Introduction
Neurological disorders are the leading cause of
disability and the second leading cause of death worldwide, and
ischemia is one of the major causes of stroke leading to
neurological disorders (1,2). Cerebral ischemia causes metabolic and
neurochemical alterations and leads to a diverse range of
neurological diseases, such as stroke, myocardial infarction, acute
heart failure, cerebral dysfunction and selective neuronal loss
(3-6).
Ischemia-reperfusion (I/R) injury is a pathological event occurring
in various disease states. It results in metabolic disorders,
excitotoxicity, calcium overload, oxidation stress and inflammatory
damage through a number of pathways (7-9)
as a result of the sudden reduction of available tissue oxygen and
nutrients (10,11).
Following transient forebrain ischemia, neuronal
loss may occur causing ‘delayed neuronal death’ (12), which may also result from
neuroinflammatory processes, such as glial activation and increased
production and release of inflammatory cytokines (13,14).
In addition to delayed neuronal death, ischemia damages the
integrity of the blood-brain barrier (BBB) and enhances microglia
activation and zinc release, leading to blood and fluid leakage
(15,16). The brain is especially sensitive to
oxidative stress from reactive oxygen species (ROS) produced as a
result of ischemic insult, because neurons have high levels of
polyunsaturated fatty acids and low levels of endogenous
antioxidant enzymes (17).
Several antioxidant substances have been shown to be
able to protect neurons from I/R injury. For example,
protocatechuic acid, a major type of benzoic acid that exists in
vegetables, fruits and numerous herbal medicines, has been revealed
to be a strong anti-oxidant that prevents Parkinson's disease
(18). It also has neuroprotective
activities on global cerebral ischemia-induced hippocampal neuron
death (16) through a combination
of the cellular mechanisms of antioxidant cytoprotection and
anti-inflammation (18). The
neuroprotective effect has also been demonstrated for stiripentol,
which reduces ischemia-induced memory impairment and neuronal death
by decreasing astrocyte damage and ameliorating BBB leakage
(19). In addition, chlorogenic
acid, naturally found in green coffee extracts and tea, has also
been revealed to attenuate cognitive impairment, and has a
neuroprotective effect against transient forebrain ischemia by
increasing the production of superoxide dismutase (SOD)2,
interleukin (IL)-4, antioxidant enzymes and anti-inflammatory
cytokines (8).
Icariin (ICA) is a major active flavonol glucoside
component that presents in the medicinal plant Epimedium
grandiflorum, and has been demonstrated to have activities
against neurodegenerative diseases, cardiovascular diseases,
osteoporosis, inflammation, oxidative stress, depression and tumors
(20,21). It improves carrageenan-induced paw
edema by modulating heme oxygenase (HO1)/nuclear factor
(erythroid-derived 2)-like 2 (Nrf2) and NF-ĸB signaling to reduce
inflammatory cytokines and increase enzymatic and non-enzymatic
antioxidants (21). In a rat
model, ICA prevents the production of amyloid β (1-42)
and inhibits the synthesis of amyloid precursor protein and β-site
APP cleaving enzyme 1 in animal models of Alzheimer's disease (AD)
(22). It also alleviates the
development of kidney fibrosis by inhibiting IL-1β/transforming
growth factor-β-mediated renal fibroblasts in rats (23). Recently, hydrophilic polyethylene
glycol monomethyl ether (mPEG) was modified to generate mPEG-ICA
nanoparticles with increased protective activity for H9c2
cardiomyocytes under oxygen-glucose deprivation conditions
(24). ICA has been demonstrated
to have potential neuroprotective activity against Aβ25-35-induced
neurotoxicity by balancing intracellular calcium homeostasis in
rats (25). It can also mitigate
pro-inflammatory responses of microglia in culture and in animal
models of cerebral ischemia, depression, Parkinson's disease and
multiple sclerosis (22). A
previous study demonstrated that ICA protects neurons from
endoplasmic reticulum stress-induced apoptosis by suppressing
IRE1α-XBP1 signaling pathway in vitro (26). However, the effect of ICA on I/R
injury of neural cells are largely unclear.
The present study aimed to investigate effect of ICA
on neural cells with I/R injury induced by oxygen-glucose
deprivation and reoxygenation (OGD-R) and possible mechanisms
underlying the protection.
Materials and methods
Animals
A total of five newborn (within 24 h of birth) male
Sprague Dawley (SD) rats, weighing 5 g, were purchased from Hunan
Silaike Jingda Laboratory Animal Co., Ltd. [license no. SCXK
(Hunan) 2020-0104] and were used immediately after arriving for
experiments. All animal experiments and animal care protocols were
approved by the Institutional Animal Care and Use Committee of
Affiliated Zhongshan Hospital, Dalian University (Dalian,
China).
Reagents and instruments
Hanks' balanced salt solution (HBSS; cat. no 88284),
Earle's balanced salt solution (EBSS; cat. no 14175095), fetal
bovine serum (FBS; cat. no 2662002), trypsin inhibitor (cat. no
J60982), Infinity Calcium Arsenazo Liquid Stable Reagent (cat. no
265-250), 2',7'-dichlorodihydrofluorescein diacetate
(H2DCFDA; cat. no. D399), CyQUANT™ lactate dehydrogenase
(LDH) Cytotoxicity Assay (cat. no. C20302), Invitrogen SOD
colorimetric activity kit (cat. no. EIASODC) and ApoDETECT Annexin
V-FITC kit (cat. no 331200) were purchased from Thermo Fisher
Scientific, Inc.; ICA [cat. no. I1286; purity ≥94% (high
performance liquid chromatography)], 0.25% trypsin (cat. no.
9002-07-7), high glucose Dulbecco's Modified Eagle's Medium (DMEM;
cat. no. D6429), poly-L-lysine (cat. no. 25988-63-0) and Cell
Counting Kit-8 (CCK-8; cat. no. 96992) were purchased from
Sigma-Aldrich (Merck KGaA); laminar flow hoods were purchased from
Global Lab Supply; CO2 incubator (NAPCO; Thermo Fisher
Scientific, Inc) was obtained from ProVendum SA; flow cytometer
(FACS LSR) was purchased from BD Biosciences; ELISA plate reader
(VANTAstar) was obtained from BMG Labtech GmbH; FS5
spectrofluorometer was obtained from Edinburgh Instruments Ltd.
Neural cell culture
Neural cells were isolated as previously reported
(27). Briefly, newborn (~24 h
old) SD rats were euthanized by intraperitoneal injection of sodium
pentobarbital (200 mg/kg body weight) followed by decapitation, and
were sterilized in 75% alcohol for 5 min. The craniums were cut
opened along the midline to isolate the brain tissue. Isolated
tissue was washed with HBSS to remove blood, soft meninges and
vascular network. The dentate gyrus was then isolated, cut into
pieces of 1-2 mm in size after washing three times with HBSS,
homogenized, filtered through a 100 mesh filter and digested with
equal volume of 0.25% trypsin at 37˚C for 20 min. Digestion was
stopped by adding two volumes of trypsin inhibitor. Digested cells
were pelleted by centrifugation at 112 x g for 5 min at room
temperature and cultured in DMEM medium with 10% FBS in 5%
CO2 at 37˚C for three passages.
