Introduction
An abdominal aortic aneurysm (AAA) is a
life-threatening disease (1,2). The
primary danger is the risk of rupture and death from hemorrhage.
The AAA may be found in up to 8% of men aged >65 years and it is
the 13th leading cause of death in the USA (3). AAA is diagnosed if the diameter of
the abdominal aorta is >1.5 times (or ≥3 cm) the normal value
(4). The single most important
predictor of rupture is the diameter of AAA, with the risk of
rupture increasing for larger aneurysms (5). The goal of medical management is to
repair the AAA before rupture. Currently, surgical treatment is the
only way to prevent the rupture of AAA and there are no reliable
pharmacological agents that limit AAA expansion (6). This is partially associated with a
lack of understanding of the underlying AAA expansion mechanisms
(7).
Animal models have been used to investigate the
mechanisms involved in AAA development and determine measures for
early prevention and treatment (8). Common AAA animal models comprise the
transient intra-arterial perfusion with porcine pancreatic elastase
(PPE) and the periaortic application of calcium chloride
(CaCl2) (9-11).
Due to the similar pathological characteristics of human AAA,
intra-arterial perfusion with elastase has been frequently used for
AAA modelling (12,13). However, several factors such as
elastase concentration and the perfusion time of elastase can also
impact the AAA formation ratio and animal mortality (14,15).
Intra-arterial perfusion with PPE was able to induce
the destruction of the medial elastic fibres, eventually causing an
AAA (16,17). To the best of the authors'
knowledge, the effects of elastase perfusion pressure on the
aneurysmal morphology and mortality in experimental animals remain
to be elucidated (18). The
present study aimed to determine if the perfusion pressure impacts
the AAA formation ratio and maximum diameter, by exploring three
different levels of perfusion pressure of elastase. The impact of
perfusion pressure on animal mortality was also investigated in the
present study. The current findings could facilitate the
understanding of more characteristics of the elastase-induced AAA
models, helping researchers to choose appropriate animal models to
explore the pathogenesis and treatment of AAA.
Materials and methods
Animals
A total of 40 male 12-15 week old Sprague Dawley
rats (weight, 550-600 g) were purchased from Vital River Laboratory
Animal Technology Co., Ltd. and housed in a standard laboratory
environment with ambient temperature of 22-25˚C, humidity of 55-65%
and a 12-h light/dark cycle. Animals had free access to standard
solid claviform food and autoclaved tap water. The procedures were
performed following the National Institute of Health (NIH) Guide
for the Care and Use of Laboratory Animals (NIH publications no.
85-23, revised in 1996) and the study was approved by the Animal
Care and Use Committee of Fuwai Hospital (Beijing, China; no.
0099-1-8-HX).
Abdominal aortic aneurysm models
The elastase-induced AAA models were prepared using
PPE (type I; 8.5 ml; 11.8 mg protein/ml; 4 U/mg protein;
Sigma-Aldrich; Merck KGaA) according to an improved method
(19). Briefly, rats were
anaesthetized with 2% pentobarbital sodium (60 mg/kg;
intraperitoneally). A laparotomy was performed via a midline
abdominal incision under sterile conditions. The infrarenal
abdominal aorta was separated from the left renal vein to the
bifurcation of the aorta. The primal and distal parts of the
isolated aorta were temporarily ligated using a 4-0 suture. In
addition, all the collateral arteries from the isolated segment
(including the lumbar artery) were ligated with a 6-0 suture. The
isolated segment was punctured using 22-GA venous indwelling needle
(BD Angiocath; BD Biosciences) and the leakproofness inside the
isolated aorta was confirmed by infusing saline solution (0.9%
NaCl). Only the rats with good leakproofness in the isolated aorta
were included in PPE or NaCl perfusion.
Perfusion pressure of porcine
pancreatic elastase
To observe the impact of perfusion pressure on AAA
morphology, the animals were randomized into four groups (10 rats
per group). The first group was named PPE-300, in which the rats
were perfused with PPE at 300 mmHg pressure. The second group was
named PPE-100 in which the rats were perfused with PPE at 100 mmHg
pressure. The third group was named PPE-0, in which the rats were
perfused with PPE without pressure. The rats in the fourth group,
which was named NaCl-300, were used as controls and perfused with
0.9% NaCl at a pressure of 300 mmHg.
The perfusion pressure of PPE or NaCl was controlled
using a pressure pump. Importantly, The PPE concentration and
perfusion time were identical among the groups; the concentration
of PPE was 47 U/ml and the perfusion time was 30 min. After
perfusion, the abdomen of the rat and the isolated aorta were
washed three times with 0.9% NaCl. The 22-GA venous indwelling
needles were subsequently removed and the puncture sites were
sutured with 7-0 Prolene™ (W8702; Johnson &
Johnson). When the blood flow was restored, the vessel diameters
were measured using a Vernier caliper (0-150 mm; Standard Gage Co.,
Inc.). Finally, the incision was closed and the rat was returned to
their cages.
Ultrasonography
The measurement of the aortic diameters was
performed using an ultrasound imaging instrument according to
previously described methods (20). To dynamically monitor the diameters
of the aneurysm, ultrasound measurements of the aorta were
performed at 7, 14 and 28 days postoperatively. In brief, the
animals were anaesthetized by isoflurane inhalation. Anesthesia was
induced with 3.5% isoflurane and was maintained using 2.0%
isoflurane. Then the animals were shaved and placed in dorsal
recumbency. Aortic imaging was performed using a Vevo®
2100 imaging system (VisualSonics, Inc.). The transducer MS-250
(13-24 MHz; VisualSonics, Inc.) was then oriented to provide axial
images from the left renal vein to the aortic bifurcation. The
maximum diameter planes were obtained for the subsequent analysis.
