Introduction
Sclerosing extramedullary hematopoietic tumor
(SEMHT) is a rare lesion, previously known as fibrous hematopoietic
tumor or myelosclerosis. Lesions of this type are associated with
chronic myeloproliferative neoplasms, particularly chronic
idiopathic myelofibrosis, in older adults (1). When chronic myeloproliferative
neoplasms lead to insufficiency in the hematopoietic function of
the bone marrow, hematopoietic tissues in locations other than the
bone marrow develop abnormally to compensate. These deposits of
extramedullary hematopoietic tissue have the propensity to form a
tumor, known as an SEMHT (2). The
clinical, radiological and morphological features of SEMHT may
complicate its differentiation from lymphoma, carcinoma and
sarcoma. In some cases, it is challenging to distinguish SEMHT from
extramedullary hematopoiesis (EMH) (3). SEMHTs are characterized by the
presentation of myelofibrosis-like components with atypical
megakaryocytes and different proportions of granulocyte and
erythrocyte precursors in a significantly sclerotic background,
with thick collagen deposition (4). Although the pathogenesis of SEMHT has
not yet been fully elucidated, it is known to involve the
inappropriate production of cytokines by megakaryocytes, which
stimulate the proliferation of fibroblasts and the production of
extracellular matrix (2). SEMHT
typically presents as a retroperitoneal mass (5), but may occur in other organs,
although rarely. The present study describes a case of SEMHT
originating in the colon that involved the peri-intestinal lymph
nodes.
Case report
A 59-year-old female patient presented to the First
Hospital of Jiaxing (Jiaxing, China) with abdominal pain in March
2022. The patient had a 1-month history of abdominal pain, diarrhea
and mucinous bloody stools. Her medical history was pertinent for
myelofibrosis 6 years ago, and she was maintained on 15 mg
lucotinib twice daily. Abdominal contrast-enhanced computed
tomography (CT) revealed cecal thickening with intestinal
obstruction, and no contrast enhancement was observed (Fig. 1A and B). Colonoscopy revealed a large necrotic
mass in the cecum, which blocked the intestinal cavity (Fig. 1C). Bone scintigraphy revealed a
superscan appearance, with a high ratio of tracer accumulation in
the bone compared with soft tissue, which was consistent with
diffuse lesions in the bone marrow hematopoietic system (Fig. 1D). The laboratory test results were
as follows: Hemoglobin level, 145 g/l; erythrocyte count,
5.01x1012/l; white blood cell count,
44.17x109/l; and platelet count, 147x109/l
(Table I). Due to the secondary
infection caused by intestinal obstruction, the white blood cell
count was increased. A bone marrow biopsy was not performed due to
the previous failure to obtain bone marrow owing to dry tap bone
marrow aspiration. Due to the huge tumor blocking the intestinal
cavity, the patient subsequently underwent surgical resection of
the colon.
 | Table IBlood analysis determined at
admission. |
Table I
Blood analysis determined at
admission.
