Introduction
Colorectal cancer is one of the most prevalent types
of cancer and the leading contributor of cancer-associated
mortality in both men and women (1). It has been estimated that colorectal
cancer resulted in 104,610 new cases and >50,000 deaths in the
United States in 2020(2). In
particular, colon cancer is considered to be a single disease
entity compared with colorectal cancer and has unique
characteristics in terms of treatment, prognostic and metastatic
profiles (3,4). According to data released by the
American Cancer Society, the incidence rate of colon cancer has
reached 10.2% whereas the mortality is ~9.2%, making it one of the
most common malignant tumors in the USA (5,6). A
previous study has suggested that the risk factors in the etiology
and pathogenesis of colon cancer include unhealthy diet, obesity,
inadequate physical activity, microbiome and family history
(7). Although substantial progress
has been made in terms of novel surgical and therapeutic strategies
for this cancer, in addition to the development of targeted
treatment methods, the overall survival rate of patients with
advanced stages of colon cancer remains low and the prognosis
remains poor (8,9). Therefore, exploration of independent
biomarkers involved in the progression of colon cancer is urgently
required.
Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase
activation protein β (YWHAB), also known as 14-3-3β/α, is a member
of the highly conserved 14-3-3 protein family (10,11).
It has been documented to regulate various cellular processes,
including cell proliferation, signal transduction, cell cycle and
apoptosis (10,11). Downregulation of YWHAB has been
reported to impart suppressive effects on the proliferation of
cervical and gastric cancer cells (12,13).
Additionally, YWHAB was previously found to be upregulated in colon
cancer cells, which is in turn associated with poorer prognosis
(14). However, the detailed role
of YWHAB in the malignant phenotypes of colon cancer and its
potential regulatory mechanism remain poorly understood.
The PI3K regulatory subunit 2 (PIK3R2) gene has been
previously identified to be an activator of the PI3K/AKT signaling
pathway in the regulation of cell proliferation and apoptosis of
various cancer types, including bladder cancer, non-small-cell lung
cancer and glioma (15,16). Accumulated evidence has
demonstrated that the downstream component of PIK3R2, AKT, can
regulate the invasion, migration and division of cancer cells,
including endometrial cancer, breast cancer and lung cancer cells
(17,18). In addition, PIK3R2 encodes p85β,
the expression of which was found to be upregulated in colon cancer
(19). Therefore, it was
hypothesized that YWHAB may regulate the malignant progression of
colon cancer by binding to PIK3R2 protein.
The present study was designed to unravel the
functional role of YWHAB in cell proliferation, cell cycle
progression and apoptosis in colon cancer, in addition to examining
its associated regulatory mechanism.
Materials and methods
Bioinformatics tools
GEPIA database version 2 (http://gepia.cancer-pku.cn/) analysis was used to
examine YWHAB expression in colon cancer tissues. The information
was obtained from The Cancer Genome Atlas normal and
Genotype-Tissue Expression data (|Log2 fold change| cutoff, 1;
q-value cutoff, 0.01) and analyzed using one-way ANOVA with Tukey's
post hoc test. The ‘COAD’ dataset corresponding to colon cancer was
used. In the Monarch Initiative database 2020 (https://monarchinitiative.org/), the term ‘YWHAB’
was searched and 560 interaction associations were obtained. Among
the 560 interaction associations, a high throughput protein
(PIK3R2) was identified based on affinity chromatography evidence
analysis data included in the database.
Cell culture
The human intestinal epithelial cell line HIEC-6 and
the DiFi colon cancer cell line were provided by BioVector NTCC,
Inc., whilst human colon cancer cell lines HCT116, HCT8 and SW620
were obtained from American Type Culture Collection. All cell lines
were incubated in DMEM (MilliporeSigma) at 37˚C with 5%
CO2. The medium was supplemented with 10% FBS
(MilliporeSigma) and 1% penicillin/streptomycin
(MilliporeSigma).
