Introduction
At present, it is estimated that 48.9 million
patients worldwide have sepsis and related diseases and 19.7% of
all deaths globally are related to sepsis (1). Sepsis involves multiple processes,
including inflammation, immunity, coagulation and neuroendocrine
responses. The pathophysiological mechanism is complex and the
clinical manifestations of patients vary (2). Consequently, there is a lack of
rapid, sensitive and specific diagnostic biomarkers. Excessive
inflammation caused by infection is the defining feature of sepsis.
Inflammatory responses induce a ‘waterfall cascade‘ reaction;
therefore, early diagnosis and treatment are key to reduce the
in-hospital mortality of patients with sepsis.
To date, ~200 sepsis-associated biomarkers have been
reported in the literature, with novel biomarkers being identified.
Commonly used markers include acute-phase proteins [C-reactive
protein (CRP) and procalcitonin], cytokines (interleukin-6,
interleukin-10 and interleukin-8), cell surface proteins (human
leukocyte antigen-DR, soluble triggering receptor expressed on
myeloid cells-1 and soluble urokinase plasminogen activator
receptor), vascular endothelial cell-associated factors (cell
adhesion molecule, angiopoietin and endothelial cell specific
molecule-1) and coagulation-associated parameters (antithrombin
III, plasminogen activator inhibitor, mean platelet volume and
platelet count). Ideal biomarkers would be useful for early
diagnosis, risk stratification, prognosis assessment and treatment
response monitoring. Most existing markers lack a theoretical basis
for routine clinical practice because of inadequate sensitivity,
specificity or clinical evaluation ability (3-5).
Early diagnosis and effective treatment not only
improve the prognosis of sepsis but also decrease the mortality and
medical costs associated with sepsis. Several studies have shown
that platelets are key players in sepsis. For example, platelets
are involved in sepsis-associated inflammation, vascular
contracture, thrombosis and delayed tissue damage following
ischemia (6-9).
Therefore, the analysis of changes in platelets and associated
factors in sepsis can be used to assess the development of the
disease. In inflammatory and infectious conditions, commonly used
platelet parameters include platelet count, mean platelet volume,
platelet distribution width, platelet large cell ratio and
plateletcrit (PCT). Changes in these parameters are not only
associated with occurrence of inflammatory diseases such as
pneumonia, ankylosing spondylitis, Hashimoto's thyroiditis, acute
appendicitis, infective endocarditis and psoriasis (10-15),
but also regularly occur when the body is infected with COVID-19,
H7N9, malaria, melioidosis and dengue (16-20).
Our previous studies showed that certain bacterial pathogenic
factors (such as suilysin, pneumolysin and streptolysin) may induce
platelet activation, leading to increased expression of CD41a and
P-selectin (also known as CD62P) on the surface of platelets
(21,22).
Given the platelet-pathogen interactions shown in
our aforementioned studies, the proposed role of activated
platelets in multiorgan injury during sepsis and therapeutic
potential of platelets in sepsis treatment (21,22),
a prospective research method was applied in the present study to
collect blood samples considered relevant based on The Third
International Consensus Definitions for Sepsis and Septic Shock
(Sepsis-3) (23). A platelet
activation detection system using flow cytometry was established,
and platelet activation specificity (P-selectin expression),
apoptosis (loss of plasma membrane asymmetry and phosphatidylserine
exposure) and mitochondrial membrane potential (Mmp)-Index values
in patients diagnosed with sepsis were analyzed. Furthermore, the
correlations between these factors and the commonly used acute
physiology and chronic health evaluation II (APACHE II) and
sequential/sepsis-related organ failure assessment (SOFA) clinical
scores were analyzed (7,24).
As biomarkers, platelet-associated parameters
include not only biomolecules expressed by platelets but also
certain biomolecules that exist in the external environment and may
affect the physiological activity of platelets. Therefore, in the
present study, tumor necrosis factor (TNF)-like weak inducer of
apoptosis (TWEAK) and angiopoietin-2 (Ang-2) were considered to be
markers that are associated with platelets (25,26).
Finally, the findings of the present study were compared with
previous literature (27-30)
and case studies (25,30-34)
to evaluate these five parameters as potential biomarkers in sepsis
and to understand the dynamic evaluation of sepsis (correlations
with clinical scores) and patient prognosis. The present results
provide a foundation for subsequent analysis in large-sample,
prospective and multicenter studies.
Materials and methods
Study population
The present study was a retrospective analysis of
prospectively collected data from The Fifth Medical Center of the
Chinese People's Liberation Army General Hospital (Beijing, China)
from September 2017 to January 2019. The criterion for inclusion
was an initial diagnosis of sepsis; patients with traumatic
coagulopathy and primary blood system disorders were excluded.
Sepsis was defined according to the Sepsis-3 criteria, which
describes sepsis as the life-threatening organ dysfunction caused
by a dysregulated host response to infection (23). Organ dysfunction was indicated by
an increase in the SOFA score ≥2 points. Septic shock was defined
as a subset of sepsis in which persisting hypotension requiring
vasopressors to maintain a mean arterial pressure of ≥65 mmHg and
serum lactate levels >2 mmol/l despite adequate volume
resuscitation, were found. Additionally, the APACHE II scoring
system was utilized, which quantifies and evaluates the degree of
abnormality in a number of physiological parameters and is widely
used because its disease severity classification system is based on
objective physiological parameters. To diagnose and clinically
assess the condition of patients, SOFA scores for sepsis and septic
shock were utilized. The severity of illness was assessed with
APACHE II and SOFA scores on the days of blood collection. The
present study was approved by the Medical Ethics Committee of The
Fifth Medical Center, Chinese People's Liberation Army General
Hospital (approval no. ky-2019-1-4; Beijing, China) and all
subjects provided written informed consent. All procedures
involving human participants were performed in accordance with The
Declaration of Helsinki.
Blood samples
From September 2017 to January 2019, the development
of initial suspected sepsis was tracked in 96 patients (age, 18-71
years; 40 males and 56 females). As controls, 25 healthy adult
volunteers (age, 25-56 years; 17 males and 8 females) were
recruited at the same time, according to local laws and regulations
(21,22). A total of ~2 ml blood was collected
within the first 48 h of hospital admission from all patients and
the blood was also collected from healthy adult volunteers. To
dynamically analyze the laboratory parameters, blood samples were
also obtained from each patient at the treatment endpoint, in
accordance with clinical assessment of the patient by the critical
care team. The end of therapy (before discharge or death) was the
preferred time for treatment endpoint blood extraction. After
patients reached the treatment endpoint, the test results of
laboratory parameters from blood samples meeting the criterion for
inclusion were analyzed. Notably, unlike previous studies (31-33)
in which blood was collected at a fixed time (typically 7 or 14
days after admission) and compared with admission samples, the
present study directly collected samples at the treatment endpoint
after fully considering the dynamic changes in the individual
condition and uncertain characteristics of prognosis of each
patient. Platelet-rich plasma (PRP) and plasma samples were
separated for further analysis. Approximately half of the blood
sample was used to separate PRP and the remaining fraction was used
to separate plasma. The anti-coagulated blood was centrifuged for
10 min at 146 x g at room temperature to obtain PRP, then
centrifuged for 10 min at 1,200 x g to obtain plasma. Plasma was
stored at -70˚C until further use.
