Introduction
Severe community-acquired pneumonia (SCAP) brings
serious public health challenges around the world. During recent
decades, the number of patients requiring intensive care management
due to SCAP has increased globally, especially among the elderly,
patients with comorbidities and the immunocompromised. A large
population-based surveillance study on hospitalized CAP patients
found that 21% of patients required intensive care unit (ICU)
admission, with 26% of them needing mechanical ventilation. SCAP
hospital mortality is still high, ranging from 25 to >50%.
Delays from hospitalization to ICU admission have been related with
increased mortality (1). In a
multi-center prospective study of SCAP in China, Influenza virus,
S. pneumoniae, Enterobacteriaceae, Legionella
pneumophila and Mycoplasma pneumoniae were the top five
most common pathogens (2). The
in-hospital mortality of patients diagnosed with SCAP with
identified and unidentified pathogens was 21.7% (43/198) and 25.9%
(20/77), respectively, in individuals >18 years (2). The incidence rate of SCAP in adults
ranged from 1.76 to 7.03 per 1,000 person-years in three cities
[General Roca (Argentina), Rivera (Uruguay) and Concepción
(Paraguay)] in South America, disclosing the high burden of disease
in the region (3). Mixed
viral-bacterial co-infections occurred in 15.4% of patients and
hospital mortality was as high as 13.7% in Singapore between
January 2014 and July 2015(4).
SCAP, characterized by its complexity and lack of
predictability, causes an increased number of mortalities each
year, especially in immunocompromised patients (5). SCAP is a common disease in
hospitalized pneumonia patients with a mortality rate of 30-50%,
which is higher in immunocompromised patients (6). Therefore, the ability to accurately
detect etiological pathogens is important for guiding optimal
antibiotic therapy and improving prognostic results (7). The current tactics of conventional
microbiological tests (CMTs) for severe community-acquired
pneumonia (SCAP) may be too complicated or impossible to use in
polymicrobial infections, and it may be difficult to identify
unexpected pathogens. Detectability of pathogens by conventional
microbiological tests (CMTs) is also limited due to the early
application of broad-spectrum or prophylactic antimicrobial drugs
and the fastidious or slow-growing pathogenic microorganisms
(8).
Metagenomic next-generation sequencing (mNGS)
provides a comprehensive method to identify nearly all potential
pathogens-viruses, bacteria, fungi, mycobacteria and parasites in a
single assay (8-11).
mNGS permits the assessment of etiological pathogens without the
need for culture. mNGS is especially applicable to rare, novel and
atypical etiologies of complicated infectious diseases (12,13).
Due to the high-throughput identification and a relatively rapid
turnaround time, mNGS has been widely applied to various clinical
diseases in previous years, including diseases of the central
nervous (14-16)
and respiratory (17) systems, the
bloodstream (18), prosthetic
joint (19) and urinary tract
(20). The present study aimed to
evaluate the potential of mNGS compared with CMTs as a first-line
diagnostic technology for SCAP in immunocompromised patients.
Materials and methods
Case definition
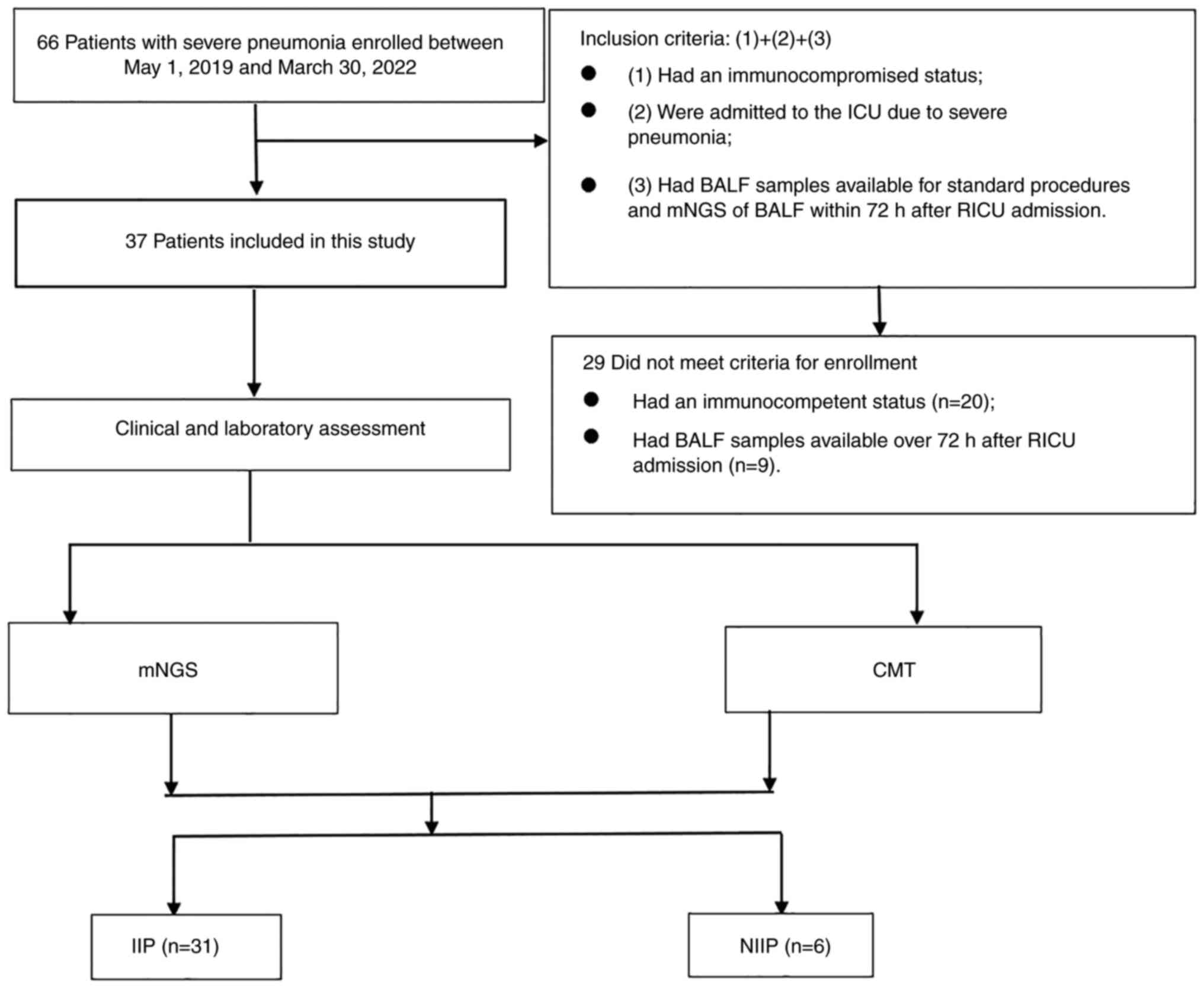
A total of 66 patients diagnosed with severe
pneumonia were admitted to Respiratory Intensive Care Unit of the
First Affiliated Hospital of Soochow University (Soochow, China)
between May 1, 2019, and March 30, 2022. Among them, 29 patients
were excluded from this study, including 20 patients who had an