Cell transfection
HCT116 cells (3x105 cells/well) were
inoculated onto six-well plates and cultured for 24 h at 37˚C with
5% CO2. Short hairpin RNA (shRNA) sequences specific to
YWHAB (shRNA-YWHAB-1; 5'-GGCTGAGCGATATGATGATAT-3' and
shRNA-YWHAB-2, 5'-TGCAGCCTACACACCCAATTC-3') were inserted into the
lentivirus expression plasmid pGCSIL-GFP, with shRNA-NC (sequence,
5'-TTCTCCGAACGTGTCACGT-3') as the corresponding negative control.
The plasmids were obtained from Sangon Biotech Co., Ltd. pcDNA3.1
containing the PIK3R2 gene (Ov-PIK3R2) were utilized to overexpress
PIK3R2 (accession no. NM_005027.4) whereas empty vector was used as
the negative control (Ov-NC), which were provided by Shanghai
GenePharma Co., Ltd. Following incubation, HCT116 cells were
transfected with shRNA-NC (25 nM), shRNA-YWHAB-1/2 (25 nM), Ov-NC
(50 nM) and Ov-PIK3R2 (50 nM), respectively, at 37˚C for 48 h using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). In another experiment, HCT116 cells were
co-transfected with shRNA-YWHAB (25 nM) and Ov-PIK3R2 (50 nM) at
37˚C for 48 h using Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). After 48 h, the cells
were harvested for other assays. Throughout all experiments, the
cells in the Control group were un-transfected HCT116 cells.
Reverse transcription-quantitative PCR
(RT-qPCR)
After the isolation of total RNA from the indicated
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), cDNA was synthesized through reverse
transcription using the iScript Reverse Transcription Supermix Kit
(Bio-Rad Laboratories, Inc.) according to the manufacturer's
protocols. Subsequently, SYBR® Premix Ex Taq™ reagent
(Takara Bio, Inc.) was applied to perform qPCR on an ABI 7500
quantitative PCR instrument (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The following thermocycling conditions were used
for qPCR: Initial denaturation at 95˚C for 10 min; followed by 40
cycles of 95˚C for 10 sec and 60˚C for 60 sec. The
2-ΔΔCq method was applied to determine the relative gene
expression with GAPDH serving as the endogenous reference (20). The following primer sequences were
used: YWHAB forward, 5'-CATGAAGGCAGTCACAGAACA-3' and reverse,
5'-CTCACGGTACTCTTTGCCCAT-3'; PIK3R2 forward,
5'-AAAGGCGGGAACAATAAGCTG-3' and reverse,
5'-CAACGGAGCAGAAGGTGAGTG-3' and GAPDH forward, 5'-
TGTGGGCATCAATGGATTTGG-3' and reverse,
5'-ACACCATGTATTCCGGGTCAAT-3'.
Western blotting
The concentration of proteins was quantified using a
BCA protein assay kit (Thermo Fisher Scientific, Inc.) after the
extraction of total proteins from the indicated cells using RIPA
lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.).
Proteins (50 µg) were loaded per lane, separated by 8% SDS-PAGE and
then transferred onto PVDF membranes. Subsequently, membranes were
blocked with 5% skim milk for 1 h at room temperature, followed by
the overnight incubation of membranes with primary antibodies
against YWHAB (cat. no. ab32560; 1:1,000; Abcam), PIK3R2 (cat. no.
