Introduction
Acute pancreatitis (AP) is characterized by
activation of pancreatic enzymes in the pancreas by a variety of
causes such as hypertriglyceridemia and cholelithiasis, followed by
local inflammatory reactions such as autodigestion, edema,
hemorrhage and necrosis in the pancreatic tissue as the main
pathological changes, with or without other disease characterized
by changes in the organ function. The common causes include biliary
stones, hyperlipidemia, alcohol (long-term heavy use),
hypercalcemia, drug use, surgery or trauma and tumors (1). Severe AP (SAP) is one of the most
common critical illnesses in surgery and intensive care unit. The
pathophysiological process with complex pathogenesis and multiple
risk factors not only causes local damage to the pancreas but also
induces systemic inflammatory response syndrome (SIRS). The initial
stage of the disease is accompanied by lung, kidney and liver
damage, and multiple organ dysfunction syndrome (MODS) and multiple
organ failure (MOF) (2). SAP is
associated with numerous complications with a mortality rate of
20-30%. Previous studies have showed that the high mortality rate
may be due to the significant correlation between infectious
complications and MODS during the development of the disease
(3,4).
Nutritional support is a key method to treat SAP
(4). On the one hand, it can
provide enough nutrition for the body in a high energy consumption
state. On the other hand, nutritional support effectively blocks
progression of SAP, such as intestinal mucosal barrier damage,
bacterial migration, large amounts of endotoxin absorption,
gastrointestinal dysfunction and serious pathological processes, so
as to gain sufficient time for further clinical treatment (5,6). In
the earlier view of nutrition support for AP, parenteral nutrition
(PN) should be adopted first, especially for SAP, because PN
provides sufficient nutrients for metabolism and minimizes
stimulation of pancreatic secretion (7). However, the adverse effects in of PN
treatment of SAP have attracted attention (8). First, PN increases the risk of
infection. Second, long-term fasting causes intestinal dysfunction,
changes in mucosal permeability, immune function decline and
intestinal bacterial growth; release of a large number of immune
mediators leads to SIRS or MODS (9).
Compared with PN, enteral nutrition (EN) is more in
line with the physiological process of the body and has more
advantages in terms of affordability. EN is more conducive to
protecting the intestinal mucosa, maintaining normal function of
the intestine and avoiding the shift of flora caused by long-term
fasting (7). Based on this, EN has
gradually been recognized and has been used in the clinical
treatment of SAP with good results (8). EN initiation time given by the
Society for SAP is 48 or 72 h after receiving treatment (10). However, a consensus on the best
cut-off time for early EN (EEN) has not yet been reached due to
concerns about its effect on the complication such as infection and
sepsis . The present systematic review and meta-analysis aimed to
analyze and compare the complications and hospital stay in patients
receiving EEN or delayed EN (DEN).
Materials and methods
Ethics
The present study was performed following the
preferred reporting items for systematic review and meta-analysis
guidelines (11). Due to the study
design, the requirement for ethical approval was waived by Ethical
Committee of 900 Hospital of The Joint Logistics Team.
Search strategy
The present systematic review and meta-analysis was
designed to compare EEN and DEN in treating AP. Pubmed (pubmed.ncbi.nlm.nih.gov/), Web of Science
(webofknowledge.com/), Embase (embase.com/) and Cochrane library (cochranelibrary.com/) were searched until Dec 1, 2022.
The grey literature was searched by Google Scholar (scholar.google.com). An additional literature search
was performed by reviewing the reference lists. These studies were
typically meta-analyses or reviews that were identified during the
literature search process and may cite articles that may be missed
by the key words search. The key words and medical sub-headings
terms included ‘acute pancreatitis’ and ‘enteral nutrition’. All
studies were downloaded and imported into Endnote X7 (Thomson
Reuters) to delete duplications and for further literature
screening.
Selection criteria
Studies were included if they satisfied the
following criteria: i) Study included patients diagnosed with AP;
ii) all patients received EN and iii) patients receiving EN were
divided and compared based on different EN time.
Study exclusion criteria were: i) No patients with
AP were involved; ii) no comparison between EEN and DEN; iii) study
was a review, comment or case report and iv) studies not published
in English.
Literature screening, quality
assessment and data extraction
Two investigators (YL and ZW) independently screened
titles and abstracts according to the inclusion and exclusion
criteria. Full-texts were further evaluated if the inclusion could
not be determined via abstract and data could not be extracted. A
third investigator (DL) was responsible for checking the results of
the other investigator and resolving discrepancies by discussing
with the other two investigators and repeating the literature
review.
In addition, two investigators (YL and DL)
independently assess the quality of the papers based on the
Newcastle-Ottawa Quality Assessment Scale (NOS); high quality was
indicated by a score of 6-9, whereas low quality scored
0-5(12).
Two investigators (YL and DL) also independently
extracted the data from the original studies and the extracted data
were recorded in an Excel (Microsoft Corporation). The data
extracted included: i) Study characteristics such as author, year
of publication, institutions, recruitment periods, country and
study type; ii) patient characteristics, such as median age, sex,
the severity of AP, enteral route, body mass index (BMI), Acute
Physiology and Chronic Health Evaluation (APACHE) II score and
number of patients; iii) associated complications such as sepsis,
necrotic collection, walled-off pancreatic necrosis, acute
respiratory distress syndrome (ARDS), MODS, SIRS and mortality in
either EEN or DEN group. Mortality was defined as the number of
deaths caused by AP or associated complications.
Statistical analysis
The relative risk (RR) was used for statistical
analysis of categorical variables, while standard mean difference
(SMD) was used for continuous variables. Both results were reported
with 95% CI. P<0.05 was considered to indicate a statistically
significant difference. Data provided as median and range (or
interquartile range) were converted to mean ± standard deviation
using the formula provided by Hozo et al (13). Data heterogeneity was evaluated
using the I2 statistic and χ2 test was used
for statistical analysis. When heterogeneity was found
(I2≥50%), the random-effects model was used; otherwise
the fixed-effect model was used. Finally, forest plots were drawn
and the funnel plots were used for evaluating publication bias. To
assess the risk of bias due to missing results in a data synthesis,
the metabias module of STATA software version 15.0 (StataCorp LP)
was used to perform Egger's test, where P<0.05 was considered to
indicate a significant publication bias. The funnel plot for
identifying underreported articles was constructed using the
metafunnel module of STATA to display the results of reporting bias
assessment. To explore the potential heterogeneity, meta-regression
was performed using the metareg module of the STATA software.
Baseline factors such as EN route and start time, study location
and design, severity of AP, APACHE II index and patient age were
analyzed to explore potential source of heterogeneity.