The images were analyzed using Vevo LAB version 1.7.1 software
(VisualSonics, Inc.) using the following formula: Dilation ratio
(%)=(aneurysmal diameter/normal aortic diameter) x100%. Normal
aortic diameters were defined as mean values corresponding to the
isolated segment. AAA was defined as a dilation ratio >150%.
Histology
At 28 days, the animals were euthanized by an
overdose of pentobarbital sodium (200 mg/kg, intraperitoneally). If
the rat's pain response disappeared and the heartbeat and
respiration stopped, the rat was judged to be dead. The abdominal
aorta tissue of rats was harvested immediately after the
administration of the anesthetic. The tissue samples were fixed in
4% methanol solution for at least 48 h at room temperature and
processed using standard procedures in graded alcohols and xylene,
and embedded in paraffin. Paraffin-embedded slices were serially
sectioned at 4 µm intervals. The slices were stained using H&E
staining and images were captured with a microscopic system (IX71;
Olympus Corporation). The maximum diameters of AAA were measured
based on H&E staining results and considered as the diameter of
the aneurysm.
Elastin degradation is an important
pathological characteristic of AAA
To observe the elastin degradation, elastic van
Gieson (EVG) staining was performed at room temperature and the
process was observed in real time under microscope (DM750; Leica
Microsystems GmbH) until the staining was successful, as previously
described (19). In addition, the
cell types in the aneurysms were identified using
immunohistochemistry (IHC). In brief, the slices were incubated
overnight at 4˚C with anti-α-smooth muscle actin (α-SMA; 1:1,000;
cat. no. ab5694; Abcam), anti-CD8 (1:500; cat. no. ab217344; Abcam)
and anti-CD68 (1:1,000; cat. no. ab213363; Abcam) primary
antibodies to identify vascular smooth muscle cells, lymphocytes
and macrophages, respectively. Signal amplification was performed
the following day using goat anti-rabbit IgG secondary antibody
(Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.; OriGene
Technologies, Inc.). Tissue images were captured using a
fluorescent inverted microscope (IX71; Olympus Corporation). The
elastin content in the abdominal aorta was analyzed using ImageJ
version 1.45 software (National Institutes of Health; https://imagej.nih.gov/ij/).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism version 8.0 (GraphPad Software, Inc.). The results are
expressed as the mean ± standard deviation. Data were analyzed
using a one-way analysis of variance followed by Tukey's highly
significant differences test for multiple comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
Operation outcomes and survival rates
of animals
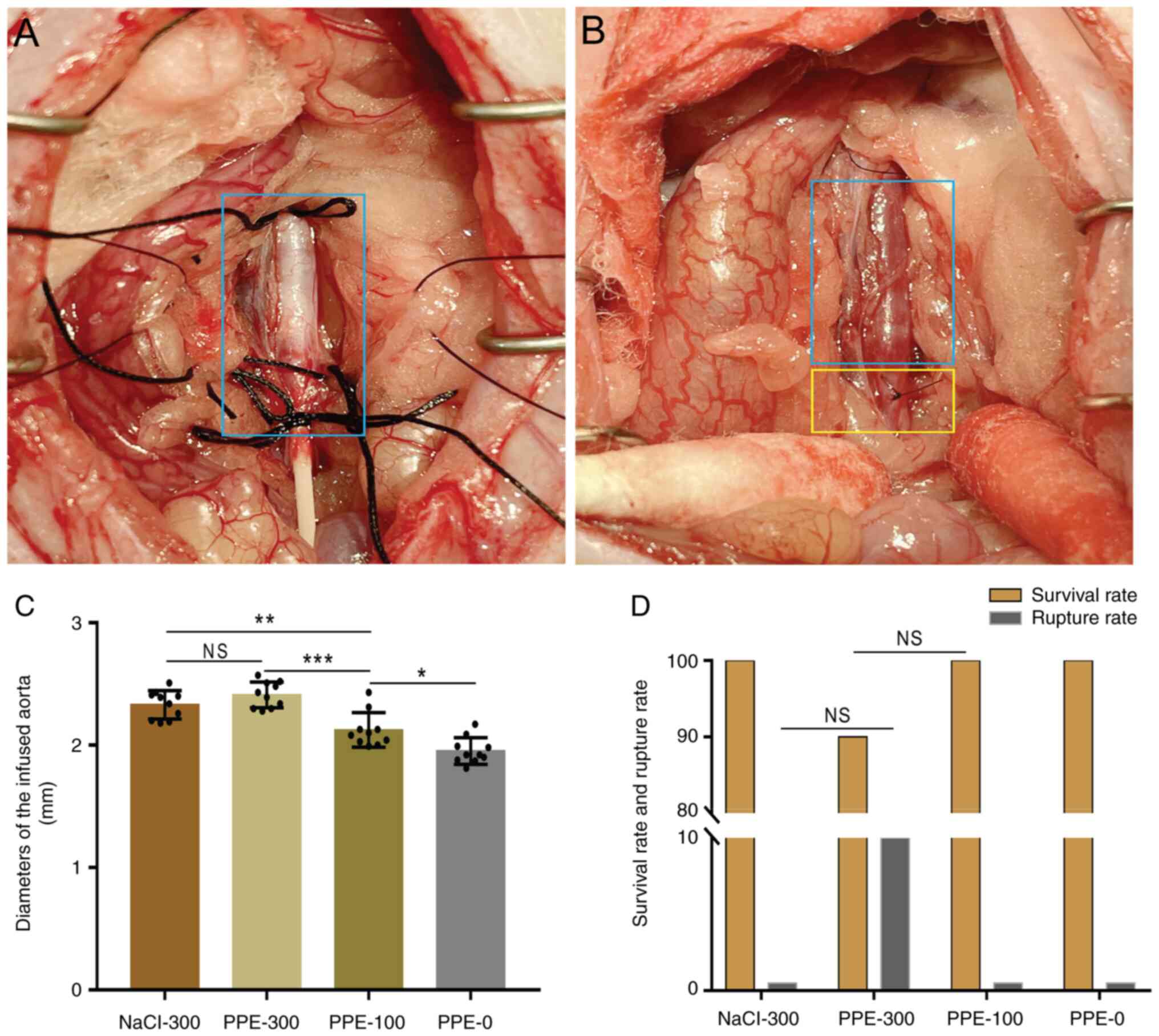
The PPE or NaCl was successfully infused in the
isolated aorta of all the experimental rats (100%) under scheduled
pressure. No aortic rupture occurred during perfusion (Fig. 1A). At the end of perfusion, the
mean diameter of the infused aorta in PPE-300 (2.41±0.01 mm) was
significantly larger than that in PPE-100 (2.12±0.21 mm) and PPE-0
(1.95±0.01 mm). The mean diameter of the infused aorta in PPE-100
was larger than that in PPE-0, while no difference was found
between PPE-300 and NaCl-300 (2.33±0.01 mm; Fig. 1B and C). Similarly, the mean diameter of the
infused aorta in NaCl-300 was larger than that in PPE-100 and
PPE-0.
During the 28-day follow-up, only one rat (10%) in
the PPE-300 group died of AAA rupture. The survival rate in
NaCl-300, PPE-300, PPE-100 and PPE-0 was 100, 90, 100 and 100%,
respectively. The rate of AAA rupture in PPE-300 was 10%, while no
AAA rupture occurred in the other three groups (Fig. 1D).
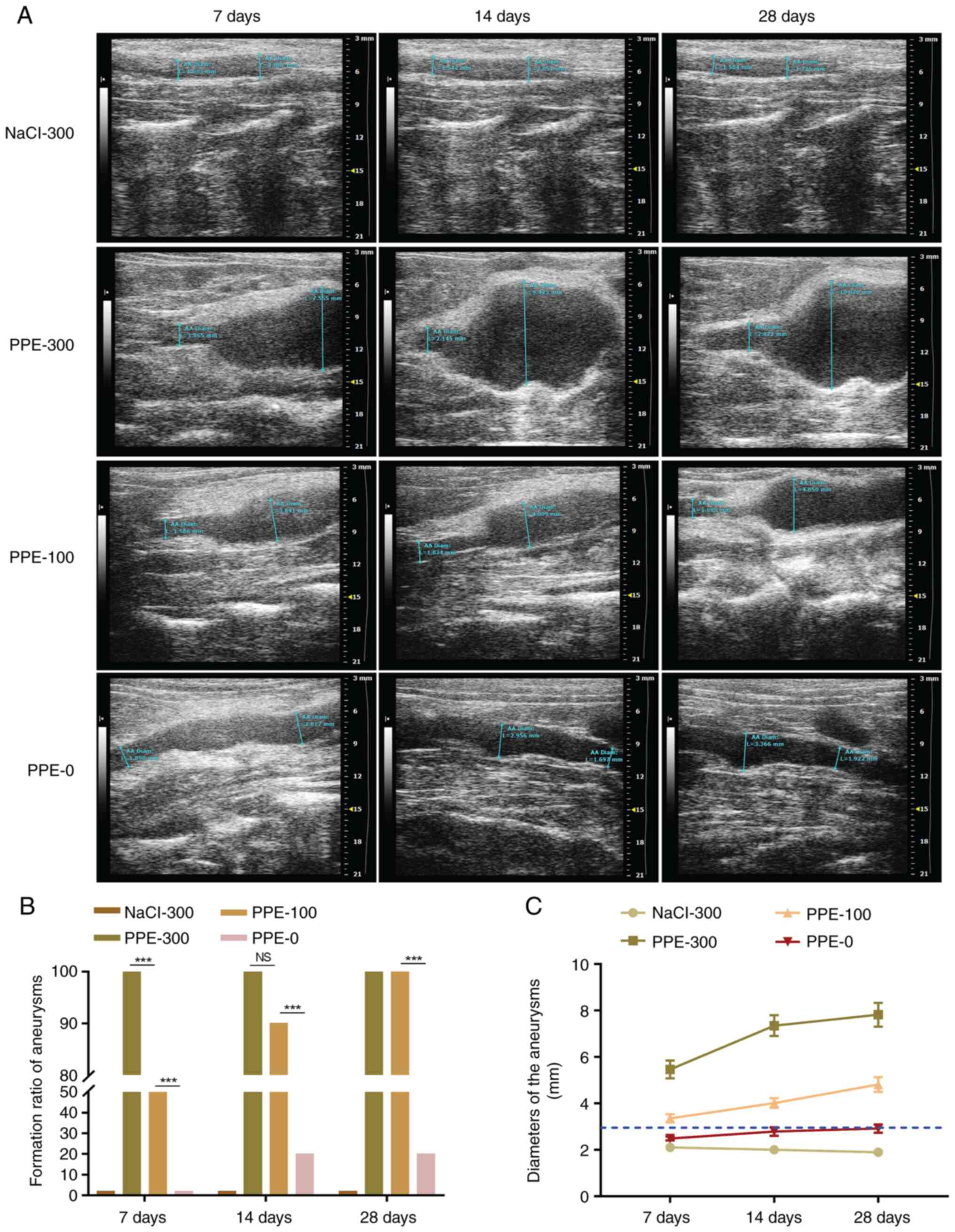
Formation ratio of AAA with time
Aortic ultrasound was performed 7, 14 and 28 days
after the operation to measure the aneurysm diameters and calculate
the formation ratio of the AAA. After 7 days, the aortic ultrasound
indicated that the diameters of the AAA increased with the
increasing of perfusion pressure (Fig.