Platelet activation
P-selectin (CD62P), an indicator of platelet
activation, is stored in α-granules of platelets and rapidly
transported to the plasma membrane upon activation. P-selectin is
hypothesized to mediate the initial adhesive interactions of
activated platelets to neutrophils and monocytes during hemostasis.
Phycoerythrin (PE)-conjugated CD62P monoclonal antibody (catalogue
number: 555524, BD Biosciences) was used to detect changes in
platelet activation, according to the manufacturer's instructions.
Briefly, 100 µl blood and 15 µl PE-conjugated CD62P monoclonal
antibody were co-incubated for 30 min at 37˚C in the dark. Blood
samples were immediately prepared for flow cytometry using OptiLyse
C No-Wash Lysing Solution (catalogue number: A11895, Beckman
Coulter, Inc.) according to the manufacturer's instructions. Before
analysis with a flow cytometer (BD Accuri C6) and FlowJo v.10.7.
(BD Biosciences), the samples were filtered with a 70-µm cell
strainer (BD Biosciences). A total of 20,000 events were acquired
and platelets were detected in the platelet-specific gate (35) and channels (FL2) by flow cytometry.
An increase in mean FL2 represented enhanced platelet activation. A
parallel incubation in which PE-labeled mouse IgG κ isotype control
(BD Biosciences) was added instead of PE-conjugated CD62P
monoclonal antibody was used as a negative control. Results were
calculated as the mean of triplicate readings for each patient.
Platelet Mmp
Depolarization of Mmp (ΔΨm) is observed during early
apoptosis (31). Changes in
platelet Mmp were determined by staining with cationic dye
5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolylcarbocyanine
iodide (JC-1; Molecular Probes; Thermo Fisher Scientific, Inc).
Specifically, 50 µl PRP was incubated with 500 µl JC-1 working
solution at 37˚C with 5% CO2 for 10 min in the dark.
Following filtration with a 70-µm cell strainer, samples were
analyzed with a flow cytometer (BD Biosciences) Accuri C6) and
FlowJo v.10.7. (BD Biosciences). A total of 20,000 events were
acquired and platelets were detected in the platelet-specific gate.
The results are presented as a ratio of the mean FL2 and FL1;
decrease in the FL2:FL1 ratio (Mmp-Index) indicated a loss in Mmp
(31). A parallel incubation in
which carbonyl cyanide m-chlorophenylhydrazone (MedChemExpress) at
25 µM was added instead of JC-1 as control.
Platelet plasma membrane
asymmetry
Platelet apoptosis is characterized by certain
morphological features, including loss of plasma membrane asymmetry
and phosphatidylserine exposure (25,36).
Changes in platelet plasma membrane asymmetry were measured using
allophycocyanin (APC)-conjugated annexin V (BD Biosciences) binding
to phosphatidylserine translocated from the inner to the outer
leaflet of the plasma membrane. For the detection of
phosphatidylserine exposure, 50 µl PRP was incubated with 5 µl
APC-conjugated annexin V and 10 µl fluorescein isothiocyanate
(FITC)-conjugated CD41a monoclonal antibody (catalogue number:
555466, BD Biosciences) for 30 min at room temperature in the dark.
Following filtration with a 70-µm cell strainer, samples were
analyzed with flow cytometer (BD Accuri C6) and FlowJo v.10.7. (BD
Biosciences). A total of 20,000 events were acquired and platelets
were detected in the platelet-specific gate (32). The gates for intact platelets were
set using the FITC-conjugated CD41a antibody. An increase in mean
FL4 indicated enhanced platelet apoptosis. The mean of triplicate
readings for each patient was recorded.
Analysis of TWEAK and Ang-2 plasma
levels
TWEAK is involved in various inflammatory responses,
including promoting apoptosis and neovascularization, as well as
inducing expression of endothelial cell adhesion molecules and
pro-inflammatory cytokines (25).
Ang-2 belongs to a family of vascular growth factors and is
associated with disrupted vascularization, promoted cell death and
neovascularization (26). The
plasma levels of TWEAK and Ang-2 were investigated using
commercially available ELISA kits (Human TWEAK ELISA kit, Catalogue
number: ELH-TWEAK; Human ANGPT2 ELISA kit, Catalogue number:
ELH-Angiopoietin2; RayBiotech, Inc.), according to the
manufacturer's instructions.
Statistical analysis
Normally distributed data are expressed as the mean
± standard deviation of triplicate readings. Differences between
groups were analyzed using unpaired Student's t-test, one-way ANOVA
followed by Tukey's multiple comparison test or mixed ANOVA
followed by Bonferroni's multiple comparison method. Receiver
operator characteristic (ROC) curves were used to identify
association between biomarkers and diagnosis (sepsis or septic
shock). Correlations between laboratory parameters and clinical
disease score were calculated using Spearman's rank correlation
coefficients. Statistical analysis was performed using SPSS
software (IBM Corp.; version 22.0) and GraphPad Prism 9 (GraphPad
Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of subjects
When combining the results of clinical diagnosis,
patient prognosis and sample collection, 39 samples from patients
without sepsis and 26 patients lacking blood samples from the
treatment endpoint were. A total of 31 patients (mean age,
66.00±15.51 years; 19 males and 12 females) with sepsis or septic
shock were included in the study (Table I). The overall mortality rate was
51.61% (16 patients were deceased). The mean APACHE II and SOFA
scores were 21.48±5.82 and 8.06±2.99, respectively. The mean
platelet count in the patients was 158.39±94.0x109/l.
The cause of disease was pulmonary infection in 24 patients,
urinary infection in two patients, abdominal infection in one
patient and bloodstream infection in four patients. The 25 healthy
controls comprised 17 males and 8 females with a mean age of
39.20±9.46 years. Additionally, considering the possible impact of
the age difference between patient and control groups on the
results, its influence was clarified through correlation analysis.
There were no significant correlations between patient age and
other platelet-associated parameters in patients with sepsis
(Fig. S1). The study design is
illustrated in Fig. 1, and
representative flow cytometry dot plots of platelet-associated
markers are shown in Fig. S2.
 | Table IDemographic and clinical
characteristics of subjects included in analysis. |
Table I
Demographic and clinical
characteristics of subjects included in analysis.
| Characteristic | Sepsis (n=19) | Septic shock
(n=12) | Control (n=25) |
|---|
| Mean age,
years | 66.94±14.52 | 64.58±17.46 | 39.20±9.46 |
| Sex, n | | | |
|
Male | 10 | 9 | 17 |
|
Female | 9 | 3 | 8 |
| Non-survivors,
n | 9 | 7 | NA |
| APACHE II
score | 21.16±6.23 | 22.00±5.33 | NA |
| SOFA score | 7.53±3.01 | 8.92±2.87 | NA |
| Mean platelet
count, x1011/l | 1.69±0.83 | 1.41±1.09 | 1.86±0.44 |
| Site of infection,
n | | | |
|
Pulmonary | 15 | 9 | NA |
|
Urinary | 1 | 1 | NA |
|
Abdominal | 1 | 0 | NA |
|
Bloodstream | 2 | 2 | NA |
Laboratory parameters of patients with
sepsis and septic shock
Parameters were significantly different between the
patients and healthy controls (P-values ranged from <0.0001 to
0.0330; Table II). ROC curve
(Fig. S3) revealed the following
area under the curve (AUC) values: Platelet activation, 1.0
(P<0.0001); Mmp-Index, 0.76 (P=0.0009); apoptosis of platelets,
0.96 (P<0.0001); plasma TWEAK level, 0.72 (P=0.0055) and plasma
Ang-2 level, 0.92 (P<0.0001). Furthermore, patients with septic
shock exhibited higher levels of phosphatidylserine on the surface
of platelets than patients with sepsis (P=0.0004).