Cellular I/R model
The in vitro I/R model of neural cells was
constructed as previously described using the OGD-R method
(28,29). Briefly, cells were cultured in DMEM
medium with 10% FBS for 2 days, washed with glucose-free EBSS and
cultured in glucose-free EBSS in an anoxic incubator. The incubator
was slowly filled with gas containing 95% N2 and 5%
CO2 to generate an oxygen-free atmosphere to mimic
ischemia condition. After culturing at 37˚C for 4 h, the cells were
transferred to high-glucose DMEM medium and cultured in 5%
CO2 at 37˚C for 12 h to mimic reperfusion process.
ICA treatment
ICA was dissolved in dimethyl sulfoxide (DMSO) to a
concentration of 50 mM and stored at -20˚C as a stock solution. It
was diluted with DMEM medium with 10% FBS before use. The medium
containing 0.1% DMSO served as the control. Immediately after the
I/R modelling, cells were adjusted to a density of 1x105
cells/ml with DMEM medium with 10% FBS containing 0 (control), 5,
10 and 15 µM ICA. Subsequently, 200 µl cells were inoculated in the
wells of 96-well plates pre-coated with poly-L-lysine and cultured
in 5% CO2 at 37˚C. The concentrations were used based on
a previous study on neural stem cells (30). Cells without OGD-R treatments were
used as control.
Cell viability assay
Cell viability was assayed using a CCK-8 cell
counting kit according to the manufacture's instruction. Briefly,
24 h after reperfusion, 20 µl CCK-8 solution was added to each well
of the plates and the plates were incubated in 5% CO2 at
37˚C for 4 h. The optical density (OD) was read at 460 nm
wavelength using an ELISA plate reader. All assays were performed
in triplicate in three independent experiments. Cell viability was
calculated as ODsample/ODcontrol x100%.
Apoptosis analysis
Apoptosis was assessed using ApoDETECT Annexin
V-FITC kit according to the manufacture's instruction. Briefly,
cells (1x106) were harvested by centrifugation at room
temperature, washed three times with 1 ml PBS, resuspended in cold
binding buffer and incubated with Annexin V-FITC and PI-PE in the
dark at room temperature for 10 min. The stained cells were
analyzed using FACS LSR flow cytometer using built-in software
according to the manufacture's protocols. All assays were performed
in triplicate in three independent experiments.
Determination of extracellular LDH
level
LDH is a cytosolic enzyme that is released into the
cell culture medium upon damage to the plasma membrane (31). LDH level was determined using
CyQUANT™ LDH Cytotoxicity Assay according to the manufacture's
instruction. Briefly, 24 h after reperfusion, 20 µl aliquots of
culture medium were taken and added with the reaction mixture from
the kit. After a 10 min incubation at room temperature, the
reaction was stopped by adding the stop solution from the kit and
the fluorescence was measured with a plate reader by using
excitation of 560 nm and emission of 590 nm. All assays were
performed in triplicate in three independent experiments. LDH level
was calculated as (fluorescence signalsample -
fluorescence signalsample
control)/(fluorescence signalstandard -
fluorescence signalstandard control) x standard
concentration X dilution.
ROS determination
For ROS production analysis, cells were incubated
with H2DCFDA diluted in serum-free DMEM to the final
concentration of 10 µmol/l. After incubation at 37˚C for 20 min,
cells were rinsed three times with serum-free DMEM medium to remove
H2DCFDA and loaded to a cytometer for analysis according
to the manufacturer's protocols. All assays were performed in
triplicate in three independent experiments.
Measurement of SOD activity
Total SOD activity was measured using an Invitrogen
SOD colorimetric activity kit according to the manufacturer's
instruction. This kit is designed to quantitatively measure all
types of SOD activity, including Cu/Zn, Mn and FeSOD types
according to the manufacturer's information. Briefly, 24 h after
treatment, cells were washed with cold PBS twice, pelleted by
centrifugation at 1,200 x g and room temperature for 5 min,
homogenized and lysed using lysis buffer in the kit. The lysates
were centrifuged at 1,200 x g at 4˚C for 10 min and the
supernatants were used for SOD activity assessment. The optical
density at 450 nm was read using a plate reader. All assays were
performed in triplicate in three independent experiments.
Ca2+ determination
Cells (106) were harvested by
centrifugation at room temperature at 1,200 x g for 5 min and
Ca2+ was reacted to Infinity Calcium Arsenazo Liquid
Stable Reagent to form a bluish-purple colored complex according to
the manufacturer's instructions. The amount of color formed was
measured by an increase in absorbance of the reaction mixture at
600 and 660 nm using FS5 spectrofluorometer. All assays were
performed in triplicate in three independent experiments.
Statistical analysis
All data were expressed as means ± standard error
obtained from three independent experiments. Statistical
comparisons among the groups were assessed using a one-way ANOVA
with post-hoc Tukey honest significant difference test. P<0.05
was considered to indicate a statistically significant
difference.
Results
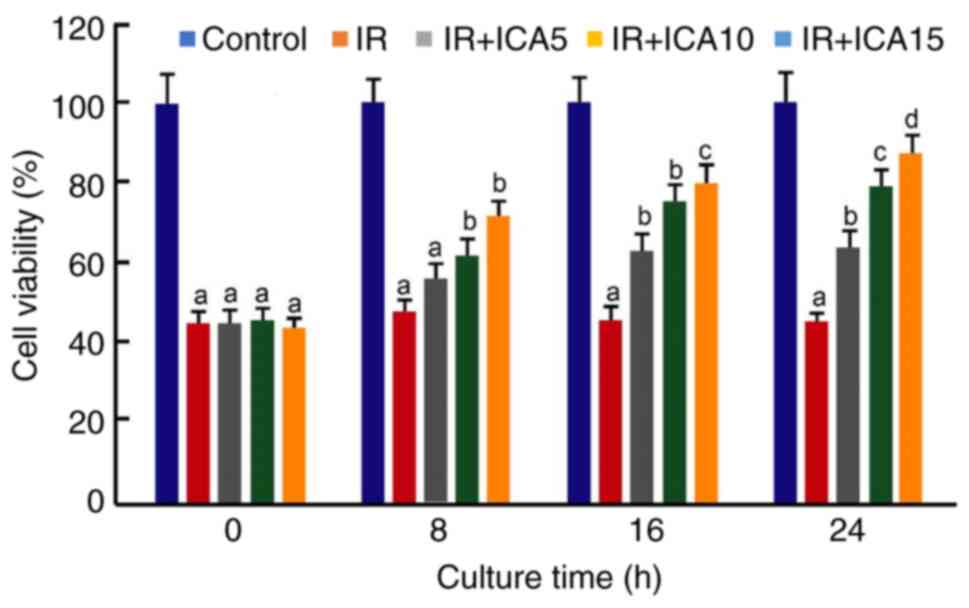
ICA increases the cell viability after
I/R
First, the effect of ICA on the viability of
cultured neural cells after I/R was investigated. CCK-8 cell
viability assays showed that after reperfusion, the viability of
cells after OGD-R was significantly reduced as compared with the
viability of control cells, indicating that the OGD-R generates
significant cellular injury. On the other hand, post-OGD-R
treatments with ICA increased the viability as the duration and
concentration of exposure to ICA increased; however, the increases
were insignificant between the low ICA concentration (ICA 5) and IR
model before 16 h exposure (P>0.05; Fig. 1). At 24 h after reperfusion, the
viabilities increased to 63.1, 79.5 and 87.1%, respectively, at the
concentrations of 5, 10 and 15 µM. The increases in the ICA treated
group were statistically significant between the concentrations at
24 h after culture (P<0.05; Fig.
