Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic
interstitial lung disease characterized by dense collagen
accumulation from alveolar epithelial cell damage and high
fibroblast proliferation. It can lead to chronic respiratory
failure, physical disability, and severe hypoxemia (1,2). The
age of onset of IPF was mainly over 50 years old, and male patients
were more than female patients in US (3). Unspecific early symptoms lead to
delayed diagnosis of IPF for years (4). Current treatments for IPF include
supplemental oxygen and anti-fibrotic drugs, such as nintedanib and
pirfenidone (5,6). However, the two drugs only modestly
reduce the rate of lung function deterioration (7). Despite these treatments, the median
2-3 survival of patients with IPF remains poor (8). Lung transplantation is the only cure
for IPF (9), but the shortage of
donor organs has resulted in only a minority of patients being able
to undergo lung transplants (10).
Therefore, the development of new mitigation strategies, treatment
methods and therapeutic targets is urgently needed for patients
with IPF.
Although the pathological mechanism of IPF has not
been elucidated, it is thought to be mediated by various
chemokines, growth factors and cytokines (11). It is important to understand the
mechanism of these factors in IPF to accelerate the progress of IPF
treatment. Insulin-like growth factor (IGF-1) signaling has been
found to be involved in the progression of pulmonary fibrosis in
human IPF tissues and mouse models in previous studies (12,13).
Meanwhile, IGF-1 requires insulin-like growth factor receptor
(IGF-1R) to exert its function. The PI3K/Akt/mTOR signaling pathway
also plays an important role in pulmonary fibrosis. Growth
factors/ligands-mediated stimulation of receptor tyrosine kinases
(RTKs) activate phosphatidylinositol-3 kinase (PI3K), which in turn
activates protein kinase B (Akt) and mammalian target of rapamycin
(mTOR). The mTOR promote collagen synthesis and proliferation in
fibroblasts, leading to pulmonary fibrosis (14,15).
Therefore, PI3K/Akt/mTOR signaling pathway act as a downstream
target of IGF-1/IGF-1R to play a role in promoting pulmonary
fibrosis.
Disabled-2 (DAB2), a member of the Disabled
gene family, encodes a mitogen-reactive phosphoprotein and is
widely expressed in human tissues, including kidney, heart, lung,
and skin. DAB2 has been reported to regulate cell-cell and
cell-fibrinogen adhesion, integrin αIIbβ3 activation, fibrinogen
uptake, and be involved in the regulation of TGF-β and Ras/MAPK/ERK
signaling pathways (16-18).
Down-regulation of DAB2 inhibits bleomycin-induced skin fibrosis
and activation of systemic sclerotic skin fibroblasts in mice
(19). Reduced expression of DAB2
protects myocardial cells from apoptosis under myocardial injury
(20,21). Differential gene expression
analysis of Gene Expression Omnibus (GEO) dataset showed that DAB2
was differentially expressed in fibrotic lung tissues of C57BL/6
mice induced by bleomycin (BLM). In the present study, we
established a mouse model of BLM-induced pulmonary fibrosis and
showed a significant up-regulation of pulmonary DAB2 in
vivo. Here, we hypothesized that DAB2 might play an important
role in IPF development. This study aims to investigate the
specific role and mechanism of DAB2 in pulmonary fibrosis and the
involvement of IGF-1R-related signaling pathways.
Materials and methods
Cell culture and transfection
MRC-5 cell line was purchased from Zhong Qiao Xin
Zhou biotechnology (Shanghai, China), and cultured in minimum
essential medium (MEM) (Solarbio, Beijing, China) containing 10%
fetal bovine serum (Tianhang Biotechnology, Zhejiang, China) at
37˚C with 5% CO2. Specific DAB2 siRNA and
IGF-1R siRNA (22) were
transfected into MRC-5 cell respectively when the cell density
reached 70%. After 24 h for transfection, cells were treated with
10 ng/ml transforming growth factor-β1 (TGF-β1) (Sino Biological,
Beijing, China) for 24 h.
Ethical approval and Animal model
Animal procedures were approved by the Ethics
Committee of the Second Affiliated Hospital of Xi'an Jiaotong
University (Approval No. 2022-781) and complied with the National
Research Council's Guide for the Care and Use of Laboratory
Animals. Healthy male C57BL/6 mice (6-8 weeks old, 19-21 g) which
were purchased from Liaoning changsheng biotechnology (China) with
consistent growth status were selected and randomly divided into
two groups (6 mice per group). Mice in each group were fed freely
at 12 h light/12 h dark, 22±1˚C, and 45-55% humidity. Anesthesia
was induced with 2-3% isoflurane and maintained with 1.5-2%
isoflurane. BLM (Yuanye Bio-Technology, Shanghai, China) was given
intratracheally into mice (2 U/kg), and the control group was given
the same amount of saline. After 21 days, the weight of the mice
was measured. A human endpoint was defined as a threshold of 20%
weight loss. Mice were sacrificed by CO2 euthanasia in a
chamber with a fill rate of 30% of the chamber volume of
CO2 per min.
Immunofluorescence (IF)
The cells were fixed in 4% paraformaldehyde
(Sinopharm Chemical Reagent, Shanghai, China) for 15 min, then
immersed in PBS for 5 min and repeated three times. The cell
sections were incubated with 0.1% tritonx-100 at room temperature
for 30 min and immersed in PBS to remove 0.1% tritonx-100 (Beyotime
Biotechnology, Shanghai, China). Before hatching the primary
antibody, the cell sections were immersed in 1% BSA (bovine serum
albumin) (Sangon Biotech, Shanghai, China) for 15 min. The dilution
ratio of the primary antibody was 1:100, and the condition was 4˚C
overnight. After the primary antibody was washed off, the secondary
antibody diluted 1:200 was added, and the cells were incubated at
room temperature in the dark for 1 h. The nuclei were stained with
DAPI (Aladdin Biochemical Technology, Shanghai, China). Finally,
half drops of anti-fluorescence quencher were added to the cell
sections. After sealing the slices, the staining was observed under
a fluorescence microscope (BX53, Olympus, Tokyo, Japan). Primary
antibodies of DAB2 (DF7792#) and α-SMA (BF9212#) were purchased
from Affinity Biosciences (China), and Cy3-labeled goat anti-rabbit
IgG (A27039#, Invitrogen, Carlsbad, CA, USA) was used as the
secondary antibodies of DAB2 and α-SMA. The experiment was
independently repeated six times, and representative pictures were
selected.
The lung tissues were dehydrated with different
concentrations of alcohol for corresponding time, and the alcohol
concentration were 70% (2 h), 80% (overnight), 90% (2 h), 100% (1
h), and 100% (1 h), respectively. After dehydration, the lung
tissues were waxed through, embedded, and sliced. After
deparaffinization, the sections were immersed in 95, 85, and 75%
ethanol for 1 min each. After removing the alcohol, the sections
were subjected to antigen repair at low heat for 10 min, followed
by immersion in 1% BSA for 15 min. The antibody was incubated at
4˚C overnight at a dilution ratio of 1:100 for the antibody used
and 1:200 for the secondary antibody. At last, the sections were
counterstained with DAPI and observed under a fluorescence
microscope (BX53). Cy3-labeled goat anti-rabbit IgG was used to
detect DAB2, and FITC-labeled goat anti-mouse IgG (ab6785#, Abcam,
Cambridge, UK) was used to detect α-SMA. The experiment was
independently repeated six times, and representative pictures were
selected.
Hematoxylin-eosin staining
After dewaxed to water, the lung tissue sections
were put in hematoxylin (Solarbio, Beijing, China) solution for 5
min and soaked in distilled water for 5 min. The sections were put
in 1% acid ethanol (99 ml 70% ethanol and 1ml concentrated
hydrochloric acid) and stay for 3 sec, then rinsed with tap water
for 20 min, and soaked in distilled water for 2 min. The sections
were stained with eosin (Sangon Biotech, Shanghai, China) solution
for 3 min. Then the stained slides were successively immersed in
75% (2 min), 85% (2 min) and 95% (2 min) ethanol and followed by
dehydration, transparent and seal. The staining was observed under
a light microscope (BX53).