Results
Literature screening
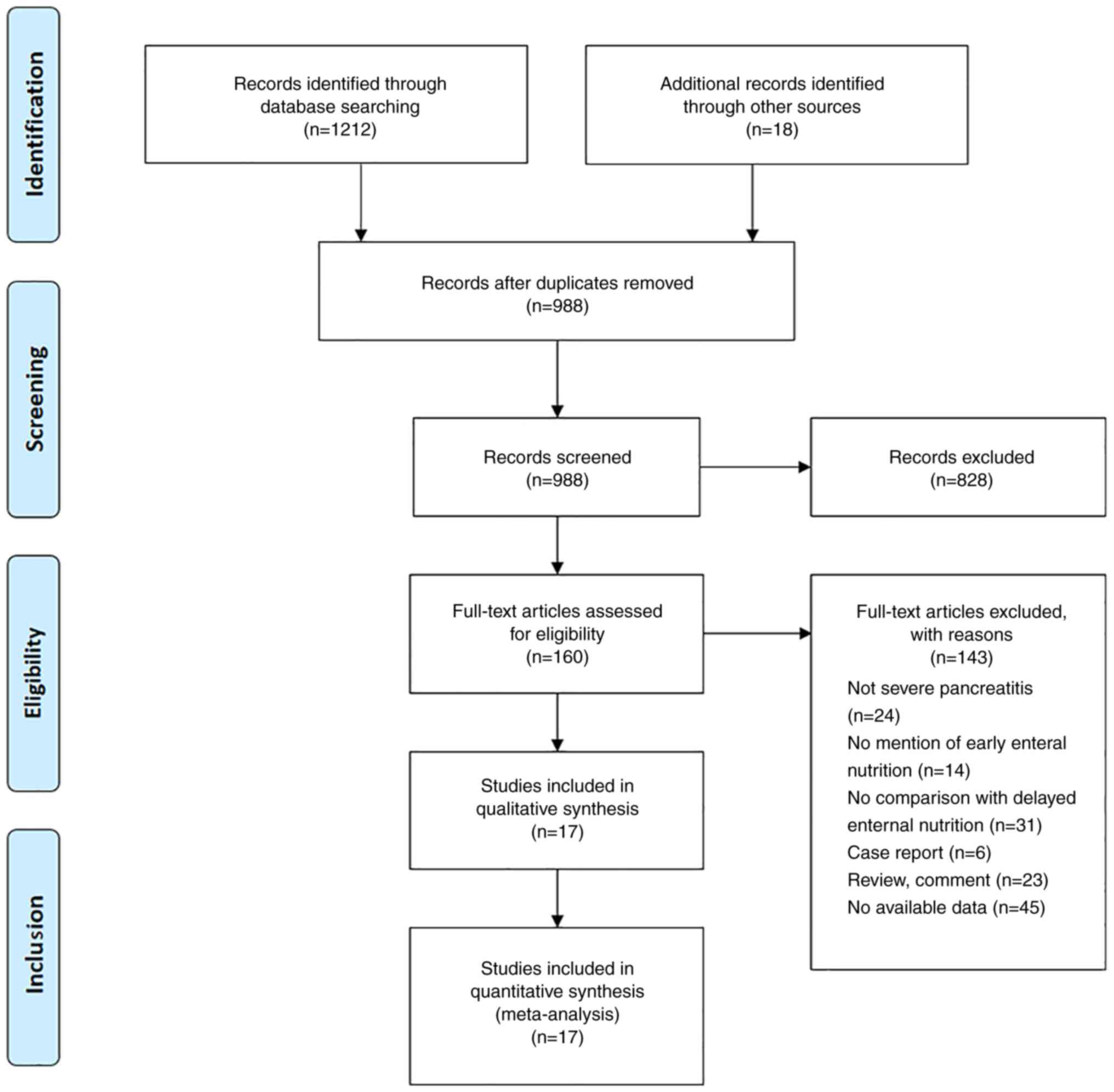
A total of 1,212 studies was identified through a
database search and 988 studies were screened by titles and
abstracts. After excluding irrelevant studies, 160 studies were
screened by full-text analysis and 17 studies were included in the
present systematic review and meta-analysis based on the
aforementioned inclusion and exclusion criteria (Fig. 1) (3,8,14-28).
Characteristics of included
studies
The characteristics of the included studies are
shown in Tables I and II. The year of publication ranged from
1997-2017, with recruitment between 1990 and 2016. The present
study included data from 12 countries, including China, Croatia,
Netherlands, New Zealand, Poland, Russia, Sweden, Canada, the
United Kingdom, Hungary, Greece and the United States. The majority
of studies (15/17) were designed as randomized control trials
(RCTs). Routes of administration were either nasojejunal or
nasogastric tube. Of the included studies, three, nine and five
studies initiated EEN support at 24, 48 and 72 h, respectively
(Table I)
 | Table ICharacteristics of included
studies. |
Table I
Characteristics of included
studies.
| First author,
year | Recruitment
period | Country | Study type | Severity of AP | EN route | EN start time,
h | NOS score | (Refs.) |
|---|
| Jin et al,
2017 | 2013-2016 | China | Retrospective | Moderate and
severe | NJ | <72 | 8 | (28) |
| Stimac et
al, 2016 | 2007-2012 | Croatia | RCT | Moderate and
severe | NJ | <72 | 8 | (27) |
| Zou et al,
2014 | 2008-2013 | China | RCT | Not stated | NJ | <72 | 8 | (26) |
| Bakker et
al, 2014 | ND | Netherlands | RCT | Severe | NJ | <48 | 9 | (3) |
|
Wereszczynska-Siemiatkowska et al,
2013 | 2001-2010 | Poland | RCT | Severe | NJ | <48 | 8 | (25) |
| Sun et al,
2013 | 2010-2011 | China | RCT | Severe | NJ | <48 | 8 | (24) |
| Petrov et
al, 2013 | 2010-2011 | New Zealand | RCT | AP | NG | <72 | 8 | (23) |
| Bakker et
al, 2009 | ND | Netherlands | Retrospective | Severe | NJ | <48 | 7 | (22) |
| Qin et al,
2008 | 2002-2006 | China | RCT | Severe | NJ | <48 | 7 | (21) |
| Petrov et
al, 2006 | 2002-2004 | Russia | RCT | Severe | NJ | <24 | 8 | (20) |
| Eckerwall et
al, 2006 | 2002-2004 | Sweden | RCT | Severe | NG | <24 | 7 | (19) |
| Louie et al,
2005 | ND | Canada | RCT | Severe | NJ | <24 | 7 | (18) |
| Gupta et al,
2003 | 1996-1998 | UK | RCT | Severe | NJ | <48 | 6 | (17) |
| Olah et al,
2002 | 1995-1996 | Hungary | RCT | AP | NJ | <48 | 7 | (16) |
| Abou-Assi et
al, 2002 | 2000 | USA | RCT | AP | NJ | <72 | 8 | (8) |
| McClave et
al, 1997 | ND | USA | RCT | Moderate | NJ | <48 | 7 | (15) |
| Kalfarentzos et
al, 1997 | 1990-1995 | Greece | RCT | Severe | NJ | <48 | 7 | (14) |
 | Table IIPatient data from the included
studies. |
Table II
Patient data from the included
studies.