2A). The AAA formation ratio in the PPE-300, PPE-100 and PPE-0
was 100, 50 and 0%, respectively (Fig.
2B). After 14 days, the formation ratio of AAA in PPE-100 and
PPE-0 increased to 90 and 20%, respectively, with a significant
difference between these values. After 28 days, the diameter of
isolated aorta in PPE-300, PPE-100, PPE-0 and NaCl-300 was
7.34±1.81, 4.02±0.40, 2.92±0.32 and 2.49±0.07 mm, respectively. The
AAA formation ratio in PPE-300, PPE-100 and PPE-0 was 100, 100 and
20%, respectively (Fig. 2B and
C). The formation ratio of AAA in
PPE-0 was significantly lower than that in PPE-300 and PPE-100
(P<0.001). No AAA was found in the NaCl-300 group during the
28-day follow-up.
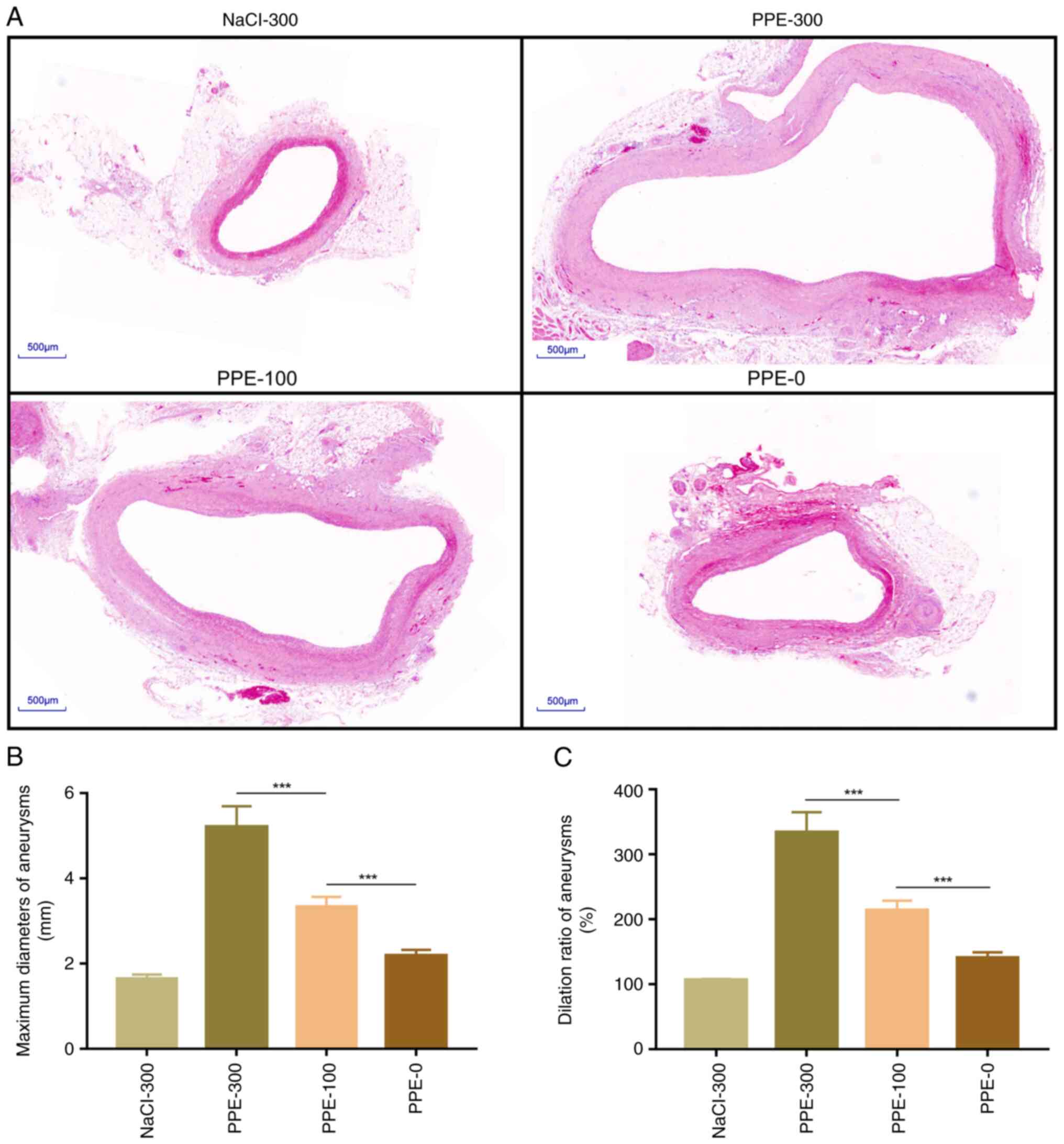
Dilation ratio of AAA based on H&E
staining
Aortic tissues were collected for histopathological
staining 28 days after the operation. The H&E staining results
showed that the normal diameter of the aorta was 1.56±0.01 mm.