Masson staining
After dewaxed to water, slices were stained with
hematoxylin solution for 6 min and followed by differentiation with
1% acid ethanol for 3 sec. Then the slices were rinsed with running
water for 20 min and soaked in distilled water for 2 min. The
moisture on the sections was blotted with absorbent paper, and the
sections were stained with ponceau red liquid dye acid complex for
1 min, which was prepared as follows: 0.7 g ponceau (Sinopharm
Chemical Reagent, Shanghai, China) and 0.3 g acid fuchsin
(Sinopharm Chemical Reagent, Shanghai, China) were dissolved in 99
ml distilled water, and 1ml glacial acetic acid was finally added.
After staining, the sections were washed with 0.2% glacial acetic
acid aqueous solution (0.2 ml glacial acetic acid and 100 ml
distilled water). Subsequently, 1% phosphomolybdic acid solution
was dropped for differentiation for 5 min, and the sections were
stained directly with aniline blue solution for 5 min. The aniline
blue solution was prepared as follows: 2 g aniline blue was
dissolved in 98 ml distilled water, and 2 ml glacial acetic acid
was finally added. After staining, the sections were dehydrated,
transparent and sealed. The staining was observed under a light
microscope (BX53).
Real-time PCR (qPCR)
The samples were lysed with 1 ml TRIpure (BioTeke
Corporation, Beijing, China) for 5 min. 200 µl chloroform was added
to the lysed samples for 3 min at room temperature. After
centrifugation, the upper liquid was collected and mixed with the
equal volume of isopropanol. Then the mixture was placed at -20˚C
overnight. The frozen sample was removed and centrifuged at 4˚C for
10 min. the supernatant was discarded, and the precipitate was
allowed to stand for 5 min. Total RNA was obtained by adding 30 µl
Rnase-free ddH2O. Reverse transcription of RNA was
performed by BeyoRT II M-MLV reverse transcriptase (Beyotime
Biotechnology, Shanghai, China) to obtain cDNA. Real-time PCR
reaction system was configured according to 2xTaq PCR MasterMix
(Solarbio, Beijing, China) and SYBR Green (Solarbio, Beijing,
China) instructions. GAPDH was used as an internal control for
normalization. The operating instrument is Exicycler™ 96 (Bioneer
Corporation, Daejeon, Korea). The instrument program was as
follows: 1) Incubation at 95˚C for 5 min, 2) Incubation at 95˚C for
10 sec, 3) Incubation at 60˚C for 20 sec, 4) Go to line 2, Cycle
40, 5) Incubation at 72˚C, for 2 min 30 sec, 6) Incubation at 40˚C
for 1 min, 7) Melting 60˚C to 94˚C, Every 1˚C for 1 sec, 8)
Incubation at 25˚C for 1-2 min, 9) Incubation at 72˚C for 30 sec.
The primer sequences were as follows: mus-Dab2 F:
5'-CCCTAATGACCCTTGATG-3'; mus-Dab2 R: 5'-GGTGGGAAAGAAGTTGAGA-3';
homo-DAB2 F: 5'-CCCTGAATGGTGATGTTG-3'; homo-DAB2 R:
5'-GGGATAATGGCTATGGAGT-3'; mus GAPDH F:
5'-TGTTCCTACCCCCAATGTGTCCGTC-3'; mus GAPDH R:
5'-CTGGTCCTCAGTGTAGCCCAAGATG-3'; homo GAPDH F:
5'-GACCTGACCTGCCGTCTAG-3'; homo GAPDH R:
5'-AGGAGTGGGTGTCGCTGT-3'.
Western blot (WB)
RIPA (Radio Immunoprecipitation Assay Lysis buffer)
and PMSF (phenylmethanesulfonylfluoride) (Solarbio, Beijing, China)
were used to extract the protein of the samples. The protein
concentration was detected by BCA protein concentration
determination kit (Solarbio, Beijing, China), and SDS-PAGE was
performed by loading 20 µl (10-20 µg) protein onto the gels, which
were composed of 5% stacking gel and four different concentrations
of separating gels (8, 10, 12, 15%). GAPDH was used as a loading
control for normalization. After electrophoresis separation, the
protein was transferred to PVDF membrane (Millipore, Billerica, MA,
US), and the transferred protein was blocked with 5% skim milk
(Sangon Biotech, Shanghai, China). Then the PVDF membrane was
incubated with diluted antibody and washed six times with TBST for
5 min each time. ECL chemiluminescence reagent (Solarbio, Beijing,
China) was added to the membrane, and the density values of the
protein bands were analyzed by Gel-Pro Analyzer 4.0 (Media
Cybernetics, Rockville, MD, US). The antibody incubation conditions
are shown in Table I.
 | Table IConditions of the antibody
incubation. |
Table I
Conditions of the antibody
incubation.
| A, Primary
antibodies |
|---|
| Name | Cat. no. | Company | Dilution ratio | Conditions |
|---|
| IGF-1 antibody | DF6096 | Affinity
Biosciences, Ltd. | 1:1,000 | 4˚C overnight |
| IGF-1R
antibody | AF7709 | Affinity
Biosciences, Ltd. | 1:1,000 | 4˚C overnight |
| p-IGF-1R
antibody | AF4397 | Affinity
Biosciences, Ltd. | 1:1,000 | 4˚C overnight |
| DAB2 antibody | bs-5999R | BIOSS | 1:1,000 | 4˚C overnight |
| Collagen I
antibody | A16891 | ABclonal Biotech
Co., Ltd. | 1:1,000 | 4˚C overnight |
| Collagen Ⅳ
antibody | AF0510 | Affinity
Biosciences, Ltd. | 1:1,000 | 4˚C overnight |
| Fibronectin
antibody | A12932 | Affinity
Biosciences, Ltd. | 1:1,000 | 4˚C overnight |
| α-SMA antibody | BF9212 | Affinity
Biosciences, Ltd. | 1:3,000 | 4˚C overnight |
| PI3K antibody | AF6241 | Affinity
Biosciences, Ltd. | 1:1,000 | 4˚C overnight |
| p-PI3K
antibody | AF3242 | Affinity
Biosciences, Ltd. | 1:1,000 | 4˚C overnight |
| AKT antibody | AF6261 | Affinity
Biosciences, Ltd. | 1:1,000 | 4˚C overnight |
| p-AKT antibody | AF0016 | Affinity
Biosciences, Ltd. | 1:1,000 | 4˚C overnight |
| GAPDH | 60004-1-Ig | Proteintech Group,
Inc. | 1:10,000 | 4˚C overnight |
| B, Secondary
antibodies |
| Name | Cat. no. | Company | Dilution ratio | Conditions |
| Goat anti rabbit
IgG-HRP | SE134 | Beijing Solarbio
Science & Technology Co., Ltd. | 1:3,000 | 37˚C 1 h |
| Goat anti rabbit
IgG-HRP | SE134 | Beijing Solarbio
Science & Technology Co., Ltd. | 1:3,000 | 37˚C 1 h |
| Goat anti rabbit
IgG-HRP | SE134 | Beijing Solarbio
Science & Technology Co., Ltd. | 1:3,000 | 37˚C 1 h |
| Goat anti rabbit
IgG-HRP | SE134 | Beijing Solarbio
Science & Technology Co., Ltd. | 1:3,000 | 37˚C 1 h |
| Goat anti rabbit
IgG-HRP | SE134 | Beijing Solarbio
Science & Technology Co., Ltd. | 1:3,000 | 37˚C 1 h |
| Goat anti rabbit
IgG-HRP | SE134 | Beijing Solarbio
Science & Technology Co., Ltd. | 1:3,000 | 37˚C 1 h |
| Goat anti rabbit
IgG-HRP | SE134 | Beijing Solarbio
Science & Technology Co., Ltd. | 1:3,000 | 37˚C 1 h |
| Goat anti mouse
IgG-HRP | SE131 | Solarbio (Beijing,
China) | 1:3,000 | 37˚C 1 h |
| Goat anti rabbit
IgG-HRP | SE131 | Beijing Solarbio
Science & Technology Co., Ltd. | 1:3,000 | 37˚C 1 h |
| Goat anti rabbit
IgG-HRP | SE131 | Beijing Solarbio
Science & Technology Co., Ltd. | 1:3,000 | 37˚C 1 h |
| Goat anti rabbit
IgG-HRP | SE131 | Beijing Solarbio
Science & Technology Co., Ltd. | 1:3,000 | 37˚C 1 h |
| Goat anti rabbit
IgG-HRP | SE131 | Beijing Solarbio
Science & Technology Co., Ltd. | 1:3,000 | 37˚C 1 h |
| Goat anti mouse
IgG-HRP | SE131 | Beijing Solarbio
Science & Technology Co., Ltd. | 1:3,000 | 37˚C 1 h |
CCK-8
MRC-5 Cells with and without transfection were
seeded in 96-well culture plates with 6ⅹ103 cells per
well and treated with 10 ng/ml TGF-β1. After 24 h, CCK-8 (Beyotime
Biotechnology, Shanghai, China) was added to the cells for 2 h
culture, and the OD value of the cells at 450 nm was determined by
800TS enzyme standard instrument (BioTek Instruments, Winooski, VT,
US).