immunocompetent status, and 9 patients who had bronchoalveolar
lavage fluid (BALF) samples available for mNGS >72 h after
Respiratory ICU (RICU) admission (Fig.
1).
Data collection and participants
The present study is retrospective, and the article
does not involve the privacy of patients. Informed consent was
obtained from all patients or their legal surrogates. Demographic
data and medical records of the 37 study subjects were summarized
in Table I. The diagnosis of SCAP
was made according to Chinese guidelines, and tuberculosis was
required to be excluded from the study (21).
 | Table ISummary of the patient population and
characteristics. |
Table I
Summary of the patient population and
characteristics.
|
Characteristics | Values |
|---|
| Age in years,
median (IQR) | 55.6 (47.5,
67.0) |
| Male, n (%) | 28 (75.7) |
| Received
broad-spectrum antibiotics before BALF (%) | 28 (75.7) |
| Laboratory
findings | |
|
C-reactive
protein in mg/l, median (IQR) | 139.3 (64.5,
186.5) |
|
Procalcitonin
in ng/ml, median (IQR) | 3.4 (0.3, 1.1) |
|
Lactate
dehydrogenase in U/l, median (IQR) | 414.1 (213.9,
535.0) |
|
White blood
cells count (109/l), median (IQR) | 9.6 (6.3,
11.5) |
|
Lymphocytes
count (109/l), median (IQR) | 0.8 (0.4, 1.0) |
|
CD4+
T cell count (106/l), median (IQR) | 203.7 (116.0,
203.0) |
|
CD8+
T cell count (106/l), median (IQR) | 259.0 (64.5,
231.0) |
| Type of
immunocompromised status, n (%) | |
|
Prolonged
corticosteroid therapya | 20 (54.1) |
|
Solid
tumor/hematological malignancy receiving chemotherapy | 12 (32.4) |
|
Solid organ
or Bone marrow transplantation | 5 (13.5) |
| Disease
severity | |
|
APACHE II
score, median (IQR) | 20.7 (18.0,
22.0) |
|
SOFA score,
median (IQR) | 5.5 (4.0, 6.0) |
|
Invasive
mechanical ventilation, n (%) | 16 (43.2) |
| Outcomes | |
|
Death within
30 days, n (%) | 12 (32.4) |
Inclusion and exclusion criteria
For inclusion, patients have to meet the following
criteria: i) were immunocompromised; ii) were admitted to the
intensive care unit (ICU) due to SCAP; and iii) had BALF samples
available for CMTs and mNGS within 3 days after RICU admission
(22). Immunocompromised status
was defined as having one of the following conditions: i) Received
repeated therapy with glucocorticoids; ii) received chemotherapy
during the last 3 months; iii) had hematological malignancies; iv)
received an organ transplant during the last 1/2 year; or v) was
diagnosed with human immunodeficiency virus infection (22).
Diagnostic tests. BALF collection and
sampling processing
Bronchoscopy was carried out under local anesthesia
for each patient. Several aliquots of 20 ml of 0.9% normal saline
were instilled into the target subsegmental bronchi. The first 20
ml was discarded as recommended (23). BALF samples were separated into
5-ml aliquots. Applying CMTs, one aliquot was used for routine
experimental examination with staining by optical microscope
[Gram's staining solution: i) Dyed with Gentian violet for 10 sec,
washed and dried; ii) dyed with iodized solution for 10 sec, washed
and dried; iii) decolorizing solution was added to decolorize for
10-20 sec, washed and dried; iv) Gaza yellow solution was used for
re-staining for 10 sec and washed; v) once dry, a light microscopic
inspection was conducted (x100 magnification). Lactic acid phenol
cotton blue staining solution: On a clean slide, 1 drop of lactic
acid phenol cotton blue staining solution and a small amount of
culture or sample was added, which was spread out with an
inoculation needle. The slide was covered, slightly warmed with an
alcohol lamp and the slide was lightly pressed to remove bubbles.