According to the >150% normal diameter criterion, the diagnostic
diameter of the AAA was >2.34 mm (Fig. 3A). The maximum diameter of the AAAs
was measured and the dilation ratio was calculated based on the
H&E results. The maximum diameter in PPE-300, PPE-100 and PPE-0
and NaCl-300 was 5.21±2.14, 3.34±0.35, 2.19±0.29 and 1.68±0.03 mm,
respectively (Fig. 3B). The
maximum dilation ratio of aneurysm in NaCl-300, PPE-300, PPE-100
and PPE-0 was 106.25±0.43, 333.91±88.07, 214.29± 14.26 and
140.20±7.81%, respectively (Fig.
3C).
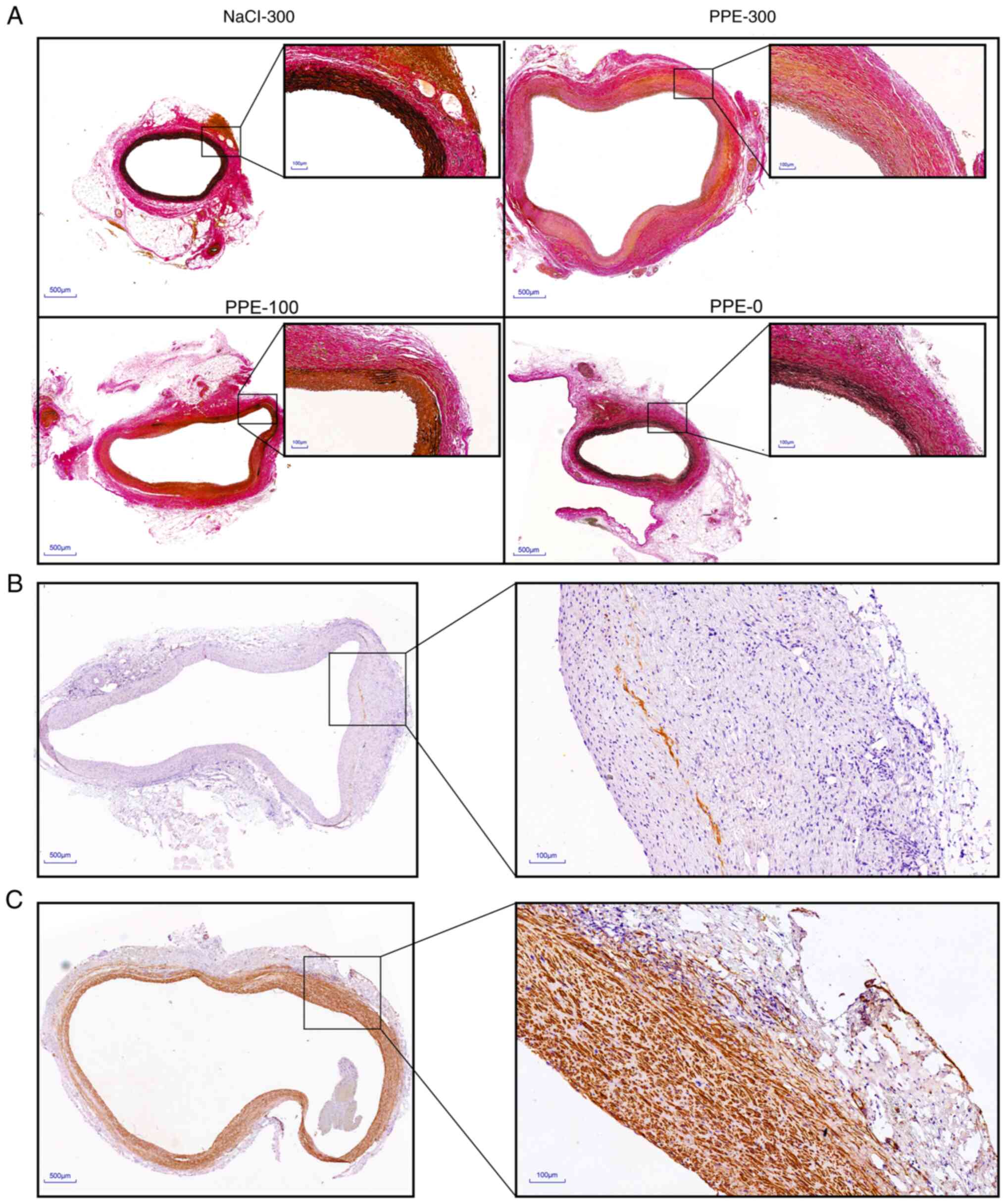
Pathological characteristics of
AAA
Elastin degradation is a pathological feature of AAA
(21). Aneurysm tissues were
obtained 28 days after surgery and EVG staining was performed to
observe the impact of elastase perfusion pressure on the elastin
degradation. The results showed that the elastin levels in PPE-300,
PPE-100, PPE-0 and NaCl-300 were 3.45±0.25, 14.54±6.31, 33.72±3.86
and 35.54±1.82%, respectively. In addition, nearly no elastin was
found in the aneurysm vessels of PPE-300 (Fig. 4A). Inflammatory cell infiltration
is an important feature of aneurysms (22). In the present study, IHC analysis
suggested that some macrophages were found in aneurysms from the
PPE-300 group and they were mainly located in the vascular
adventitia areas (Fig. 4B).