Statistical analysis
All data analysis was completed on Graphpad 8.0.
Unpaired t-test was used to test the differences between the two
groups, and Ordinary One Way ANOVA was used to analyze the gray
values of protein bands. Significant post hoc effects were revealed
by the Tukey's post hoc test. qPCR results were analyzed by
2-ΔΔCT method. All data were presented as mean ±
standard deviation (SD) with at least three biological repetitions
in each group. P-value less than 0.05 was considered statistically
significant.
Results
The expression of DAB2 was
significantly up-regulated in the BLM-induced fibrotic lung
tissue
Screening of the candidate gene DAB2 was briefly
described as following: two datasets from GEO database including
GSE42301 and GSE8553 were used for analyzing the differentially
up-regulated genes (|Log2 fold change (FC)|>1, P<0.05) in
bleomycin-induced fibrotic lungs of C57BL/6 mice. Genes with
incomplete annotation information and duplicated genes were removed
from this project. There are 30 overlapping genes between GSE8553
and GSE42301. IGF activity is known to maintain human lung
homeostasis and involve in relevant pulmonary diseases with an
inflammatory component, including pulmonary fibrosis. Previous
studies have demonstrated the potential of treating pulmonary
fibrosis by targeting IGF-1R. Here, we aim to further investigate
the molecular mechanisms involved in IGF-1R signaling in idiopathic
pulmonary fibrosis. The differentially down-regulated genes (|Log2
FC|>1, P<0.05) in IGF-1R knockout mice were analyzed based on
GEO dataset GSE47065. Only 4 genes (DAB2, TYROBP, FCER1G, and
SFRP1) were overlapped among GSE8553, GSE42301 and GSE47065 as
shown in the Fig. S1. Genes with
known function in lung fibrosis or without any references in
regulating fibrosis were not further considered, and thus DAB2 was
screen out in this project. Here, in the Fig. 1, we included some data of
bioinformatics analysis based on GEO database to support our
findings of the differentially expressed DAB2 in pulmonary
fibrosis.
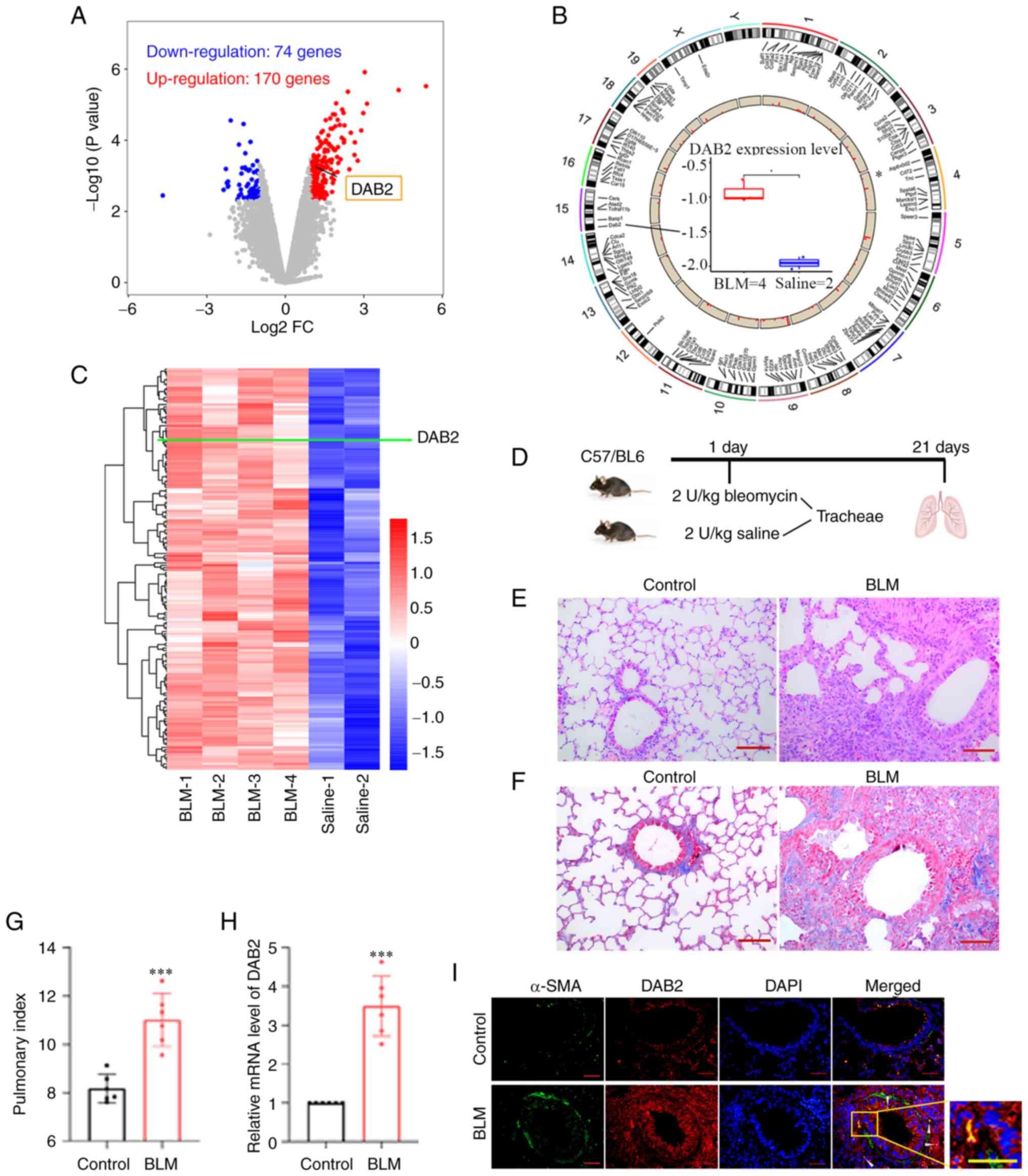 | Figure 1Expression of DAB2 is significantly
upregulated in the bleomycin-induced fibrotic lung tissue. (A-C)
Results from differential gene expression analysis based on Gene
Expression Omnibus database. Lung samples from mice treated with
saline (Control group) or bleomycin (BLM group) were set in
GSE8553. (A) Volcano plot showing 244 differentially expressed
genes with blue dots representing downregulated genes
(Log2FC<-1; P<0.005) and red dots representing
upregulated genes (Log2FC>1; P<0.005). (B)
Chromosome mapping of these differentially expressed genes
including DAB2, which is located on chromosome 15. Boxplot in the
center shows the pulmonary expression of DAB2 in Control and BLM
mice. *P<0.05. (C) Hierarchical clustering heatmap of
differentially upregulated genes, in which the row represents genes
and the column represents samples. The green line points out the
position of DAB2. (D) Schematic diagram of experimental paradigm
for bleomycin-induced pulmonary fibrosis model in mice. (E)
Hemoxylin and eosin staining of lung tissue sections of mice (scale
bar, 100 µm). (F) Masson staining of lung tissue sections of mice
(scale bar, 100 µm). (G) Mouse pulmonary index. (H) Relative mRNA
level of DAB2 in lung tissues. ***P<0.001 vs.