Light microscopic examination (x100 magnification) was then
conducted], and for cultures of fungi and bacteria [Culture of
bacteria: i) After inoculation, all types of agar plates (blood
plate, chocolate plate and MacConkey plate) were placed in the
incubator for 24 h according to relevant culture requirements, and
then the results were observed; ii) the bacteria on various plates
were observed for color, transparency, bulge state and edge state,
and attention was focused on distinguish between contaminated
bacteria and pathogenic bacteria; iii) samples from a suspicious
single independent bacterial colony were placed on a clean slide
that had been dropped with physiological saline. The samples were
then directly smeared thinly until they naturally dried. The
manufacturer's instructions were followed. A microscope was used to
observe the smear after is had dried. Fungal culture: The collected
BALF samples were inoculated on Sabouraud medium and placed in a
Heraeus CO2 constant temperature incubator for 48 h to 1
week, and then the growth and colony morphology were observed using
the naked eye], and PCR [i) DNA source: Exfoliated cells and
secretions of nasopharynx; ii) method of extraction is based on the
full-automatic nucleic acid extraction instrument produced by Bio
Perfectus Technologies, performed as elaborated in the reagent
specification; iii) Hot Start DNA Polymerase, manufactured by
Beijing XABT Biotechnology Co., Ltd., was used; iv) Sequences of
the forward and reverse primers: Influenza virus forward,
5'-AGAGACTTGAAGATGTCTTTGC-3' and reverse,
5'-GCTCTGTCCATGTTATTTGGATC-3'; influenza virus forward,
5'-GAAAAATTACACTGTTGGTTCGG-3' and reverse,
5'-AGCGTTCCTAGTTTTACTTGCAT-3'; adenovirus forward,
5'-GCCGCAGTGGTCTTACATGCACATC-3' and reverse,
5'-CAGCACGCCGCGGATGTCAAAGT-3'; respiratory syncytial virus forward,
5'-AGCACTTATATGTTAACAAATAG-3' and reverse,
5'-TGGGAAGAAAGATACTGATCC-3'; parainfluenza virus (PIV-1) forward,
5'-ATTTCTGGAGATGTCCCGTAGGAGAAC-3' and reverse,
5'-CACATCCTTGAGTGATTAAGTTTGATG-3'; PIV-3 forward,
5'-TCGAGGTTGTCAGGATATAG-3' and reverse,
5'-CTTTGGGAGTTGAACACAGTT-3'; v) thermocycling conditions: Initial
annealing step at 95˚C for 5 min, denaturation step at 95˚C for 15
sec, annealing and elongation at 60˚C for 45 sec. Fluorescence
signals were collected at the last 45th sec. 45 cycles were
performed.] for virus detection (including influenza virus) were
carried out in every sample. All these routine laboratory methods
were known as CMTs. mNGS for the other aliquots were performed
parallel with the conventional microbiological testing.
Concerning the serology tests, peripheral blood
specimens were used for the detection of immunoglobulin antibodies
of influenza A/B, parainfluenza virus, human rhinovirus,
adenovirus, Coxsackie virus A/B, L. pneumophila, M.
pneumoniae and C. pneumoniae by using commercial ELISA
kits (cat. no. YZB/SPA 5210-2009; Figure Bioengineering Co., Ltd.)
according to the manufacturer's instructions. These diagnostic
tests were performed by the First Affiliated Hospital of Soochow
University, and they were a part of the clinical treatment.
DNA extraction and sequencing. The collected
BALF was tested for the gene of pathogenic microorganisms using
mNGS. Bronchoalveolar fluid (600 µl) samples were mixed with
proteinase kinase enzyme (cat. no. DP316; Tiangen Biotech, Co.,
Ltd.) and glass beads (0.5 mm diameter; zirconia/silica cat. no.
11079105z; Thistle Scientific), before being vortexed at 1509.3 x g
for 30 min at 4˚C. The TIANamp Micro DNA kit (cat. no. DP316;
Tiangen Biotech, Co., Ltd.) was used for extracting the total DNA.
The DNA extraction and library construction were performed using an
NGS automatic DNA library system (cat. no. MAR002; MatriDx Biotech
Corp.) and a total DNA library preparation kit (cat. no. MD001T;
MaxtriDx Biotech Corp.). Libraries were then quantified by
quantitative PCR using a KAPA Library Quantification 75-cycle
sequencing kit (cat. no. 20024906; Illumina, Inc.). The library
concentration had to pass the quality control cut-off (>50 pmol
l-1). A total of 10-20 million 50-bp single-end reads were obtained
for each library.
Bioinformation pipeline. In order to generate
high-quality data, the raw data were needed to remove adapter,
low-quality, low complexity and short reads (<35 bp), with an
in-house program. Then, the human sequences were excluded by
mapping reads to the human reference genome (hg19) with the
application of the Burrows-Wheeler Alignment (http://bio-bwa.sourceforge.net). The remaining
data were aligned to a microbial genomedatabase (National Center
for Biotechnology Information; ftp://ftp.ncbi.nlm.nih.gov/genomes). The reference
database used for the present study contained 11,910 bacteria,
7,103 viruses, 1,046 fungi and 305 parasites that are all
associated with human diseases (8).
Infectious pathogens were defined as that meeting
either of the following criteria (20): i) >30% relative abundance at the
genus level, regardless of culture or smear result, and there was
robust evidence of pathogenicity in the lungs based on the clinical
literature; ii) culture and mNGS identified the same microbes, the
number of unique reads was ≥50 from a single species (24) and there was robust evidence of
pathogenicity in the lungs based on the clinical literature. Oral
colonization microorganisms were not considered infectious
pathogens regardless of their relative abundance unless proven
otherwise, or they were deemed significant by the managing
physician. These were based on strict clinical criteria (24), combined with multiple clinician
adjudication, to rigorously discriminate infection from
colonization and contamination. The 37 patients were categorized
into two groups: i) Identified infectious pathogens (IIP) group;
ii) and non-identified infectious pathogens (NIIP) group, according
to the final diagnosis.
