Introduction
Sepsis, an infection-caused systemic inflammatory
response syndrome, is a common, clinically acute and severe disease
(1). Millions of patients
worldwide suffer from sepsis every year, and the mortality rate
ranges from 30 to 70% (2). The
pathogenesis of sepsis is very complex. At present, it is
hypothesized that certain infection factors rapidly activate the
collective non-specific immune system and release a great number of
pro-inflammatory factors, such as TNF-α and IL-6, inducing the
occurrence and development of an uncontrolled inflammatory
reaction, which can cause multiple organ dysfunction (3-5).
The heart is an especially susceptible target organ
of sepsis (6). A study has shown
that 40-50% of patients with sepsis have organic myocardial damage
in the early stage, manifesting as arrhythmia, hypotension and
heart failure, and ~20% of patients also have concealed myocardial
damage (7). The mechanisms of
myocardial injury caused by sepsis are complex, including the
involvement of inflammatory factors, the dysfunction of myocardial
energy metabolism, the dysregulation of nitric oxide, the notably
increased production of oxygen free radicals, increased apoptosis
and excessive activation of the renin-angiotensis system (8-10).
Among them, endotoxin, also known as lipopolysaccharide (LPS),
activates the immune response of monocytes-macrophages and produces
a large number of inflammatory factors, which play a key role in
myocardial injury (11). TNF-α
causes myocardial injury by activating sphingomyelinase and
proteolytic enzyme, as well as inhibiting calcium influx (12). Injection of TNF-α in healthy
individuals leads to decreased myocardial contractility and
reversible ventricular enlargement (13). IL-6 and IL-1β cause myocardial
injury by activating nitric oxide synthase and type 2
cyclooxygenase (COX) (14).
Non-steroidal anti-inflammatory drugs, which have
been confirmed to alleviate postoperational pain, possess
therapeutic actions, such as decreasing the activity of COX-1 and
COX-2(15). Parecoxib can
effectively cross the blood-brain barrier and improve acute mild
and moderate pancreatitis by functioning as a selective
COX-2-specific inhibitor (16,17).
In addition, parecoxib can upregulate IFN-γ levels in the plasma of
brain tumors to further improve systemic T helper type 1 (Th1)
immune responses (18). In
addition, it also possesses analgesic effects, for example, by
reducing IL-1β, IL-6 and TNF-α levels in the spinal cords of mice
(19). The MAPK signaling pathway
has been demonstrated to serve an important role in septic
cardiomyopathy (20).
Nevertheless, the impacts and mechanisms of parecoxib on septic
cardiomyopathy remain unclear. Therefore, the present study focused
on examining the effects and processes of parecoxib in LPS-treated
H9c2 rat cardiomyocytes through a number of in vitro
experiments. The findings indicated that parecoxib might represent
a unique therapeutic target for the treatment of sepsis brought on
by LPS.
Materials and methods
Cell culture and treatment
To investigate the mechanism of sepsis-induced
myocardial injury, H9c2 rat cardiomyocytes were selected for use in
the present study (21,22). Dulbecco's modified Eagle's medium
(DMEM; Thermo Fisher Scientific, Inc.) containing 10% FBS (Thermo
Fisher Scientific, Inc.) was used to cultivate H9c2 cells, which
were supplied by the American Type Culture Collection, at 37˚C with
5% CO2. Parecoxib (50, 100 and 200 µM, diluted with
DMSO; ChemeGen) was administered to H9c2 cells at 37˚C for 30 min
following stimulation with LPS (10 µg/ml; Sigma-Aldrich; Merck
KGaA) for 24 h at 37˚C (23). The
groups were as follows: Control (H9c2 cells without LPS treatment),
LPS (H9c2 cells with LPS treatment), LPS + parecoxib low (50 µM),
LPS + parecoxib medium (100 µM) and LPS + parecoxib high (200
µM).
Cell counting kit (CCK)-8 assay
H9c2 cells from various groups (Control, LPS and LPS
+ parecoxib low/medium/high) were injected into 96-well plates
(5x103 cells/well). The culture plate was placed in an
incubator for preculture at 37˚C with 5% CO2 for 48 h. A
total of 10 µl of CCK-8 solution (MilliporeSigma) was added to each
well. Then, the culture plate was incubated for a further 2 h. The
Swiss-made Tecan Infinite M200 microplate reader was used to detect
the absorbance (or optical density, OD) value at 490 nm.
5-Ethynyl-2'-deoxyuridine (EdU)
assay
Cell proliferation capacity was measured using a
Cell-Light EdU Apollo567 In Vitro Kit (Guangzhou RiboBio
Co., Ltd.). H9c2 cells (4x104 cells/well) from different
groups were inoculated into 24-well plates. The medium was removed
by adding 20 µM of EdU working solution at 25˚C for 2 h. After
being fixed with 4% paraformaldehyde at 25˚C for 15 min, DAPI (100
ng/ml; Sigma-Aldrich) was added to each well and the cells were
incubated at 25˚C for 30 min in the dark. Images were observed
using a fluorescence microscope (Nikon Corporation).