1), suggesting that ICA protects neural cells from I/R injury,
although the highest cell viability after treatment with 15 µM ICA
was still lower compared with that of control at 24 h after culture
(P<0.05; Fig. 1). Since
exposure to ICA for 24 h generated significant improvement of cell
viability after I/R, the cells at this time point were used for
subsequent analysis.
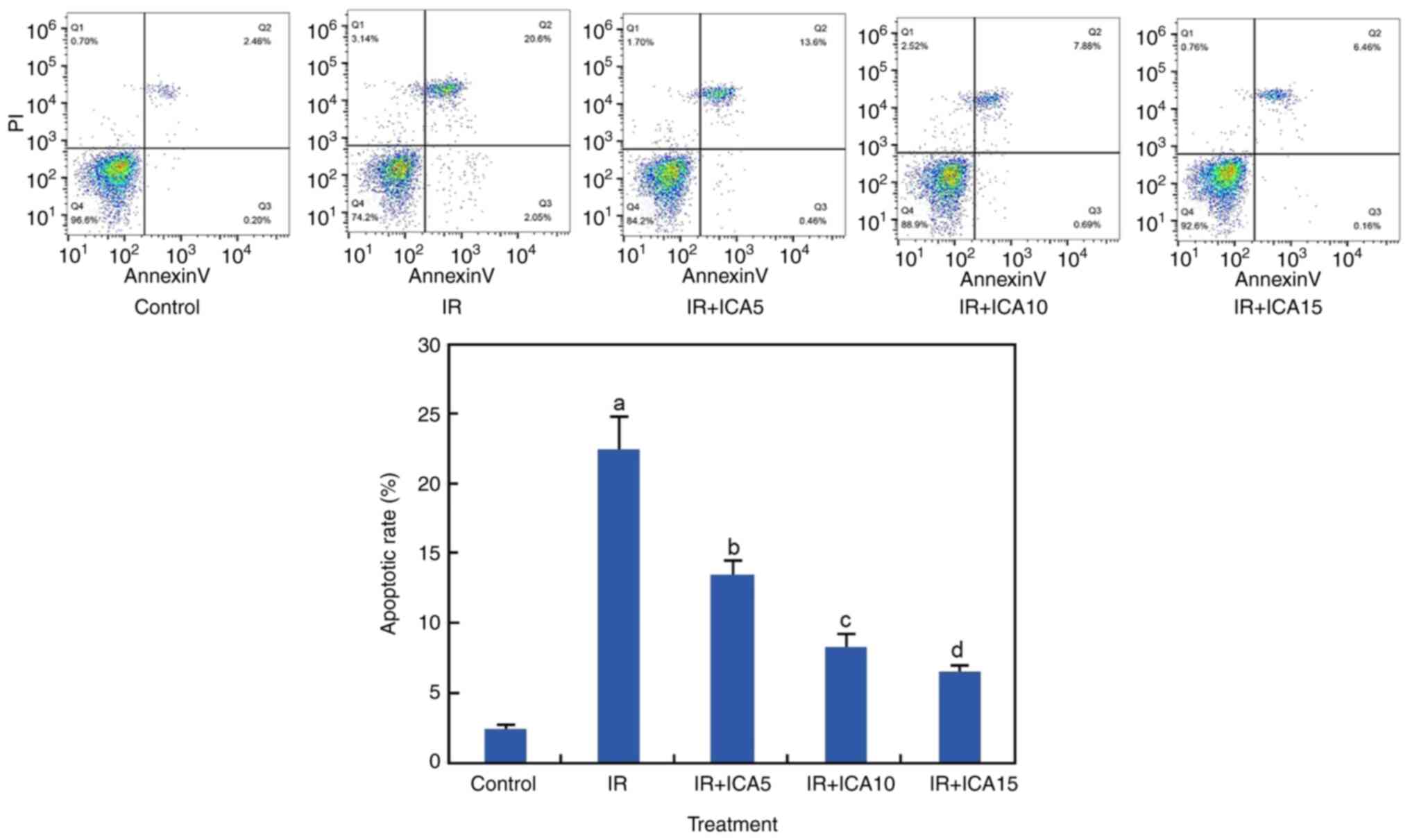
ICA reduces apoptosis after I/R
Apoptosis is one of the major mechanisms leading to
cell death. To investigate if apoptosis was induced after OGD-R
induced I/R damage, apoptosis was assessed using Annexin V-FITC
staining method. At 24 h after I/R, significant increases in
apoptotic rates were observed in the OGD-R treated neural cells as
compared with untreated cells (P<0.05; Fig. 2). However, apoptosis was
significantly reduced to 14.1, 8.4 and 6.6% when the OGD-R-treated
cells were exposed to 5, 10 and 15 µM ICA, respectively (P<0.05;
Fig. 2).
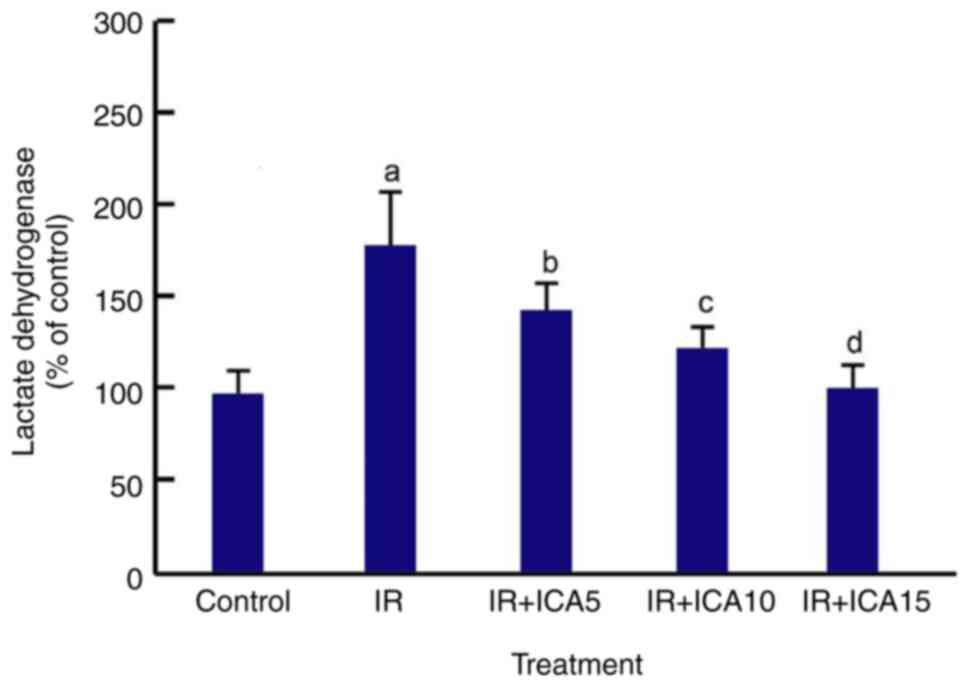
ICA reduces extracellular LDH
activity
LDH is released from cells when cell membrane is
damaged as a result of various cytotoxicities. The extracellular
LDH level in the culture medium of the cells was measured 24 h
after OGD-R and ICA treatments using CyQUANT LDH cytotoxicity
assay. The results showed that 24 h after OGD-R treatment, the
extracellular LDH activity was significantly increased as compared
with control (440.5 vs. 230.3 U/l) but decreased after being
exposed to ICA at the three ICA concentrations (P<0.05; Fig. 3).