 | Table IILaboratory parameters. |
Table II
Laboratory parameters.
| | P-value |
|---|
| Parameter | Sepsis | Septic shock | Control | Sepsis vs.
control | Septic shock vs.
control | Sepsis vs. septic
shock |
|---|
| Platelet activation
(CD62P-PE) | 1,196.9±543.5 | 1,215.2±723.6 | 328.9±16.8 | <0.0001 | <0.0001 | 0.9940 |
| Mmp-Index | 2.9±1.0 | 2.1±1.4 | 3.8±0.8 | 0.0330 | <0.0001 | 0.0490 |
| Apoptosis (Annexin
V-APC) | 398.4±48.7 | 498.8±125.2 | 333.6±24.3 | 0.0060 | <0.0001 | 0.0004 |
| TWEAK, pg/ml | 158.6±63.1 | 165.0±52.6 | 273.4±145.9 | 0.0050 | 0.0070 | 0.9560 |
| Ang-2, ng/ml | 5.8±4.7 | 7.6±4.9 | 1.4±0.6 | 0.0040 | <0.0001 | 0.0020 |
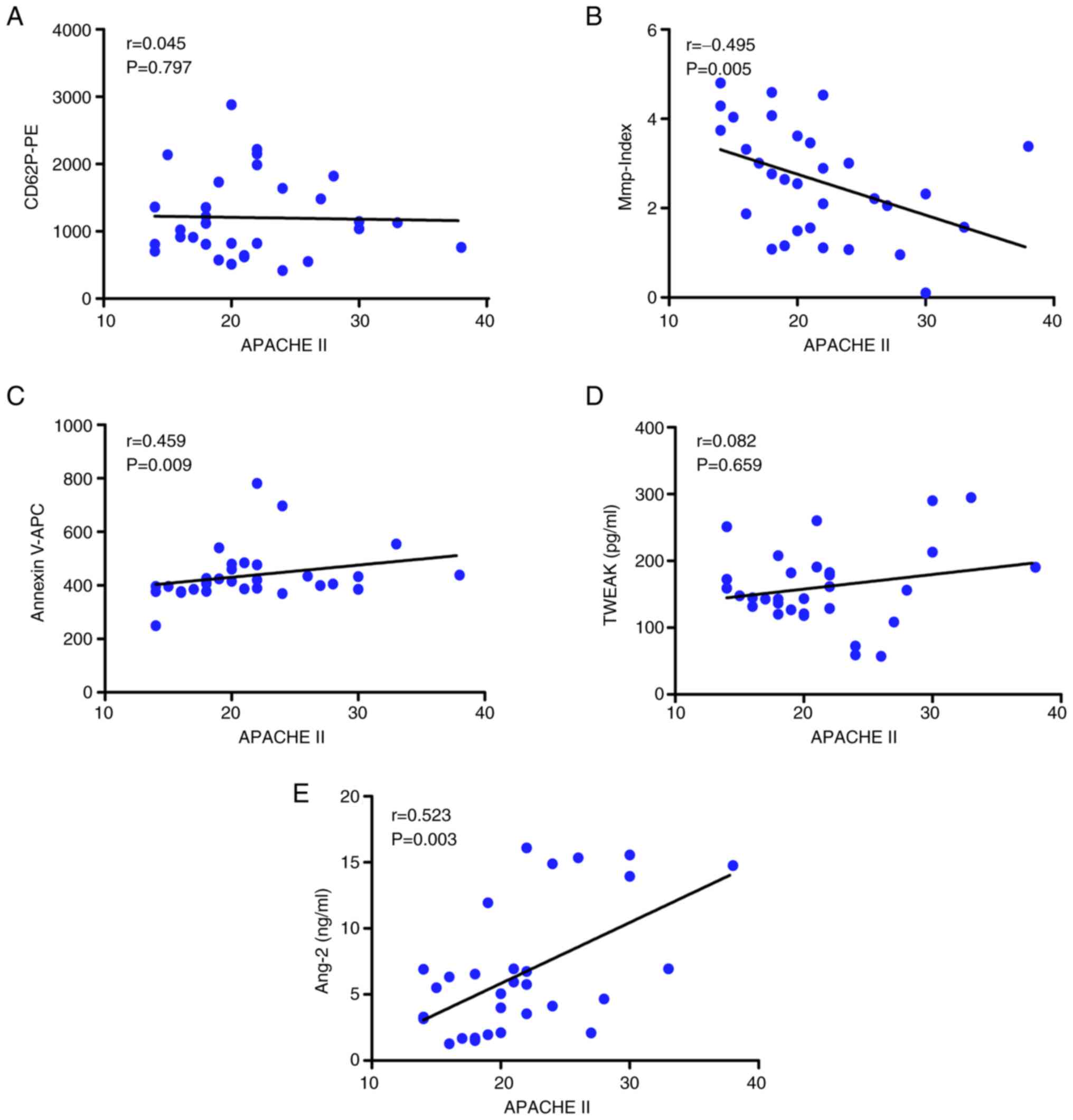
Correlation between laboratory
parameters and APACHE II scores
When stratifying patients with sepsis and septic
shock by clinical condition, the APACHE II score of patients was
significantly correlated with platelet Mmp-Index (r=-0.495,
P=0.005; Fig. 2B),
phosphatidylserine exposure (r=0.459, P=0.009; Fig. 2C) and Ang-2 levels (r=0.523,
P=0.003; Fig. 2E). Therefore, the
changes in these three parameters reflected disease status and were
correlated with APACHE II score. By contrast, there were no
significant correlations with APACHE II score for the other two
parameters, namely platelet activation (r=0.045, P=0.797) and
plasma TWEAK levels (r=0.082, P=0.659; Fig. 2A and D).