| Blood parameter | Value | Laboratory range |
|---|
| Hemoglobin level,
g/l | 145 | 115-150 |
| Erythrocyte count,
x1012/l | 5.01 | 3.80-5.10 |
| White blood cell
count, x109/l | 44.17 | 3.50-9.50 |
| Platelet count,
x109/l | 147 | 125-350 |
On macro-examination, the cecal mass was found to
measure 6x5x5 cm. The tumor extended from the mucosa to the serosa,
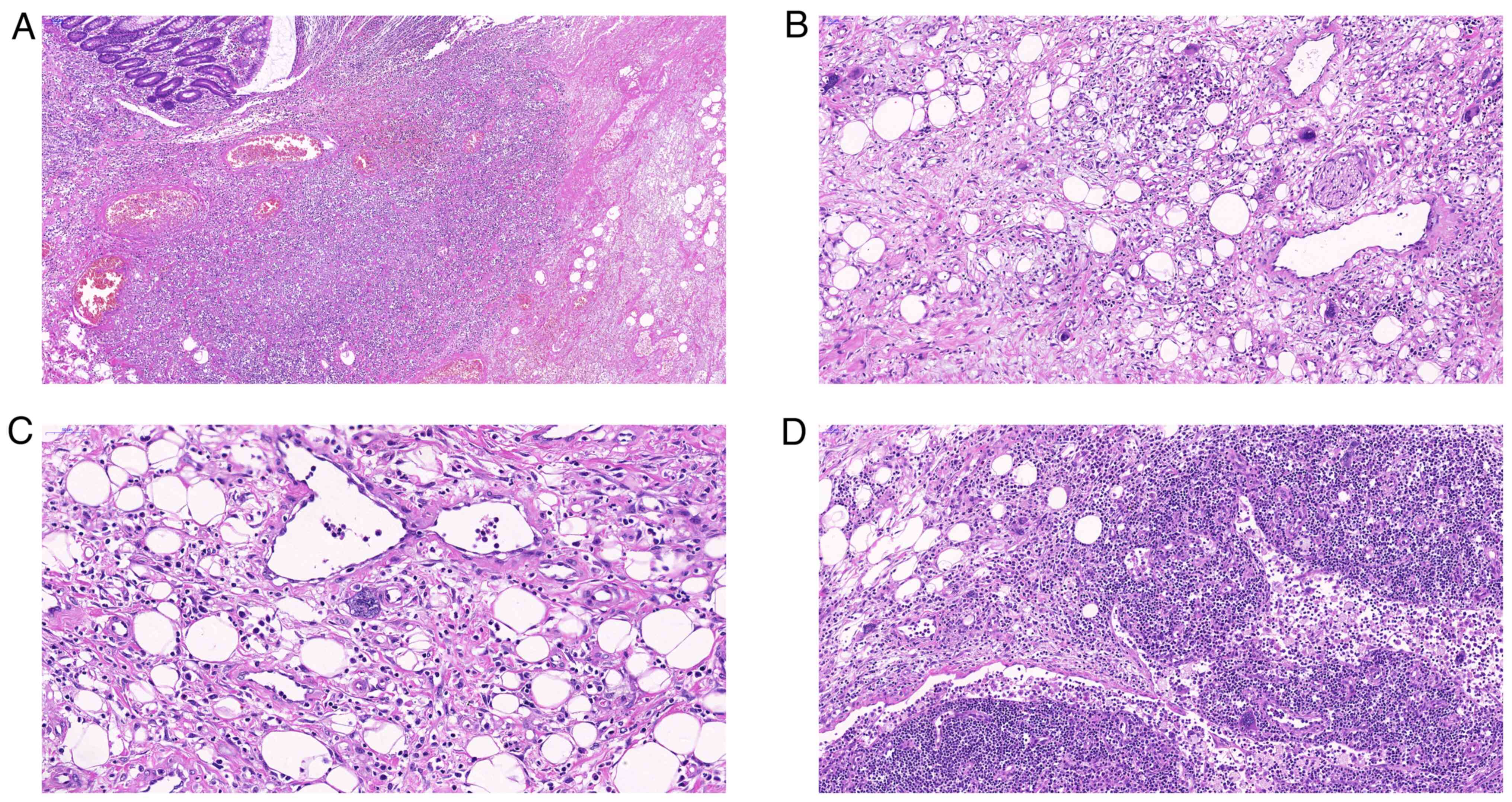
with evident necrosis. Microscopically, extensive hemorrhage,
necrosis and thrombosis were observed in the intestinal wall
(Fig. 2). The serous layer of the
colon exhibited obvious interstitial fibrous hyperplasia and myxoid
degeneration. In the fibromyxoid background, scattered atypical
megakaryocytes and a small number of erythrocytes and granulocyte
precursors were observed. Atypical megakaryocytes were also noted