TUNEL assay
H9c2 cells from the different groups were cultivated
for 48 h at 25˚C and fixed in 100% methanol at 25˚C for 2 h to test
for apoptosis. Following the addition of 3%
H2O2 for 10 min at 25˚C, the cells were then
treated with equilibration buffer from the TUNEL cell apoptosis
detection kit (cat. no. KGA704; Nanjing KeyGen Biotech Co., Ltd.)
for 10 min at 25˚C, Terminal Transferase (cat. no. 3333566001;
Roche Diagnostics GmbH) for 15 min at 25˚C and Anti-Digoxigenin-POD
(poly) (cat. no. 11633716001; Roche Diagnostics GmbH) for 30 min at
25˚C. Cells were incubated with DAB (10 mg/ml; Sigma-Aldrich; Merck
KGaA) at 25˚C for 30 min. Slices were rinsed several times with
bi-distilled water and sealed using neutral balsam (cat. no.
10004160; Sinopharm Chemical Reagent Co., Ltd.) and imaged using a
fluorescence microscope (Olympus Corporation). The cell numbers
were counted in three random fields of view. The number of cells in
the visual field was roughly 100-200.
ELISA
To detect cell inflammation, the expression levels
of TNF-α, IL-1β and IL-6 in H9c2 cell culture supernatants were
determined using Rat TNF-α (cat. no. EK-M27765), IL-1β (cat. no.
EK-R36877) and IL-6 (cat. no. EK-R36902) ELISA kits (all purchased
from EK-Bioscience Biotechnology Co., Ltd.), according to the
manufacturer's protocol. A standard curve was constructed based on
the concentration and OD value of the standard, and then the sample
concentration was calculated using the standard curve equation.
Western blotting
H9c2 cell protein was extracted using RIPA lysis
buffer (Beyotime Institute of Biotechnology), and a BCA kit was
used for protein determination (Beyotime Institute of
Biotechnology). Protein (45 µg per lane) was separated by SDS-PAGE
(12%) and then transferred to PVDF membranes (MilliporeSigma). The
membranes were blocked with 5% skim milk for 60 min at 4˚C.
Subsequently, membranes were treated overnight with primary
antibodies at 4˚C, namely anti-proliferating cell nuclear antigen
(PCNA; 1:2,000 dilution; cat. no. 10205-2-AP; ProteinTech Group,
Inc.), anti-Ki-67 (1:2,000 dilution; cat. no. 27309-1-AP;
ProteinTech Group, Inc.), anti-Bax (1:2,000 dilution; cat. no.
50599-2-Ig; ProteinTech Group, Inc.), anti-Bcl-2 (1:2,000 dilution;
cat. no. 12789-1-AP; ProteinTech Group, Inc.), anti-Cleaved
Caspase-3 (1:1,000 dilution; cat. no. ab2302; Abcam), anti-Cleaved
Caspase-9 (1:1,000 dilution; cat. no. ab2324; Abcam),
anti-phosphorylated (p)-JNK (1:2,000 dilution; cat. no. 80024-1-RR;
ProteinTech Group, Inc.), anti-JNK (1:2,000 dilution; cat. no.
24164-1-AP; ProteinTech Group, Inc.), anti-p-p38 (1:2,000 dilution;
cat. no. 28796-1-AP; ProteinTech Group, Inc.), anti-p38 (1:2,000
dilution; cat. no. 14064-1-AP; ProteinTech Group, Inc.), anti-p-ERK
(1:2,000 dilution; cat. no. 28733-1-AP; ProteinTech Group, Inc.),
anti-ERK (1:2,000 dilution; cat. no. 16443-1-AP; ProteinTech Group,
Inc.) and anti-β-actin (1:5,000 dilution; cat. no. 20536-1-AP;
ProteinTech Group, Inc.). β-actin was regarded as the endogenous
control. Then, the membranes were then treated for 1 h at room
temperature with an HRP-labeled secondary antibody: Goat
Anti-Rabbit IgG (H+L) HRP [1:5,000 dilution; cat. no. GAR007;
MultiSciences (Lianke) Biotech Co., Ltd.]. Lastly, protein blots
were visualized using an enhanced chemiluminescence kit (ECL;
MilliporeSigma) and quantification was performed using ImageJ
Software (National Institutes of Health; version 4.3).
Statistical analysis
Data was presented as the mean ± the standard
deviation. All experiments were repeated three times. One-way ANOVA
followed by Tukey's post hoc test was used to assess the
differences between groups. Data was analyzed using GraphPad Prism
(GraphPad Software, Inc.; version 5.0). P<0.05 was considered to
indicate a statistically significant difference.