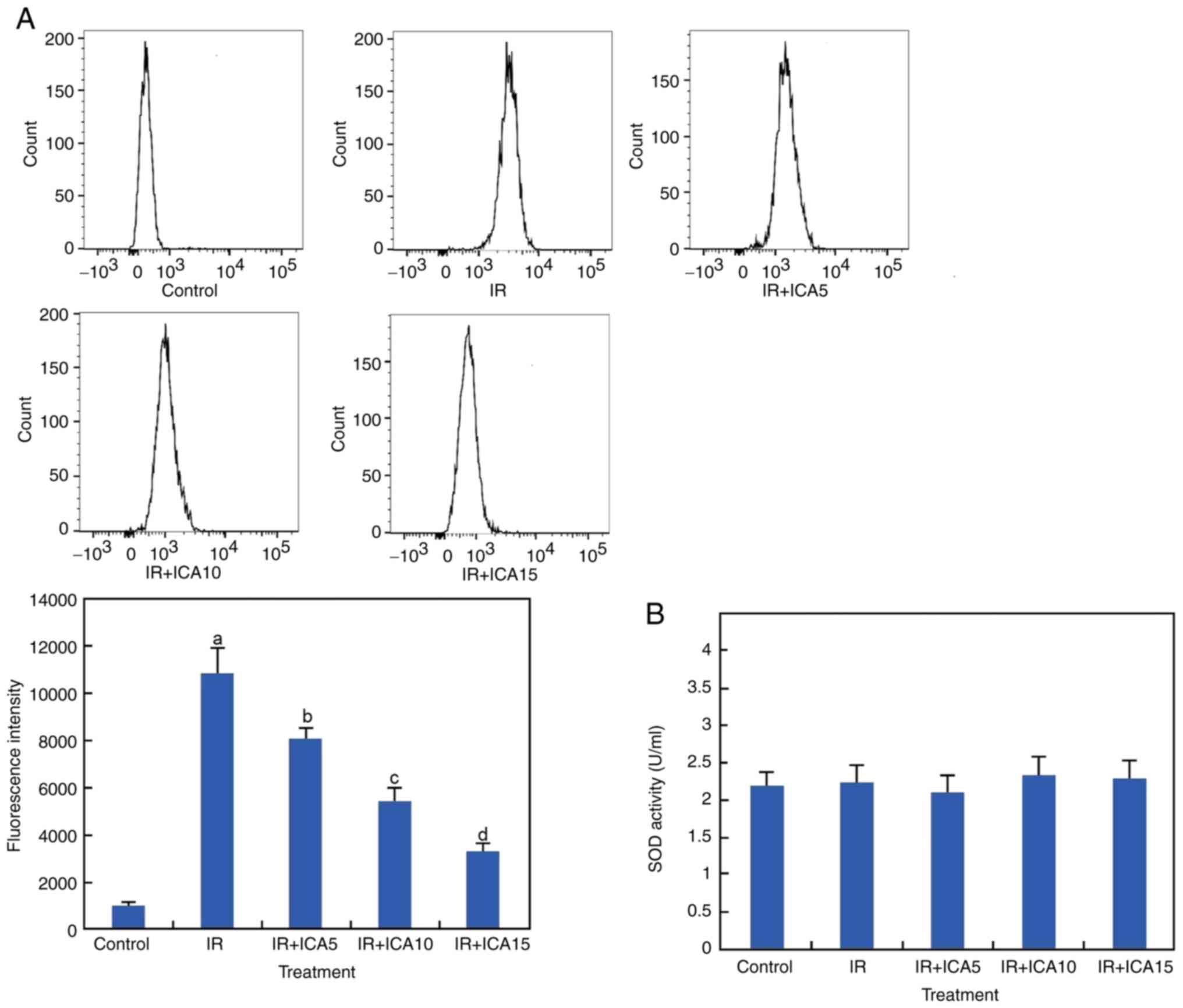
ICA reduces ROS production but not
total SOD activity
ROS induction is a common consequence of I/R, and
the production of ROS after OGD-R and ICA treatment was measured
using H2DCFDA as a fluorescent probe. The results showed
that the ROS level was significantly increased after the cells were
subjected to OGD-R as compared with control and was significantly
decreased after ICA treatments at the three concentrations
(P<0.05; Fig. 4A). To examine
the effect of ICA on OGD/R-induced oxidative stress, the SOD
activity was also examined. It was revealed that the SOD activity
did not change significantly after these treatments (P<0.05;
Fig. 4B).
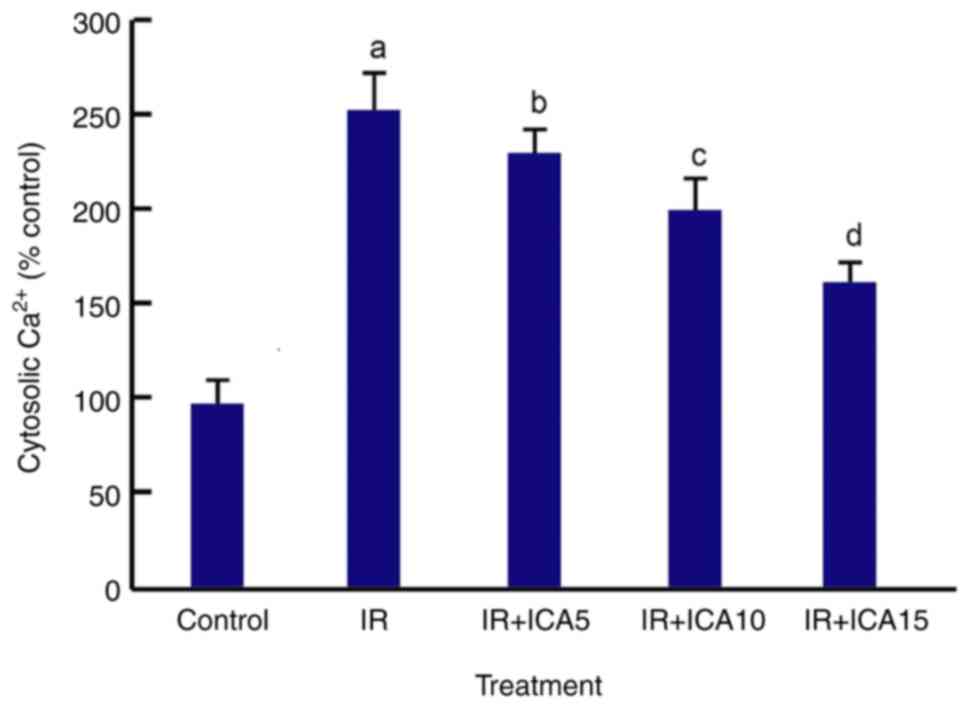
ICA reduces cytosolic Ca2+
level
One of the constant early responses to hypoxia in
almost all cell types is an increase in intracellular
Ca2+ due to the activation of various plasma membrane
Ca2+ ion channels (32). Analysis using a dual wavelength
fluorescence spectrophotometer showed that the cytosolic
Ca2+ level increased significantly after OGD-R
treatment, and reduced after the cells were exposed to ICA in a
dose-dependent manner (P<0.05; Fig.