control. (I) Immunofluorescence staining of lung tissue sections of
mice, the white arrow indicates the location where DAB2 is
co-expressed with α-SMA (scale bar, 50 µm). Data were presented as
mean ± SD with six biological repetitions in each group. DAB2,
Disabled-2; FC, fold-change; BLM, bleomycin; SMA, smooth muscle
actin. |
The differentially expressed genes in a mouse model
of BLM-induced pulmonary fibrosis were identified based on GEO
dataset (GSE8553), which contained 4 cases of BLM-induced lung
samples (GSM212539, GSM212540, GSM212548, GSM212549) and 2 cases of
Saline-treated control samples (GSM212543, GSM212552). After
bioinformatics analysis, 244 differentially expressed genes
including 74 down-regulated genes and 170 up-regulated genes that
met criteria (|Log2 FC|>1, P<0.005) were obtained
and showed in a volcano map (Fig.
1A). The chromosomal distribution of these genes was displayed
in Fig. 1B. The differentially
up-regulated genes were showed by a hierarchical clustering heatmap
(Fig. 1C). The pulmonary
expression of DAB2 was significantly up-regulated in the BLM group
(Fig. 1A-C).
Herein, pulmonary fibrosis was induced by direct
tracheal injection of BLM into the lungs of mice, and an equal
volume of normal saline was administered in the same manner as a
control (Fig. 1D). HE (Fig. 1E) and Masson (Fig. 1F) staining showed the
histopathological changes of the lung, and there was obvious
pulmonary fibrosis in the BLM group. The lung index analysis showed
that BLM induced a higher lung index (P=0.0002) (Fig. 1G). All above results proved the
successful establishment of the mouse model of IPF. After
verification by qPCR, we found that the expression level of DAB2
mRNA was significantly increased in BLM group (P<0.0001)
(Fig. 1H). The IF staining was
observed extensive expression of DAB2 in the BLM group (Fig. 1I). In conclusion, the expression of
DAB2 was up-regulated in the BLM-induced pulmonary fibrosis model
of mice.
Knockdown of DAB2 inhibited
TGF-β1-induced proliferation of MRC-5 cells
In order to clarify the biological function of DAB2,
we explored its expression in MRC-5 cells and its effect on cell
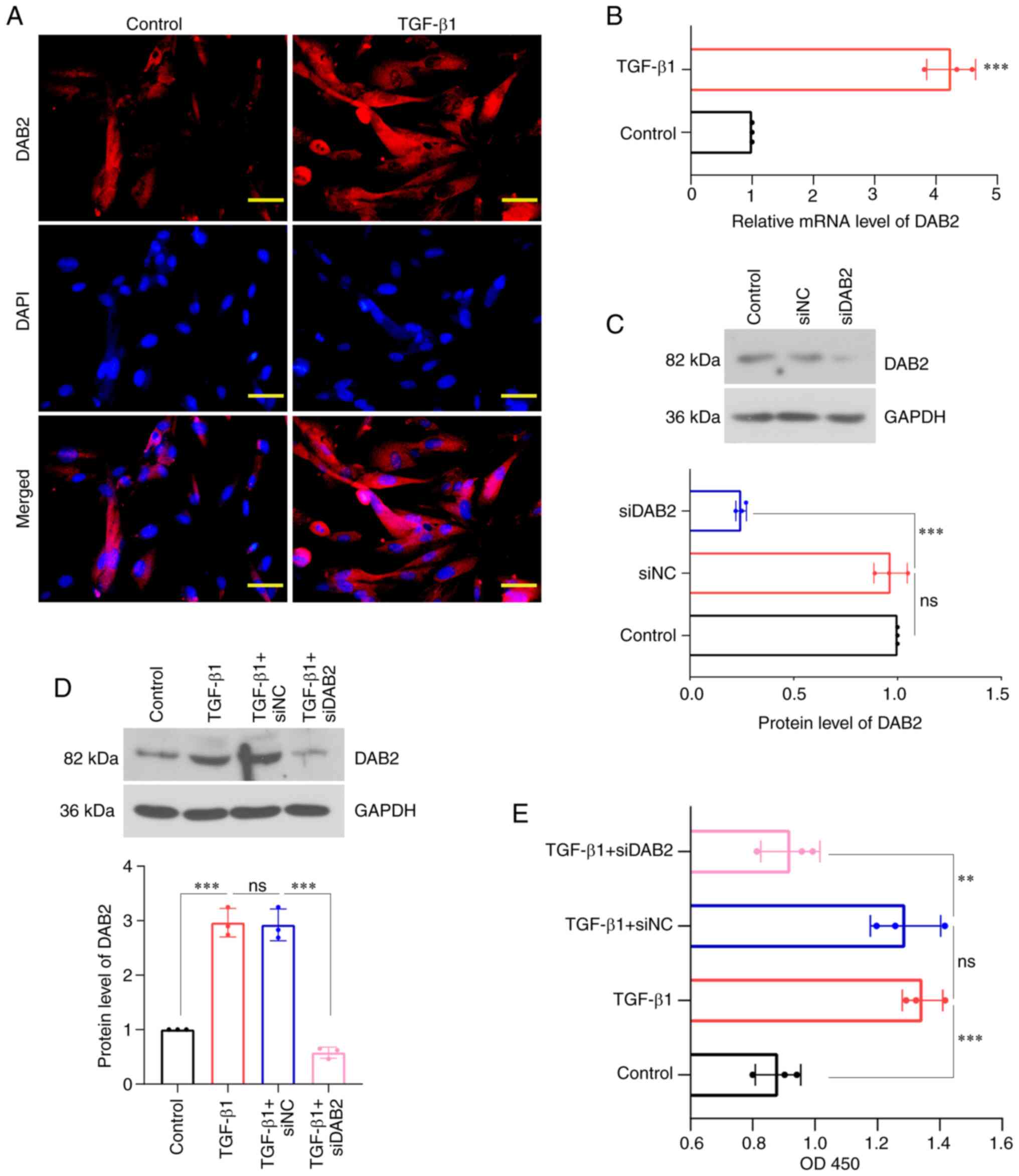
proliferation. We revealed that the expression of DAB2 was
significantly observed in cells treated with TGF-β1 (Fig. 2A), and the relative level of DAB2
mRNA was increased in TGF-β1 group compared with control (Fig. 2B). WB was used to detect protein
level of DAB2 in cells transfected with specific siRNA of DAB2
(Fig. 2C, D). The content of DAB2
was found to be significantly decreased in DAB2-knockdown cells
with and without TGF-β1 treatment (P<0.0001). After TGF-β1
treatment, it was found that the cell concentration of
DAB2-knockdown cells was significantly decreased compared with that
of cells transfected with siNC (Fig.