However, macrophages were not observed in the other three groups
(data not shown). Unexpectedly, some intimal hyperplasia was found
in the aneurysms in the PPE-300 group. IHC staining showed that the
cells in the intimal hyperplasia areas mainly consisted of vascular
smooth muscle cells (Fig. 4C).
Discussion
The present study explored the impact of elastase
perfusion pressure on the aneurysm morphology. Based on the present
results, two conclusions can be made. First, the elastase perfusion
pressure could impact the formation ratio of AAA at an early stage.
Second, the perfusion of elastase with high pressure could increase
the maximum diameters of AAA in rats. Moreover, increasing the
elastase perfusion pressure up to 300 mmHg did not increase the
mortality of the animals. In addition, the current findings
indicated that perfusion of elastase with high pressure represents
a method to create a considerably large AAA. The present study
contributes to refining elastase-induced AAA models and can also
help researchers to select appropriate models for their
studies.
Perfusion time and elastase concentration are
important factors affecting elastase-induced aneurysm models
(15,16). In the present study, identical
perfusion times and elastase concentrations were applied to
different groups, which contributed to the observation of the
impact of elastase perfusion pressure on the aneurysmal morphology.
The perfusion time of elastase reported in previous studies varied
from 7-120 min (23). Of course,
the perfusion time of elastase is always different in different
animal species and the commonly used perfusion time of elastase in
rats is 30 min (24). Given that
the mortality increases with the increase of perfusion time, the
perfusion time used in the present study was set at 30 min.
Elastase concentration used in previous studies varied from 5-200
U/ml (25). In the present study,
a commercial PPE solution without any dilution was used.
The elastase perfusion pressure is also influenced
by the leakproofness of the isolated aorta (26). In the present study, the lateral
branches of the isolated aorta, including the lumbar arteries, were
completely ligated before perfusion to obtain good leakproofness
and maintain perfusion pressure. Given that the normal blood
pressure of rats was about 100 mmHg, the commonly used elastase
perfusion pressure in previous studies was always 100 mmHg
(27,28). The current study compared three
different perfusion pressures of elastase including the commonly
used values (100 mmHg). The present results suggested that elastase
perfusion pressure can significantly affect aneurysm morphology.
First, the formation ratio of AAA was proportional to the elastase
perfusion pressure at 7 days postoperatively. All rats (100%) in
the high-pressure group (PPE-300) reached the criterion of aneurysm
7 days post-operation (>150% normal vessel diameter), while the
formation ratio of the aneurysm in medium-(PPE-100) and
low-pressure (PPE-0) group were 50 and 0%, respectively. In
addition, the formation ratio in medium and low perfusion pressure
showed an increasing trend, reaching 90 and 20% at 14 days,
respectively. Moreover, their formation ratios were up to 100 and
20%, respectively, 28 days after the operation. The perfusion of
elastase with high pressure could accelerate AAA formation. Second,
the elastase perfusion pressure could also impact the maximum
diameters of the aneurysm. The present findings suggested that the
maximum aneurysm diameters in the high-pressure group were
significantly larger than those in the medium- and low-pressure
groups. Similarly, the maximum diameters of aneurysms in the
medium-pressure were larger than those in the low-pressure group.
These results suggest that the perfusion of elastase with high
pressure is a potential method to create a considerably large-size
AAA. To the best of the authors' knowledge, large-size AAA models
have not been reported in the literature and these need to be
explored in the future.
In addition to aneurysm morphology, elastin
degradation and infiltration of inflammatory cells are important
characteristics of AAA (29). The
present results showed that the degree of elastin degradation was
proportional to the elastase perfusion pressure. As expected,
almost no elastic fibers were found in aneurysms from the
high-pressure group, which can be explained by the excessive
dilation of the aorta under high pressure and more elastase
permeation into vessels. Elastic fibers showed significant
degradation in the medium-pressure group (100 mmHg). At 100 mmHg,
the degree of elastin degradation in the present study was more
evident than that reported in the previous studies (30,31).
This divergence may be explained by the leakproofness of the
isolated aorta and the difference in elastase concentrations.
As for the inflammatory cells, some macrophages were
found in the vessels of the high-pressure group, which were mainly
localized in the adventitia areas, while there were almost no
inflammatory cells in the other groups. This phenomenon may be
associated with the advanced stage of AAA because there is always a
significant inflammatory reaction at the early stage of AAA
progression (32). Interestingly,
some intimal hyperplasia was found in the aneurysms in the
high-pressure group. IHC staining showed that the cells in the
intimal hyperplasia area were mainly vascular smooth muscle cells.