5).
Discussion
Using cultured neural cells, the present study
revealed that OGD-R could mimic I/R to reduce cell viability,
induce apoptosis, increase LDH release and ROS production. These
cellular damages were alleviated after the cells were exposed to
ICA in a dose-dependent manner. This suggested that ICA has
protective activity and may be explored clinically for its
therapeutic functions in I/R-related diseases and complications
after the protective activity is validated with in vivo
models.
ICA is a prenylated flavonol glycoside of the
Epimedium herb and has been shown to have various
pharmacological activities against neurodegenerative diseases,
cardiovascular diseases, osteoporosis, inflammation, oxidative
stress depression and cancer (20,33,34).
It reduces I/R-induced gap junctional intercellular communication
injury by regulating the synthesis of gap junctional protein
connexin 43(35). Since ICA
regulates the expression of sirtuin 1 to inhibit the synthesis of
amyloid-β protein and improves other amyloid-β cascade pathogenesis
related to AD, it is considered to be candidate therapeutic agent
for AD and other neurodegenerative diseases (36). In addition, due to its
anti-inflammatory and anti-oxidant properties, it is recognized as
a potential natural compound to slow the progression of CNS
disorders, such as neurodegenerative diseases (37).
The present study investigated the protective
activity of ICA using cultured neural cells following OGD-R
treatment. As a well-established method, OGD-R (I/R mimic) has been
used to generate an in vitro cellular model of I/R injury
(38,39). The present results confirmed that
generated injury in the cultured neural cells. After the OGD-R
treatment, cell viability was significantly reduced and apoptosis
was increased, and there was increased production of ROS. These
results are consistent with previous studies showing that OGD-R
induces oxidative stress in astrocytes as a result of increased ROS
production, reduces cell viability (39) and increases LDH release and
apoptosis in neuroblastoma cells (40).
Several molecules have been shown to protect neural
cells from I/R injury, mainly by reducing oxidative stress,
apoptosis and autophagy. The protection might be achieved by
blocking NF-κB signaling and activating Nrf2/HO1, Akt and
mTOR/p70S6K/4E-BP-1 pathways (40), downregulating the CaMKKβ/AMPK/mTOR
signaling pathway (41) or by
inhibiting the TLR2/4 signaling pathways (42). The present study revealed that
post-OGD-R ICA treatment increased cell viability and reduced
apoptosis, suggesting that ICA reduced OGD-R-induced cytotoxicity.
As a consequence, less LDH was released, suggesting that the cells
had more intact plasma membranes after I/R as compared with
untreated cells, because I/R damage is especially associated with
increased LDH activity (43).
However, it remains to be investigated how ICA regulates the
expression of apoptosis- and LDH-related genes to regulate the
apoptosis pathways. Whether ICA modulates aforementioned pathways
such as the Nrf2/HO1, Akt and mTOR/p70S6K/4E-BP-1 pathways and the
CaMKKβ/AMPK/mTOR signaling pathway to reduce I/R injury warrants
further investigation. Among them, Nrf2 signaling is of particular
interest due to its role in stress response and cellular protection
(44,45).
Recent studies show that brain injury may occur
after transient or permanent focal cerebral ischemia as a result of
complex series of pathophysiological events, such as ROS injury
caused by oxidative stress, ion balance disorder, excitotoxicity,
apoptosis and inflammation and brain edema (46,47).
As a consequence, clinical management of stroke still faces various
challenges (48). Although
therapeutic approaches including mechanical or thrombolytic
reperfusion, arteriogenesis, pharmacological neuroprotection,
ischemic preconditioning and regeneration have been attempted, an
improved method of differentiation between hemodynamic and
molecular factors contributing to the manifestation of ischemic
injury is considered to be important for therapeutic interventions
(49). At the molecular level,
increased neutrophil-derived neurovascular matrix
metalloproteinase-9 activity is an important mechanism underlying
the exacerbation of ischemic brain injury by systemic inflammation
that contributes to the poor clinical outcome in stroke patients
(50).
Brain tissue has high concentrations of unsaturated
fatty acids and consumes large amount of oxygen. It is therefore
sensitive to oxidative stress injury (51). During cerebral I/R, excessive
production of ROS, if not scavenged sufficiently or timely, would
result in lipid peroxidation and damage the membrane structure of
nerve cells (52,53). It will also aggravate brain tissue
damage associated with hypoxia/reoxygenation-induced apoptosis in
cultured forebrain neurons, suggesting that oxidative stress might
be responsible for hypoxia-induced neurotoxicity (54). On other hand, increased synthesis
of intracellular SOD attenuates the hypoxia-reoxygenation injury by
scavenging intracellular-free superoxide anion and protecting
mitochondria from damage (55).
Hypoxic postconditioning has also been attempted to reduce the cell
loss since a hypoxic postconditioning containing three cycles of 5
min of reoxygenation and 5 min of rehypoxia applied before 6 h of
reoxygenation reduces ROS generation, cardiomyocyte death and
mitochondrial Ca2+ overload (56). However, SOD activity was not
changed after ICA treatment, implying that other mechanisms
including lipid synthesis de novo and prevention of lipid
oxidation may be involved in the reduced cell death, likely
including reduced production of ROS (57,58),
which is main cause of lipid peroxidation (59).
To further probe the mechanism underlying observed
ICA-mediated neuroprotection, ROS production was assessed. ROS is a
major by-product of aerobic metabolism and is often increased after
the reperfusion process after prolonged ischemia (5). ROS induce apoptosis by oxidizing the
inhibitor of apoptosis signal-regulating kinase and upregulating
the expression of FasL, a well-known and well-characterized
death-inducing ligand, or they can bind to the tumor necrosis
factor receptor to activate caspase (60,61).
In the present study, a significant increase in ROS production was
observed in the neural cells after OGD-R treatment, suggesting that
overproduction of ROS is likely a cause of cell damage. On the
other hand, ROS generation was reduced by ICA treatment in the
OGD-R treatment cells, suggesting that ICA inhibits the production
of ROS or facilitates the clearance of ROS and functions as an
antioxidant. As a flavone compound, ICA is likely to have
antioxidant activity through the scavenging of free radicals, or by
chelating metal ions or by inhibiting the enzymatic systems
responsible for producing free radicals (62). In a recent study, ICA was
demonstrated to have radical scavenging activities using a
2,2'-diphenyl-1-picrylhydrazyl radical scavenging assay (63). It is hypothesized that the phenol
functional groups in ICA could undergo H-atom abstraction for
stable and delocalized radical species (63).