Results
Parecoxib ameliorates inflammatory
responses of LPS-induced H9c2 cells
H9c2 cells were pretreated with parecoxib at 37˚C
for 24 h at doses of 50, 100 and 200 µM following 24-h LPS (10
µg/ml) stimulation. Then, ELISA was conducted to evaluate the
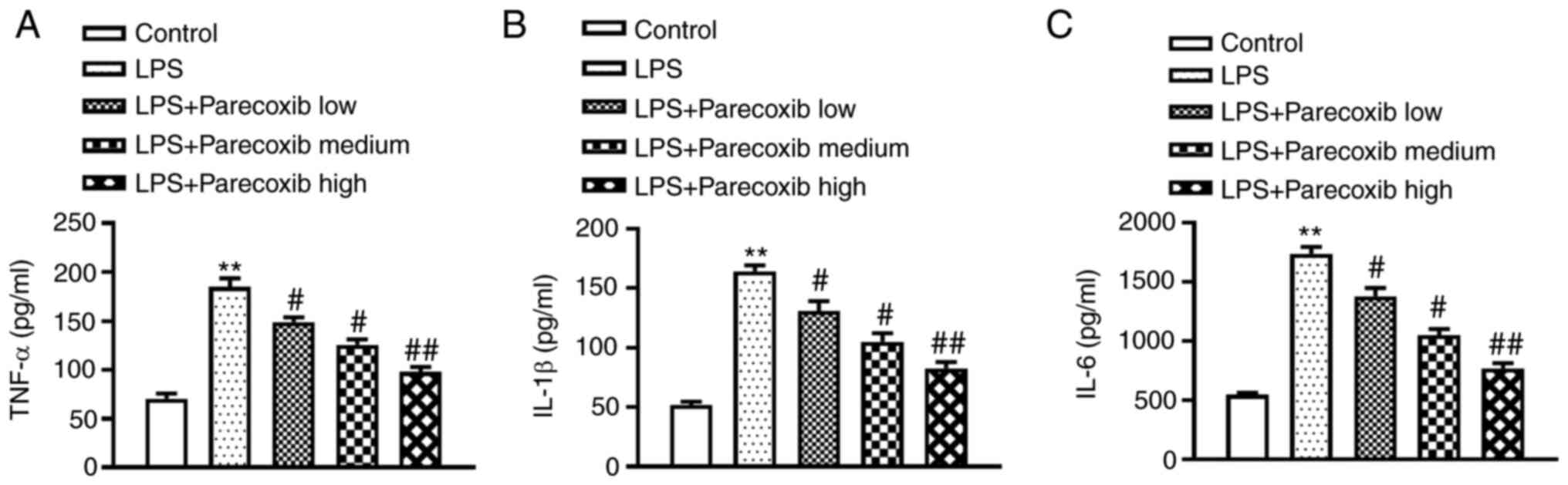
impacts of parecoxib on inflammatory factor levels. As shown in
Fig. 1, TNF-α, IL-1β and IL-6
protein levels were significantly upregulated in H9c2 cells induced
by LPS, while parecoxib treatment significantly ameliorated the
inflammatory responses of LPS-treated H9c2 cells. Parecoxib
treatment notably ameliorated the inflammatory responses of
LPS-treated H9c2 cells in a dose-dependent manner.
Parecoxib increases the proliferation
of LPS-induced H9c2 cells
To improve understanding of the effects of parecoxib
on the proliferation of H9c2 cells treated with LPS, a CCK-8 assay
was performed. Fig. 2A illustrates
that relative to the control group, LPS significantly decreased
H9c2 cell viability, while parecoxib significantly reversed the
LPS-induced decrease in H9c2 cell viability. Similarly, the EdU
assay indicated that parecoxib reversed the inhibitory effects of
LPS on H9c2 cell proliferation, as shown in Fig. 2B. Furthermore, western blotting was