The pathogen for SCAP was defined as follows: i) The
final etiology result was assessed by the attending physician teams
based on clinical features, microbiological results and response to
the treatment; and ii) when disputes arise, the physician group
discussed and decided the final results.
Statistical analysis
Continuous variables are reported as the mean and
standard deviation when they are normally distributed and as the
median and interquartile range (IQR) when they have a skewed
distribution, according to the Shapiro-Wilk test. Categorical
variables are expressed as frequencies and percentages. Pathogens
identified by CMTs or mNGS should be totally identical to those
confirmed by the physician group with the reference standards, and
the reference standards were combined with clinical composite
diagnosis and determination of microbiological etiology. The paired
McNemar χ2 test was used to compare the diagnostic
efficiency of mNGS vs. CMTs. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
utilized using SPSS 26.0 (IBM Corp.).
Results
Patient characteristics
During the study period, 66 patients were admitted
to RICU due to SCAP, among whom 29 patients were excluded either
because mNGS was not performed within 72 h of their admittance or
because they did not meet the criteria of immunocompromised status.
Thus, 37 patients [median age, 55.6 years; IQR, 47.5, 67.0 years;
males, 28 (75.7%)] met the inclusion criteria and were included in
the final analysis. Subsequently, 20 patients (54.1%) received
prolonged corticosteroid therapy for autoimmune diseases, 12
patients (32.4%) were treated with chemotherapy due to solid tumor
or hematological malignancy, whereas 5 patients (13.5%) were
treated with solid organs or bone marrow transplantation (Table I). The CD4+ T cell count
was 203.7x106/l (IQR, 116.0, 203.0), and CD8+
T cell count was 259.0x106/l (IQR, 64.5, 231.0). The
median APACHE II score was 20.7 (IQR, 18.0, 22.0), and the median
SOFA score was 5.5 (IQR 4.0, 6.0). Of the 37 patients, 28 (75.7%)
patients received broad-spectrum antibiotics treatment prior to
BALF collection. Separately, the average values of the C-reactive
protein (≤6 mg/l), procalcitonin (0-0.5 ng/ml), lactate
dehydrogenase (120-250 U/l), white blood cell counts
(3.5x109-9.5x109 cells/l), and lymphocyte
counts (1.1x109-3.2x109 cells/l) of the 37
patients were found to be 139.3 mg/l (IQR 64.5, 186.5 mg/l), 3.4
ng/ml (0.3, 1.1 ng/ml), 414.1 U/L (213.9, 535.0 U/L),
9.6x109 cells/l (6.3, 11.5x109 cells/L),
0.8x109 cells/l (0.4, 1.0x109 cells/l). Then,
16 (43.2%) patients underwent tracheal intubation and received
mechanical ventilation when the BALF was collected. Overall, 12
(32.4%) died within 30 days of being admitted to the ICU (Table I).
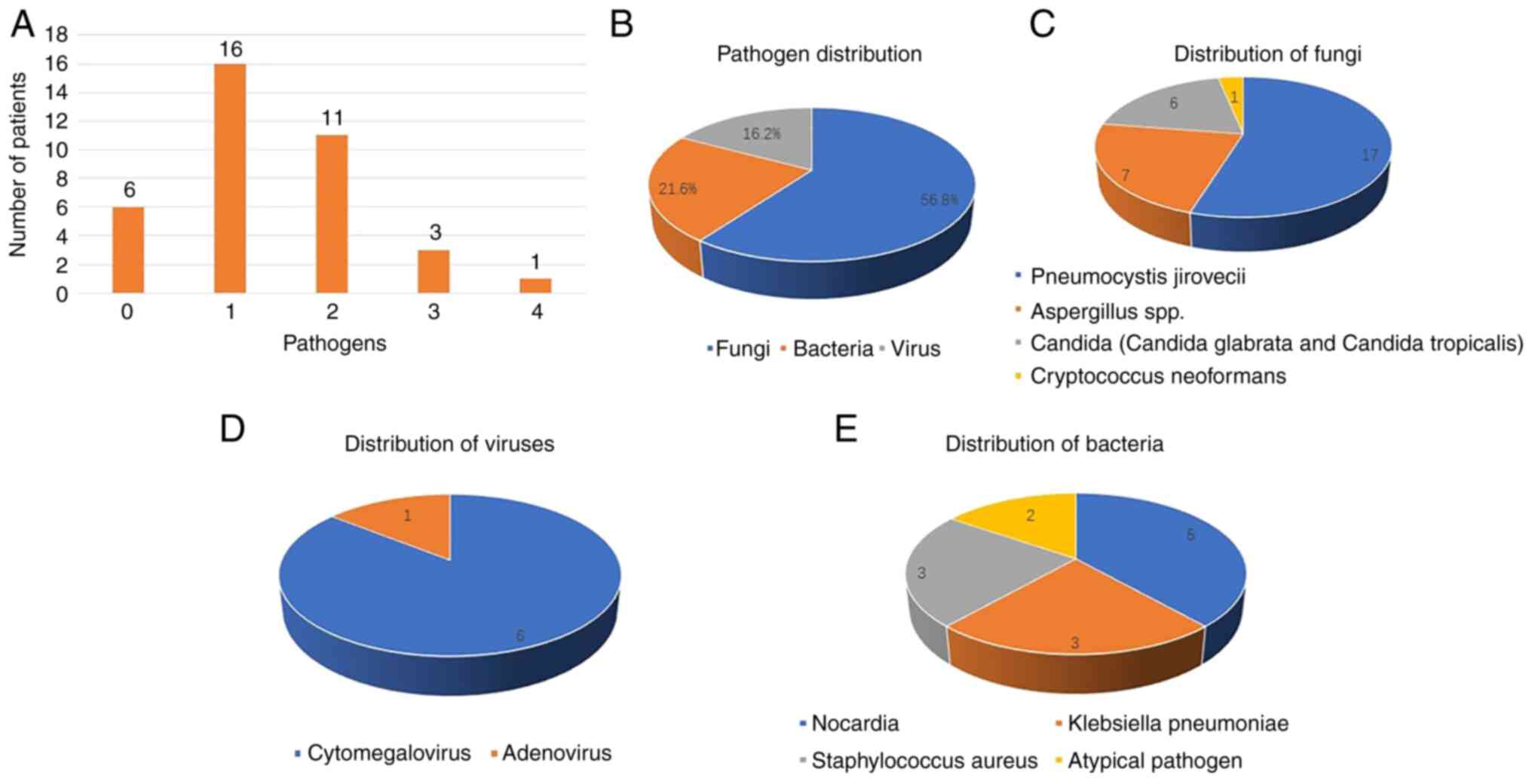
Pneumonia pathogens
Based on a retrospective review of clinical
manifestations, the diagnosis of SCAP also required judgement by an
experienced team of experts. Among the 37 patients enrolled in the
present study, pathogenic pathogens have been clearly defined in 31
patients, and not been defined in the other 6 patients. Among these
31 patients, 16 (43.2%) had monomicrobial infections, while 15
(40.5%) had polymicrobial infections (including 11 patients with
two pathogens, 3 patients with three pathogens and 1 patient with
four pathogens). A total of 21 (56.8%) patients had fungal
infections [Pneumocystis jirovecii (n=17),