SOD is one of the most important antioxidant enzymes
that scavenges ROS and eliminates oxidative stress caused by
excessive ROS (64,65). The activity of SOD was also
assessed after OGD-R and ICA treatments. However, no change in SOD
content was observed after these treatments, suggesting that SOD
does not contribute to reduce OGD-R-induced ROS production in the
neutral cells. However, the result is in contrast with a previous
study, where SOD activity was diminished in neurons when ICA was
applied during I/R process (66).
The difference in SOD expression between the studies may be due to
difference in ICA treatment methods, although the exact reason that
SOD did not change after ICA treatment in the present study is not
clear. Therefore, ICA may exert its antioxidation activity via
other mechanisms. For example, ICA may regulate Nrf2 to generate
antioxidant response, as observed in porcine oocyte (57) and further studies are needed to
investigate whether ICA activates Nrf2 signaling to generate
antioxidant activity. In addition, ICA was shown to activate
AMPK-SIRT3 signaling pathway, and it may mitigate the antioxidant
activity via mitochondrial ROS homeostasis by AMPK-SIRT3 signaling
pathway (58).
Ca2+ is one of the most important
secondary messengers in cells with complex biological functions,
including regulating the release of neurotransmitters and neuron
excitability (67). Maintenance of
intracellular Ca2+ homeostasis is achieved through the
integrated and coordinated function of Ca2+ transport
molecules and Ca2+ buffers, mainly in the endoplasmic
reticulum and mitochondria (68)
since increase in intracellular Ca2+ is often cytotoxic
(69). Increasing evidence
indicates that Ca2+ overload is a pathological factor
associated with AD (70) and brain
ischemia (68). There are 3
channels related to Ca2+ intake and release on
endoplasmic reticulum: Ryanodine receptor (RyR), inositol
1,4,5-trisphosphate receptor (IP3R) and sarco (endo) plasmic
reticulum calcium-ATPases that pumps Ca2+ from the
cytoplasm to the endoplasmic reticulum. Under normal circumstances,
Ca2+ in the endoplasmic reticulum cavity is released to
the cytoplasm mainly through RyR and IP3R and pumped into the
endoplasmic reticulum from the cytoplasm to achieve dynamic
equilibrium (71,72). However, this homeostasis can be
disrupted during the I/R process, leading to calcium overload and
increased concentration of Ca2+ in the cytoplasm, thus
causing cell damage (73). To
examine if the change in Ca2+ concentration is involved
in ICA-mediated protection of the neural cells, cytosolic
Ca2+ level was determined after OGD-R and ICA treatment.
Increased Ca2+ was observed after I/R as compared with
untreated cells. On the other hand, ICA treatment reduced the
increase, suggesting that ICA may influence the release or intake
of Ca2+ to balance Ca2+ after OGD-R. This is
consistent with the results from the previous studies showing that
ICA reduces the calcium content to protect MC3T3-E1 cells from
hydrogen peroxide-induced damage (74). Several mechanisms are likely to
underly this regulation although the exact mechanisms remain
unclear. ICA may bind with IP3R in the endoplasmic reticulum to
antagonize IP3R-mediated calcium release from ER calcium pool
(75), or block the calcium
channels to reduce Ca2+ being pumped into cytoplasm
(76). Further studies are needed
to elucidate the molecular mechanisms underlying ICA-mediated
Ca2+ homeostasis.
Taken together, data from the present study with an
in vitro model show that ICA protects neural cells from I/R
injury and that this protection activity is likely achieved through
its antioxidation activity and ability to maintaining cellular
Ca2+ homeostasis. Further studies are needed to further
elucidate the molecular mechanisms underlying the neuroprotection
activity, including de novo lipid synthesis and lipid
oxidation, which were not assessed in the present study. Studies
with in vivo I/R model are needed to further validate the
protective activity and develop approaches for potential clinical
use of ICA as a therapeutic strategy for ischemia-related
diseases.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KN and RG contributed to project conceptualization,
investigation and data analysis. KN and RG also performed data
collection, analysis, methodology development and investigation All
authors have read and approved the final manuscript. KN and RG
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Affiliated Zhongshan Hospital, Dalian University (Dalian, China;
approval no. HSH221D). All methods were performed in accordance
with the relevant guidelines and regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kuriakose D and Xiao Z: Pathophysiology
and treatment of stroke: Present status and future perspectives.
Int J Mol Sci. 21(7609)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Herpich F and Rincon F: Management of
acute ischemic stroke. Crit Care Med. 48:1654–1663. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li Y, Li S and Li D: Breviscapine
alleviates cognitive impairments induced by transient cerebral
ischemia/reperfusion through its anti-inflammatory and anti-oxidant
properties in a rat model. ACS Chem Neurosci. 11:4489–4498.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kirino T and Sano K: Selective
vulnerability in the gerbil hippocampus following transient
ischemia. Acta Neuropathol. 62:201–208. 1984.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wu MY, Yiang GT, Liao WT, Tsai AP, Cheng
YL, Cheng PW, Li CY and Li CJ: Current mechanistic concepts in
ischemia and reperfusion injury. Cell Physiol Biochem.
46:1650–1667. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sacks BA, Rosenthal DI and Hall FM:
Capsular visualization in lipohemarthrosis of the knee. Radiology.
122:31–32. 1977.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Yan HF, Tuo QZ, Yin QZ and Lei P: The
pathological role of ferroptosis in ischemia/reperfusion-related
injury. Zool Res. 41:220–230. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee TK, Kang IJ, Kim B, Sim HJ, Kim DW,
Ahn JH, Lee JC, Ryoo S, Shin MC, Cho JH, et al: Experimental
pretreatment with chlorogenic acid prevents transient
ischemia-induced cognitive decline and neuronal damage in the
hippocampus through anti-oxidative and anti-inflammatory effects.
Molecules. 25(3578)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Puyal J, Ginet V and Clarke PG: Multiple
interacting cell death mechanisms in the mediation of
excitotoxicity and ischemic brain damage: A challenge for
neuroprotection. Prog Neurobiol. 105:24–48. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Drossos G, Lazou A, Panagopoulos P and
Westaby S: Deferoxamine cardioplegia reduces superoxide radical
production in human myocardium. Ann Thorac Surg. 59:169–172.
1995.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Mahy GE: The effects of clomipramine on
depression in Barbadian patients. West Indian Med J. 27:75–80.
1978.PubMed/NCBI
|
|
13
|
Lee TK, Kim H, Song M, Lee JC, Park JH,
Ahn JH, Yang GE, Kim H, Ohk TG, Shin MC, et al: Time-course pattern
of neuronal loss and gliosis in gerbil hippocampi following mild,
severe, or lethal transient global cerebral ischemia. Neural Regen
Res. 14:1394–1403. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Victoria ECG, Toscano ECB, Oliveira FMS,
de Carvalho BA, Caliari MV, Teixeira AL, de Miranda AS and Rachid
MA: Up-regulation of brain cytokines and metalloproteinases 1 and 2
contributes to neurological deficit and brain damage in transient
ischemic stroke. Microvasc Res. 129(103973)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ju F, Ran Y, Zhu L, Gao H, Xi X, Yang Z
and Zhang S: Increased BBB permeability enhances activation of
microglia and exacerbates loss of dendritic spines after transient
global cerebral ischemia. Front Cell Neurosci.