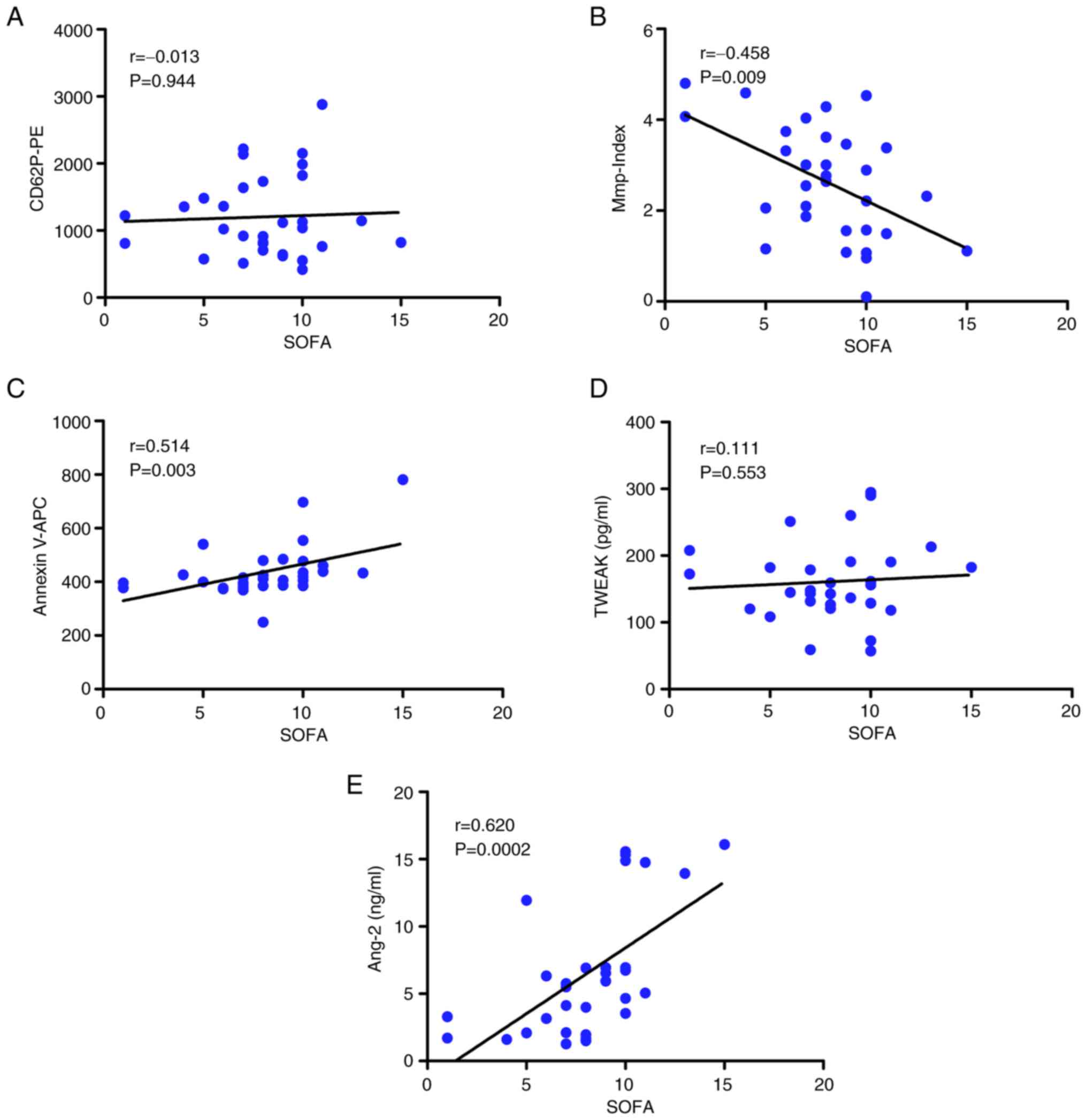
Correlation between laboratory
parameters and SOFA scores
The SOFA score of patients (sepsis and septic shock
groups) was significantly correlated with platelet Mmp-Index
(r=-0.458, P=0.009; Fig. 3B),
phosphatidylserine exposure (r=0.514, P=0.003; Fig. 3C) and Ang-2 levels (r=0.620,
P=0.0002; Fig. 3E). SOFA score was
not significantly correlated with platelet activation (r=-0.013,
P=0.944) or plasma TWEAK levels (r=0.111, P=0.553; Fig. 3A and D).
Correlation between laboratory
parameters and clinical disease outcome
The prognostic determination in sepsis is key;
therefore the present study analyzed the correlation between
specific detection indicators and clinical outcomes to provide a
basis for assessing clinical progression of the disease.
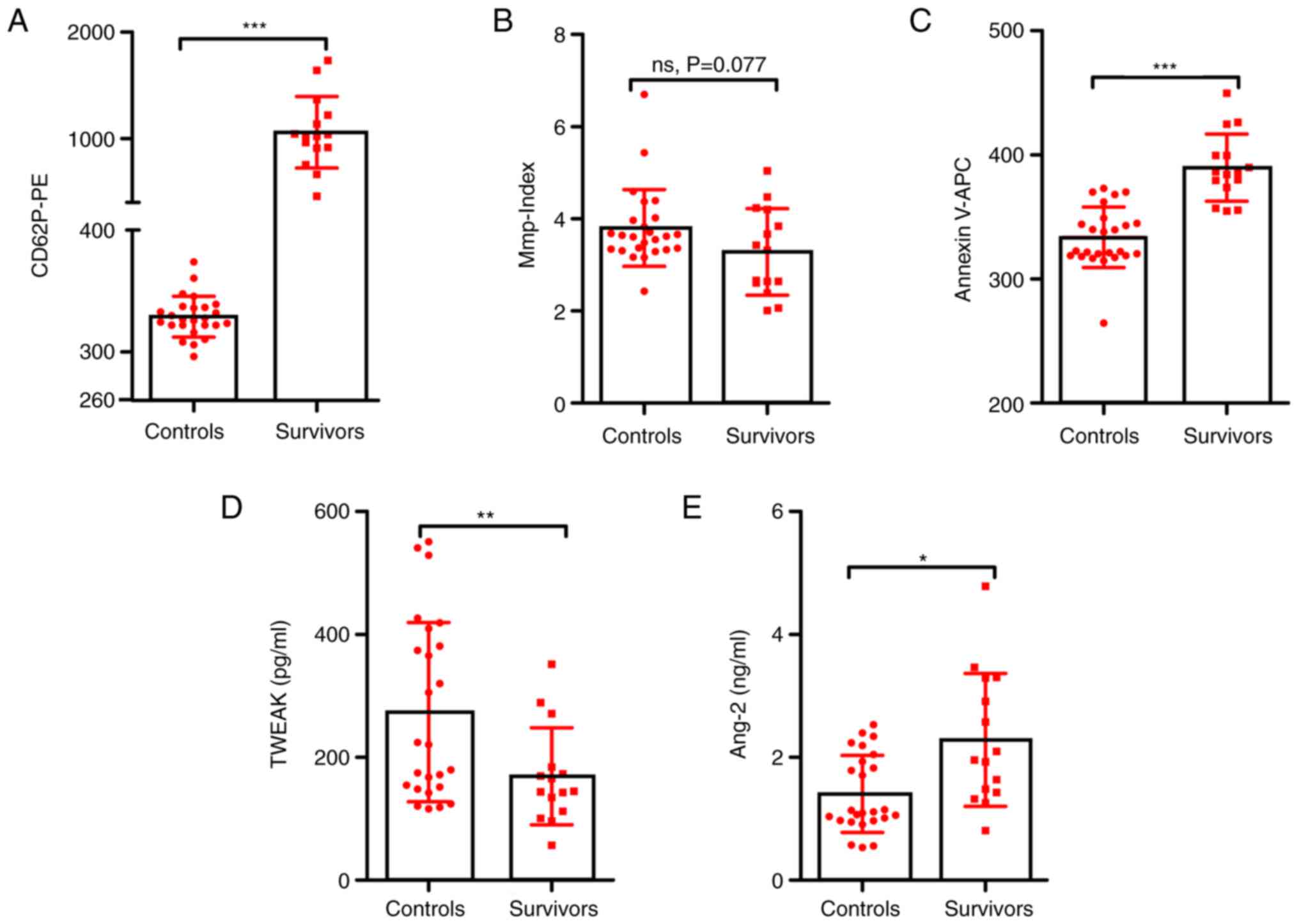
Laboratory parameters of all participants were
compared between the surviving patients and the healthy (control)
group. There were significant between-group differences in platelet
activation (1,060.13±336.18 vs. 328.94±16.82, P<0.001; Fig. 4A), phosphatidylserine exposure
(389.68±27.15 vs. 333.55±24.27, P<0.001; Fig. 4C) and plasma levels of TWEAK
(169.04±79.03 vs. 273.41±145.98, P=0.006; Fig. 4D) and Ang-2 (2.28±1.08 vs.