| | ENN group | DEN group | |
|---|
| First author,
year | Sample size, n | Male, % | Age, years, mean
±SD/median (range) | BMI,
kg/m2 | APACHE II | Sample size | Male, % | Age, years, mean
±SD/median (range) | BMI,
kg/m2 | APACHE II | (Refs.) |
|---|
| Jin et al,
2017 | 35 | 23(66) | 43.7±15.6 | 26.3±3.7 | 10.8±5.6 | 52 | 45(87) | 45.7±14.4 | 26.2±3.5 | 11.4±7.2 | (28) |
| Stimac et
al, 2016 | 107 | 64(60) | 69.0
(28.0-88.0) | ND | 9.84±3.26 | 107 | 57(53) | 72.0
(26.0-90.0) | ND | 9.8±3.2 | (27) |
| Zou et al,
2014 | 46 | 26(56) | 46.5
(34.6-59.3) | 25.8
(22.5-26.9) | 6.0 (3.0–9.0) | 47 | 26(55) | 48.0
(34.0-60.0) | 24.5
(21.9-26.1) | 8.0 (5.0-10.0) | (26) |
| Bakker et
al, 2014 | 101 | 55(54) | 65.0±160 | ND | 11.0±4.0 | 104 | 45(43) | 65.0±15.0 | ND | 11.0±5.0 | (3) |
|
Wereszczynska-Siemiatkowska et al,
2013 | 97 | 72(74) | 49.0
(39.0-56.0) | ND | 4.0 (2.0-7.0) | 100 | 61(61) | 50.0
(41.0-62.5) | ND | 5.0 (2.0-7.5) | (25) |
| Sun et al,
2013 | 30 | 18(60) | 43.0
(34.5-51.0) | 24.6
(23.5-26.8) | 9.5 (8.5-11) | 30 | 20(67) | 45.0
(35.0-52.0) | 25.8
(23.9-28.8) | 10.0
(8.0-11.5) | (24) |
| Petrov et
al, 2013 | 17 | 10(59) | 41.0
(34.0-60.0) | 26.0
(24.0-30.0) | 6.0 (2.0-9.0) | 18 | 8(44) | 55.0
(36.0-70.0) | 25.0
(23.0-28.0) | 6.0 (3.0-11.0) | (23) |
| Bakker et
al, 2009 | 184 | ND | ND | ND | ND | 112 | ND | ND | ND | ND | (22) |
| Qin et al,
2008 | 36 | 11(31) | 54.3±13.1 | 21.1±2.3 | 8.8±0.5 | 38 | 12(32) | 58.4 (19.1) | 22.7±1.8 | 8.9±0.7 | (21) |
| Petrov et
al, 2006 | 35 | 27(77) | 51.0
(42.0-67.0) | ND | 12 (10-14) | 34 | 24(71) | 52.0
(41.0-70.0) | ND | 12.5
(11.0-16.0) | (20) |
| Eckerwall et
al, 2006 | 24 | 10(42) | 71.0
(58.0-80.0) | 27.0
(25.0-30.0) | 9.0 (8.0-10.0) | 26 | 14(54) | 68.0
(60.0-80.0) | 28.0
(27.0-30.0) | 10.0
(8.0-13.0) | (19) |
| Louie et al,
2005 | 10 | ND | ND | ND | ND | 18 | ND | ND | ND | ND | (18) |
| Gupta et al,
2003 | 8 | 4(50) | 65.0
(56.0-89.0) | ND | 8.0 (6.0-12.0) | 9 | 3(33) | 57.0
(38.0-86.0) | ND | 10.0
(7.0-14.0) | (17) |
| Olah et al,
2002 | 41 | 33(80) | 42.0 | ND | ND | 48 | 42(88) | 43.8 | ND | ND | (16) |
| Abou-Assi et
al, 2002 | 26 | 16(62) | 48.0±3.0 | 26.6±1.3 | ND | 27 | 13(48) | 50.0±3.0 | 25.7±1.6 | ND | (8) |
| McClave et
al, 1997 | 16 | ND | 47.6±4.0 | ND | 17.5±4.1 | 16 | ND | 45.1±4.2 | ND | 22.4±5.0 | (15) |
| Kalfarentzos et
al, 1997 | 18 | 8(44) | 63.0±10.7 | ND | 12.7±2.6 | 20 | 7(35) | 67.2±8.9 | ND | 11.8±1.9 | (14) |
A total of 1,637 patients with AP was included in
the present study (Table II). Of
these, 831 patients received EEN, while 806 patients received DEN.
The median proportion of male patients was 58.4 and 55.0% in the
EEN and DEN groups, respectively. The median age was 53.2 (range,
41-71) and 54.8 (range, 43.8-72) years in the EEN and DEN groups,
respectively. Median BMI and APACHE II scores were similar between
the two groups.
The majority of studies were assessed as high
quality with a score >7; however Gupta et al (17) was assessed with NOS score of 6.
Clinical outcome between EEN and DEN
groups
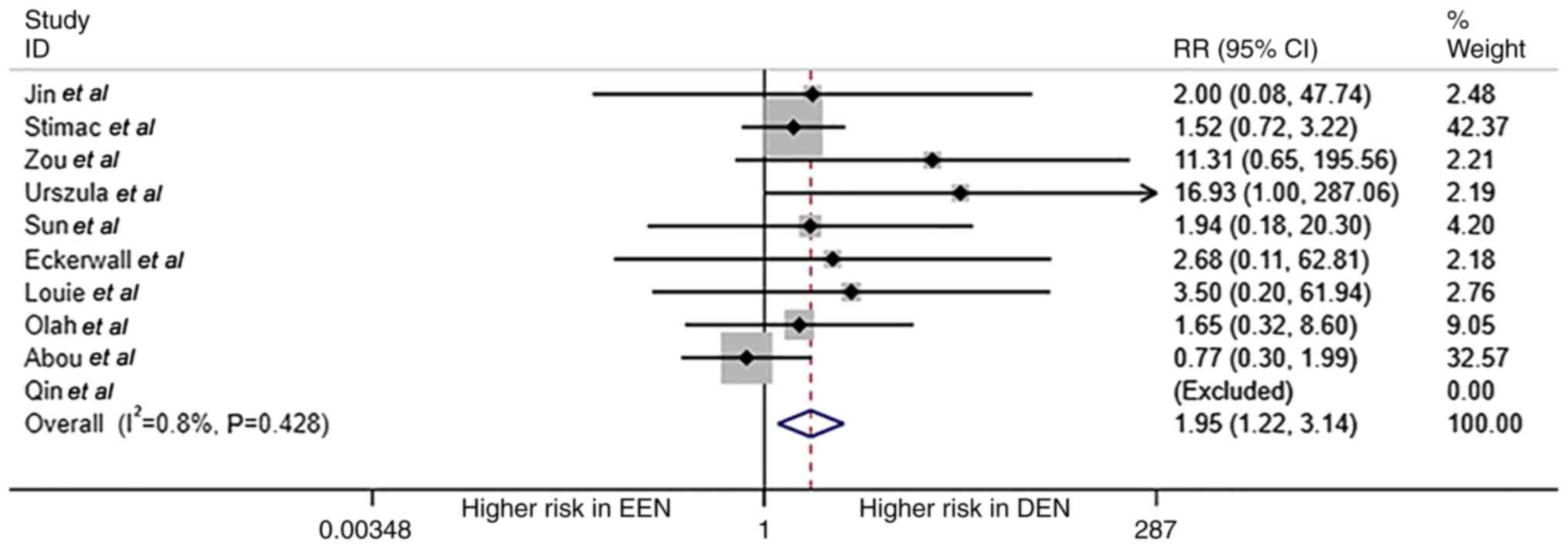
Overall mortality of patients with AP between EEN
and DEN groups is shown in Fig. 2.
A total of 10 studies reported mortality data; in the study by Qin
et al, (21) the mortality
in both EEN and DEN groups was zero. Thus, only nine studies
reported valid data on mortality with a total of 28 patients with
AP-associated death. Patients in the DEN group had a higher risk
for mortality compared with the EEN group (RR=1.95; 95% CI,
1.21-3.14; P=0.006; I2=0.8%; fixed effect model).