12(236)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kho AR, Choi BY, Lee SH, Hong DK, Lee SH,
Jeong JH, Park KH, Song HK, Choi HC and Suh SW: Effects of
protocatechuic Acid (PCA) on global cerebral ischemia-induced
hippocampal neuronal death. Int J Mol Sci. 19(1420)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fecchio C, Palazzi L and de Laureto PP:
α-Synuclein and polyunsaturated fatty acids: Molecular basis of the
interaction and implication in neurodegeneration. Molecules.
23:2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang Z, Li G, Szeto SSW, Chong CM, Quan
Q, Huang C, Cui W, Guo B, Wang Y, Han Y, et al: Examining the
neuroprotective effects of protocatechuic acid and chrysin on in
vitro and in vivo models of Parkinson disease. Free Radic Biol Med.
84:331–343. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Shin MC, Lee TK, Lee JC, Kim HI, Park CW,
Cho JH, Kim DW, Ahn JH, Won MH and Lee CH: Therapeutic effects of
stiripentol against ischemia-reperfusion injury in gerbils focusing
on cognitive deficit, neuronal death, astrocyte damage and blood
brain barrier leakage in the hippocampus. Korean J Physiol
Pharmacol. 26:47–57. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
He C, Wang Z and Shi J: Pharmacological
effects of icariin. Adv Pharmacol. 87:179–203. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
El-Shitany NA and Eid BG: Icariin
modulates carrageenan-induced acute inflammation through HO-1/Nrf2
and NF-kB signaling pathways. Biomed Pharmacother.
120(109567)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jin J, Wang H, Hua X, Chen D, Huang C and
Chen Z: An outline for the pharmacological effect of icariin in the
nervous system. Eur J Pharmacol. 842:20–32. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wang M, Wang L, Zhou Y, Feng X, Ye C and
Wang C: Icariin attenuates renal fibrosis in chronic kidney disease
by inhibiting interleukin-1β/transforming growth factor-β-mediated
activation of renal fibroblasts. Phytother Res. 35:6204–6215.
2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zheng Y, Lu L, Yan Z, Jiang S, Yang S,
Zhang Y, Xu K, He C, Tao X and Zhang Q: mPEG-icariin nanoparticles
for treating myocardial ischaemia. Artif Cells Nanomed Biotechnol.
47:801–811. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li L, Tsai HJ, Li L and Wang XM: Icariin
inhibits the increased inward calcium currents induced by
amyloid-beta (25-35) peptide in CA1 pyramidal neurons of neonatal
rat hippocampal slice. Am J Chin Med. 38:113–125. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mo ZT, Liao YL, Zheng J and Li WN: Icariin
protects neurons from endoplasmic reticulum stress-induced
apoptosis after OGD/R injury via suppressing IRE1α-XBP1 signaling
pathway. Life Sci. 255(117847)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ahlemeyer B and Baumgart-Vogt E: Optimized
protocols for the simultaneous preparation of primary neuronal
cultures of the neocortex, hippocampus and cerebellum from
individual newborn (P0.5) C57Bl/6J mice. J Neurosci Methods.
149:110–120. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Goldberg MP and Choi DW: Combined oxygen
and glucose deprivation in cortical cell culture: Calcium-dependent
and calcium-independent mechanisms of neuronal injury. J Neurosci.
13:3510–3524. 1993.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Flammang TJ, Yerokun T, Bryant MS, Couch
LH, Kirlin WG, Lee KJ, Ogolla F, Ferguson RJ, Talaska G and Hein
DW: Hemoglobin adduct and hepatic- and urinary bladder-DNA adduct
levels in rapid and slow acetylator Syrian inbred hamsters
administered 2-aminofluorene. J Pharmacol Exp Ther. 260:865–871.
1992.PubMed/NCBI
|
|
30
|
Ma D, Zhao L, Zhang L, Li Y, Zhang L and
Li L: Icariin promotes survival, proliferation, and differentiation
of neural stem cells in vitro and in a rat model of Alzheimer's
disease. Stem Cells Int. 2021(9974625)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kumar P, Nagarajan A and Uchil PD:
Analysis of cell viability by the lactate dehydrogenase assay. Cold
Spring Harb Protoc:. 2018, 2018 doi: 10.1101/pdb.prot095497.
|
|
32
|
Gelband CH and Gelband H: Ca2+ release
from intracellular stores is an initial step in hypoxic pulmonary
vasoconstriction of rat pulmonary artery resistance vessels.
Circulation. 96:3647–3654. 1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang Z, Wang D, Yang D, Zhen W, Zhang J
and Peng S: The effect of icariin on bone metabolism and its
potential clinical application. Osteoporos Int. 29:535–544.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Song L, Chen X, Mi L, Liu C, Zhu S, Yang
T, Luo X, Zhang Q, Lu H and Liang X: Icariin-induced inhibition of
SIRT6/NF-kappaB triggers redox mediated apoptosis and enhances
anti-tumor immunity in triple-negative breast cancer. Cancer Sci.
111:4242–4256. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang YW, Morita I, Zhang L, Shao G, Yao
XS and Murota S: Screening of anti-hypoxia/reoxygenation agents by
an in vitro method. Part 2: Inhibition of tyrosine kinase
activation prevented hypoxia/reoxygenation-induced injury in
endothelial gap junctional intercellular communication. Planta Med.
66:119–123. 2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ali S, Ansari S, Ehtesham NZ, Azfer MA,
Homkar U, Gopal R and Hasnain SE: Analysis of the evolutionarily
conserved repeat motifs in the genome of the highly endangered
central Indian swamp deer Cervus duvauceli branderi. Gene.
223:361–367. 1998.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Khezri MR and Ghasemnejad-Berenji M:
Icariin: A potential neuroprotective agent in Alzheimer's Disease
and Parkinson's disease. Neurochem Res. 47:2954–2962.
2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Alluri H, Anasooya Shaji C, Davis ML and
Tharakan B: Oxygen-glucose deprivation and reoxygenation as an in
vitro ischemia-reperfusion injury model for studying blood-brain
barrier dysfunction. J Vis Exp. (e52699)2015.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Liu L, Zhao Z, Yin Q and Zhang X: TTB
protects astrocytes against oxygen-glucose
deprivation/reoxygenation-induced injury via activation of
Nrf2/HO-1 signaling pathway. Front Pharmacol.
10(792)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhi SM, Fang GX, Xie XM, Liu LH, Yan J,
Liu DB and Yu HY: Melatonin reduces OGD/R-induced neuron injury by
regulating redox/inflammation/apoptosis signaling. Eur Rev Med
Pharmacol Sci. 24:1524–1536. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sun B, Ou H, Ren F, Huan Y, Zhong T, Gao M
and Cai H: Propofol inhibited autophagy through
Ca2+/CaMKKβ/AMPK/mTOR pathway in OGD/R-induced neuron injury. Mol
Med. 24(58)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhou JM, Gu SS, Mei WH, Zhou J, Wang ZZ
and Xiao W: Ginkgolides and bilobalide protect BV2 microglia cells
against OGD/reoxygenation injury by inhibiting TLR2/4 signaling
pathways. Cell Stress Chaperones. 21:1037–1053. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Stankovic Stojanovic K and Lionnet F:
Lactate dehydrogenase in sickle cell disease. Clin Chim Acta.