ab180967; 1:2000; Abcam), p21 (cat. no. ab109520; 1:1,000; Abcam),
cyclin D1 (cat. no. 26939-1-AP; 1:1,000; ProteinTech Group, Inc.),
Bcl-2 (cat. no. ab32124; 1:1,000; Abcam), Bax (cat. no. ab32503;
1:1,000; Abcam), phosphorylated (p-) PI3K (cat. no. ab278545;
1:1,000; Abcam), PI3K (cat. no. ab32089; 1:1,000; Abcam), p-AKT
(cat. no. ab81283; 1:5,000; Abcam), AKT (cat. no. ab8805; 1:500;
Abcam) or GAPDH (cat. no. ab9485; 1:2,500; Abcam) at 4˚C. GAPDH was
used as a loading control for normalization. On the next day, the
membranes were incubated with HRP-labeled Goat Anti-Rabbit
secondary antibodies (cat. no. ab6721; 1:2,000; Abcam) for another
2 h at room temperature. Finally, the proteins were visualized
using the ECL Detection Reagent (Shanghai Yeasen Biotechnology Co.,
Ltd.). ImageJ software (version 1.41; National Institutes of
Health) was used to semi-quantify the bands of each protein.
Cell Counting Kit-8 (CCK-8) assay
HCT116 cells were seeded into 96-well plates at a
density of 5x103 cells and incubated for 24 h at 37˚C.
Subsequently, 10 µl CCK-8 solution (Dojindo Molecular Technologies,
Inc.) was added into each well for further incubation at 37˚ with
5% CO2 for 4 h. Finally, the absorbance was determined
using a microplate reader (λ=450 nm).
Flow cytometry
A total of 1x106 HCT116 cells were seeded
into 24-well plates for incubation at 37˚C. When the cells grew to
cover ~85% of the flask, they were harvested using trypsin without
EDTA, centrifuged (5 min; 175 x g) at room temperature and washed
twice with 3 ml PBS. Ethanol (70%) was used for cell fixation for 1
h at 4˚C. Cells were then centrifuged at 850 x g for 5 min at room
temperature and washed twice with 3 ml PBS. Subsequently, 50 µg/ml
PI (Thermo Fisher Scientific, Inc.) and 100 µg/ml RNase A (Thermo
Fisher Scientific, Inc.) were added to the 1x106 cells
in a 100 µl cell suspension and incubated at 4˚C for 30 min.
Finally, cell cycle was evaluated by flow cytometry (CytoFLEX;
Beckman Coulter, Inc.) with the Flowjo 10 (FlowJo LLC) analysis
software.
TUNEL
A Click-iT™ Plus TUNEL assay kit (cat. no. C10617;
Invitrogen; Thermo Fisher Scientific, Inc.) was used to detect cell
apoptosis using standard protocols. After rinsing with PBS three
times, HCT116 cells were fixed with 4% paraformaldehyde for 15 min
at room temperature and permeabilized with 0.25% Triton-X 100 for
20 min at room temperature. TUNEL and FITC-deoxyuridine
triphosphate solution (Roche Diagnostics GmbH) were added to the
cells, which were incubated at 37˚C for 60 min in the dark. DAPI
(0.5 µg/ml; Beijing Solarbio Science & Technology Co., Ltd.)
was used to stain the nuclei for 5 min at room temperature and the
cells were mounted in an anti-fade reagent (Beijing Solarbio
Science & Technology Co., Ltd.). Finally, images of the
positive apoptotic cells in a total of five fields of view per
sample were captured using a fluorescence microscope
(magnification, x200).
Co-immunoprecipitation (Co-IP)
assay
Total proteins from HCT116 cells were isolated using
the RIPA lysis buffer (Beyotime Institute of Biotechnology) and
quantified using BCA kits (Beyotime Institute of Biotechnology).
The supernatant was centrifuged at 14,000 x g at 4˚C for 10 min to
obtain whole-cell extracts. Subsequently, 400 µl whole-cell
extracts (2x106 cells) were preincubated with 25 µg
magnetic beads on a rotator for 2 h at 4˚C to clear non-specific
bead binding. For immunoprecipitation, the extracts were incubated
with 2 µg YWHAB (cat. no. A71754-050; 1:100; EpiGentek Group, Inc.)
or PIK3R2 (cat. no. ab180967; 1:80; Abcam) antibodies overnight at
4˚C. Afterwards, 50 µg Protein G/A agarose beads (cat. no. 88803;
Invitrogen; Thermo Fisher Scientific, Inc.) and 80 µl lysate were
added, followed by incubation for 4 h at 4˚C. Following rinsing
with PBS, the beads were resuspended in 5X SDS-PAGE loading buffer
and boiled for 5 min at 100˚C to release the protein from the beads
by centrifugation at 2,000 x g for 1 min at 4˚C. Western blotting
was then performed on these immunoprecipitation products to detect
PIK3R2 and YWHAB.