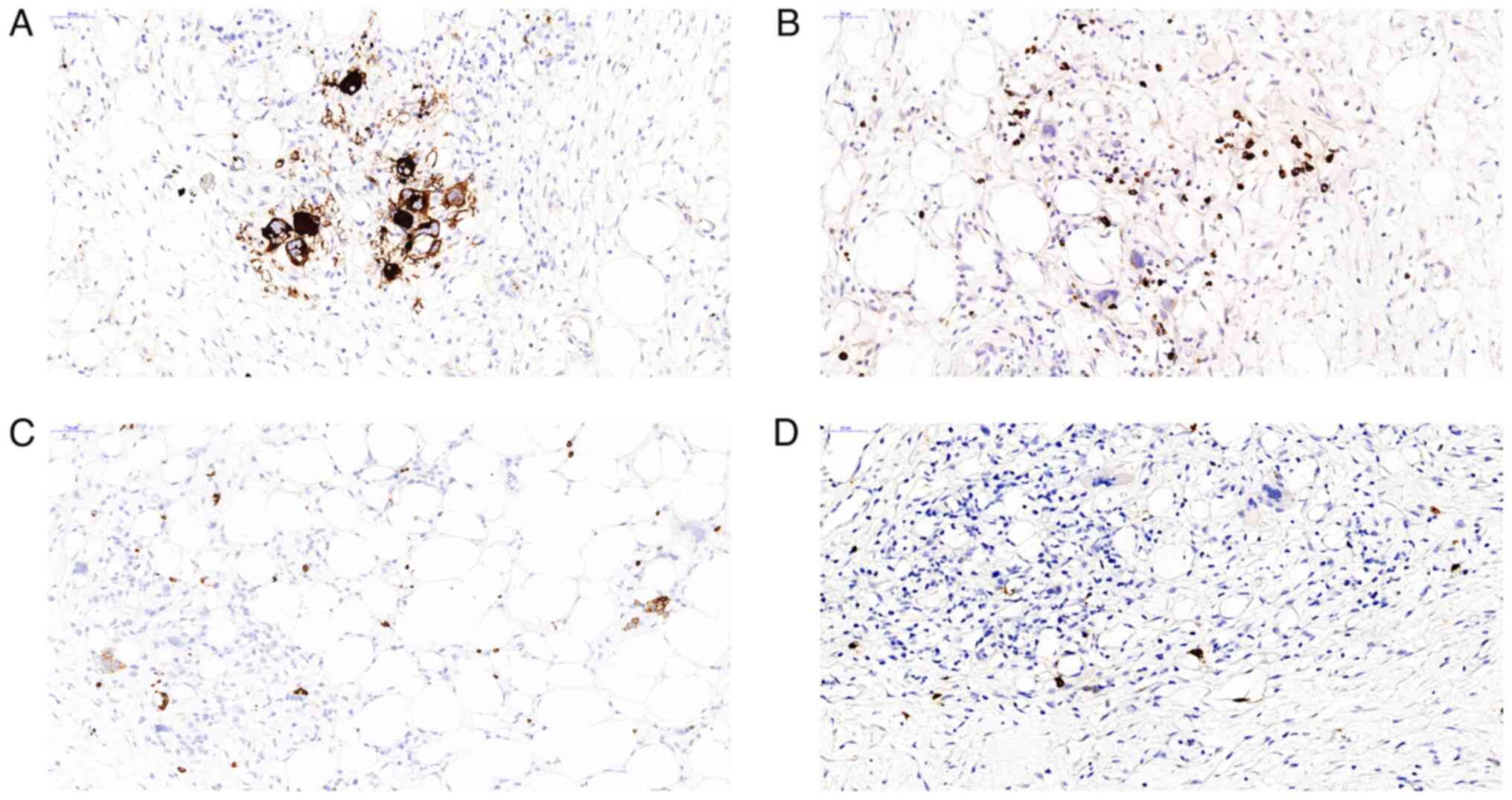
in several peri-intestinal lymph nodes. Immunohistochemical
staining for CD61 revealed the presence of atypical megakaryocytes
(Fig. 3A). Myeloperoxidase (MPO)
and glycophorin A immunohistochemical staining differentiated
granulocyte and erythrocyte precursors, respectively (Fig. 3B and C), while CD117 staining was observed in a
small number of cells (Fig. 3D).
Immunohistochemical staining for cytokeratin, smooth muscle actin
and desmin yielded negative results. Based on these findings and
the history of myelofibrosis, the patient was diagnosed with SEMHT.
After surgery, the patient continued to take 15 mg lucotinib twice
daily for myelofibrosis treatment. Four months after the surgery,
the patient developed abdominal pain and underwent abdominal CT
examination, which indicated intestinal edema and ascites.