458:99–102. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shaw P and Chattopadhyay A: Nrf2-ARE
signaling in cellular protection: Mechanism of action and the
regulatory mechanisms. J Cell Physiol. 235:3119–3130.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kumar M, Singh G, Kushwah AS, Surampalli
G, Singh TG and Gupta S: Arbutin protects brain against middle
cerebral artery occlusion-reperfusion (MCAo/R) injury. Biochem
Biophys Res Commun. 577:52–57. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lindblom RPF, Tovedal T, Norlin B,
Hillered L, Englund E and Thelin S: Mechanical Reperfusion
following prolonged global cerebral ischemia attenuates brain
injury. J Cardiovasc Transl Res. 14:338–347. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Dirnagl U, Iadecola C and Moskowitz MA:
Pathobiology of ischaemic stroke: An integrated view. Trends
Neurosci. 22:391–397. 1999.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hossmann KA: Pathophysiology and therapy
of experimental stroke. Cell Mol Neurobiol. 26:1057–1083.
2006.PubMed/NCBI View Article : Google Scholar
|
|
50
|
McColl BW, Rothwell NJ and Allan SM:
Systemic inflammation alters the kinetics of cerebrovascular tight
junction disruption after experimental stroke in mice. J Neurosci.
28:9451–9462. 2008.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Bazinet RP and Laye S: Polyunsaturated
fatty acids and their metabolites in brain function and disease.
Nat Rev Neurosci. 15:771–785. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Li X, Cheng S, Hu H, Zhang X, Xu J, Wang R
and Zhang P: Progranulin protects against cerebral
ischemia-reperfusion (I/R) injury by inhibiting necroptosis and
oxidative stress. Biochem Biophys Res Commun. 521:569–576.
2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang Q, Tompkins KD, Simonyi A, Korthuis
RJ, Sun AY and Sun GY: Apocynin protects against global cerebral
ischemia-reperfusion-induced oxidative stress and injury in the
gerbil hippocampus. Brain Res. 1090:182–189. 2006.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lievre V, Becuwe P, Bianchi A, Koziel V,
Franck P, Schroeder H, Nabet P, Dauça M and Daval JL: Free radical
production and changes in superoxide dismutases associated with
hypoxia/reoxygenation-induced apoptosis of embryonic rat forebrain
neurons in culture. Free Radic Biol Med. 29:1291–1301.
2000.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Liu J, Hou J, Xia ZY, Zeng W, Wang X, Li
R, Ke C, Xu J, Lei S and Xia Z: Recombinant PTD-Cu/Zn SOD
attenuates hypoxia-reoxygenation injury in cardiomyocytes. Free
Radic Res. 47:386–393. 2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Sun HY, Wang NP, Kerendi F, Halkos M, Kin
H, Guyton RA, Vinten-Johansen J and Zhao ZQ: Hypoxic
postconditioning reduces cardiomyocyte loss by inhibiting ROS
generation and intracellular Ca2+ overload. Am J Physiol Heart Circ
Physiol. 288:H1900–H1908. 2005.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yoon JW, Lee SE, Park YG, Kim WJ, Park HJ,
Park CO, Kim SH, Oh SH, Lee DG, Pyeon DB, et al: The antioxidant
icariin protects porcine oocytes from age-related damage in vitro.
Anim Biosci. 34:546–557. 2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hu Y and Ma X: Icariin treatment protects
against gentamicin-induced ototoxicity via activation of the
AMPK-SIRT3 pathway. Front Pharmacol. 12(620741)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Juan CA, Perez de la Lastra JM, Plou FJ
and Perez-Lebena E: The chemistry of reactive oxygen species (ROS)
revisited: Outlining their role in biological macromolecules (DNA,
Lipids and Proteins) and induced pathologies. Int J Mol Sci.
22(4642)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wang X, Lu X, Zhu R, Zhang K, Li S, Chen Z
and Li L: Betulinic acid induces apoptosis in differentiated PC12
Cells Via ROS-Mediated mitochondrial pathway. Neurochem Res.
42:1130–1140. 2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Kaminskyy VO and Zhivotovsky B: Free
radicals in cross talk between autophagy and apoptosis. Antioxid
Redox Signal. 21:86–102. 2014.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Angeloni C, Barbalace MC and Hrelia S:
Icariin and Its metabolites as potential protective phytochemicals
against Alzheimer's Disease. Front Pharmacol.
10(271)2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Mensah A, Chen Y, Asinyo BK, Howard EK,
Narh C, Huang J and Wei Q: Bioactive Icariin/β-CD-IC/Bacterial
cellulose with enhanced biomedical potential. Nanomaterials
(Basel). 11(387)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Lu R, Zhang T, Wu D, He Z, Jiang L, Zhou M
and Cheng Y: Production of functional human CuZn-SOD and EC-SOD in
bitransgenic cloned goat milk. Transgenic Res. 27:343–354.
2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Fridovich I: Superoxide radical and
superoxide dismutases. Annu Rev Biochem. 64:97–112. 1995.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Li L, Zhou QX and Shi JS: Protective
effects of icariin on neurons injured by cerebral
ischemia/reperfusion. Chin Med J (Engl). 118:1637–1643.
2005.PubMed/NCBI
|
|
67
|
Grzybowska EA: Calcium-Binding proteins
with disordered structure and their role in secretion, storage, and
cellular signaling. Biomolecules. 8(42)2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Verkhratsky A and Toescu EC: Endoplasmic
reticulum Ca(2+) homeostasis and neuronal death. J Cell Mol Med.
7:351–361. 2003.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Roderick HL and Cook SJ: Ca2+ signalling
checkpoints in cancer: Remodelling Ca2+ for cancer cell
proliferation and survival. Nat Rev Cancer. 8:361–375.
2008.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Wu AJ, Tong BC, Huang AS, Li M and Cheung
KH: Mitochondrial calcium signaling as a therapeutic target for
Alzheimer's Disease. Curr Alzheimer Res. 17:329–343.
2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Santulli G, Nakashima R, Yuan Q and Marks
AR: Intracellular calcium release channels: An update. J Physiol.
595:3041–3051. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Lawal TA, Todd JJ, Witherspoon JW,
Bönnemann CG, Dowling JJ, Hamilton SL, Meilleur KG and Dirksen RT:
Ryanodine receptor 1-related disorders: An historical perspective
and proposal for a unified nomenclature. Skelet Muscle.
10(32)2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Harukuni I and Bhardwaj A: Mechanisms of
brain injury after global cerebral ischemia. Neurol Clin. 24:1–21.
2006.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Sun JB, Wang Z and An WJ: Protection of
icariin against hydrogen peroxide-induced MC3T3-E1 cell oxidative
damage. Orthop Surg. 13:632–640. 2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Zima AV and Blatter LA:
Inositol-1,4,5-trisphosphate-dependent Ca(2+) signalling in cat
atrial excitation-contraction coupling and arrhythmias. J Physiol.
555:607–615. 2004.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Jiang W, Zeng M, Cao Z, Liu Z, Hao J,
Zhang P, Tian Y, Zhang P and Ma J: Icariin, a novel blocker of
sodium and calcium channels, eliminates early and delayed
afterdepolarizations, as well as triggered activity, in rabbit
cardiomyocytes. Front Physiol. 8(342)2017.PubMed/NCBI View Article : Google Scholar
|