1.40±0.62, P=0.01; Fig. 4E), but
not in platelet Mmp-Index values (3.2807±0.94 vs. 3.80±0.84,
P=0.077; Fig. 4B). However, in
patients with sepsis, no significant correlations were detected
between patient age and platelet activation (r=-0.147, P=0.437),
Mmp-Index values (r=-0.128, P=0.499), phosphatidylserine exposure
(r=0.264, P=0.158) or plasma levels of TWEAK (r=0.312, P=0.093) or
Ang-2 (r=0.148, P=0.436; Fig.
S1). These results indicated that notable changes in the
parameters had occurred within a short period after disease onset
in the survivor group and between-group differences were not
attributable to age difference.
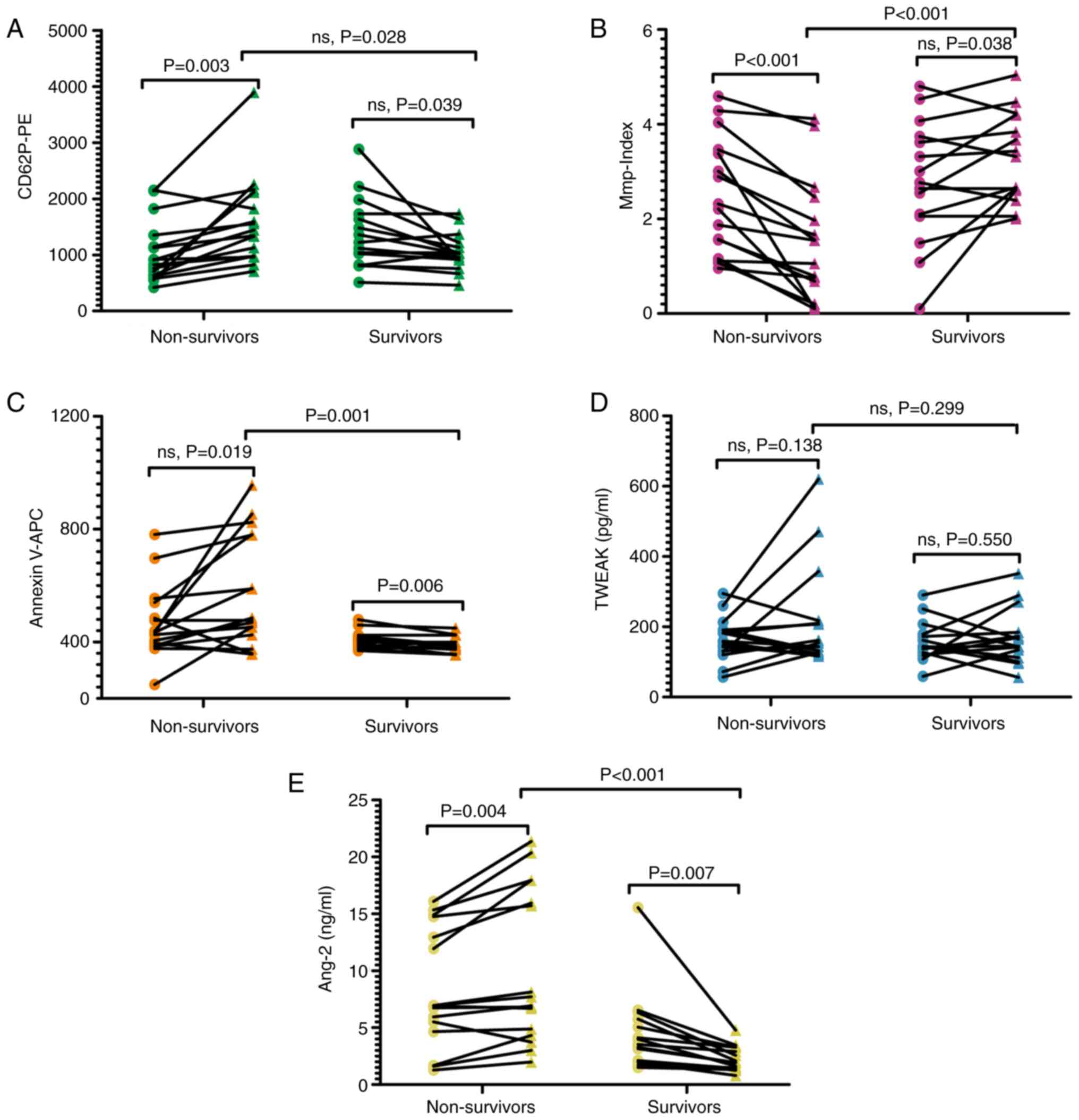
Samples were grouped by clinical disease outcomes in
the treatment endpoint group and a mixed ANOVA with clinical
disease outcomes (level 2, survivors and non-survivors) as a
between-subject comparison and sample extraction time (level 2,
admission and treatment endpoint) as a within-subject comparison
was used (Tables SI and SII). The results revealed significant
group-by-time interactions for platelet activation level (F=17.188,
P<0.001), Mmp-Index (F=24.855, P<0.001), phosphatidylserine
exposure (F=8.410, P=0.007) and Ang-2 (F=21.055, P<0.001). No
significant group-by-time interactions for TWEAK (F=1.036, P=0.317)
were found (Tables SII). Simple
effects analysis was performed for clinical outcome at each level
of time, with an α level of 0.0125 for each test. The results
revealed significant differences in platelet Mmp-Index
(P<0.001), phosphatidylserine exposure (P=0.001) and plasma
Ang-2 levels (P<0.001) between survivors and non-survivors. By
contrast, platelet activation (P=0.028) and plasma TWEAK levels
(P=0.299) were not significantly different between survivors and
non-survivors (Fig. 5A-E; Table SI). Furthermore, among the
survivors, the admission and treatment endpoint groups were
compared; phosphatidylserine exposure (P=0.006) and plasma Ang-2
levels (P=0.007) were significantly different (Fig. 5C and E). However, there were no significant
differences in platelet activation (P=0.039) and Mmp-Index values
(P=0.038) or plasma TWEAK levels (P=0.550; Fig. 5A, B and D).