Aspergillus spp. (n=7), Candida glabrata (n=3), Candida
tropicalis (n=3) and Cryptococcus neoformans (n=1)], 8 (21.6%)
patients had bacterial infections [Nocardia (n=5),
Klebsiella pneumoniae (n=3), Staphylococcus aureus
(n=3) and Atypical pathogen (n=2)] and 6 (16.2%) patients had viral
infections [cytomegalovirus (CMV; n=6) and adenovirus (n=1)]
(Fig. 2).
Diagnostic performance of CMTs and
mNGS
Among the 37 patients, an accurate and complete
microbiological diagnosis of mNGS was obtained for 31 patients, and
3 out of 6 patients with a non-infectious aetiology had negative
mNGS results (data not shown), corresponding to 96.8% sensitivity
and 33.3% specificity, positive and negative predictive values were
88.2 and 66.6%, while the positive and negative likelihood ratio
were 1.45 and 0.1, respectively. Regarding CMTs, pathogens were
detected in 13 patients by CMTs only, among whom 7 (18.9%) patients
had fungal pneumonia [Candida glabrata (n=3), Candida
tropicalis (n=2), Aspergillus spp. (n=2)], 5 (13.5%)
patients had bacterial infections [Nocardia (n=3), atypical
pathogen (n=1), Staphylococcus aureus (n=1)] and 1 (2.7%)
patient had a viral infection [CMV (n=1)]. The sensitivity and
specificity were 38.7 and 83.3%, the positive and negative
predictive values were 92.3 and 20.8%, while the positive and
negative likelihood ratios were 2.3 and 0.74, respectively.
Compared with that of CMTs, the overall diagnostic accuracy of mNGS
was higher and it was statistically significantly different [86.5%
(32/37) vs. 45.9% (17/37); P<0.001] (Table II).
 | Table IIDiagnostic performance comparison of
mNGS and CMTs. |
Table II
Diagnostic performance comparison of
mNGS and CMTs.
| Parameter | mNGS | CMTs |
|---|
| Positive
detection | | |
|
IIP | 30 | 12 |
|
NIP | 4 | 1 |
| Negative
detection | | |
|
IIP | 1 | 19 |
|
NIP | 2 | 5 |
| Sensitivity, % | 96.80 | 38.70 |
| Specificity, % | 33.30 | 83.30 |
| Positive predictive
value, % | 88.20 | 92.30 |
| Negative predictive
value, % | 66.60 | 20.80 |
| LR+ | 1.45 | 2.3 |
| LR- | 0.1 | 0.74 |
| Accuracy, % | 86.50 | 45.90a |
Discussion
Precise and timely responsible pathogen diagnosis is
essential for SCAP in immunocompromised individuals (25). Despite current advanced diagnostic
techniques, ~60% infectious diseases fail to identify pathogens
(26). CMTs have drawbacks in
their abilities of detection and sensitivity, due to the
characteristics of being time-consuming, technically intensive and
error-prone (27,28). mNGS, which is independent of the
etiological hypothesis, can theoretically permit the identification
of all known pathogenic microbes (29,30).
To the best of our knowledge, the present research is the first
study to apply mNGS to the diagnosis of pathogenic microbes in
immunocompromised SCAP adult patients. The present study reported
our experience in the evaluation of immunocompromised patients,
with the majority of the focus on those receiving long-term steroid
therapy and chemotherapy with febrile illness or invasive
infections by mNGS.
In the present study, of the 37 immunocompromised
SCAP patients, 31 patients (83.8%) were identified as having
infectious pathogens based on mNGS and CMTs. In total, >40%
patients (40.5%) had polymicrobial etiology, with Pneumocystis
jirovecii (45.9%) and Aspergillus spp. (18.9%) as the
most common pathogens of mixed infection; however, to the best of
our knowledge, none were reported in previous studies (31,32).