Subsequently, enteral nutrition and antibiotic treatment were
administered to the patient in the hospital. After half a month of
treatment, the symptoms experienced by the patient were
ameliorated.
IHC staining was performed on a BenchMark XT (Roche
Diagnostics), an automatic IHC staining device. All procedures were
performed as per the manufacturer's protocols. The endogenous
peroxides and protein were blocked using the Endogenous Biotin
Blocking kit (cat. no. ab64212; Abcam) at 37˚C for 4 min. The
following primary antibodies were used: Anti-CD61 (cat. no.
ab179473; 1:800 dilution), anti-MPO (cat. no. ab208670; 1:1,000
dilution), anti-glycophorin A (cat. no. ab129024; 1:2,500
dilution), anti-CD117 (cat. no. ab32363; 1:400 dilution), anti-CK
(cat. no. ab215838; 1:100 dilution), anti-SMA (cat. no. ab5694;
1:100 dilution) and anti-desmin (cat. no. ab32362; 1:2,000
dilution) (all Abcam). Primary antibodies were added and incubated
for 16 min at 37˚C. A light microscope was used for
observation.
Discussion
SEMHT is a rare condition associated with chronic
myeloproliferative neoplasms. This tumor is mainly detected in
individuals in their 50s or 60s (6). SEMHT may occur at various sites,
including the thyroid gland, lung, lymph nodes, gastrointestinal
tract, kidney, skin and breast (7). The clinical symptoms depend on the
location of the SEMHT and mainly include abdominal pain, headache,
appearance of a skin mass and lacrimal gland swelling (2,8-10).
At present, few cases of SEMHT have been reported, and the majority
of SEMHT cases have a history of myelofibrosis. Few cases are
associated with essential thrombocytosis, acute B-cell
lymphoblastic leukemia or myelodysplastic syndrome, and the
patients without myelofibrosis who develop SEMHT are young
individuals (6,7). SEMHT presents with single or multiple
tumor foci that vary in size and can be as large as 16 cm in
diameter. In terms of gross pathology, SEMHT appears rubbery, pink,
white or yellowish-brown with clear boundaries. SEMHT can be
sometimes lobulated and has the ability to infiltrate the
surrounding tissues (8).
Histopathologically, SEMHT is characterized by
atypical megakaryocytes, erythrocytes and granulocyte precursors
distributed in the sclerotic or fibromyxoid stroma (2). SEMHT can also include adipose tissue
trapped within the tumor (8).
Usually, the fibrous stroma is dominant; therefore, it is
challenging to identify the hematopoietic components in
conventional hematoxylin and eosin-stained sections. Due to the
marked fibrotic background, the observed hematopoietic components
may only be giant atypical megakaryocytes (11). In this context, megakaryocytes are
scattered throughout the matrix and may appear alone or in clusters
(12). Atypical megakaryocytes are
characterized by hyperchromatic, multilobulated, giant,
ink-blot-like nuclei with a slightly eosinophilic cytoplasm
(8). In addition to the enlarged
atypical megakaryocytes, loose aggregates of immature granulocyte
precursors and scattered erythroid precursor cells are present,
which are most apparent near to blood vessels (9).
It may be a challenging to recognize the presence of
hematopoietic cells in SEMHT only through morphological examination
as these cells lack specificity. Immunohistochemistry can help to
highlight the hematopoietic components of SEMHT and guide diagnosis
when similar malignant lesions are suspected. Atypical
megakaryocytes stain positively for CD61, CD41, CD31 and factor
VIII. Granulocytes stain positively for MPO, and erythrocyte
precursors stain positively for glycoprotein A and hemoglobin
(8). CD163 staining reveals the
accompanying histiocytes rather than the expanded megakaryocyte
forms. However, the immunohistochemical staining of vimentin in
SEMHT should be avoided because it highlights a large number of
accompanying fibroblasts that have been shown to be polyclonal
(9).