Statistical analysis
All experiments were repeated at least three times.
All data collected from experiments are presented as the mean ±
standard deviation and were analyzed using the GraphPad Prism 8.0
software (GraphPad Software, Inc.). One-way ANOVA with Tukey's post
hoc test was applied to assess differences among different groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
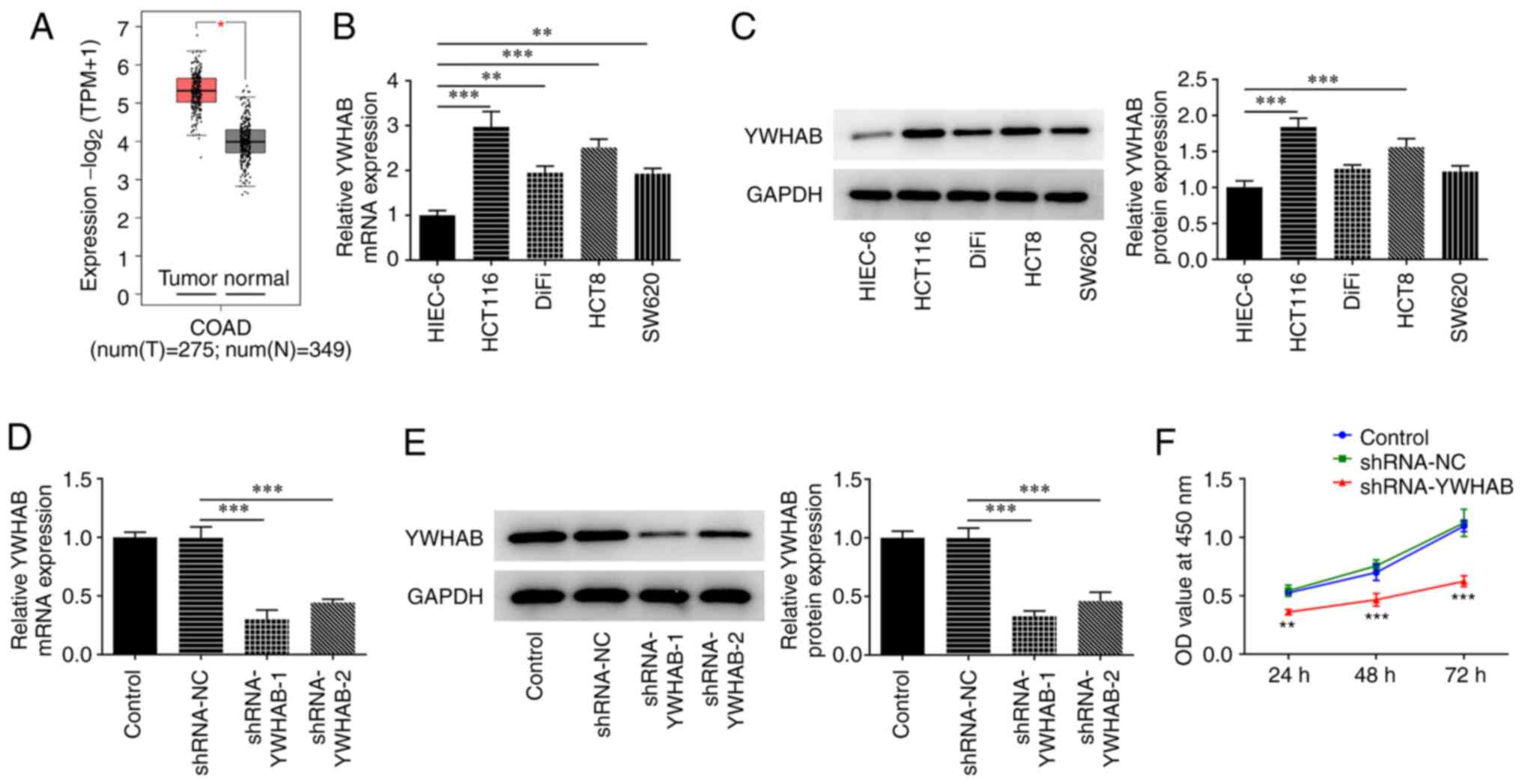
YWHAB expression is increased in colon
cancer cells but knocking it down inhibits cell proliferation
The GEPIA database revealed that the expression of
YWHAB in colon cancer tissues was increased compared with that in
normal tissues from healthy controls (Fig. 1A). YWHAB expression was examined at
both mRNA and protein levels by RT-qPCR and western blotting,
respectively. As shown in Fig. 1B
and C, the mRNA and protein
expression levels of YWHAB were markedly increased in the majority
of the colon cancer cell lines tested compared with those in the