The spectrum of pathogens in immunocompromised patients with SCAP
is different compared with those in immunocompetent SCAP
individuals, with mixed pulmonary infections often reported
(31,32). mNGS demonstrated the ability to
detect the causative agents of mixed infection, especially in a
mixed infection of bacteria, fungi and viruses. The CMTs (such as
culture, serology and molecular methods) performed poorly in
detecting mixed infections by comparison.
In contrast to previous studies (8,22),
broad-spectrum antibiotics were commonly administered in a large
proportion of these patients (28/37, 75.7%) before ICU admission,
and CMTs showed a relatively poor level of overall diagnostic
performance compared with that of mNGS. We hypothesize that the use
of broad-spectrum antibiotics had led to a decline in the detection
rate of CMTs, while mNGS was not affected by antibiotics. It is
noteworthy that the present study confirmed the potential
advantages of mNGS in the identification of fungi and viral
infection, especially Pneumocystis jiroveci and CMV. The
current study demonstrated the high sensitivity of mNGS compared
with CMTs in immunocompromised patients with SCAP, making it
recommended as a front-line test for microbiological diagnosis in
suspected infections or as a ‘rule-out’ method to exclude infection
in the immunocompromised patients. mNGS would be most valuable when
physicians are unable to find presumed causative pathogens or when
a full work-up of CMTs are unavailable in the clinical testing
centers. The distinct advantage of mNGS may contribute to more
comprehensive evaluation of empiric antibiotics therapy and more
effective adjustments for these immunocompromised SCAP
patients.
Bacteria were identified in 21.6% patients in which
Nocardia (13.5% of 37 patients) were the most frequent
pathogen in the bacteria etiology. Klebsiella pneumoniae and
Staphylococcus aureus were identified in 8.1%
immunocompromised patients with SCAP, respectively. Fungi (56.8% of
37 patients) were the most common pathogenic pathogens in
immunosuppressive individuals. Pneumocystis jirovecii (45.9%
of 37 patients) remained the most frequent pathogen in the fungi
etiology. Aspergillus spp. (18.9% of 37 patients) and
Candida (Candida glabrata 8.1% and Candida
tropicalis 8.1%) were identified in immunocompromised patients
with SCAP, respectively. A higher number of patients in the present
study developed Pneumocystis jirovecii compared with other
studies (26,27). It may be related to the serious
immunosuppression condition of the patients that were included in
the present study.
Respiratory viruses play an increasing role in
immunocompromised patients with SCAP; for example, influenza virus
and covid-19 cause SCAP in immunocompromised patients (33,34).
In the present study, cytomegalovirus (16.2% of 37 patients) was
the most common viruses in the patients. Therefore, the management
strategy of SCAP in immunocompromised patients has to involve the
detection of respiratory viruses such as cytomegalovirus (35,36).
Since the outbreak of coronavirus disease-19, mNGS
technology has been widely developed in China. The cost of mNGS is
~3,600 RMB, and the cost of CMTS (such as culture technology,
antibody detection and PCR) is ~2,000 RMB or more (34). Although mNGS is more expensive when
compared with CMTs, the shorter time taken to identify the pathogen
can often bring major adjustments to treatment and, thus, reduce
the hospitalization days and expenses (27). mNGS technology is already being
performed in numerous hospitals in China. In the Department of
Pulmonary and Critical Care Medicine of The First Affiliated
Hospital of Soochow University, mNGS testing can be performed
independently. For hospitals unable to carry out the technology
themselves, it is convenient to entrust a third-party biological
company for testing. To the best of our knowledge, there is no
relevant research on the clinical benefit to cost ratio. According
to our clinical experience, there is no significant difference in
the cost of mNGS and CMTs. Therefore, the present study recommends
mNGS as a powerful tool.
The current study has several limitations, as
follows: i) this study was conducted in one single center, where
there was a small sample size in addition to potential selection
bias; ii) patients were recruited with highly heterogeneous
immunosuppression, including autoimmune diseases with prolonged
corticosteroid therapy, solid tumors or hematological malignancies
receiving chemotherapy and patients with solid organ or bone marrow
transplantation, which may be associated with different pathogen
profiles and diagnostic efficiencies of mNGS; iii) due to the lack
of standard criteria, the interpretation of mNGS results may
influence the diagnostic results. Some bacteria and viruses with
relatively low abundance may be regarded as non-pathogens or
contamination; and iv) the respiratory tract is an open airway, and
therefore there are some confounding variables that may affect
results. In fact, the respiratory tract is susceptible to the
influence of various colonizing bacteria. Patients with
immunosuppressive pneumonia have different basic conditions and
complications, and there are indeed many confounding factors.
However, the diagnosis of pneumonia pathogens depends not only on
the microbiological diagnosis, but also on the clinical
characteristics and treatment effects. Ultimately, the
comprehensive judgment of clinicians is required. Therefore, the
final determination of pneumonia pathogens strictly complies with
the comprehensive judgment of the expert group.
In conclusion, mNGS is a revolutionary technology in
the microbiological diagnosis of SCAP in immunocompromised
patients. mNGS demonstrated its notable advantages in detecting
pathogenic microbes, mixed infections and rare pathogens in these
patients. The present study indicated that mNGS could quickly offer
etiological evidence for SCAP in immunocompromised adult patients.