2E).
DAB2-knockdown inhibited
fibrogenesis
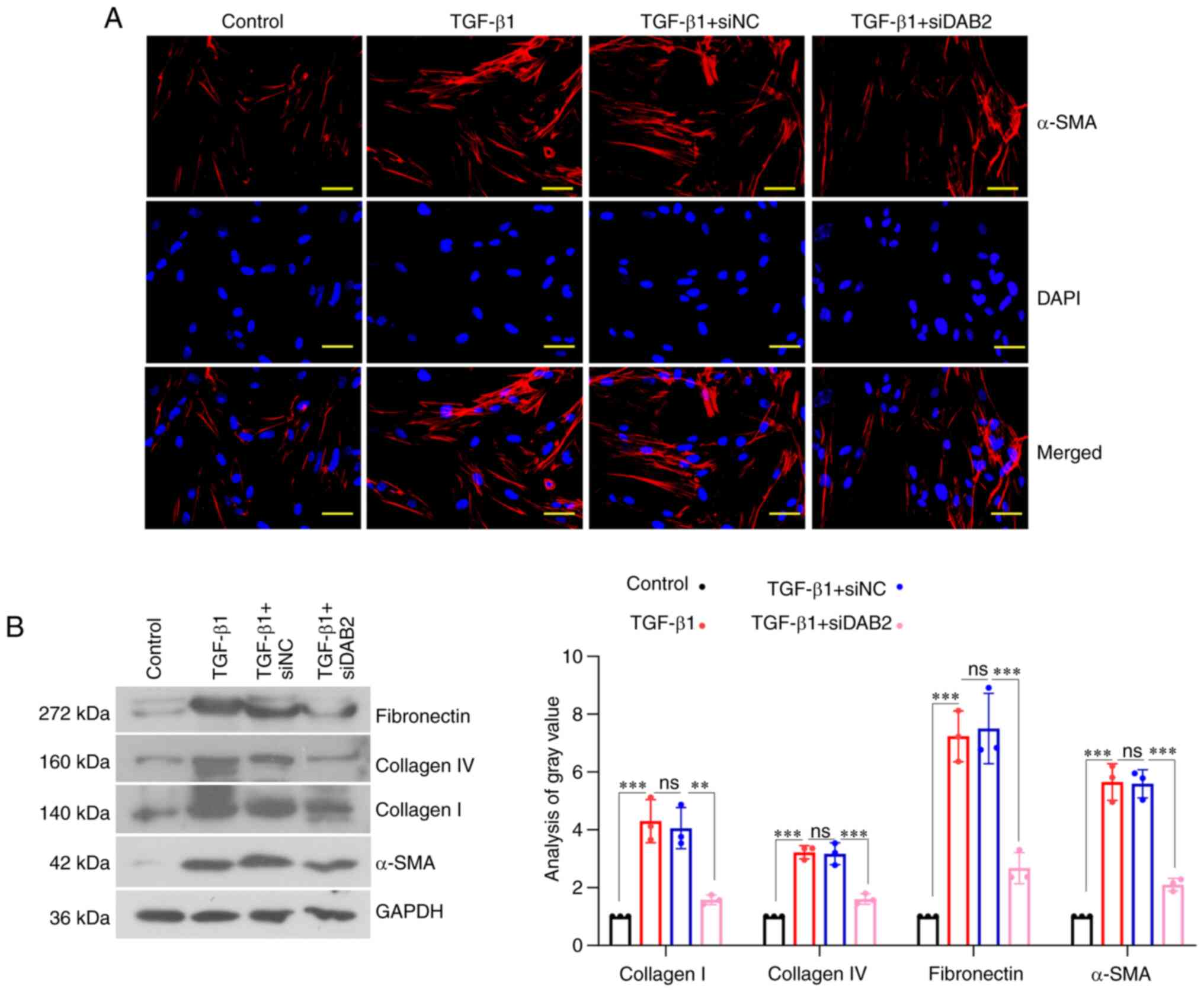
The results from IF staining revealed that the
expression of α-SMA in cells with DAB2 knockdown was significantly
reduced compared with cells with siNC (Fig. 3A). In terms of the effect of DAB2
on fibrogenesis, WB was used to detect the levels of related
proteins in the process of fibrosis. We found that the expression
of Collagen I, Collagen IV, Fibronectin, and α-SMA in
DAB2-knockdown cells was decreased compared with siNC group
(Fig. 3B). Therefore,
DAB2-knockdown could inhibit fibrogenesis.
Knockdown of DAB2 inhibited PI3K/AKT
signaling pathway
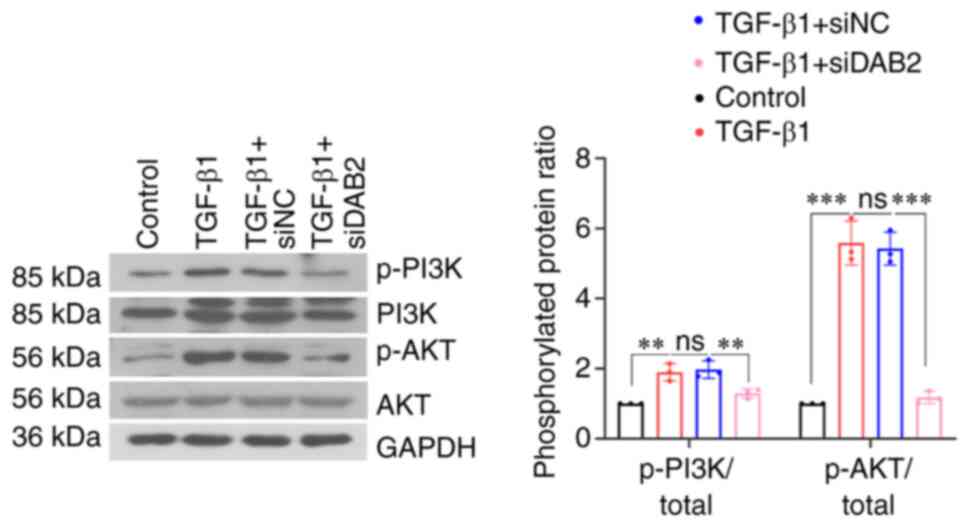
A decrease in the phosphorylation levels of PI3K and
AKT was found in MRC-5 cells with DAB2 knockdown (Fig. 4). It indicated that knockdown of
DAB2 in MRC-5 cells inhibited phosphorylation of PI3K and AKT and
thus suppressed PI3K/AKT signaling pathway.