The genetic analysis of patients with SEMHT has
revealed the presence of the JAK2 V617F point mutation (6). As cytoplasmic tyrosine kinases,
proteins of the JAK family participate in cytokine receptor
superfamily signaling. The activation of JAK signaling and
downstream gene transcription is a cytokine-mediated signal
transduction pathway. JAK is essential for normal hematopoiesis,
and its activation mainly involves the response of JAK2 to type 1
cytokine ligands, including thrombopoietin, granulocyte-macrophage
stimulating factor and erythropoietin (4). V617F is the most common mutation in
the JAK2 gene in chronic myeloproliferative neoplasms, with the
exception of chronic myeloid leukemia, and is a sign of clonal
proliferation (4). A significant
difference in complications between JAK2 V617F negative and
positive cases has been reported in patients with chronic
myeloproliferative neoplasms. Specifically, the incidence of
fibrosis, bleeding, thrombosis and associated complications is
significantly higher in patients with JAK2 point mutations that in
those with wild-type JAK2(4).
Chromosomal aberrations can lead to disease progression to the
secondary myelofibrosis or accelerated phase, and leukemic
transformation in some patients with chronic myeloproliferative
neoplasms. Aberrations of chromosomes 1q and 9p have been found to
be positively associated with disease progression to the
accelerated phase (13). In
addition, the acquisition of one or more aberrations involving
chromosome 5, 7 or 17p has been indicated to be associated
specifically with progression to acute myeloid leukemia, and to
significantly affect overall survival (14). Unfortunately, data on the
chromosome mutation status of the present patient are not
available.
As previously mentioned, SEMHT rarely occurs in the
colon. A previous study reported considerable thickening of the
serosa, which almost completely surrounded the small and large
bowels and was accompanied by diffuse mesenteric sclerotic fibrosis
and scattered large, atypical megakaryocytes (15). However, in the present case, the
SEMHT was mainly located in the serosal layer of the intestinal
tube. Another distinctive feature of the present case is that
extensive bleeding, necrosis and thrombosis of the bowel were
additionally observed. These pathological changes may explain the
mucinous bloody stools with which the patient presented. In
addition, atypical megakaryocytes were found in the peri-intestinal
lymph nodes, suggesting that the lymph nodes were also involved.
This phenomenon is consistent with a previous study, which observed
atypical megakaryocytes in dilated lymph node sinuses (16).
The development of extramedullary hematopoietic
tissue is known as EMH and is usually accompanied by chronic
myeloproliferative neoplasms. When the hematopoietic function of
the bone marrow is insufficient, EMH acts as a compensatory
response (2). In this context,
since SEMHT and EMH have similar clinical features, clinicians may
find it challenging to differentiate between these two conditions
(3). EMH occurs not only in
chronic myeloproliferative neoplasms, but also in other diseases,
including hereditary spherocytosis, hemoglobinopathies, thalassemia
and sickle cell anemia (7). EMH
commonly affects the spleen, liver and lymph nodes, where it is
accompanied by hepatosplenomegaly or lymphadenopathy (8). Moreover, EMH may produce one or more
normal blood components in parts of the body other than in the bone
marrow, similar to SEMHT. The difference is that SEMHT is an
extramedullary dissemination of the neoplastic bone marrow
proliferation. These lesions resemble their tumor counterparts in
the bone marrow rather than the normal myeloid tissue that
undergoes trilineage hematopoiesis and maturation (9). The presence of atypical
megakaryocytes in SEMHT is a diagnostic hallmark for distinguishing
between the two entities, as is not found in EMH (7). Additionally, SEMHT forms a hard gray
mass and exhibits prominent fibrous stroma, similar to
myelofibrosis (17). Furthermore,
compared with EMH, SEMHT usually has less cellular, more atypical
megakaryocytes, and a more mucoid matrix (2,7).
The specific details of the pathogenesis of SEMHT
are currently unclear. However, research has shown that
myelofibrosis can lead to an increase in the number of circulating
stem cells in the peripheral blood. The adhesion of integrin
molecules may lead to the deposition of these stem cells in
peripheral organs, which can lead to the emergence of ectopic
hematopoietic foci (12). Previous
studies have shown that an inappropriate cytokine response is
associated with the myelofibrosis observed in chronic
myeloproliferative neoplasms (4,11).