In the group of non-survivors, there were significant differences
in platelet activation (P=0.003) and Mmp-Index values (P<0.001)
and plasma Ang-2 (P=0.004; Fig.
5A, B and E), but not in phosphatidylserine exposure
(P=0.019) or plasma levels of TWEAK (P=0.138; Fig. 5C and D).
This suggested that platelet activation and
Mmp-Index and plasma Ang-2 in patients in non-survivors was
significantly different compared with when they were admitted to
hospital. Additionally, due to the limited sample size of the
present study, the potential impact of sex differences was
analyzed. Sex had no effect on the results (Fig. S4; Tables SIII and SIV).
Discussion
Although critical care technologies have improved,
the prevalence and fatality rates of sepsis are still increasing
(1,2). A frequent complication of sepsis is
the development of organ dysfunction (1,2).
Platelets are implicated in endothelial damage and take part in the
pathogenesis of tissue damage in sepsis. For example, in
sepsis-induced acute kidney injury, leukocytes and platelet
adhesion dysfunction induce renal microvascular alterations
(8). The pathogenesis of sepsis is
complicated and has not yet been fully elucidated. As an
independent risk factor, thrombocytopenia is used to predict
prognosis of critically ill patients, and platelets play an
important role in thrombocytopenia, sepsis inflammation and blood
coagulation (37). Therefore,
platelet-associated parameters may provide effective biomarkers for
disease prognosis.
Platelet activation and sepsis are connected and
activated platelets undergo apoptosis (3,6,25).
The present study involved three apoptosis-associated parameters,
namely platelet Mmp-Index, phosphatidylserine exposure and plasma
TWEAK levels. The mitochondria-mediated intrinsic pathway and death
receptor-mediated extrinsic pathway are the primary initiators of
apoptosis. During the early stages of apoptosis induced by the
intrinsic pathway, Mmp depolarization is often observed, leading to
changes in certain morphological features, including loss of plasma
membrane asymmetry and phosphatidylserine exposure (38). TWEAK belongs to the TNF receptor
superfamily and induces platelet apoptosis via the extrinsic
pathway (25). Therefore, in
addition to analysis of platelet Mmp-Index values and
phosphatidylserine exposure, TWEAK was assessed.
To the best of our knowledge, the present study is
the first to demonstrate that the detected parameters had
specificity as biomarkers for diagnosing sepsis in all patients.
Additionally, correlations were observed between phosphatidylserine
exposure, platelet Mmp-Index values, plasma Ang-2 levels and
clinical scores.
ROC curve analysis demonstrated that the platelet
activation level was more powerful than the other parameters for
prediction of sepsis and septic shock in patients. The AUC of the
platelet activation level was 1.0 with an optimal cut-off of 359.5.
By comparison, AUCs of platelet Mmp-Index, apoptosis of platelets,
and plasma levels of TWEAK and Ang-2 were 0.76, 0.96, 0.72 and
0.92, respectively. This analysis supports the conclusion that
platelet activation may be a useful risk factor in sepsis.
Meta-analyses have shown that the cut-off value of plasma Ang-2 as
a biomarker is 3.2 ng/ml (33,39).
The present study found a cut-off value of plasma Ang-2 of 2.85
ng/ml. This discrepancy may be due to the criteria for volunteers
that were included. Therefore, further large-sample, multicenter
studies are required to verify these results.
In the present study, P-selectin expression on
platelets and plasma TWEAK levels were not correlated with clinical
score. One possible explanation for these results is that the
enrolled patients had different basic diseases. In patients with
sepsis who undergo direct hemoperfusion with polymyxin B
immobilized cartridge treatment, TNF expression and serum release
are enhanced during inflammation (25). However, animal experiments suggest
that TWEAK is stably expressed in multiple tissues, and its mRNA
expression level is downregulated in autoimmune disease (34,35).
In line with these results, the present study showed a decrease in
plasma TWEAK levels between the admission and treatment endpoint
groups Certain studies have shown that, although TWEAK is
associated with TNF superfamily ligands and metabolic status of
patients, there is no correlation between TWEAK and body mass
index, age, sex or underlying disease (25,40).
This indicates that decreased plasma TWEAK levels are a general
feature of sepsis. TWEAK levels have been shown to be independent
of disease stage (34,40).
The results of the current study are different from
those reported by Gründler et al (31). In their study, significant recovery
of platelet Mmp-Index was observed in the group of survivors (0.235
to 0.9), whereas persistently low platelet Mmp-Index values
(<0.5) were recorded in non-survivors group. The inconsistencies
between these results may be associated with multiple factors.
Firstly, the criteria for assessing the study groups were
different. In the present study, data was collected from the
treatment endpoint, which is different from the 7th day of
admission to the hospital used in the aforementioned study.
Secondly, the grouping of patients between was different. The
present study used the Sepsis-3 criteria, in which severe sepsis is
not included as a concept.
There is an interaction between endothelial cells
and platelets. The structural stability of endothelial cells is
associated with changes in platelet function (41). Among the endothelial angiopoietins
associated with stability of the vascular endothelium structure,
Ang-1 and Ang-2 have been studied in depth (42,43).
There are several reports on Ang-2/Ang-1, Ang-1/TEK tyrosine kinase
(Tie-2) and Ang-2/Tie-2 as prognostic biomarkers (41,44,45).
The extensive loss of vascular endothelial function is related to
the severity of disease (44-46).
Kümpers et al (32)
injected lipopolysaccharide (4 ng/kg) into 22 healthy volunteers
and found that the Ang-2 levels in their blood reached a peak of
4.5 h after injection. Ang-2 levels also peaked earlier than
endothelial cell-specific adhesion molecules, such as E-selectin
and intercellular adhesion molecule-1, demonstrating that Ang-2
responded faster than other sepsis-associated biomarkers and
therefore had advantages in the early diagnosis of sepsis. The
results of the present study demonstrated that patients who
survived had significantly lower post-treatment plasma Ang-2 levels
compared with those measured on admission to the hospital. These
results are consistent with previous studies (32,41,45),
suggesting that the use of plasma Ang-2 alone for prognostic
evaluation is effective. Additionally, a meta-analysis by Liu et
al (46) indicated that the
sensitivity of Ang-2 level alone (82%) for sepsis diagnosis is
higher than the sensitivities of the current commonly used
(46,47) clinical markers PCT (79%) and CRP
(75%).
Here, the results on platelet phosphatidylserine
exposure were notable. This parameter is associated with the
pathological mechanism of sepsis, which is evident by its
correlation with clinical scores. However, there was no significant
difference in the detection value of phosphatidylserine exposure in
patients with sepsis with clinical outcomes at admission. Levels of
phosphatidylserine in platelets was found to be significantly lower
in survivors than in non-survivors. This suggested that sepsis
aggravated apoptosis of platelets in patients with different
clinical outcomes. Therefore, the potential use of platelet
phosphatidylserine exposure as a biomarker for prognosis of sepsis
needs to be verified with a larger number of samples.
Because of the limited sample size, the patients and
controls in the present study were not completely matched for sex
and age. However, there were no significant correlations between
patient age or sex and other platelet-associated parameters in
patients with sepsis. Certain reports have indicated that
expression levels of cytokines, such as TWEAK, are correlated with
the sex and age of patients with sepsis (40,41,44).