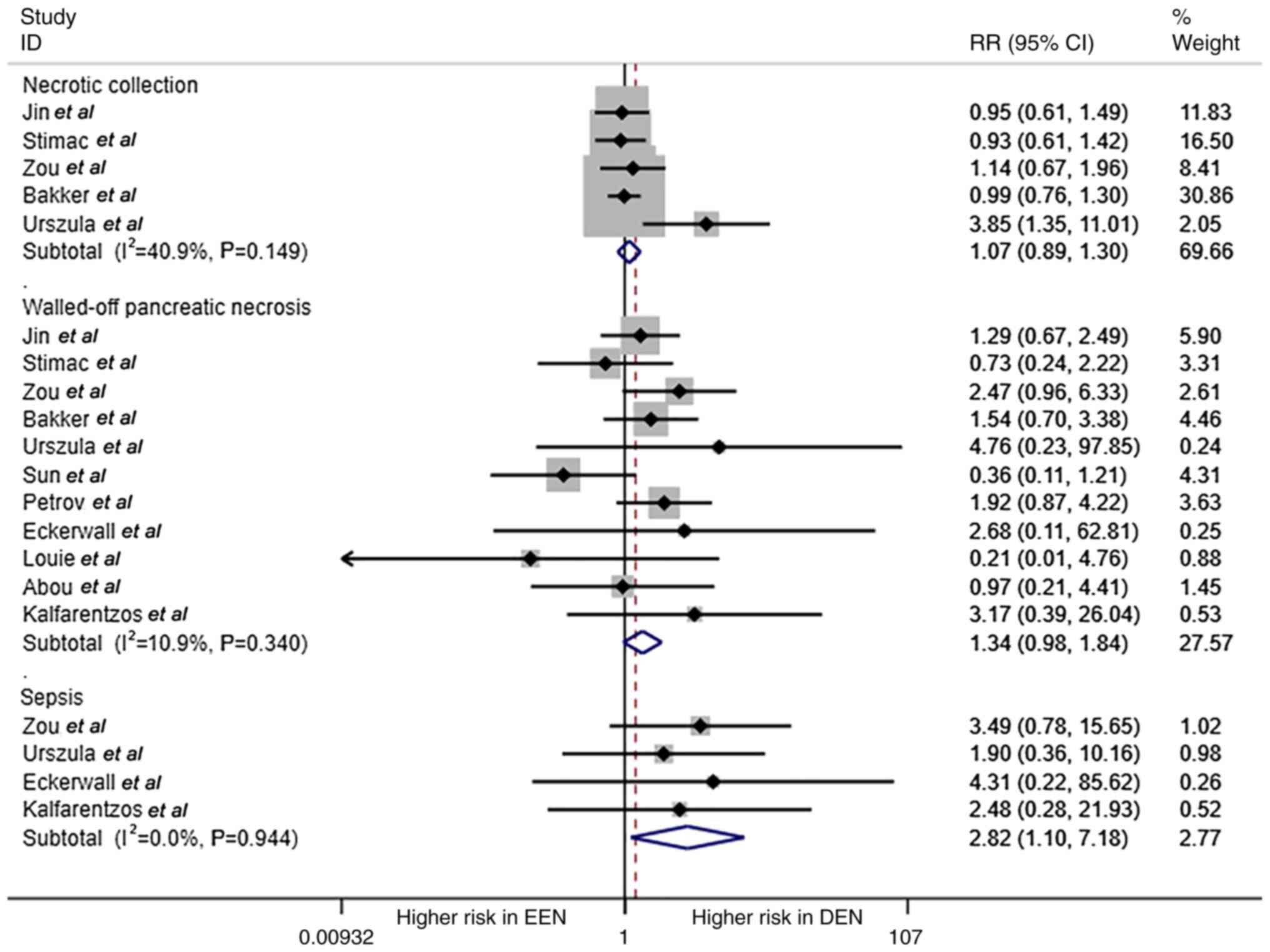
The comparison of necrotic collection, walled-off
pancreatic necrosis and sepsis is shown in Fig. 3. Patients with AP in the DEN group
had a higher probability of sepsis compared with that in the EEN
group (RR=2.82; 95% CI, 1.10-7.18; P=0.03; I2=0%; fixed
effect model); however, there was no significance in terms of
necrotic collection and walled-off pancreatic necrosis between the
two groups (RR=1.074 and 1.342, respectively; all P>0.05).
ARDS, MODS and SIRS are shown in Fig. 4. No statistical differences were
found in these complications of AP (RR=1.01, 1.27 and 1.07,
respectively; all P>0.05).
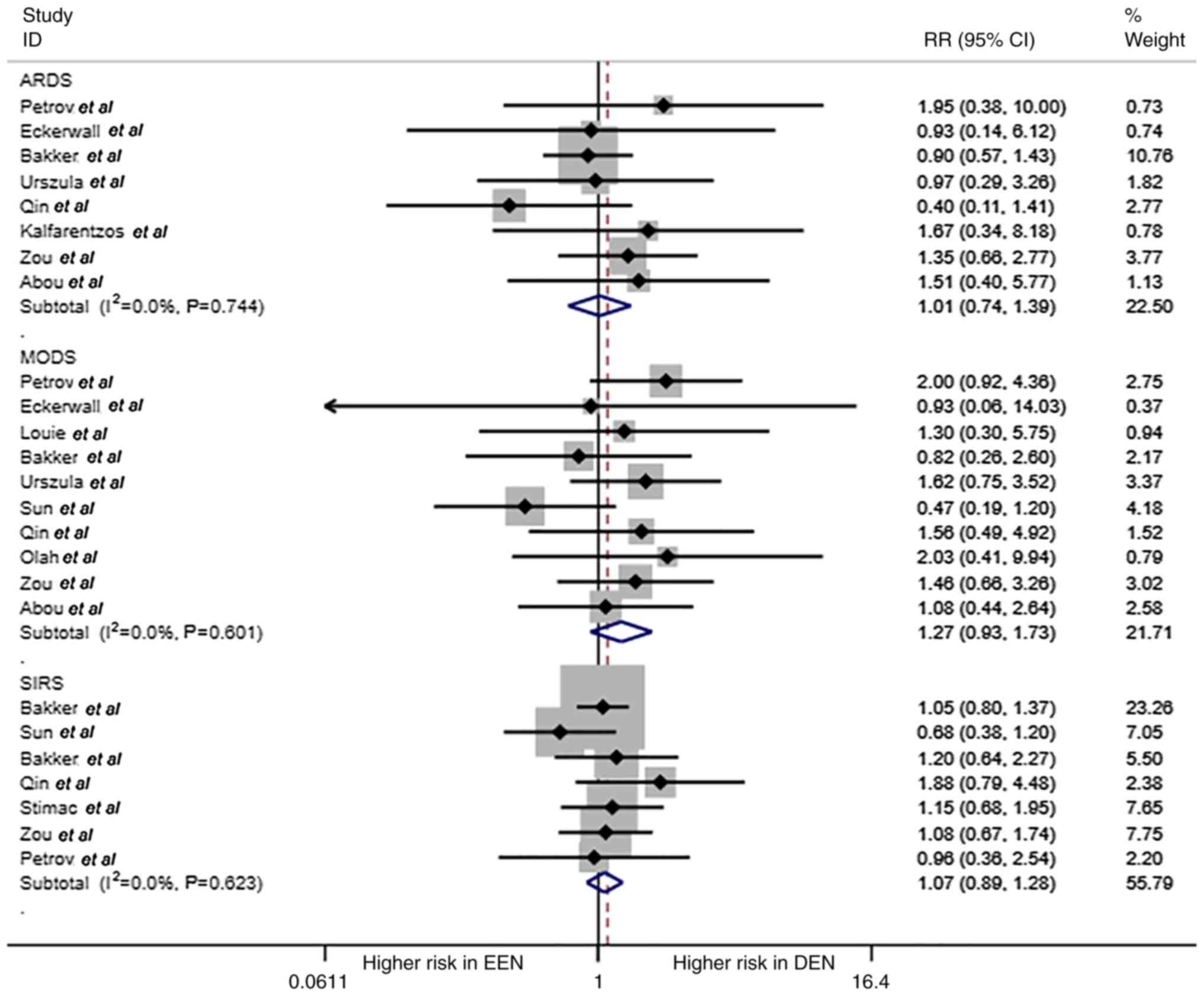 | Figure 4ARDS, MODS, SIRS for EEN and DEN. The
forest plot shows the RR of ARDS, MODS, SIRS of EEN vs. control