HIEC-6 human intestinal epithelial cell line. In particular, HCT116
cells exhibited the highest expression levels of YWHAB among all
colon cancer cell lines analyzed in the present study. Therefore,
HCT116 cells were selected for subsequent experiments. To silence
YWHAB expression, shRNA-YWHAB were transfected into HCT116 cells.
RT-qPCR and western blotting were then performed to evaluate
transfection efficacy. Compared with those in cells transfected
with shRNA-NC, the mRNA and protein expression levels of YWHAB were
significantly decreased in cells transfected with shRNA-YWHAB
(Fig. 1D and E). shRNA-YWHAB-1 exhibited superior
transfection efficacy, as demonstrated by markedly lower expression
levels of YWHAB in HCT116 cells compared with those in the
shRNA-YWHAB-2 group (Fig. 1D and
E). The effect of YWHAB knockdown
on the proliferation of HCT116 cells was next assessed using a
CCK-8 assay. The results revealed that the proliferation of HCT116
cells was significantly reduced at 24, 48 and 72 h after
transfection with shRNA-YWHAB compared with that in cells
transfected with shRNA-NC (Fig.
1F).
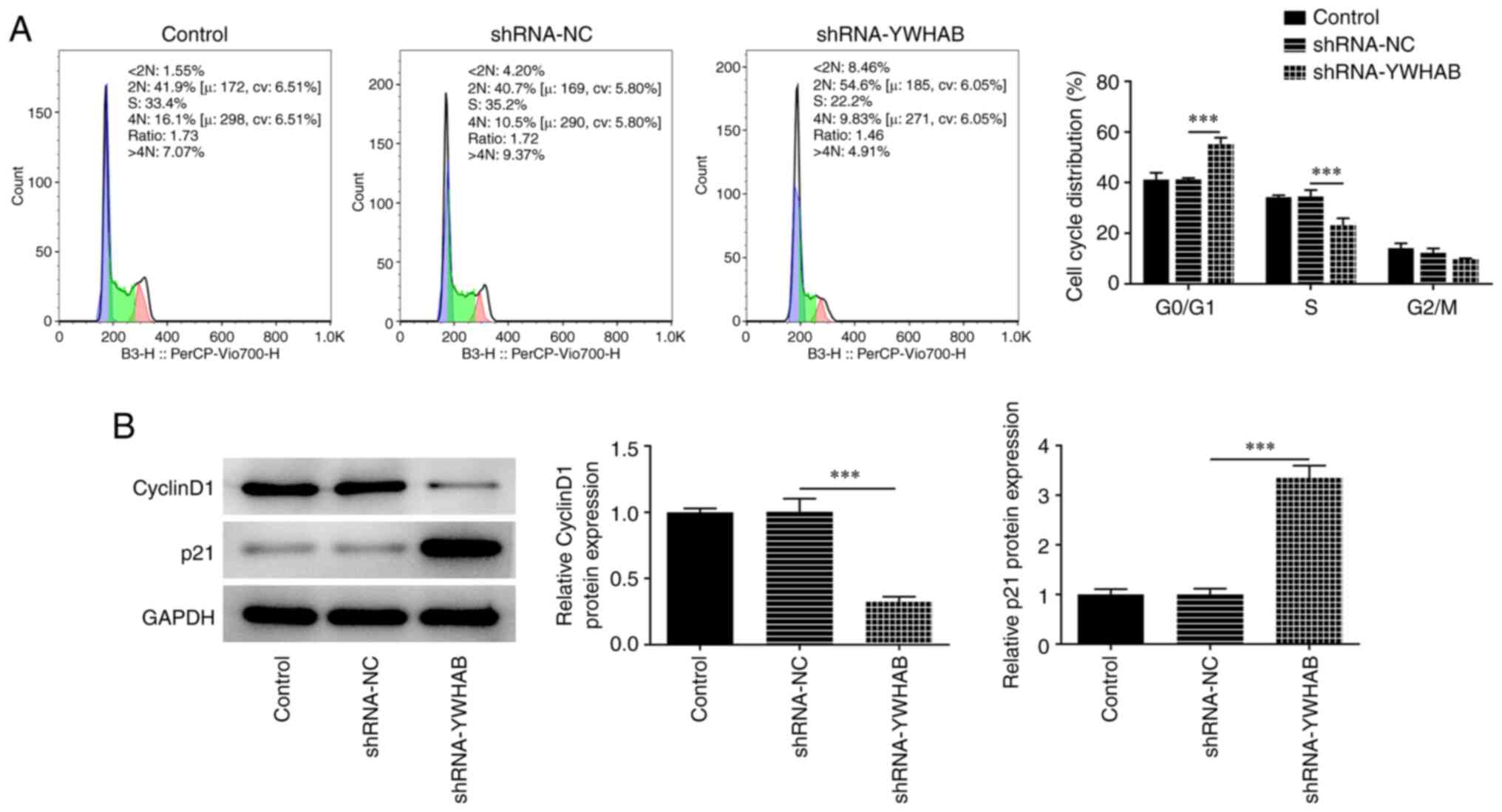
YWHAB knockdown promotes cell cycle
arrest in colon cancer cells at the G0/G1
phase
Compared with those in the shRNA-NC group, the
numbers of cells in the G0/G1 phase were
significantly elevated whilst the numbers of cells at the S and
G2/M phases were markedly reduced after knocking down
YWHAB expression in HCT116 cells (Fig.
2A). Subsequently, western blotting was used to determine the
expression levels of the G1-S cell-cycle transition
regulator cyclin D1 and G1-checkpoint CDK inhibitor p21,
where the results demonstrated that cyclin D1 protein expression
was significantly decreased, while p21 protein expression was
notably increased in the YWHAB knockdown group (Fig. 2B). These aforementioned results
suggest that YWHAB knockdown can induce cell cycle arrest in colon
cancer cells.