DAB2 might be a downstream target of
IGF-1R signaling
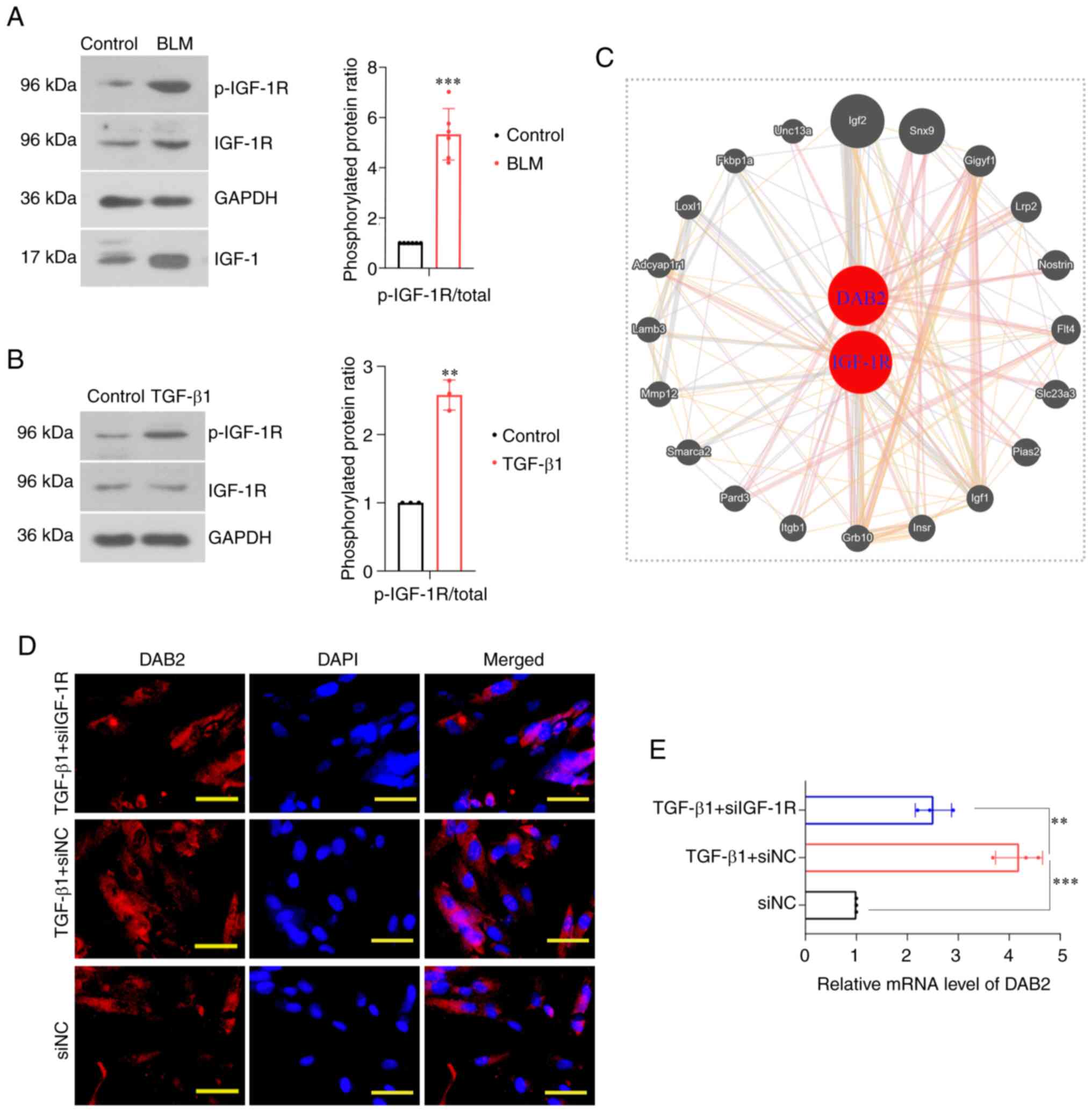
In the constructed mouse model, the protein levels
of IGF-1, p-IGF-1R and IGF-1R were detected by WB. It revealed that
the phosphorylation level of IGF-1R was significantly increased
(P<0.001) and protein level of IGF-1 was increased (P<0.001)
(Fig. 5A). In vitro cell
experiments, it was found that the phosphorylation level of IGF-1R
was increased in cells induced by TGF-β1 (P=0.0012) (Fig. 5B). Protein-protein interaction
(PPI) network analysis based on the GeneMANIA database (http://genemania.org/) showed a potential genetic
interaction between DAB2 and IGF-1R (Fig. 5C). We further knocked down IGF-1R
in MRC-5 cells and examined the expression level of DAB2 in this
cell. IF staining showed that the number of positive cells in the
IGF-1R-knockdown group was significantly lower than that in the
TGF-β1 treatment group without IGF-1R- knockdown (Fig. 5D). QPCR found that the relative
mRNA level of DAB2 also decreased (P=0.0021) (Fig. 5E).
Discussion
IPF is one of the most aggressive forms of
idiopathic interstitial pneumonia and the most common form of
interstitial lung diseases, resulting in decline in lung function
and progressive respiratory failure (23). Previous studies have found that the
pathogenesis of IPF involves numerous growth factors, cytokines and
signaling pathways (15), whether
these factors can be used as targets for the treatment of IPF and
the specific mediator of these signaling pathways in IPF have been
the focus of contemporary research.
In studies on fibrotic diseases (19,24),
the regulatory effect of DAB2 on fibrogenesis has been revealed. In
this study, we will initially reveal the important role of DAB2 in
pulmonary fibrotic diseases. DAB2 expression has been shown to be
upregulated in skin fibrosis models (19). Herein, we confirmed the
up-regulation of DAB2 expression in BLM-induced pulmonary fibrosis
in mouse. In vitro, we observed that the deletion of DAB2
caused the down-regulation of fibrosis marker genes, indicating
that DAB2 may play an important role in pulmonary fibrosis. From
the above elaboration, we predict that the expression of DAB2 is
up-regulated in fibrotic tissues, and the degree of fibrosis and
the expression of related genes were significantly inhibited after
knockdown of DAB2. It further presumes that the general inhibitory
effect of DAB2 in fibrotic diseases. In addition, most studies have
reported the inhibitory effect of DAB2 on cell proliferation
(25,26). We found that DAB2-knockdown
inhibited the proliferation of MRC-5 cells, which is in agreement
with previous research reports.
DAB2, as an adapter molecule, can regulate related
signaling pathways to play physiological roles (27). For example, DAB2 can activate
TGFβ-R to associate with Smad signaling pathway (18). In the Wnt signaling pathway, DAB2
plays a regulatory role by linking its PTB domain to the domain of
Disheveled (28). However, whether
DAB2 is involved in IGF-1R-related pathways is still unclear. In
addition, numerous studies have reported the pro-fibrotic function
of IGF-1/IGF-1R. In a streptozotocin (STZ)-induced mouse diabetes
model, activation of IGF-1 induced fibrogenesis in diabetic
kidneys, and inhibition of IGF-1R could ameliorate
tubelointerstitial fibrosis (29).
Deficiency of IGF-1R attenuated the acute inflammatory response in
bleomycin-induced lung injury (30). IGF-1R-deficient mice exhibited an
improved degree of pulmonary fibrosis (31). In our study, we found that IGF-1
and IGF-1R were upregulated in BLM-induced pulmonary fibrosis. It
had an elevated phosphorylation level of IGF-1R in vitro. Above all
suggested that IGF-1/IGF-1R has a crucial role in pulmonary
fibrosis. It was worth mentioning that the same promoting effect of
DAB2 and IGF-1/IGF-1R in pulmonary fibrosis led us to speculate the
possible interaction mechanism between DAB2 and IGF-1/IGF-1R.
Therefore, the interactions between DAB2 and its upstream (and
downstream) molecules were explored preliminarily in our study.
While studying the function of DAB2, we found that
the phosphorylation levels of AKT was decreased in DAB2-knockdown
cells. Interestingly, DAB2 has been reported to bind AKT through
proline-rich domain (PRD) (32).
Another report revealed it played an essential role in AKT
recruitment. AKT is a central mediator in the PI3K-AKT-mTOR
signaling pathway and indispensable in this signaling pathway
(33). It confirmed that DAB2 is
able to regulate this signaling pathway through AKT. However, there
is another possibility that DAB2 can directly act on PI3K, since
the phosphorylation level of PI3K is significantly inhibited in
DAB2-knockdown cells. As described in introduction, IGF-1/IGF-1R
can activate this pathway to affect pulmonary fibrosis. In the
current study, the results from immunofluorescence staining and
real-time PCR showed that the expression level of DAB2 was
significantly inhibited in IGF-1R-knockdown cells, suggesting that
DAB2 might be positively regulated by IGF-1R. The lack of a more
effective quantification, such as flow cytometry, for the
difference in DAB2 expression levels might be a limitation of the
study. Though microscopy analysis may not be the most effective
measurement, the present results clearly showed the difference in
DAB2 expression in the absence of IGF-1R. As a consequence, IGF-1R
has some effect on DAB2 and PI3K/Akt/mTOR signaling pathway. In the
regards of exploring the relationship among them, although the
interaction between IGF-1R and DAB2 is still unclear, it is
possible that IGF-1/IGF-1R may regulate PI3K/Akt/mTOR signaling
pathway through DAB2.