treatment (DEN). ARDS, acute respiratory distress syndrome; MODS,
multiple organ dysfunction syndrome; MOF, multiple organ failure;
EEN, early enteral nutrition; DEN, delayed EN; RR, relative
risk. |
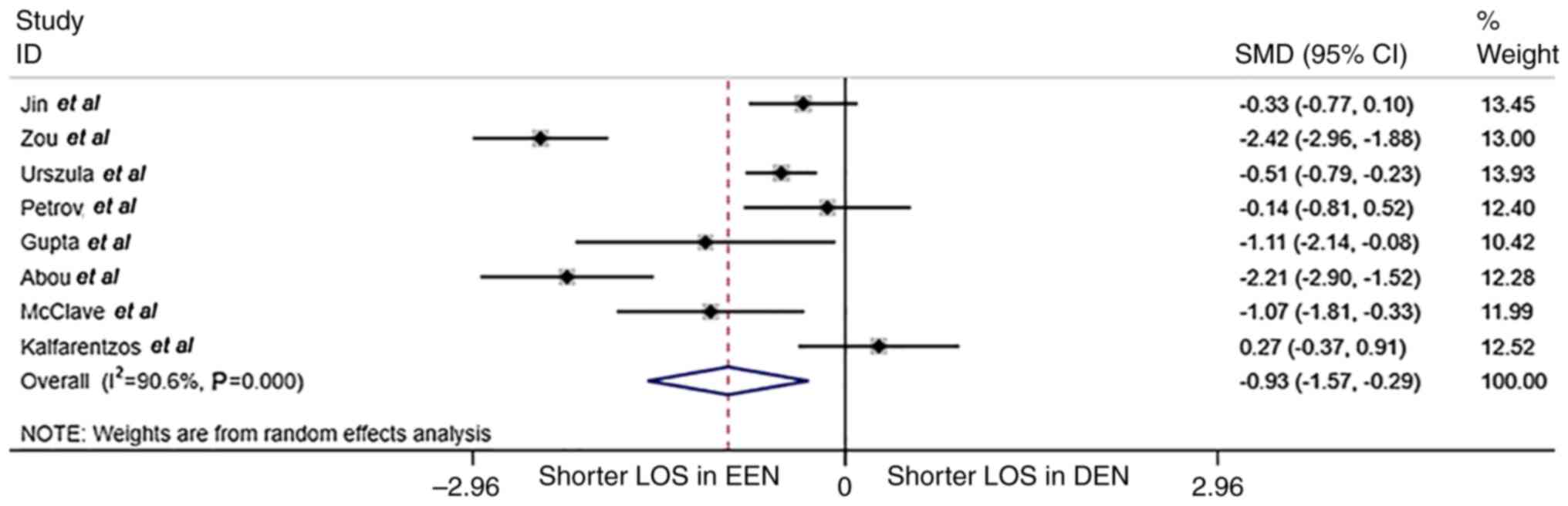
The comparison of hospital stay is shown in Fig. 5. The median duration of hospital
stay was significantly shorter in the EEN group compared with that
in the DEN group (17.4 vs. 20.0 days; SMD=-0.93; 95% CI, -1.57-
-0.29; P<0.001; I2=90.4%; randomized effect
model).
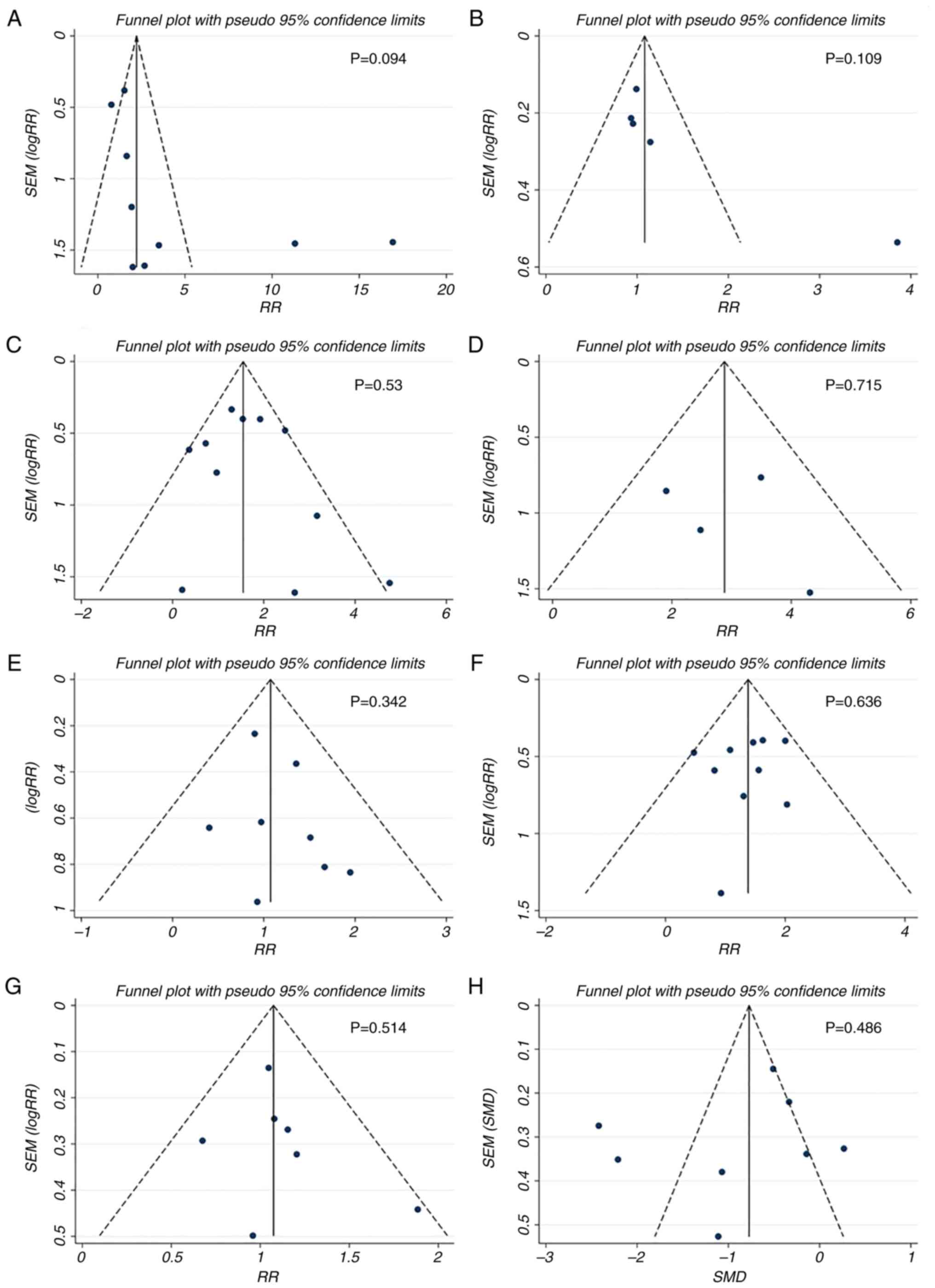
Publication bias analysis
Egger's test for the comparisons of mortality,
necrotic collection, walled-off pancreatic necrosis, sepsis, ARDS,
MODS, SIRS and hospital stay showed no significant difference
(P>0.05; data not shown). The funnel plots of these comparisons
showed favorable symmetry (Fig.