In the future, additional clinical trials need to be carried out to
evaluate the clinical usage of mNGS further. In addition, the data
analysis strategy could be further improved.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by Young Scientific and
Technical Talents Lift Project of Jiangsu Association for Science
and Technology, Gusu Youth Medical Talent (grant no. GSWS2020017),
The Third Level ‘333’ High Level Talent Training Project of Jiangsu
Province, Jiangsu Provincial Key Medical Key Discipline Laboratory
(grant no. ZDXK202201), The Clinical Medicine Center of Suzhou
(grant no. Szzx201502), Suzhou Key Laboratory for Respiratory
Medicine (grant no. SZS201617), and The National Natural Science
Foundation of China (grant no. NSFC82000023).
Availability of data and materials
The datasets generated and/or analysed during the
current study are not publicly available due to our team using part
of the data for writing another manuscript, but are available from
the corresponding author on reasonable request.
Authors' contributions
XZ wrote the manuscript, analyzed and interpreted
the patient data. YQ was responsible for acquisition of data, and
analyzed and interpreted the patient data. WL and JAH were
responsible for designing the study, and the analysis and
interpretation of the data. All authors have read and approved the
final manuscript. XZ and WL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of the First Affiliated Hospital of Soochow University
(approval no. [2023] Ethical Research NO.073). Informed consent was
obtained from all patients or their legal surrogates.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Spasovska K, Grozdanovski K, Milenkovic Z,
Bosilkovski M, Cvetanovska M, Kuzmanovski N, Kapsarov K and
Atanasovska E: Evaluation of severity scoring systems in patients
with severe community acquired pneumonia. Rom J Intern Med.
59:394–402. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Qu J, Zhang J, Chen Y, Huang Y, Xie Y,
Zhou M, Li Y, Shi D, Xu J, Wang Q, et al: Etiology of severe
community acquired pneumonia in adults identified by combined
detection methods: A multi-center prospective study in China. Emerg
Microbes Infect. 11:556–566. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lopardo GD, Fridman D, Raimondo E,
Albornoz H, Lopardo A, Bagnulo H, Goleniuk D, Sanabria M and
Stamboulian D: Incidence rate of community-acquired pneumonia in
adults: A population based prospective active surveillance study in
three cities in South America. BMJ. 8(e019439)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Quah J, Jiang B, Tan PC, Siau C and Tan
TY: Impact of microbial Aetiology on mortality in severe
community-acquired pneumonia. BMC Infect Dis.
18(451)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wu XJ, Gu SC, Cai Y, Zhai TS and Zhan QY:
Etiology of severe community-acquired pneumonia in
immunocompromised patients. Zhonghua Jie He He Hu Xi Za Zhi.
44:892–896. 2021.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
6
|
Montull B, Menendez R, Torres A, Reyes S,
Mendez R, Zalacain R, Capelastegui A, Rajas O, Borderías L,
Martin-Villasclaras J, et al: Predictors of severe sepsis among
patients hospitalized for community-acquired pneumonia. PLoS One.
11(e0145929)2016.PubMed/NCBI
|
|
7
|
Ewig S, Birkner N, Strauss R, Schaefer E,
Pauletzki J, Bischoff H, Schraeder P, Welte T and Hoeffken G: New
perspectives on community-acquired pneumonia in 388 406 patients.
Results from a nationwide mandatory performance measurement
programme in healthcare quality. Thorax. 64:1062–1069.
2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Miao Q, Ma Y, Wang Q, Pan J, Zhang Y, Jin
W, Yao Y, Su Y, Huang Y, Wang M, et al: Microbiological diagnostic
performance of metagenomic next-generation sequencing when applied
to clinical practice. Clin Infect Dis. 67 (Suppl 2):S231–S240.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gu W, Miller S and Chiu CY: Clinical
metagenomic next-generation sequencing for pathogen detection. Annu
Rev Pathol. 14:319–338. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Simnera PJ, Miller S and Carroll KC:
Understanding the promises and hurdles of metagenomic
next-generation sequencing as a diagnostic tool for infectious
diseases. Clin Infect Dis. 66:778–788. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wilson MR, O'Donovan BD, Gelfand JM,
Sample HA, Chow FC, Betjemann JP, Shah MP, Richie MB, Gorman MP,
Hajj-Ali RA, et al: Chronic meningitis investigated via metagenomic
next-generation sequencing. JAMA Neurol. 75:947–955.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Goldberg B, Sichtig H, Geyer C, Ledeboer N
and Weinstock GM: Making the leap from research laboratory to
clinic: Challenges and opportunities for next-generation sequencing
in infectious disease diagnostics. MBio. 6:e01888–e018815.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schlaberg R, Chiu CY, Miller S, Procop GW
and Weinstock G: Professional Practice Committee and Committee on
Laboratory Practices of the American Society for Microbiology;
Microbiology Resource Committee of the College of American
Pathologists. Validation of metagenomic next-generation sequencing
tests for universal pathogen detection. Arch Pathol Lab Med.