Therefore, these inconsistencies need to be further evaluated in a
study with a larger number of samples.
In summary, sepsis is a complicated disease due to
the combined effects of bacterial toxins, inflammatory mediators,
reactive oxygen species and imbalances between energy supply and
demand (1,4-6).
Here, the dynamic monitoring of phosphatidylserine exposure,
platelet Mmp-Index values and plasma Ang-2 levels was effective for
prognosis assessment of sepsis.
Supplementary Material
Correlation of laboratory parameters
with patient age at admission. Platelet (A) activation, (B)
Mmp-Index and (C) apoptosis. Plasma (D) TWEAK and (E) Ang-2 levels.
P<0.05 was considered to indicate a statistically significant
difference; r-values indicate correlation coefficient. Mmp,
mitochondrial membrane potential; TWEAK, tumor necrosis factor-like
weak inducer of apoptosis; Ang-2, angiopoietin-2; APC,
allophycocyanin; PE, phycoerythrin.
Representative flow cytometry dot
plots of platelet-associated markers. G1 gate was set based on the
reaction of the platelets with CD62P-PE. Q2 gate was set based on
the reaction of the platelets with FITC-conjugated CD41a
antibody/APC-conjugated annexin V. P1 and P2 gates were set based
on the reaction of the platelets with cationic dye JC-1. Healthy
indicates the healthy control group. SSC-A, side scatter-area; APC,
allophycocyanin; PE, phycoerythrin; FITC, fluorescein
isothiocyanate; Mmp, mitochondrial membrane potential.
ROC curves of laboratory parameters
for the diagnosis of sepsis and septic shock. Platelet (A)
activation, (B) Mmp-Index and (C) apoptosis. Plasma (D) TWEAK and
(E) Ang-2 levels. ROC, receiver operator curve; AUC, area under the
curve; Mmp, mitochondrial membrane potential; TWEAK, tumor necrosis
factor-like weak inducer of apoptosis; Ang-2, angiopoietin-2; APC,
allophycocyanin; PE, phycoerythrin. P<0.05 was considered to
indicate a statistically significant difference.
Laboratory parameters of males and
females in the patient group. Platelet (A) activation, (B)
Mmp-Index and (C) apoptosis. Plasma (D) TWEAK and (E) Ang-2 levels.
The solid circles and triangles represent admission and treatment
endpoint, respectively. Simple effects analysis was conducted at
each level of sample extraction time (admission and treatment
endpoint), with an α level of 0.0125 for each test. Mmp,
mitochondrial membrane potential; TWEAK, tumor necrosis factor-like
weak inducer of apoptosis; Ang-2, angiopoietin-2; ns, not
significant.
Analysis of platelet-related
parameters between survivors and non-survivors.
Repeated measures of platelet-related
parameters between groups (non-survivors vs. survivors).
Analysis of platelet-related
parameters between males and females.
Repeated measures of platelet-related
parameters between group factor (male vs. female).
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Science
and Technology Major Project (grant no. 2018ZX10713002-002-004),
the National Natural Science Foundation of China (grant no.
81571959) and the Hebei Medical Science Research Project Plan
(grant no. 20220408).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CZ, YY and YL designed the study and revised the
manuscript. CZ and XS confirm the authenticity of all the raw data.
CZ, YY and XS contributed to acquisition, analysis and
interpretation of the data. CZ and YY wrote the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of The Fifth Medical Center, Chinese People's Liberation
Army General Hospital (approval no. ky-2019-1-4; Beijing, China)
and all subjects provided their written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rudd KE, Johnson SC, Agesa KM, Shackelford
KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer
S, et al: Global, regional, and national sepsis incidence and
mortality, 1990-2017: Analysis for the Global Burden of Disease
Study. Lancet. 395:200–211. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
An G and Cockrell RC: Agent-based modeling
of systemic inflammation: A pathway toward controlling sepsis.
Methods Mol Biol. 2321:231–257. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kataria Y and Remick D: Sepsis Biomarkers.
Methods Mol Biol. 2321:177–189. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cantey JB and Lee JH: Biomarkers for the
diagnosis of neonatal sepsis. Clin Perinatol. 48:215–227.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Odum JD, Wong HR and Stanski NL: A
Precision medicine approach to biomarker utilization in pediatric
sepsis-associated acute kidney injury. Front Pediatr.
9(632248)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dewitte A, Lepreux S, Villeneuve J,
Rigothier C, Combe C, Ouattara A and Ripoche J: Blood platelets and
sepsis pathophysiology: A new therapeutic prospect in critically
[corrected] ill patients. Ann Intensive Care. 7(115)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Al Saleh K and Al Qahtani RM: Platelet
count patterns and patient outcomes in sepsis at a tertiary care
center: Beyond the APACHE score. Medicine (Baltimore).
100(e25013)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bouglé A and Duranteau J: Pathophysiology
of sepsis-induced acute kidney injury: The role of global renal
blood flow and renal vascular resistance. Contrib Nephrol.
174:89–97. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ge S, Ma Y, Xie M, Qiao T and Zhou J: The
role of platelet to mean platelet volume ratio in the
identification of adult-onset still's disease from sepsis. Clinics
(Sao Paulo). 76(e2307)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
You S, Sun X, Zhou Y, Zhong C, Chen J,
Zhai W and Cao Y: The prognostic significance of white blood cell
and platelet count for inhospital mortality and pneumonia in acute
ischemic stroke. Curr Neurovasc Res. 18:427–434. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Beyan C and Beyan E: Is mean platelet
volume and inflammatory activity really correlated in patients with
ankylosing spondylitis. Mod Rheumatol. 30(410)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Demir AD: Relationship of the platelet
distribution width/platelet count ratio with thyroid antibody
levels in patients with Hashimoto's thyroiditis. J Int Med Res.
49(3000605211043241)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shen G, Li S, Shao Z, Liu L, Liu Q, Yu H,
Wang H and Mei Z: Platelet indices in patients with acute
appendicitis: A systematic review with meta-analysis. Updates Surg.
73:1327–1341. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhou Y, Wei F and Liu C: Mean platelet
volume/platelet count ratio predicts long-term mortality in
patients with infective endocarditis. Biomark Med. 14:823–827.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li L, Yu J and Zhou Z: Platelet-associated
parameters in patients with psoriasis: A PRISMA-compliant
systematic review and meta-analysis. Medicine (Baltimore).
100(e28234)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jiang SQ, Huang QF, Xie WM, Lv C and Quan
XQ: . The association between severe COVID-19 and low platelet
count: Evidence from 31 observational studies involving 7613
participants. Br J Haematol. 190:e29–e33. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen Y, Yang Y, Cheng J, Lu J and Hu W:
Platelet count and mortality of H7N9 infected patients in
Guangdong, China. Platelets. 31:268–271. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Leli C, Di Matteo L, Gotta F, Vay D,
Cavallo V, Mazzeo R, Busso S, Carrabba L and Rocchetti A: Clinical
utility of platelet count for screening of malaria. New Microbiol.