6), indicating no significant publication bias for these
comparisons.
Subgroup analysis
The comparison of complications between EEN and DEN
at different cut-off times is shown in Table III. DEN was associated with a
3.89-fold increase in risk of mortality compared with that in the
EEN group with a cut-off of 48 h (95% CI, 1.25-12.17; P=0.019).
Although there was a higher risk of complications such as
walled-off pancreatic necrosis, sepsis, ARDS, MODS and SIRS in
patients with DEN, there was no significant difference due to small
sample size.
 | Table IIISubgroup analysis of complications at
different time points. |
Table III
Subgroup analysis of complications at
different time points.
| | Enteral nutrition
time point, h |
|---|
| | <24 | <48 | <72 |
|---|
| Outcome | Number of
studies | RR (95% CI) | P-value | Number of
study | RR (95% CI) | P-value | Number of study,
n | RR (95% CI) | P-value |
|---|
| Mortality | 2 | 3.14
(0.37-26.34) | 0.29 | 4 | 3.89
(1.25-12.17) | 0.02 | 4 | 1.50
(0.87-2.59) | 0.14 |
| Walled-off
pancreatic necrosis | 3 | 1.64
(0.86-3.36) | 0.17 | 4 | 1.18
(0.67-2.09) | 0.57 | 4 | 1.35
(0.86-2.12) | 0.20 |
| Sepsis | 1 | 4.31
(0.21-85.61) | 0.34 | 2 | 2.1
(0.56-7.92) | 0.27 | 1 | 3.49
(0.78-15.65) | 0.10 |
| Necrotic
collection | NA | - | - | 2 | 1.17
(0.90-1.52) | 0.24 | 3 | 0.98
(0.6-1.29) | 0.93 |
| Acute respiratory
distress syndrome | 2 | 1.43
(0.42-4.83) | 0.56 | 4 | 0.86
(0.58-1.27) | 0.45 | 2 | 1.39
(0.74-2.61) | 0.35 |
| Multiple organ
dysfunction syndrome | 3 | 1.74
(0.89-3.38) | 0.10 | 5 | 1.09
(0.70-1.71) | 0.68 | 2 | 1.28
(0.71-2.33) | 0.41 |
| Systemic
inflammatory response syndrome | NA | - | - | 4 | 1.01
(0.85-1.21) | 0.64 | 3 | 1.10
(0.91-1.54) | 0.59 |
Heterogeneity of the comparison of
hospital stays
The heterogeneity of the comparison of hospital
stays was considerable (Fig. 5.
Therefore, meta-regression was performed. Of baseline factors, only
the severity of AP showed a significant impact on the outcome
(Coefficient=0.903; P=0.025; Table
IV). The difference in hospital stay was more significant in
patients with AP compared with that in patients with SAP/moderate
AP; this contributed to the heterogeneity of hospital stays.
 | Table IVMeta-regression. |
Table IV
Meta-regression.
| Variable | Coef. | Standard error | P-value | Lower 95% CI | Upper 95% CI |
|---|
| EN route | 0.901 | 1.071 | 0.432 | -1.720 | 3.523 |
| EN start time | -0.691 | 0.689 | 0.355 | -2.377 | 0.995 |
| Study location | 0.076 | 0.203 | 0.721 | -0.421 | 0.573 |
| Study design | -0.689 | 1.066 | 0.542 | -3.298 | 1.919 |
| Severity of acute
pancreatitis | 0.903 | 0.302 | 0.025 | 0.161 | 1.643 |
| Acute Physiology
and Chronic Health Evaluation II index | 0.032 | 0.084 | 0.716 | -0.183 | 0.248 |
| Patient age | 0.019 | 0.045 | 0.683 | -0.092 | 0.131 |
Discussion
The present study is an up-to-date systematic review
and meta-analysis of studies comparing the complications observed
in patients with AP and supported with EEN or DEN. The present
meta-analysis showed that patients with AP and supported with EEN
have a lower risk of mortality and sepsis, and therefore have a
shorter hospital stay.
SAP is a disease with rapid onset and progression
and high mortality (10). At
present, several conservative medical treatments are performed in
the early stage of the disease (18,28).
In addition to early fluid resuscitation and organ function
support, nutritional support treatment is a key example of
conservative medical treatment (4,6).
Patients with SAP are mostly in a state of high catabolism with
severe negative nitrogen balance coupled with prolonged
gastrointestinal decompression and often suffer from water and
electrolyte imbalances and malnutrition, which cause complications
such as arhythmia and affect the prognosis of the disease (29,30).
In the case of patients with high metabolism, it is important to
provide adequate energy support. The sequential pathological
processes of SAP include acute reaction, systemic infection and
residual infection period. The acute stage of SAP is characterized
by a ‘cascade of inflammation’ (10). In theory, EN improves
inflammation-associated intestinal wall, pancreatic edema and
peripancreatic effusion and can reduce intra-abdominal pressure
(31). At SAP onset, the
intestinal mucosa undergoes cell shedding and apoptosis of villi,
the height of villi and thickness of the intestinal mucosa are
significantly reduced, the tight junctions between cells loosen,
the morphology and function of the intestinal mucosa are damaged
and the intestinal flora is shifted (31). Secondary systemic infection and
local necrotic tissue infection may also occur (32); therefore, intestinal barrier
function is very important in the occurrence and development of
SAP. It is reported that 80% of patients with SAP have secondary
infections of the pancreas and peripancreatic tissue (7,10).
The pathogens of secondary infection are mostly Escherichia
coli, Enterococcus and certain anaerobic bacteria, all
of which are intestinal-derived strains. After infection occurs,
the stress response is aggravated, patient condition worsens and
the occurrence of systemic complications is increased (33,34).
EN at the appropriate timepoint of SAP course is not
only more aligned with the normal physiology of the human body but
also decreases bacterial translocation, while ensuring that the
intestine have sufficient rest time and preserving the integrity of
intestinal mucosa function and structure, which can significantly
decrease morbidity and mortality to benefit patients with SAP
(5,7). While EN provides nutrients needed by
the body, it also decreases occurrence of metabolic complications
and catheter-associated infections. At the same time, it can
effectively maintain functional integrity of gastrointestinal
mucosa, decrease bacterial translocation and intestinal infection,
promote gastrointestinal peristalsis, increase intestinal mucosal
perfusion blood flow and decrease incidence and mortality of MODS
(6,7,34).
Therefore, early nutritional support is important in the treatment
of SAP (35). The initiation of EN
in patients with SAP is associated with gastrointestinal function.