conducted to assess the effects of parecoxib on
proliferation-related protein levels. As expected, PCNA and Ki-67
protein levels were significantly reduced in the H9c2 cells treated
with LPS, while parecoxib significantly reversed the inhibitory
effects of LPS (Fig. 2C). These
data suggested that parecoxib reversed the decrease in
proliferation of H9c2 cells treated with LPS.
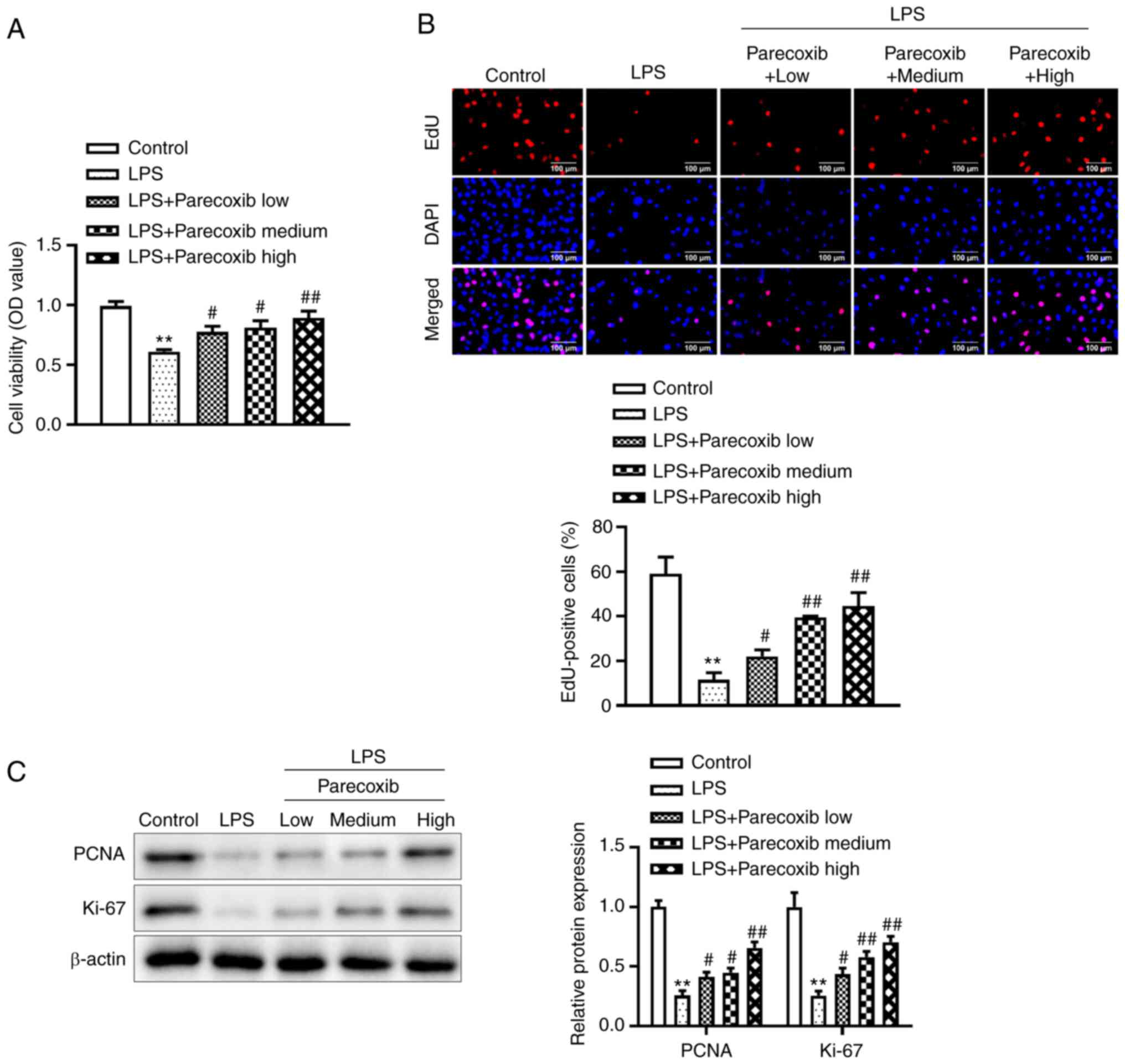 | Figure 2Proliferation of H9c2 cells treated
with LPS is increased by parecoxib. (A) Using the Cell Counting
Kit-8 assay, the viability of H9c2 cells stimulated by LPS and
varying concentrations of parecoxib was evaluated. (B) EdU assay
was used to measure the proliferation of H9c2 cells treated with
parecoxib and stimulated with LPS (scale bar, 100 µm). (C) Using
western blotting, the expression levels of proliferation-related
proteins, such as PCNA and Ki-67, in H9c2 cells from various groups
were assessed. All results are shown as the mean ± SD. n=3.
**P<0.01 vs. the control group;
#P<0.05, ##P<0.01 vs. the LPS-only
group. LPS, lipopolysaccharide; EdU, 5-Ethynyl-2'-deoxyuridine; OD,
optical density; PCNA, proliferating cell nuclear antigen. |
Parecoxib inhibits the apoptosis of
LPS-induced H9c2 cells
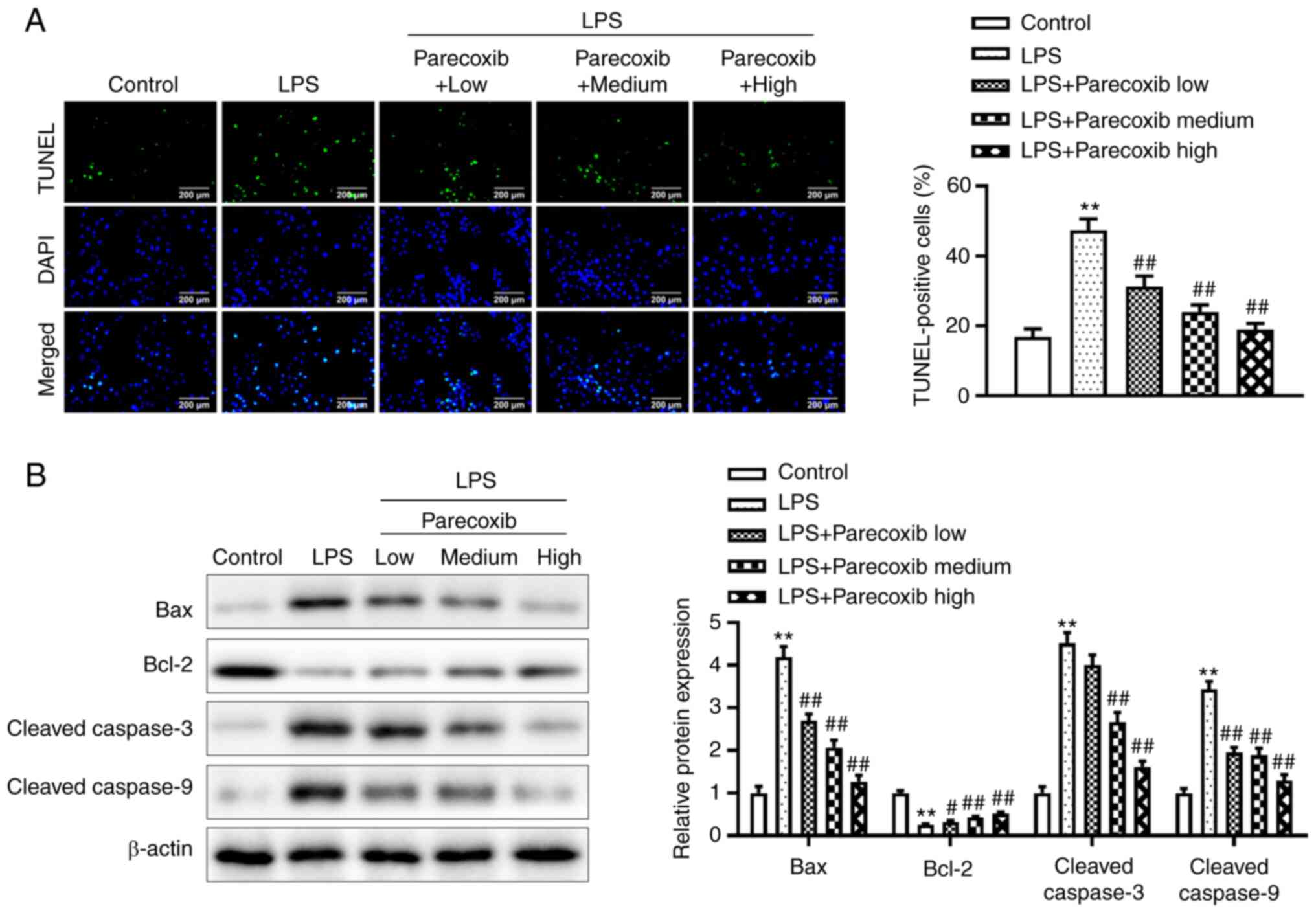