The present work is a preliminary study revealing
the potential contribution of DAB2 to the development of IPF. Here,
we mainly focused on in vitro studies and revealed the
profibrotic role of DAB2 in TGF-β1-induced MRC-5 cells, which
provides a novel direction for better understanding the
pathogenesis of pulmonary fibrosis. This study failed to elucidate
the role of DAB2 in IPF in vivo and more studies need to be
carried out in animal models of pulmonary fibrosis. The lack of
clinical samples supporting the expression of DAB2 in IPF is also a
limitation of the study. Another innovative discovery of this study
is that DAB2 might be regulated by the upstream IGF-1R signaling
and intervened the downstream PI3K/Akt signaling, which suggests
that IGF-1/IGF-1R may affect pulmonary fibrosis via DAB2-mediated
PI3K/AKT signaling. Obviously, our study is far from sufficient to
unveil the regulatory mechanisms among DAB2, IGF-1/IGF-1R and
PI3K/Akt/mTOR signaling pathway. The underlying mechanisms of DAB2
in IPF and the interaction with IGF-1/IGF-1R signaling pathway need
further investigation both in vitro and in vivo. A
knockout mouse model for IGF-1R or DAB2 would be helpful to
evidence the role of DAB2 and the potential interaction with
IGF-1/IGF-1R in IPF development. Although the absence of IGF-1R or
DAB2 knockout mice could be a limitation of the present study, the
findings of the present study provide novel insights into the
pathogenesis of IPF and a potential therapeutic target for
pulmonary diseases, which are of significance for basic and
clinical research fields of IPF.
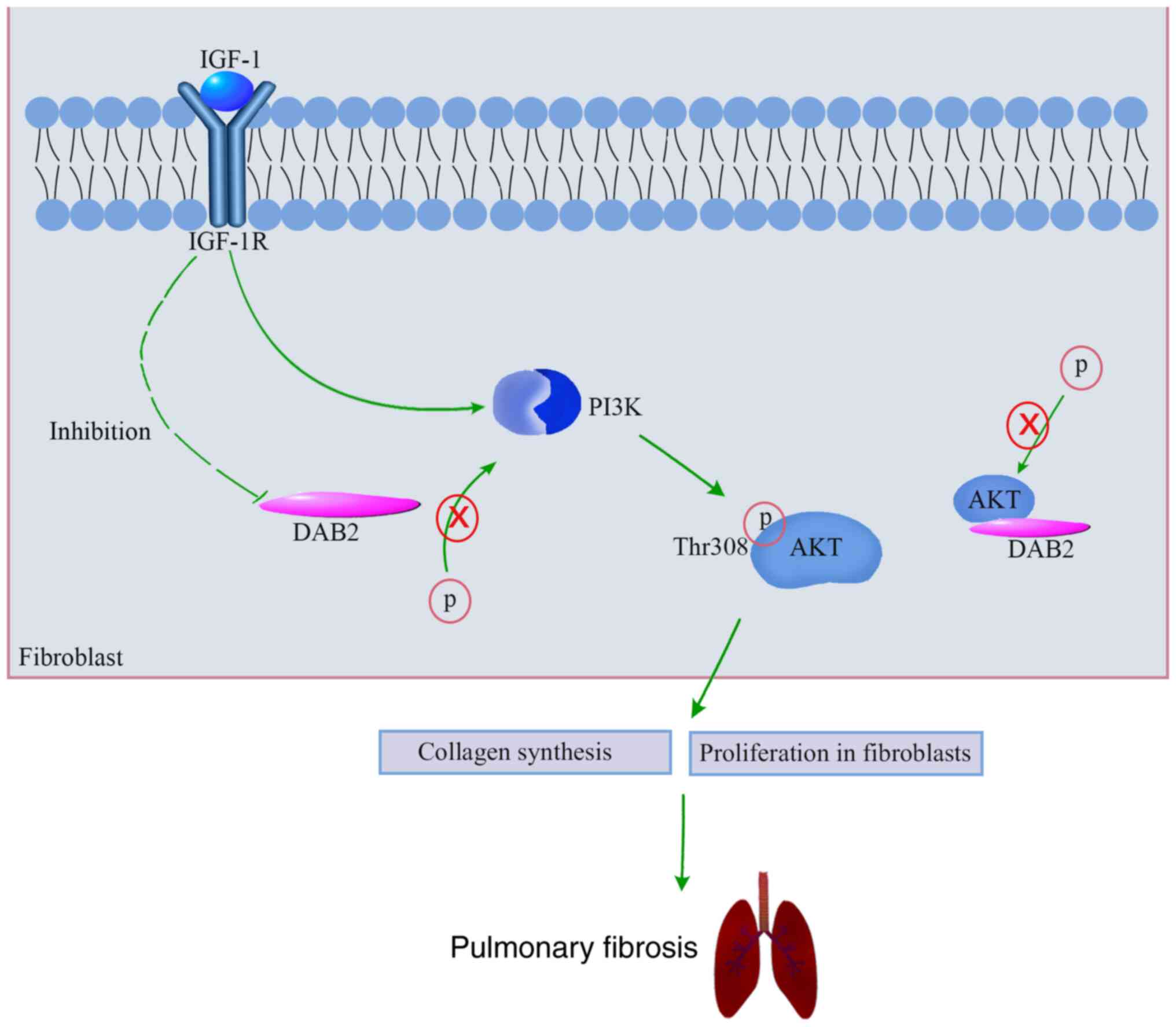
Our results revealed an upregulation of DAB2 in lung
tissues of mice with bleomycin-induced pulmonary fibrosis and in
TGF-β1-induced MRC-5 cells. In vitro studies elucidated that
DAB2 could promote the expression of fibrosis-related genes and the
proliferation of fibroblasts. It suggests that DAB2 might play an
important role in promoting pulmonary fibrosis. In addition, our
results showed the activation of IGF-1/IGF-1R signaling in in
vivo and in vitro models of pulmonary fibrosis. We
further demonstrated that IGF-1R positively regulated the
expression of DAB2, and DAB2 positively regulated the
phosphorylation of PKT and AKT in vitro. It suggests that
DAB2 may act as an intermediate between IGF-1/IGF-1R and PKT/AKT
signaling pathway and thus inducing fibrogenesis. A schematic
summarizing the mechanism of promoting effect of DAB2 in pulmonary
fibrosis is shown in Fig. 6. Our
study provides more possibilities for the pathogenesis of pulmonary
fibrosis and a new therapeutic target for the treatment of
pulmonary fibrosis.
Supplementary Material
Venn diagram of screening the
candidate gene DAB2. GSE8553, GSE42301 and GSE47065, which were
from the GEO datasets, were used to screen the candidate gene DAB2.
GSE8553 and GSE42301 represented the differentially upregulated
genes [|Log2 fold-change (FC)|>1; P<0.05] in
bleomycin-induced fibrotic lungs of C57BL/6 mice. GSE47065 showed
the differentially downregulated genes (|Log2 FC|>1; P<0.05)
in IGF-1R knockout mice. A total of four genes, including DAB2,
TYROBP, FCER1G and SFRP1 were overlapped among the three GEO
datasets. GEO, gene expression omnibus; DAB2, Disabled-2 actin;
IGF-1, insulin-like growth factor; IGF-1R, IGF-1 receptor.
Acknowledgements
Not applicable.
