury by
suppressing oxidative stress and inflammation via inhibition of
TXNIP/NLRP3 and NF-κB pathways. Int J Mol Med. 42:1460–1472.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kwak SB, Koppula S, In EJ, Sun X, Kim YK,
Kim MK, Lee KH and Kang TB: Artemisia extract suppresses NLRP3 and
AIM2 inflammasome activation by inhibition of ASC phosphorylation.
Mediators Inflamm. 2018(6054069)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yang Y, Wang H, Kouadir M, Song H and Shi
F: Recent advances in the mechanisms of NLRP3 inflammasome
activation and its inhibitors. Cell Death Dis.
10(128)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Qu Y, Misaghi S, Newton K, Maltzman A,
Izrael-Tomasevic A, Arnott D and Dixit VM: NLRP3 recruitment by
NLRC4 during Salmonella infection. J Exp Med. 213:877–885.
2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jiang Y, Lin Y, Hu YJ, Song XJ, Pan HH and
Zhang HJ: Caffeoylquinic acid derivatives rich extract from
Gnaphalium pensylvanicum willd. Ameliorates hyperuricemia
and acute gouty arthritis in animal model. BMC Complement Altern
Med. 17(320)2017.PubMed/NCBI View Article : Google Scholar
|