The TUNEL assay was adopted for determining the
effects of parecoxib on the apoptosis of H9c2 cells induced by LPS.
As shown in Fig. 3A, relative to
the control group, LPS significantly promoted the apoptosis of H9c2
cells. Notably, parecoxib treatment significantly reduced the
promoting effects of LPS on the apoptosis of H9c2 cells in a
dose-dependent manner. Additionally, western blotting was conducted
to investigate apoptosis-related protein levels. As shown in
Fig. 3B, LPS significantly
increased Bax, Cleaved caspase-3 and Cleaved caspase-9 protein
levels, and also decreased Bcl-2 protein levels, while parecoxib
significantly reversed the effects of LPS. These results indicated
that parecoxib inhibited the apoptosis of H9c2 cells induced by
LPS.
Parecoxib suppresses the expression of
the MAPK signaling pathway of LPS-treated H9c2 cells
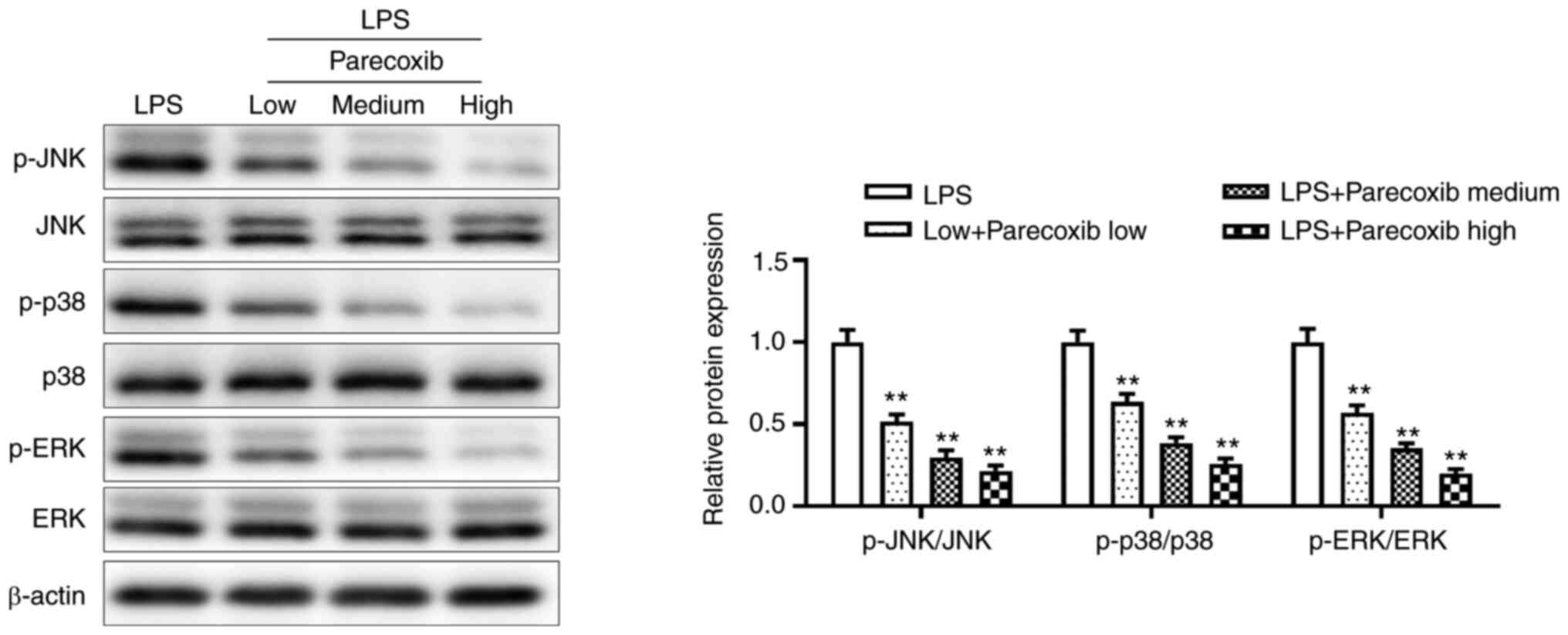
To ascertain whether parecoxib protects against
myocardial injury by regulating the MAPK signaling pathway, western
blotting was performed. As shown in Fig. 4, parecoxib significantly inhibited
the protein expression of the MAPK signaling pathway, compared with
the LPS-only group. These results indicated that parecoxib
suppressed the expression of the MAPK signaling pathway of H9c2
cells induced by LPS.