43:89–92. 2020.PubMed/NCBI
|
|
19
|
Kirby P, Smith S, Ward L, Hanson J and
Currie BJ: Clinical Utility of Platelet Count as a Prognostic
Marker for Melioidosis. Am J Trop Med Hyg. 100:1085–1087.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shah D, Khataniar M, Sawhney A, Nautiyal
M, Desai R and Kakar A: An observational study to see the
correlation between trends of platelet counts and immature platelet
fraction in dengue infection. Trop Doct. 51:378–381.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang S, Zheng Y, Chen S, Huang S, Liu K,
Lv Q, Jiang Y and Yuan Y: Suilysin-induced platelet-neutrophil
complexes formation is triggered by pore formation-dependent
calcium Influx. Sci Rep. 6(36787)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang S, Wang J, Chen S, Yin J, Pan Z, Liu
K, Li L, Zheng Y, Yuan Y and Jiang Y: Effects of suilysin on
streptococcus suis-induced platelet aggregation. Front Cell Infect
Microbiol. 6(128)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang H, Kang X, Shi Y, Bai ZH, Lv JH, Sun
JL and Pei HH: SOFA score is superior to APACHE-II score in
predicting the prognosis of critically ill patients with acute
kidney injury undergoing continuous renal replacement therapy. Ren
Fail. 42:638–645. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nagai M, Hirayama K, Ebihara I, Higuchi T,
Imaizumi M, Maruyama H, Miyamoto Y, Kakita T, Ogawa Y, Fujita S, et
al: Serum TNF-related and weak inducer of apoptosis levels in
septic shock patients. Ther Apher Dial. 15:342–348. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Michels M, van der Ven AJ, Djamiatun K,
Fijnheer R, de Groot PG, Griffioen AW, Sebastian S, Faradz SM and
de Mast Q: Imbalance of angiopoietin-1 and angiopoetin-2 in severe
dengue and relationship with thrombocytopenia, endothelial
activation, and vascular stability. Am J Trop Med Hyg. 87:943–946.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Karabulut B and Alatas SO: Diagnostic
Value of neutrophil to lymphocyte ratio and mean platelet volume on
early onset neonatal sepsis on term neonate. J Pediatr Intensive
Care. 10:143–147. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
McDonald B and Dunbar M: Platelets and
Intravascular Immunity: Guardians of the vascular space during
bloodstream infections and sepsis. Front Immunol.
10(2400)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mangalesh S, Dudani S and Malik A:
Platelet Indices and their kinetics predict mortality in patients
of sepsis. Indian J Hematol Blood Transfus. 24:1–9. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tunjungputri RN, van de Heijden W, Urbanus
RT, de Groot PG, van der Ven A and de Mast Q: Higher platelet
reactivity and platelet-monocyte complex formation in Gram-positive
sepsis compared to Gram-negative sepsis. Platelets. 28:595–601.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gründler K, Angstwurm M, Hilge R, Baumann
P, Annecke T, Crispin A, Sohn HY, Massberg S and Kraemer BF:
Platelet mitochondrial membrane depolarization reflects disease
severity in patients with sepsis and correlates with clinical
outcome. Crit Care. 18(R31)2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kümpers P, van Meurs M, David S, Molema G,
Bijzet J, Lukasz A, Biertz F, Haller H and Zijlstra JG: Time course
of angiopoietin-2 release during experimental human endotoxemia and
sepsis. Crit Care. 13(R64)2009.PubMed/NCBI View
Article : Google Scholar
|
|
33
|
Yamakawa K, Ogura H, Koh T, Ogawa Y,
Matsumoto N, Kuwagata Y and Shimazu T: Platelet mitochondrial
membrane potential correlates with severity in patients with
systemic inflammatory response syndrome. J Trauma Acute Care Surg.
74:411–417. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Abós B, Pérez-Fernández E, Morel E,
Perdiguero P and Tafalla C: Pro-Inflammatory and B Cell Regulating
Capacities of TWEAK in Rainbow Trout (Oncorhynchus mykiss).
Front Immunol. 12(748836)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Manu V, Chakrabarty BK and Singh S:
Analysis of platelet-activating factors in severe sepsis by flow
cytometry and its correlation with clinical sepsis scoring system:
A pilot study. Med J Armed Forces India. 75:429–36. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lhermusier T, Chap H and Payrastre B:
Platelet membrane phospholipid asymmetry: From the characterization
of a scramblase activity to the identification of an essential
protein mutated in Scott syndrome. J Thromb Haemost. 9:1883–1891.
2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Vinholt PJ: The role of platelets in
bleeding in patients with thrombocytopenia and hematological
disease. Clin Chem Lab Med. 57:1808–1817. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hanafi MMM, Afzan A, Yaakob H, Aziz R,
Sarmidi MR, Wolfender JL and Prieto JM: In Vitro Pro-apoptotic and
anti-migratory effects of Ficus deltoidea L. plant extracts
on the human prostate cancer cell lines PC3. Front Pharmacol.
8(895)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Cao Y, Ma W, Liu Z, Pei Y, Zhu Y, Chen F,
Zou L, Jiang Y, Liu X, Huang J, et al: Early predictive value of
platelet function for clinical outcome in sepsis. J Infect.
84:628–636. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Roderburg C, Benz F, Schüller F, Pombeiro
I, Hippe HJ, Frey N, Trautwein C, Luedde T, Koch A, Tacke F and
Luedde M: Serum levels of TNF receptor ligands are dysregulated in
sepsis and predict mortality in Critically Ill patients. PLoS One.
11(e0153765)2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Melendez E, Whitney JE, Norton JS,
Silverman M, Harju-Baker S, Mikacenic C, Wurfel MM and Liles WC:
Systemic Angiopoietin-1/2 dysregulation in pediatric sepsis and
septic shock. Int J Med Sci. 16:318–323. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hussain RM, Neiweem AE, Kansara V, Harris
A and Ciulla TA: Tie-2/Angiopoietin pathway modulation as a
therapeutic strategy for retinal disease. Expert Opin Investig
Drugs. 28:861–869. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Khan M, Aziz AA, Shafi NA, Abbas T and
Khanani AM: Targeting Angiopoietin in Retinal Vascular Diseases: A
Literature review and summary of clinical trials involving
faricimab. Cells. 9(1869)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wright JK, Hayford K, Tran V, Al Kibria
GM, Baqui A, Manajjir A, Mahmud A, Begum N, Siddiquee M, Kain KC
and Farzin A: Biomarkers of endothelial dysfunction predict sepsis
mortality in young infants: A matched case-control study. BMC
Pediatr. 18(118)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Fang Y, Li C, Shao R, Yu H, Zhang Q and
Zhao L: Prognostic significance of the
angiopoietin-2/angiopoietin-1 and angiopoietin-1/Tie-2 ratios for
early sepsis in an emergency department. Crit Care.
19(367)2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu Y, Hou JH, Li Q, Chen KJ, Wang SN and
Wang JM: Biomarkers for diagnosis of sepsis in patients with
systemic inflammatory response syndrome: A systematic review and
meta-analysis. Springerplus. 5(2091)2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Matowicka-Karna J: Markers of
inflammation, activation of blood platelets and coagulation
disorders in inflammatory bowel diseases. Postepy Hig Med Dosw.
70:305–312. 2016.PubMed/NCBI View Article : Google Scholar
|