Funding
Funding: This research was funded by the Natural Science Basic
Research Plan of Shaanxi Province (grant no. 2018JM7076).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CLL conceptualized, wrote, reviewed and edited the
manuscript, acquired funding and supervised the study. XLL carried
out the investigation, experiments and data analysis, and wrote the
original draft. XJQ performed the investigation, experiments and
data analysis. LZ carried out the experiments and data analysis.
CLL and XLL confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Second Affiliated Hospital of Xi'an Jiaotong University
(approval no. 2022-781).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Richeldi L, Collard HR and Jones MG:
Idiopathic pulmonary fibrosis. Lancet. 389:1941–1952.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kekevian A, Gershwin ME and Chang C:
Diagnosis and classification of idiopathic pulmonary fibrosis.
Autoimmun Rev. 13:508–512. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Raghu G, Chen SY, Hou Q, Yeh WS and
Collard HR: Incidence and prevalence of idiopathic pulmonary
fibrosis in US adults 18-64 years old. Eur Respir J. 48:179–186.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Raghu G, Rochwerg B, Zhang Y, Garcia CA,
Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, et
al: An Official ATS/ERS/JRS/ALAT clinical practice guideline:
Treatment of idiopathic pulmonary fibrosis. an update of the 2011
clinical practice guideline. Am J Respir Crit Care Med. 192:e3–19.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Adamali HI and Maher TM: Current and novel
drug therapies for idiopathic pulmonary fibrosis. Drug Des Devel
Ther. 6:261–272. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bonella F, Stowasser S and Wollin L:
Idiopathic pulmonary fibrosis: Current treatment options and
critical appraisal of nintedanib. Drug Des Devel Ther. 9:6407–6419.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shenderov K, Collins SL, Powell JD and
Horton MR: Immune dysregulation as a driver of idiopathic pulmonary
fibrosis. J Clin Invest. 131(e143226)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sgalla G, Iovene B, Calvello M, Ori M,
Varone F and Richeldi L: Idiopathic pulmonary fibrosis:
Pathogenesis and management. Respir Res. 19(32)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
American Thoracic Society. Idiopathic
pulmonary fibrosis. Diagnosis and treatment. International
consensus statement. American Thoracic Society (ATS), and the
European Respiratory Society (ERS). Am J Respir Crit Care Med.
161:646–664. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Spagnolo P, Tzouvelekis A and Bonella F:
The management of patients with idiopathic pulmonary fibrosis.
Front Med (Lausanne). 5(148)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kelly M, Kolb M, Bonniaud P and Gauldie J:
Re-evaluation of fibrogenic cytokines in lung fibrosis. Curr Pharm
Des. 9:39–49. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hernandez DM, Kang JH, Choudhury M,
Andrianifahanana M, Yin X, Limper AH and Leof EB: IPF pathogenesis
is dependent upon TGFβ induction of IGF-1. FASEB J. 34:5363–5388.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Aston C, Jagirdar J, Lee TC, Hur T, Hintz
RL and Rom WN: Enhanced insulin-like growth factor molecules in
idiopathic pulmonary fibrosis. Am J Respir Crit Care Med.
151:1597–1603. 1995.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alzahrani AS: PI3K/Akt/mTOR inhibitors in
cancer: At the bench and bedside. Semin Cancer Biol. 59:125–132.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ma H, Liu S, Li S and Xia Y: Targeting
growth factor and cytokine pathways to treat idiopathic pulmonary
fibrosis. Front Pharmacol. 13(918771)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tsai HJ and Tseng CP: The adaptor protein
Disabled-2: New insights into platelet biology and integrin
signaling. Thromb J. 14 (Suppl 1)(S28)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cheong SM, Choi H, Hong BS, Gho YS and Han
JK: Dab2 is pivotal for endothelial cell migration by mediating
VEGF expression in cancer cells. Exp Cell Res. 318:550–557.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hocevar BA, Smine A, Xu XX and Howe PH:
The adaptor molecule Disabled-2 links the transforming growth
factor beta receptors to the Smad pathway. EMBO J. 20:2789–2801.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mei X, Zhao H, Huang Y, Tang Y, Shi X, Pu
W, Jiang S, Ma Y, Zhang Y, Bai L, et al: Involvement of Disabled-2
on skin fibrosis in systemic sclerosis. J Dermatol Sci. 99:44–52.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang BW, Fang WJ and Shyu KG: MicroRNA-145
regulates disabled-2 and Wnt3a expression in cardiomyocytes under
hyperglycaemia. Eur J Clin Invest: 48, 2018 doi:
10.1111/eci.12867.
|
|
21
|
Lin CM, Fang WJ, Wang BW, Pan CM, Chua SK,
Hou SW and Shyu KG: (-)-Epigallocatechin Gallate Promotes MicroRNA
145 Expression against Myocardial Hypoxic Injury through
Dab2/Wnt3a/β-catenin. Am J Chin Med. 48:341–356. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ma W, Kang Y, Ning L, Tan J, Wang H and
Ying Y: Identification of microRNAs involved in gefitinib
resistance of non-small-cell lung cancer through the insulin-like
growth factor receptor 1 signaling pathway. Exp Ther Med.
14:2853–2862. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Barratt SL, Creamer A, Hayton C and
Chaudhuri N: Idiopathic Pulmonary Fibrosis (IPF): An Overview. J
Clin Med. 7(201)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fu L, Rab A, Tang LP, Rowe SM, Bebok Z and
Collawn JF: Dab2 is a key regulator of endocytosis and
post-endocytic trafficking of the cystic fibrosis transmembrane
conductance regulator. Biochem J. 441:633–643. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tseng CP, Ely BD, Li Y, Pong RC and Hsieh
JT: Regulation of rat DOC-2 gene during castration-induced rat
ventral prostate degeneration and its growth inhibitory function in
human prostatic carcinoma cells. Endocrinology. 139:3542–3553.
1998.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sheng Z, Sun W, Smith E, Cohen C, Sheng Z
and Xu XX: Restoration of positioning control following Disabled-2
expression in ovarian and breast tumor cells. Oncogene.
19:4847–4854. 2000.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Finkielstein CV and Capelluto DG:
Disabled-2: A modular scaffold protein with multifaceted functions
in signaling. Bioessays. 38 (Suppl 1):S45–S55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hocevar BA, Mou F, Rennolds JL, Morris SM,
Cooper JA and Howe PH: Regulation of the Wnt signaling pathway by
disabled-2 (Dab2). EMBO J. 22:3084–3094. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dong R, Yu J, Yu F, Yang S, Qian Q and Zha
Y: IGF-1/IGF-1R blockade ameliorates diabetic kidney disease
through normalizing Snail1 expression in a mouse model. Am J
Physiol Endocrinol Metab. 317:E686–E698. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pineiro-Hermida S, Lopez IP, Alfaro-Arnedo
E, Torrens R, Iñiguez M, Alvarez-Erviti L, Ruíz-Martínez C and
Pichel JG: IGF1R deficiency attenuates acute inflammatory response
in a bleomycin-induced lung injury mouse model. Sci Rep.
7(4290)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chung EJ, Kwon S, Reedy JL, White AO, Song
JS, Hwang I, Chung JY, Ylaya K, Hewitt SM and Citrin DE: IGF-1
Receptor signaling regulates type II pneumocyte senescence and
resulting macrophage polarization in lung fibrosis. Int J Radiat
Oncol Biol Phys. 110:526–538. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Koral K and Erkan E: PKB/Akt partners with
Dab2 in albumin endocytosis. Am J Physiol Renal Physiol.
302:F1013–1024. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Goldbraikh D, Neufeld D, Eid-Mutlak Y,
Lasry I, Gilda JE, Parnis A and Cohen S: USP1 deubiquitinates Akt
to inhibit PI3K-Akt-FoxO signaling in muscle during prolonged
starvation. EMBO Rep. 21(e48791)2020.PubMed/NCBI View Article : Google Scholar
|