Discussion
As severe sepsis progresses, sepsis-induced cardiac
dysfunction, one of its symptoms, is the primary factor
contributing to the rise in mortality among patients with sepsis,
and cardiovascular dysfunction leads to the decline of quality of
life and a poor prognosis (24).
In addition, the pathogenesis of sepsis remains unclear, and the
inflammatory hypothesis was once considered to be the main
explanation for the progression of sepsis (25). A study has found that TNF-α and ILs
exert notable impacts on the inflammatory responses of the body,
and that these inflammatory factors can cause myocardial injury via
various mechanisms, such as activating sphingomyelinase on the cell
membrane, inhibiting calcium transport inside and outside the cell
membrane, and reducing the release of sarcoplasmic reticulum
Ca2+ ions (26).
Moreover, TNF-α, IL-1β and IL-6 activate myocardial
protein hydrolase, and subsequently degrade cardiac troponin and
other key contractile proteins, resulting in myocardial cell
contractility damage (27). It has
been reported that continuous infusion of TNF-α interferes with the
synthesis of cardiac proteins and eventually reduces the synthesis
of myocardial fiber and sarcoplasmic proteins, which may be related
to the effects of TNF-α on mRNA translation efficiency (28). In addition, an increasing number of
studies have shown that pro-inflammatory factors and
anti-inflammatory factors are involved in the progression of sepsis
(29-31).
When the dynamic balance of pro-inflammatory and anti-inflammatory
reactions is disrupted, the body becomes injured (32). The present study demonstrated the
protective effects of parecoxib on cardiomyopathy inflammatory
responses. Parecoxib inhibited TNF-α, IL-1β and IL-6 levels in H9c2
cells induced by LPS. Additionally, Lancel et al (33) found that myocardial cell apoptosis
directly leads to sepsis-induced myocardial dysfunction. As
expected, parecoxib reduced LPS-treated H9c2 apoptosis in the
present study.
The MAPK signaling pathway plays an important role
in myocardial injury resulting from sepsis, by affecting the
expression of numerous inflammatory genes (34). MAPKs are serine and threonine
kinases that are activated in signaling cascades and subsequently
move from the cytoplasm to the nucleus so as to regulate the
activity of transcription factors, transmit signals into the
nucleus and participate in cell differentiation, proliferation and
death (35). LPS, the pathogenic
factor of Gram-negative bacteria, activates the MAPK signaling
pathway, increases the expression of MAPK2/3 in lymphocytes,
promotes the rapid synthesis of TNF-α in ribosomes and enhances the
cellular immunity mediated by Th1 cells, which further improves
humoral immunity (36). Moreover,
a study has shown that SB 203580 reduces the production of
pro-inflammatory factors by inhibiting p38 MAPK activation in
septic mice (37). In addition,
several drugs can play a protective role in septic organs by
inhibiting the MAPK signaling pathway (38). For example, Carthamus tinctorius
L. improves H9c2 cardiomyocytes by suppressing JNK1/2-NFκB
signaling (39). Gas6 has also
been shown to attenuate TNF-α expression and apoptosis in
LPS-induced H9c2 cells by inhibiting the MAPK pathway (40). Therefore, the development of
MAPK-specific inhibitors is currently an area of research for the
treatment of septic cardiomyopathy. The current study demonstrated
that in LPS-induced H9c2 cells, parecoxib decreased the activation
of the MAPK signaling pathway.
In conclusion, parecoxib protected against septic
cardiomyopathy by promoting the proliferation and inhibiting the
apoptosis and inflammation of LPS-induced H9c2 cells via the
inactivation of MAPK. The present study therefore provides a
possible treatment strategy for septic cardiomyopathy using
parecoxib.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Beijing Medical
and Health Fund (grant no. B19155Dt).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX conceived the study. XQ performed the experiments
and drafted the manuscript. SX contributed to data analysis. QC and
JZ designed the methodology and performed data extraction. XQ and
JX revised the manuscript and confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Salomão R, Ferreira BL, Salomão MC, Santos
SS, Azevedo LCP and Brunialti MKC: Sepsis: Evolving concepts and
challenges. Braz J Med Biol Res. 52(e8595)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Candel FJ, Borges Sá M, Belda S, Bou G,
Del Pozo JL, Estrada O, Ferrer R, González Del Castillo J,
Julián-Jiménez A, Martín-Loeches I, et al: Current aspects in
sepsis approach. Turning things around. Rev Esp Quimioter.