The present study hypothesized that the reason for the intimal
hyperplasia in these vessels may be associated with the loss of
elastin and the abnormal hemodynamic changes. A previous study
reported that aortic wall smooth muscle cell proliferation may
limit the progression of AAA (33). Therefore, the present study
hypothesized that the intimal hyperplasia in the aneurysms from the
high-pressure group may have been a protective reaction under the
abnormal hemodynamic changes.
In the present study, only one rat in the
high-pressure group underwent an aneurysm rupture and died 5 days
after the operation, which was confirmed by exploratory laparotomy.
No deaths occurred in the other three groups. The mortality rate in
the present study was lower than that reported in the literature
(34). Mortality can be associated
with several factors including the operation method, perfusion time
and elastase concentration (14).
The present study hypothesized that, given the lateral vessels of
the isolated aorta were completely ligated, there was limited
elastase flowing into the blood cycle, which contributed to a
decrease in animal mortality. In addition, the abdomen of the rats
was washed three times with saline solution after perfusion.
Similarly, the elastase solution inside the isolated aorta was also
removed and washed three times with saline solution. The operation
method was a factor that impacted animal mortality. A method
similar to that described by Hu et al (19) was used in the current study, which
can also partly explain the lower mortality.
The present study has some limitations. First, the
follow-up was 28 days. During follow-up, the elastase perfusion
pressure was found to impact the formation ratio at an early stage
and the maximum diameters of the AAAs. However, the effects of
elastase perfusion pressure on the morphology of aneurysms need to
be further explored in long-term follow-up studies. Second, an
inflammatory reaction in the early stages of AAA formation cannot
be confirmed. To continuously monitor the changes in aneurysm
diameters using non-invasive ultrasound, no aneurysm samples were
obtained during the early stages of AAA formation, which prevented
observing the possible infiltration of inflammatory cells in the
vessels. Third, the current findings were obtained in rats, if
other animals could present similar results remain to be
elucidated. Fourth, the study sample size is limited and the
current findings still need to be further confirmed by studies with
larger samples.
The diameter of the aneurysm is proportional to the
pressure of the perfusion, which more insights into the
characteristics of elastase-induced AAA models. The current
findings indicated that for some intervention studies a constant
perfusion pressure should be maintained to avoid bias in the study
results due to perfusion pressure.
Acknowledgements
The authors would like to thank Professor Laiyuan
Wang from the Key Laboratory of Cardiovascular Epidemiology &
Department of Epidemiology, Fuwai Hospital, National Center for
Cardiovascular Diseases, Chinese Academy of Medical Sciences and
Peking Union Medical College (Beijing, China) for his useful
suggestions for the present study.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81870350).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
XL performed the experiments. CQ analyzed the data.
JF prepared the manuscript. LT analyzed and interpreted the
results. YZ revised the manuscript and CS designed the study. XL
and CQ confirm the authenticity of all the raw data. All authors
were involved in discussions and commented on the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Use Committee of Fuwai Hospital (Beijing, China; no.
0099-1-8-HX).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sakalihasan N, Limet R and Defawe OD:
Abdominal aortic aneurysm. Lancet. 365:1577–1589. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chaikof EL, Dalman RL, Eskandari MK,
Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH,
Nguyen LL, et al: The society for vascular surgery practice
guidelines on the care of patients with an abdominal aortic
aneurysm. J Vasc Surg. 67:2–77.e2. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Clancy K, Wong J and Spicher A: Abdominal
aortic aneurysm: A case report and literature review. Perm J.
23(18.218)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Schanzer A and Oderich GS: Management of
abdominal aortic aneurysms. N Engl J Med. 385:1690–1698.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tchana-Sato V, Sakalihasan N and Defraigne
JO: Ruptured abdominal aortic aneurysm. Rev Med Liege. 73:296–299.
2018.PubMed/NCBI(In French).
|
|
6
|
Wang YD, Liu ZJ, Ren J and Xiang MX:
Pharmacological therapy of abdominal aortic aneurysm: An update.
Curr Vasc Pharmacol. 16:114–124. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Davis FM, Daugherty A and Lu HS: Updates
of recent aortic aneurysm research. Arterioscler Thromb Vasc Biol.
39:e83–e90. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Patelis N, Moris D, Schizas D, Damaskos C,
Perrea D, Bakoyiannis C, Liakakos T and Georgopoulos S: Animal
models in the research of abdominal aortic aneurysms development.
Physiol Res. 66:899–915. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Anidjar S, Salzmann JL, Gentric D, Lagneau
P, Camilleri JP and Michel JB: Elastase-induced experimental
aneurysms in rats. Circulation. 82:973–981. 1990.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xue C, Zhao G, Zhao Y, Chen YE and Zhang
J: Mouse abdominal aortic aneurysm model induced by perivascular
application of elastase. J Vis Exp: 10.3791/63608, 2022.
|
|
11
|
Wang Y, Krishna S and Golledge J: The
calcium chloride-induced rodent model of abdominal aortic aneurysm.
Atherosclerosis. 226:29–39. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Quintana RA and Taylor WR: Cellular
mechanisms of aortic aneurysm formation. Circ Res. 124:607–618.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sénémaud J, Caligiuri G, Etienne H,
Delbosc S, Michel JB and Coscas R: Translational relevance and
recent advances of animal models of abdominal aortic aneurysm.