141:776–786. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wilson MR, Sample HA, Zorn KC, Arevalo S,
Yu G, Neuhaus J, Federman S, Stryke D, Briggs B, Langelier C, et
al: Clinical metagenomic sequencing for diagnosis of meningitis and
encephalitis. N Engl J Med. 380:2327–2340. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Simner PJ, Miller HB, Breitwieser FP,
Pinilla Monsalve G, Pardo CA, Salzberg SL, Sears CL, Thomas DL,
Eberhart CG and Carroll KC: Development and optimization of
metagenomic next-generation sequencing methods for cerebrospinal
fluid diagnostics. J Clin Microbiol. 56:e00472–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang Y, Ai JW, Cui P, Zhang WH, Wu HL and
Ye MZ: A cluster of cases of pneumocystis pneumonia identified by
shotgun metagenomics approach. J Infect. 78:158–169.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pan T, Tan R, Qu H, Weng X, Liu Z, Li M
and Liu J: Next-generation sequencing of the BALF in the diagnosis
of community-acquired pneumonia in immunocompromised patients. J
Infect. 79:61–74. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Blauwkamp TA, Thair S, Rosen MJ, Blair L,
Lindner MS, Vilfan LD, Kawli T, Christians FC, Venkatasubrahmanyam
S, Wall GD, et al: Analytical and clinical validation of a
microbial cell-free DNA sequencing test for infectious disease. Nat
Microbiol. 4:663–674. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ivy MI, Thoendel MJ, Jeraldo PR,
Greenwood-Quaintance KE, Hanssen AD, Abdel MP, Chia N, Yao JZ,
Tande AJ, Mandrekar JN and Patel R: Direct detection and
identification of prosthetic joint infection pathogens in synovial
fluid by metagenomic shotgun sequencing. J Clin Microbiol.
56:e00402–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Burnham P, Dadhania D, Heyang M, Chen F,
Westblade LF, Suthanthiran M, Lee JR and De Vlaminck I: Urinary
cell-free DNA is a versatile analyte for monitoring infections of
the urinary tract. Nat Commun. 9(2412)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cao B, Huang Y, She DY, Cheng QJ, Fan H,
Tian XL, Xu JF, Zhang J, Chen Y, Shen N, et al: Diagnosis and
treatment of community-acquired pneumonia in adults: 2016 clinical
practice guidelines by the Chinese Thoracic Society, Chinese
Medical Association. Clin Respir J. 12:1320–1360. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Peng JM, Du B, Qin HY, Wang Q and Shi Y:
Metagenomic next-generation sequencing for the diagnosis of
suspected pneumonia in immunocompromised patients. J Infect.
82:22–27. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen X, Ding SZ, Lei C, Qin JL, Guo T,
Yang DH, Yang M, Qing J, He WL, Song M, et al: Blood and
bronchoalveolar lavage fluid metagenomic next-generation sequencing
in pneumonia. Can J Infect Dis Med Microbiol.
2020(6839103)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li Y, Sun B, Tang X, Liu YL, He HY, Li XY,
Wang R, Guo F and Tong ZH: Application of metagenomic
next-generation sequencing for bronchoalveolar lavage diagnostics
in critically ill patients. Eur J Clin Microbiol Infect Dis.
39:369–374. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang P, Chen Y, Li S, Li C, Zhang S,
Zheng W, Chen Y, Ma J, Zhang X, Huang Y and Liu S: Metagenomic
next-generation sequencing for the clinical diagnosis and prognosis
of acute respiratory distress syndrome caused by severe pneumonia:
A retrospective study. PeerJ. 8(e9623)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Phua J, Ngerng W, See K, Tay C, Kiong T,
Lim H, Chew M, Yip H, Tan A, Khalizah H, et al: Characteristics and
outcomes of culture-negative versus culture-positive severe sepsis.
Crit Care. 17(R202)2013.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Han D, Li Z, Li R, Tan P, Zhang R and Li
JM: mNGS in clinical microbiology laboratories: On the road to
maturity. Crit Rev Microbiol. 45:668–685. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Duan H, Li X, Mei A, Li P, Liu Y, Li X, Li
W, Wang C and Xie S: The diagnostic value of metagenomic
next-generation sequencing in infectious diseases. BMC Infect Dis.
21(62)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Grumaz S, Stevens P, Grumaz C, Decker SO,
Weigand MA, Hofer S, Brenner T, von Haeseler A and Sohn K:
Next-generation sequencing diagnostics of bacteremia in septic
patients. Genome Med. 8(73)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Abril MK, Barnett AS, Wegermann K,
Fountain E, Strand A, Heyman BM, Blough BA, Swaminathan AC,
Sharma-Kuinkel B, Ruffin F, et al: Diagnosis of capnocytophaga
canimorsus sepsis by whole-genome next-generation sequencing. Open
Forum Infect Dis. 3(ofw144)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu X, Li Y, Zhang M, Li M, Zhang R, Lu X,
Gao W and Li Q, Xia Y, Pan P and Li Q: Etiology of Severe
community-acquired pneumonia in adults based on metagenomic
next-generation sequencing: A prospective multicenter study. Infect
Dis Ther. 9:1003–1015. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sun T, Wu X, Cai Y, Zhai T, Huang L, Zhang
Y and Zhan Q: Metagenomic next-generation sequencing for pathogenic
diagnosis and antibiotic management of severe community-acquired
pneumonia in immunocompromised adults. Front Cell Infect Microbiol.
11(661589)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Griffiths P and Reeves M: Pathogenesis of
human cytomegalovirus in the immunocompromised host. Nat Rev
Microbiol. 19:759–773. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee ARYB, Wong SY, Chai LYA, Lee SC, Lee
MX, Muthiah MD, Tay SH, Teo CB, Tan BKJ, Chan YH, et al: Efficacy
of covid-19 vaccines in immunocompromised patients: Systematic
review and meta-analysis. BMJ. 376(e068632)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fulkerson HL, Nogalski MT,
Collins-McMillen D and Yurochko AD: Overview of human
cytomegalovirus pathogenesis. Methods Mol Biol. 2244:1–18.
2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bateman CM, Kesson A, Powys M, Wong M and
Blyth E: Cytomegalovirus infections in children with primary and
secondary immune deficiencies. Viruses. 13(2001)2021.PubMed/NCBI View Article : Google Scholar
|