31:298–315. 2018.PubMed/NCBI
|
|
3
|
Huang M, Cai S and Su J: The pathogenesis
of sepsis and potential therapeutic targets. Int J Mol Sci.
20(5376)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
van der Poll T, van de Veerdonk FL,
Scicluna BP and Netea MG: The immunopathology of sepsis and
potential therapeutic targets. Nat Rev Immunol. 17:407–420.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gotts JE and Matthay MA: Sepsis:
Pathophysiology and clinical management. BMJ.
353(i1585)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fattahi F and Ward PA: Complement and
sepsis-induced heart dysfunction. Mol Immunol. 84:57–64.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shi X, Liu Y, Zhang D and Xiao D: Valproic
acid attenuates sepsis-induced myocardial dysfunction in rats by
accelerating autophagy through the PTEN/AKT/mTOR pathway. Life Sci.
232(116613)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li F, Lang F, Zhang H, Xu L, Wang Y, Zhai
C and Hao E: Apigenin alleviates endotoxin-induced myocardial
toxicity by modulating inflammation, oxidative stress, and
autophagy. Oxid Med Cell Longev. 2017(2302896)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lado-Abeal J, Martinez-Sánchez N, Cocho
JA, Martín-Pastor M, Castro-Piedras I, Couce-Pico ML, Saha AK and
López M: Lipopolysaccharide (LPS)-induced septic shock causes
profound changes in myocardial energy metabolites in pigs.
Metabolomics. 14(131)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hsu WT, Galm BP, Schrank G, Hsu TC, Lee
SH, Park JY and Lee CC: Effect of renin-angiotensin-aldosterone
system inhibitors on short-term mortality after sepsis: A
population-based cohort study. Hypertension. 75:483–491.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang SM, Liu GQ, Xian HB, Si JL, Qi SX and
Yu YP: LncRNA NEAT1 alleviates sepsis-induced myocardial injury by
regulating the TLR2/NF-κB signaling pathway. Eur Rev Med Pharmacol
Sci. 23:4898–4907. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu H, Liu J, Li W, Liu G and Li Z:
LncRNA-HOTAIR promotes TNF-α production in cardiomyocytes of
LPS-induced sepsis mice by activating NF-κB pathway. Biochem
Biophys Res Commun. 471:240–246. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun LJ, Qiao W, Xiao YJ, Cui L, Wang X and
Ren WD: Naringin mitigates myocardial strain and the inflammatory
response in sepsis-induced myocardial dysfunction through
regulation of PI3K/AKT/NF-κB pathway. Int Immunopharmacol.
75(105782)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Han D, Li X, Li S, Su T, Fan L, Fan WS,
Qiao HY, Chen JW, Fan MM, Li XJ, et al: Reduced silent information
regulator 1 signaling exacerbates sepsis-induced myocardial injury
and mitigates the protective effect of a liver X receptor agonist.
Free Radic Biol Med. 113:291–303. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Essex MN, Xu H, Parsons B, Xie L and Li C:
Parecoxib relieves pain and has an opioid-sparing effect following
major gastrointestinal surgery. Int J Gen Med. 10:319–327.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ling Y, Chen J, Tao M, Chu X and Zhang X:
A pilot study of nimotuzumab combined with cisplatin and 5-FU in
patients with advanced esophageal squamous cell carcinoma. J Thorac
Dis. 4:58–62. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tan JH, Zhou L, Kan HP and Zhang GW:
Parecoxib improves the outcomes of acute mild and moderate
pancreatitis: A 3-year matched cohort study based on a prospective
database. Pancreas. 48:1148–1154. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Eberstål S, Badn W, Fritzell S,
Esbjörnsson M, Darabi A, Visse E and Siesjö P: Inhibition of
cyclooxygenase-2 enhances immunotherapy against experimental brain
tumors. Cancer Immunol Immunother. 61:1191–1199. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chen J, Cong X, Zhan X, Zhou Z and Zheng
W: Effects of parecoxib on pain threshold and inflammatory factors
IL-1β, IL-6 and TNF-α in spinal cord of rats with bone cancer pain.
J Coll Physicians Surg Pak. 29:528–531. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang J, Wang L, Xie W, Hu S, Zhou H, Zhu
P and Zhu H: Melatonin attenuates ER stress and mitochondrial
damage in septic cardiomyopathy: A new mechanism involving BAP31
upregulation and MAPK-ERK pathway. J Cell Physiol. 235:2847–2856.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen Z, Cao Z, Gui F, Zhang M, Wu X, Peng
H, Yu B, Li W, Ai F and Zhang J: TMEM43 protects against
sepsis-induced cardiac injury via inhibiting ferroptosis in mice.