In the early stage of SAP, severe stress response and inflammatory
stimulation lead to intestinal ischemia, hypoxia and increased
permeability. Therefore, premature EN support not only fails to
ensure good digestion but also leads to further damage to the
intestine, thereby increasing the likelihood of endotoxin
translocation of intestinal bacterial agents (36). Suitable EN support is beneficial to
maintain integrity of intestinal mucosal cell structure and
function, repair and maintain mechanical, biological, immune and
chemical barrier function of the intestinal mucosa, decrease
bacterial translocation and intestinal infection and improve the
prognosis (6).
For patients with more severe AP, the clinician
might delay the initiation of EN, and this might lead to patient
selection bias. However, according to the RCT design of most
included studies, the type of intervention (ENN or DNN) was not
decided based on severity. The disease severity parameters in ENN
and DNN groups were comparable among the included studies.
Therefore, the selection bias was not present.
For patients with SAP who are considered for EN
treatment, the optimal time to initiate EN remains unclear.
European Society for Parenteral Enteral Nutrition recommends that
EN should be started within 24 h of hospital admission (37), while the American Society for
Parenteral Enteral Nutrition clinical guidelines recommend that EN
be started within 48 h (38).
Moreover, certain studies consider it safe and feasible to start EN
within 3 days (39), while others
have indicated that the best time to start EN is when the internal
bowel function starts to recover 3-5 days after jejunostomy or
conservative treatment for 3-5 days (40). Pontell et al (41) showed that following intestinal
ischemia-reperfusion, intestinal peristalsis is weakened and
longitudinal muscles of the intestine are severely damaged, which
may aggravate destruction of the intestinal barrier and cause
bacteria shifting. In clinical practice, it is not easy to start EN
within 48 h so it is important to record and evaluate EN.
Reperfusion is performed following intestinal mucosal blood loss in
patients with SAP at the initial stage, which induces activation of
inflammatory factors and produces inflammatory response syndrome,
resulting in MODS (5,10). European Society for Parenteral
Enteral Nutrition (37) has
discussed the needs of PN and EN and concluded that EN should be
administered within 24 h of admission to obtain the best treatment
effect. Relevant studies have proved that EN can only be applied in
a relatively narrow ‘diagnostic window’ to achieve the expected
treatment efficacy (14,15). Within 48 h of admission, EN can be
used to control the inflammatory response, decrease bacterial
translocation and protect the gastrointestinal mucosal barrier
(3,24). A number of studies have also
confirmed that the initiation of EN in patients with SAP 48 h after
admission significantly increases incidence of pancreatic
infection, MODS and mortality compared with EN started within 48 h
(25). The present data analysis
further confirmed that it is more appropriate to initiate EN within
48 h, while it is more harmful to start it >48 h after hospital
admission. Hegazi et al (42) demonstrated that initial nutritional
support can significantly decrease patient mortality and optimize
the prognosis of patients with SAP. The most appropriate mode of
nutrition for the human body is digestion and absorption of
nutrients via intestinal nutrition, which not only effectively
decrease intestinal infections and bacterial translocation, but
also encourages patients to restore body nutrition support as soon
as possible (10). A meta-analysis
by Qi et al (43)
investigated EEN (defined as EN initiated within 24 h of hospital
admission) and DEN. Their analysis included eight studies and found
no significant difference in risk of mortality, infectious
complications and pancreatic-associated infection but MOF was less
common in patients treated with EEN. By contrast, the present
meta-analysis included 17 studies and explored EEN (defined as EN
initiated at 24, 48 or 72 h after hospital admission) and DEN. The
present analysis showed that starting EN at 48 h significantly
decreased mortality, sepsis and length of hospital stay. In light
of the previously published meta-analysis (43), the present results indicated that,
as the time point for EEN start, 48 h may be more beneficial to
decrease disease-related mortality.
The present meta-analysis had limitations. Firstly,
due to the lack of peer-reviewed topical studies, the subgroup
analysis did not find the best time point for EEN. Secondly,
heterogeneity in hospital stay data may be present and the hospital
stay should be further investigated in future studies.
The present systematic review suggested that EEN
decreased complication in patients with AP and therefore provides a
safe approach to improve recovery. The best time point for EEN is
still debated but 24-72 h are safe time points for EEN.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and DL contributed to the study conception and
design. YL, DL and ZW wrote the manuscript and analyzed and
interpreted data. YL and DL confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Requirement for ethical approval of the present
study was waived by Ethical Committee of 900 Hospital of The Joint
Logistics Team.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Working Group IAP/APA Acute Pancreatitis
Guidelines. IAP/APA evidence-based guidelines for the management of
acute pancreatitis. Pancreatology. 13 (Suppl 2):e1–e15.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Muddana V, Whitcomb DC and Papachristou
GI: Current management and novel insights in acute pancreatitis.
Expert Rev Gastroenterol Hepatol. 3:435–444. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bakker OJ, van Brunschot S, van Santvoort
HC, Besselink MG, Bollen TL, Boermeester MA, Dejong CH, van Goor H,
Bosscha K, Ahmed Ali U, et al: Early versus on-demand nasoenteric
tube feeding in acute pancreatitis. N Engl J Med. 371:1983–1993.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Greenberg JA, Hsu J, Bawazeer M, Marshall
J, Friedrich J, Nathens A, Coburn N, May G, Pearsall E and McLeod
R: Clinical practice guideline: Management of acute pancreatitis.
Can J Surg. 59:128–140. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Forsmark CE, Vege SS and Wilcox CM: Acute
pancreatitis. N Eng J Med. 375:1972–1981. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Reintam Blaser A, Starkopf J, Alhazzani W,
Berger MM, Casaer MP, Deane AM, Fruhwald S, Hiesmayr M, Ichai C,
Jakob SM, et al: Early enteral nutrition in critically ill
patients: ESICM clinical practice guidelines. Intensive Care Med.
43:380–398. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Al-Omran M, Albalawi ZH, Tashkandi MF and
Al-Ansary LA: Enteral versus parenteral nutrition for acute
pancreatitis. Cochrane Database Syst Rev.
2010(CD002837)2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Abou-Assi S, Craig K and O'Keefe SJ:
Hypocaloric jejunal feeding is better than total parenteral
nutrition in acute pancreatitis: Results of a randomized
comparative study. Am J Gastroenterol. 97:2255–2262.
2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mora J, Casas M, Cardona D and Farré A:
Effect of enteral versus parenteral nutrition on inflammatory
markers in severe acute pancreatitis. Pancreas.
35(292)2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
ASPEN Board of Directors and the Clinical
Guidelines Task Force. Guidelines for the use of parenteral and
enteral nutrition in adult and pediatric patients. JPEN J Parenter
Enteral Nutr. 26 (Suppl):1SA–138SA. 2002.PubMed/NCBI
|
|
11
|
Moher D, Liberati A, Tetzlaff J, Altman DG
and PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med.
6(e1000097)2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stang A: Critical evaluation of the
newcastle-ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hozo SP, Djulbegovic B and Hozo I:
Estimating the mean and variance from the median, range, and the
size of a sample. BMC Med Res Methodol. 5(13)2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kalfarentzos F, Kehagias J, Mead N,
Kokkinis K and Gogos CA: Enteral nutrition is superior to
parenteral nutrition in severe acute pancreatitis: Results of a
randomized prospective trial. Br J Surg. 84:1665–1669.
1997.PubMed/NCBI
|
|
15
|
McClave SA, Greene LM, Snider HL, Makk LJ,
Cheadle WG, Owens NA, Dukes LG and Goldsmith LJ: Comparison of the
safety of early enteral vs parenteral nutrition in mild acute
pancreatitis. JPEN J Parenter Enteral Nutr. 21:14–20.