Arterioscler Thromb Vasc Biol. 37:401–410. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yamaguchi T, Yokokawa M, Suzuki M,
Higashide S, Katoh Y, Sugiyama S and Misaki T: Factors influencing
mortality in the rat elastase-induced-aneurysm model. J Surg Res.
94:81–83. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nie M, Yan Y, Li X, Feng T, Zhao X, Zhang
M and Zhao Q: Effect of low-pressurized perfusion with different
concentration of elastase on the aneurysm formation rate in the
abdominal aortic aneurysm model in rabbits. Biomed Res Int.
2016(6875731)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Busch A, Chernogubova E, Jin H, Meurer F,
Eckstein HH, Kim M and Maegdefessel L: Four surgical modifications
to the classic elastase perfusion aneurysm model enable
haemodynamic alterations and extended elastase perfusion. Eur J
Vasc Endovasc Surg. 56:102–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamaguchi T, Yokokawa M, Suzuki M,
Higashide S, Katoh Y, Sugiyama S and Misaki T: Shortened elastase
infusion time in the elastase-induced rat aneurysm model. J Surg
Res. 85:158–162. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu Z, Wang Q, Ren J, Assa CR, Morgan S,
Giles J, Han Q and Liu B: Murine abdominal aortic aneurysm model by
orthotopic allograft transplantation of elastase-treated abdominal
aorta. J Vasc Surg. 62:1607–1614.e2. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hu G, Dong Z and Fu W: A novel
modification of the murine elastase infusion model of abdominal
aortic aneurysm formation. Ann Vasc Surg. 42:246–253.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Knipp BS, Ailawadi G, Sullivan VV, Roelofs
KJ, Henke PK, Stanley JC and Upchurch GR Jr: Ultrasound measurement
of aortic diameters in rodent models of aneurysm disease. J Surg
Res. 112:97–101. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Suh MK, Batra R, Carson JS, Xiong W, Dale
MA, Meisinger T, Killen C, Mitchell J and Baxter BT: Ex vivo
expansion of regulatory T cells from abdominal aortic aneurysm
patients inhibits aneurysm in humanized murine model. J Vasc Surg.
72:1087–1096.e1. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu B, Granville DJ, Golledge J and
Kassiri Z: Pathogenic mechanisms and the potential of drug
therapies for aortic aneurysm. Am J Physiol Heart Circ Physiol.
318:H652–H670. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Azuma J, Asagami T, Dalman R and Tsao PS:
Creation of murine experimental abdominal aortic aneurysms with
elastase. J Vis Exp. (1280)2009.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Daugherty A and Cassis LA: Mouse models of
abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol.
24:429–434. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Munezane T, Hasegawa T, Suritala Tanaka A,
Okada K and Okita Y: Activation of transglutaminase type 2 for
aortic wall protection in a rat abdominal aortic aneurysm
formation. J Vasc Surg. 52:967–974. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu R, Huang J, Ge Y, Liu S, Huang T, Cai
H, Pan B, Zhang Q, Yang P, Liao M, et al: Inhibition of
phosphatidylinositol 3-kinase γ by IPI-549 attenuates abdominal
aortic aneurysm formation in mice. Eur J Vasc Endovasc Surg.
60:254–263. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yu J, Liu R, Huang J, Wang L and Wang W:
Inhibition of phosphatidylinositol 3-kinease suppresses formation
and progression of experimental abdominal aortic aneurysms. Sci
Rep. 7(15208)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wen H, Wang M, Gong S, Li X, Meng J, Wen
J, Wang Y, Zhang S and Xin S: Human umbilical cord mesenchymal stem
cells attenuate abdominal aortic aneurysm progression in
sprague-dawley rats: Implication of vascular smooth muscle cell
phenotypic modulation. Stem Cells Dev. 29:981–993. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang Y, Jia L, Xie Y, Cai Z, Liu Z, Shen
J, Lu Y, Wang Y, Su S, Ma Y and Xiang M: Involvement of
macrophage-derived exosomes in abdominal aortic aneurysms
development. Atherosclerosis. 289:64–72. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fan Y, Li N, Liu C, Dong H and Hu X:
Excessive methionine supplementation exacerbates the development of
abdominal aortic aneurysm in rats. J Vasc Res. 56:230–240.
2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lu H, Du W, Ren L, Hamblin MH, Becker RC,
Chen YE and Fan Y: Vascular smooth muscle cells in aortic aneurysm:
From genetics to mechanisms. J Am Heart Assoc.
10(e023601)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sinha I, Sinha-Hikim AP, Hannawa KK, Henke
PK, Eagleton MJ, Stanley JC and Upchurch GR Jr:
Mitochondrial-dependent apoptosis in experimental rodent abdominal
aortic aneurysms. Surgery. 138:806–811. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hoshina K, Koyama H, Miyata T, Shigematsu
H, Takato T, Dalman RL and Nagawa H: Aortic wall cell proliferation
via basic fibroblast growth factor gene transfer limits progression
of experimental abdominal aortic aneurysm. J Vasc Surg. 40:512–518.
2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lewis DA, Ding YH, Dai D, Kadirvel R,
Danielson MA, Cloft HJ and Kallmes DF: Morbidity and mortality
associated with creation of elastase-induced saccular aneurysms in
a rabbit model. AJNR Am J Neuroradiol. 30:91–94. 2009.PubMed/NCBI View Article : Google Scholar
|