Cells. 11(2992)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Huang Y, Zhang N, Xie C, You Y, Guo L, Ye
F, Xie X and Wang J: Lipocalin-2 in neutrophils induces ferroptosis
in septic cardiac dysfunction via increasing labile iron pool of
cardiomyocytes. Front Cardiovasc Med. 9(922534)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lei S, Zhang Y, Su W, Zhou L, Xu J and Xia
ZY: Remifentanil attenuates lipopolysaccharide-induced oxidative
injury by downregulating PKCβ2 activation and inhibiting autophagy
in H9C2 cardiomyocytes. Life Sci. 213:109–115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang WX, He BM, Wu Y, Qiao JF and Peng
ZY: Melatonin protects against sepsis-induced cardiac dysfunction
by regulating apoptosis and autophagy via activation of SIRT1 in
mice. Life Sci. 217:8–15. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Alvarez S, Vico T and Vanasco V: Cardiac
dysfunction, mitochondrial architecture, energy production, and
inflammatory pathways: Interrelated aspects in endotoxemia and
sepsis. Int J Biochem Cell Biol. 81:307–314. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen H, Wang X, Yan X, Cheng X, He X and
Zheng W: RETRACTED: LncRNA MALAT1 regulates sepsis-induced cardiac
inflammation and dysfunction via interaction with miR-125b and p38
MAPK/NFκB. Int Immunopharmacol. 55:69–76. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang Z, Bu L, Yang P, Feng S and Xu F:
Alleviation of sepsis-induced cardiac dysfunction by overexpression
of Sestrin2 is associated with inhibition of p-S6K and activation
of the p-AMPK pathway. Mol Med Rep. 20:2511–2518. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yücel G, Zhao Z, El-Battrawy I, Lan H,
Lang S, Li X, Buljubasic F, Zimmermann WH, Cyganek L, Utikal J, et
al: Lipopolysaccharides induced inflammatory responses and
electrophysiological dysfunctions in human-induced pluripotent stem
cell derived cardiomyocytes. Sci Rep. 7(2935)2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Muller-Werdan U, Buerke M, Ebelt H,
Heinroth KM, Herklotz A, Loppnow H, Ruß M, Schlegel F, Schlitt A,
Schmidt HB, et al: Septic cardiomyopathy-a not yet discovered
cardiomyopathy? Exp Clin Cardiol. 11:226–236. 2006.PubMed/NCBI
|
|
30
|
Zhou N, Zeng MN, Li K, Yang YY, Bai ZY,
Zheng XK and Feng WS: An integrated metabolomic strategy for the
characterization of the effects of Chinese yam and its three active
components on septic cardiomyopathy. Food Funct. 9:4989–4997.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shang X, Lin K, Yu R, Zhu P, Zhang Y, Wang
L, Xu J and Chen K: Resveratrol protects the myocardium in sepsis
by activating the phosphatidylinositol 3-kinases
(PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway and
inhibiting the nuclear factor-κB (NF-κB) signaling pathway. Med Sci
Monit. 25:9290–9298. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Buerke U, Carter JM, Schlitt A, Russ M,
Schmidt H, Sibelius U, Grandel U, Grimminger F, Seeger W,
Mueller-Werdan U, et al: Apoptosis contributes to septic
cardiomyopathy and is improved by simvastatin therapy. Shock.
29:497–503. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lancel S, Joulin O, Favory R, Goossens JF,
Kluza J, Chopin C, Formstecher P, Marchetti P and Neviere R:
Ventricular myocyte caspases are directly responsible for
endotoxin-induced cardiac dysfunction. Circulation. 111:2596–2604.
2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhou R, Yang X, Li X, Qu Y, Huang Q, Sun X
and Mu D: Recombinant CC16 inhibits NLRP3/caspase-1-induced
pyroptosis through p38 MAPK and ERK signaling pathways in the brain
of a neonatal rat model with sepsis. J Neuroinflammation.
16(239)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhang FL, Zhou BW, Yan ZZ, Zhao J, Zhao
BC, Liu WF, Li C and Liu KX: 6-Gingerol attenuates macrophages
pyroptosis via the inhibition of MAPK signaling pathways and
predicts a good prognosis in sepsis. Cytokine.
125(154854)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Koch L, Frommhold D, Buschmann K, Kuss N,
Poeschl J and Ruef P: LPS- and LTA-induced expression of IL-6 and
TNF-α in neonatal and adult blood: Role of MAPKs and NF-κB.
Mediators Inflamm. 2014(283126)2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yego ECK and Dillman JF III: Cytokine
regulation by MAPK activated kinase 2 in keratinocytes exposed to
sulfur mustard. Toxicol In Vitro. 27:2067–2075. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Luo Y, Che W and Zhao M: Ulinastatin
post-treatment attenuates lipopolysaccharide-induced acute lung
injury in rats and human alveolar epithelial cells. Int J Mol Med.
39:297–306. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tien YC, Lin JY, Lai CH, Kuo CH, Lin WY,
Tsai CH, Tsai FJ, Cheng YC, Peng WH and Huang CY: 9Carthamus
tinctorius L. prevents LPS-induced TNFalpha signaling
activation and cell apoptosis through JNK1/2-NFkappaB pathway
inhibition in H9c2 cardiomyoblast cells. J Ethnopharmacol.
130:505–513. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li M, Ye J, Zhao G, Hong G, Hu X, Cao K,
Wu Y and Lu Z: Gas6 attenuates lipopolysaccharide-induced TNF-α
expression and apoptosis in H9C2 cells through NF-κB and MAPK
inhibition via the Axl/PI3K/Akt pathway. Int J Mol Med. 44:982–994.
2019.PubMed/NCBI View Article : Google Scholar
|