Clonal populations of megakaryocytes release calmodulin, epidermal
growth factor, transforming growth factor and platelet-derived
growth factor, which stimulate the proliferation of nonclonal
fibroblasts and promote the production of extracellular matrix, and
the occurrence of myelofibrosis may be caused by this inappropriate
cytokine expression (11). The
mechanism of SEMHT formation may be similar that of myelofibrosis,
and involve the induction of fibroblast proliferation and matrix
production by megakaryocytes. The fibrous matrix produced by the
fibroblasts causes collagen deposition, which can lead to sclerotic
changes in tumors (1,18). In terms of the hypothetical
mechanism, the progression of SEMHT may resemble that of the
transfer process (primary tumor to metastasis). The cytogenetic or
clonal analysis of megakaryocytes may be used to verify this
mechanism (18), as there is
evidence to suggest that the extramedullary proliferation of
hematopoietic cells in primary myelofibrosis indicates the spread
of tumor clones rather than compensatory EMH (9). At present, to the best of our
knowledge, there is no literature on the association between drugs
and SEMHT. In some cases, patients have been treated with
ruxolitinib for myelofibrosis and SEMHT subsequently occurred
during the treatment (15,16). The occurrence of extramedullary
complications may reflect differences in drug distribution, cell
trafficking or metabolism in extramedullary regions. With prolonged
survival times, the increased duration of aggressive disease and
the associated hypercytokinemia may also lead to the development
and progression of these rare end-stage complications (15).
The prognosis of patients with SEMHT is variable and
usually depends on the underlying disease. In some cases, the
survival period is short, which may be associated with advanced
disease; however, the disease can also remain stable for a long
time without aggressive local therapy (8). Notably, chronic myeloproliferative
neoplasms present with extramedullary neoplasms, which serve as a
marker of the acute phase. SEMHT itself may be regarded as an
advanced manifestation of myelofibrosis. Adverse prognostic factors
for SEMHT include skin involvement, thrombocytopenia, leukocytosis,
anemia and old age. An abnormal karyotype in the nucleus, which is
associated with acute transformation, has been recognized as an
independent risk factor for poor prognosis (8). SEMHT may respond to hydroxyurea and
low-dose radiation therapy; however, research on the topic has
found that the effectiveness of the response is short-lived
(17).
In conclusion, the present study describes a novel
case of SEMHT. The identification of these rare lesions is
important when diagnosing tumors with anaplastic morphology in
order to guide appropriate treatment. In addition, it is important
to have a complete medical history for the patient, because when
the medical history is insufficient, the pathological and
radiological data may be challenging to evaluate. The present case
thus highlights the requirement for physicians to consider SEMHT as
a differential diagnosis for gastrointestinal masses, particularly
when a history of previous hematological disease is present.
Acknowledgements
Not applicable.
Funding
Funding: The study was financially supported by a grant from the
Jiaxing Municipal Science and Technology Project (grant no.
2021AD30160).
Availability of data and materials
All data used and/or analyzed during this study are
included in this published article.
Authors' contributions
ZZ and QZ obtained and analyzed the patient's
information and wrote the manuscript. XL and LZ collected and
analyzed the patient data. JW and ZG contributed to data extraction
and quality assessment. SH and HL analyzed and interpreted the
imaging findings. ZZ and WW designed the study and reviewed the
manuscript. All authors contributed to the manuscript and all
authors read and approved the final manuscript. ZZ and QZ confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of the First Hospital of Jiaxing (Jiaxing, China;
approval no. 2022-LY-243).
Patient consent for publication
The patient provided written informed consent for
the publication of this case report and all the accompanying
images.