1997.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Oláh A, Pardavi G, Belágyi T, Nagy A,
Issekutz A and Mohamed GE: Early nasojejunal feeding in acute
pancreatitis is associated with a lower complication rate.
Nutrition. 18:259–262. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gupta R, Patel K, Calder PC, Yaqoob P,
Primrose JN and Johnson CD: A randomised clinical trial to assess
the effect of total enteral and total parenteral nutritional
support on metabolic, inflammatory and oxidative markers in
patients with predicted severe acute pancreatitis (APACHE II >
or =6). Pancreatology. 3:406–413. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Louie BE, Noseworthy T, Hailey D, Gramlich
LM, Jacobs P and Warnock GL: 2004 MacLean-Mueller prize enteral or
parenteral nutrition for severe pancreatitis: A randomized
controlled trial and health technology assessment. Can J Surg.
48:298–306. 2005.PubMed/NCBI
|
|
19
|
Eckerwall GE, Axelsson JB and Andersson
RG: Early nasogastric feeding in predicted severe acute
pancreatitis: A clinical, randomized study. Ann Surg. 244:959–965.
2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Petrov MS, Kukosh MV and Emelyanov NV: A
randomized controlled trial of enteral versus parenteral feeding in
patients with predicted severe acute pancreatitis shows a
significant reduction in mortality and in infected pancreatic
complications with total enteral nutrition. Dig Surg. 23:336–344.
2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Qin HL, Zheng JJ, Tong DN, Chen WX, Fan
XB, Hang XM and Jiang YQ: Effect of Lactobacillus plantarum enteral
feeding on the gut permeability and septic complications in the
patients with acute pancreatitis. Eur J Clin Nutr. 62:923–930.
2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bakker OJ, van Santvoort HC, Besselink MG,
Fischer K, Bollen TL, Boermeester MA and Gooszen HG: 473 Timing of
enteral nutrition in patients with predicted severe acute
pancreatitis: An early start is associated with a reduction in
bacteremia. Gastroenterology. 136:A75–A76. 2009.
|
|
23
|
Petrov MS, McIlroy K, Grayson L, Phillips
AR and Windsor JA: Early nasogastric tube feeding versus nil per os
in mild to moderate acute pancreatitis: A randomized controlled
trial. Clin Nutr. 32:697–703. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sun JK, Mu XW, Li WQ, Tong ZH, Li J and
Zheng SY: Effects of early enteral nutrition on immune function of
severe acute pancreatitis patients. World J Gastroenterol.
19:917–922. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wereszczynska-Siemiatkowska U,
Swidnicka-Siergiejko A, Siemiatkowski A and Dabrowski A: Early
enteral nutrition is superior to delayed enteral nutrition for the
prevention of infected necrosis and mortality in acute
pancreatitis. Pancreas. 42:640–646. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zou L, Ke L, Li W, Tong Z, Wu C, Chen Y,
Li G, Li N and Li J: Enteral nutrition within 72 h after onset of
acute pancreatitis vs delayed initiation. Eur J Clin Nutr.
68:1288–1293. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Stimac D, Poropat G, Hauser G, Licul V,
Franjic N, Valkovic Zujic P and Milic S: Early nasojejunal tube
feeding versus nil-by-mouth in acute pancreatitis: A randomized
clinical trial. Pancreatology. 16:523–528. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jin M, Zhang H, Lu B, Li Y, Wu D, Qian J
and Yang H: The optimal timing of enteral nutrition and its effect
on the prognosis of acute pancreatitis: A propensity score matched
cohort study. Pancreatology. 17:651–657. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Barreto SG, Habtezion A, Gukovskaya A,
Lugea A, Jeon C, Yadav D, Hegyi P, Venglovecz V, Sutton R and
Pandol SJ: Critical thresholds: Key to unlocking the door to the
prevention and specific treatments for acute pancreatitis. Gut.
70:194–203. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Boxhoorn L, Voermans RP, Bouwense SA,
Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC and
Besselink MG: Acute pancreatitis. Lancet. 396:726–734.
2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Narayanan S, Bhutiani N, Adamson D and
Jones C: Pancreatectomy, islet cell transplantation, and nutrition
considerations. Nutr Clin Pract. 36:385–397. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kuan LL, Dennison AR and Garcea G:
Association of visceral adipose tissue on the incidence and
severity of acute pancreatitis: A systematic review. Pancreatology.
20:1056–1061. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ge P, Luo Y, Okoye CS and Chen H, Liu J,
Zhang G, Xu C and Chen H: Intestinal barrier damage, systemic
inflammatory response syndrome, and acute lung injury: A
troublesome trio for acute pancreatitis. Biomed Pharmacother.
132(110770)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hu F, Lou N, Jiao J, Guo F, Xiang H and
Shang D: Macrophages in pancreatitis: Mechanisms and therapeutic
potential. Biomed Pharmacother. 131(110693)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Horibe M, Iwasaki E, Nakagawa A, Matsuzaki
J, Minami K, Machida Y, Tamagawa H, Takimoto Y, Ueda M, Katayama T,
et al: Efficacy and safety of immediate oral intake in patients
with mild acute pancreatitis: A randomized controlled trial.
Nutrition. 74(110724)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chen X, Yang K, Jing G, Yang J and Li K:
Meta-analysis of efficacy of rhubarb combined with early enteral
nutrition for the treatment of severe acute pancreatitis. JPEN J
Parenter Enteral Nutr. 44:1066–1078. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Marik PE: What is the best way to feed
patients with pancreatitis? Curr Opin Crit Care. 15:131–138.
2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kotani J, Usami M, Nomura H, Iso A,
Kasahara H, Kuroda Y, Oyanagi H and Saitoh Y: Enteral nutrition
prevents bacterial translocation but does not improve survival
during acute pancreatitis. Arch Surg. 134:287–292. 1999.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Working Party of the British Society of
Gastroenterology; Association of Surgeons of Great Britain and
Ireland; Pancreatic Society of Great Britain and Ireland;
Association of Upper GI Surgeons of Great Britain and Ireland. UK
guidelines for the management of acute pancreatitis. Gut. 54 Suppl
3(Suppl 3):iii1–iii9. 2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sun JK, Li WQ, Ke L, Tong ZH, Ni HB, Li G,
Zhang LY, Nie Y, Wang XY, Ye XH, et al: Early enteral nutrition
prevents intra-abdominal hypertension and reduces the severity of
severe acute pancreatitis compared with delayed enteral nutrition:
a prospective pilot study. World J Surg. 37:2053–2060.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Pontell L, Sharma P, Rivera LR, Thacker M,
Tan YH, Brock JA and Furness JB: Damaging effects of
ischemia/reperfusion on intestinal muscle. Cell Tissue Res.
343:411–419. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hegazi R, Raina A, Graham T, Rolniak S,
Centa P, Kandil H and O'Keefe SJ: Early jejunal feeding initiation
and clinical outcomes in patients with severe acute pancreatitis.
JPEN J Parenter Enteral Nutr. 35:91–96. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Qi D, Yu B, Huang J and Peng M:
Meta-analysis of early enteral nutrition provided within 24 hours
of admission on clinical outcomes in acute pancreatitis. JPEN J
Parenter Enteral Nutr. 42:1139–1147. 2018.PubMed/NCBI View Article : Google Scholar
|