Authors' information
Dr Zhibo Zuo; ORCID: 0000-0002-3222-4530 (https://orcid.org/0000-0002-3222-4530).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deniz K, Kahriman G, Koçyiğit İ, Ökten T
and Ünal A: Sclerosing extramedullary hematopoietic tumor. Turk J
Haematol. 35:209–210. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Woodward Z, Robertson T, Sim S and
Tollesson G: Intracranial sclerosing extramedullary haematopoietic
tumour mimicking meningioma in a patient with myelofibrosis: Case
report. J Clin Neurosci. 88:268–270. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Karaarslan S, Nese N, Oncel G, Ozsan N,
Akalin T, Kaplan H, Buyukkececi F and Hekimgil M: Sclerosing
extramedullary hematopoietic tumor mimicking intra-abdominal
sarcoma. J Pathol Transl Med. 49:335–338. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gualco G, Ojopi EB, Chioato L, Cordeiro
DL, Negretti F and Bacchi CE: Postsplenectomy sclerosing
extramedullary hematopoietic tumor with unexpected good clinical
evolution: Morphologic, immunohistochemical, and molecular analysis
of one case and review of the literature. Appl Immunohistochem Mol
Morphol. 18:291–295. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gu MJ: Sclerosing extramedullary
hematopoietic tumor presenting as an inguinal mass in a patient
with primary myelofibrosis: A diagnostic pitfall. Int J Clin Exp
Pathol. 8:3381–3383. 2015.PubMed/NCBI
|
|
6
|
Sezgin GC, Deniz K, Karahan Öİ and Gürsoy
Ş: Hepatobiliary and pancreatic: Sclerosing extramedullary
hematopoietic tumor involving hepatic hilum. J Gastroenterol
Hepatol. 34(817)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang D, Castro E, Rao A and McPhaul CM:
Sclerosing extramedullary hematopoietic tumor: A case report. J
Investig Med High Impact Case Rep: Sep 10, 2020 (Epub ahead of
print).
|
|
8
|
Dema S, Lazar F, Barna R, Dobrescu A, Dema
ALC, Popa O, Ionita I and Taban SM: Sclerosing extramedullary
hematopoietic tumor (SEHT) mimicking a malignant bile duct
tumor-case report and literature review. Medicina (Kaunas).
57(824)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
LeBlanc RE, Lester L, Kwong B and Rieger
KE: JAK2-positive cutaneous myelofibrosis presenting as sclerosing
extramedullary hematopoietic tumors on the scalp: Case presentation
and review of the literature. J Cutan Pathol. 42:858–862.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ghazi NG, Bowman AM and Shields MD:
Bilateral lacrimal system involvement by sclerosing extramedullary
hematopoietic tumor. Ophthalmic Plast Reconstr Surg. 22:296–298.
2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sukov WR, Remstein ED, Nascimento AG,
Sethi S and Lewin M: Sclerosing extramedullary hematopoietic tumor:
Emphasis on diagnosis by renal biopsy. Ann Diagn Pathol.
13:127–131. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yuen HK, Mahesh L, Tse RK, Yau KC, Chan N
and Lam DS: Orbital sclerosing extramedullary hematopoietic tumor.
Arch Ophthalmol. 123:689–691. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Klampfl T, Harutyunyan A, Berg T,
Gisslinger B, Schalling M, Bagienski K, Olcaydu D, Passamonti F,
Rumi E, Pietra D, et al: Genome integrity of myeloproliferative
neoplasms in chronic phase and during disease progression. Blood.
118:167–176. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rumi E, Harutyunyan A, Elena C, Pietra D,
Klampfl T, Bagienski K, Berg T, Casetti I, Pascutto C, Passamonti
F, et al: Identification of genomic aberrations associated with
disease transformation by means of high-resolution SNP array
analysis in patients with myeloproliferative neoplasm. Am J
Hematol. 86:974–979. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Babushok DV, Nelson EJ, Morrissette JJD,
Joshi S, Palmer MB, Frank D, Cambor CL and Hexner EO: Myelofibrosis
patients can develop extramedullary complications including renal
amyloidosis and sclerosing hematopoietic tumor while otherwise
meeting traditional measures of Ruxolitinib response. Leuk
Lymphoma. 60:852–855. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Barouqa M and McPhail ED: Sclerosing
extramedullary hematopoietic tumor in chronic myeloproliferative
neoplasms. Blood. 139(3345)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kwon Y, Yu E, Huh J, Lee SK and Ro JY:
Sclerosing extramedullary hematopoietic tumor involving lesser
omentum and ligamentumteres in liver explant. Ann Diagn Pathol.
8:227–232. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang X, Bhuiya T and Esposito M:
Sclerosing extramedullary hematopoietic tumor. Ann Diagn Pathol.
6:183–187. 2002.PubMed/NCBI View Article : Google Scholar
|