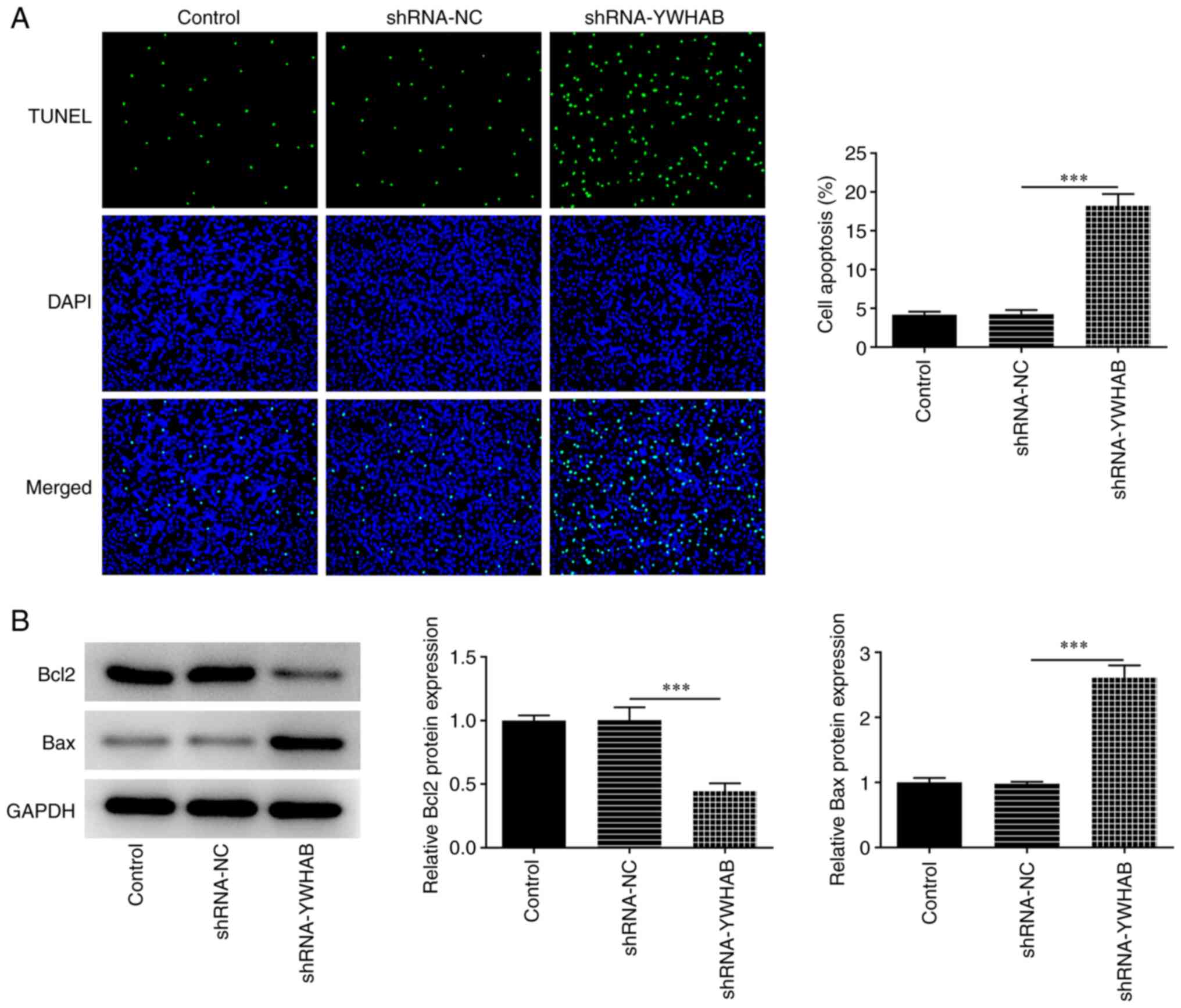
YWHAB knockdown promotes cell
apoptosis
YWHAB knockdown significantly enhanced the apoptosis
level of HCT116 cells compared with that in the shRNA-NC group,
thus suggesting that YWHAB knockdown exerted apoptotic effects on
HCT116 cells (Fig. 3A).
Additionally, the expression of apoptosis regulators were
determined by western blotting. The results revealed that Bcl2
expression was significantly decreased whilst Bax expression was
significantly increased in HCT116 cells after transfection with
shRNA-YWHAB compared with those in the shRNA-NC group (Fig. 3B).
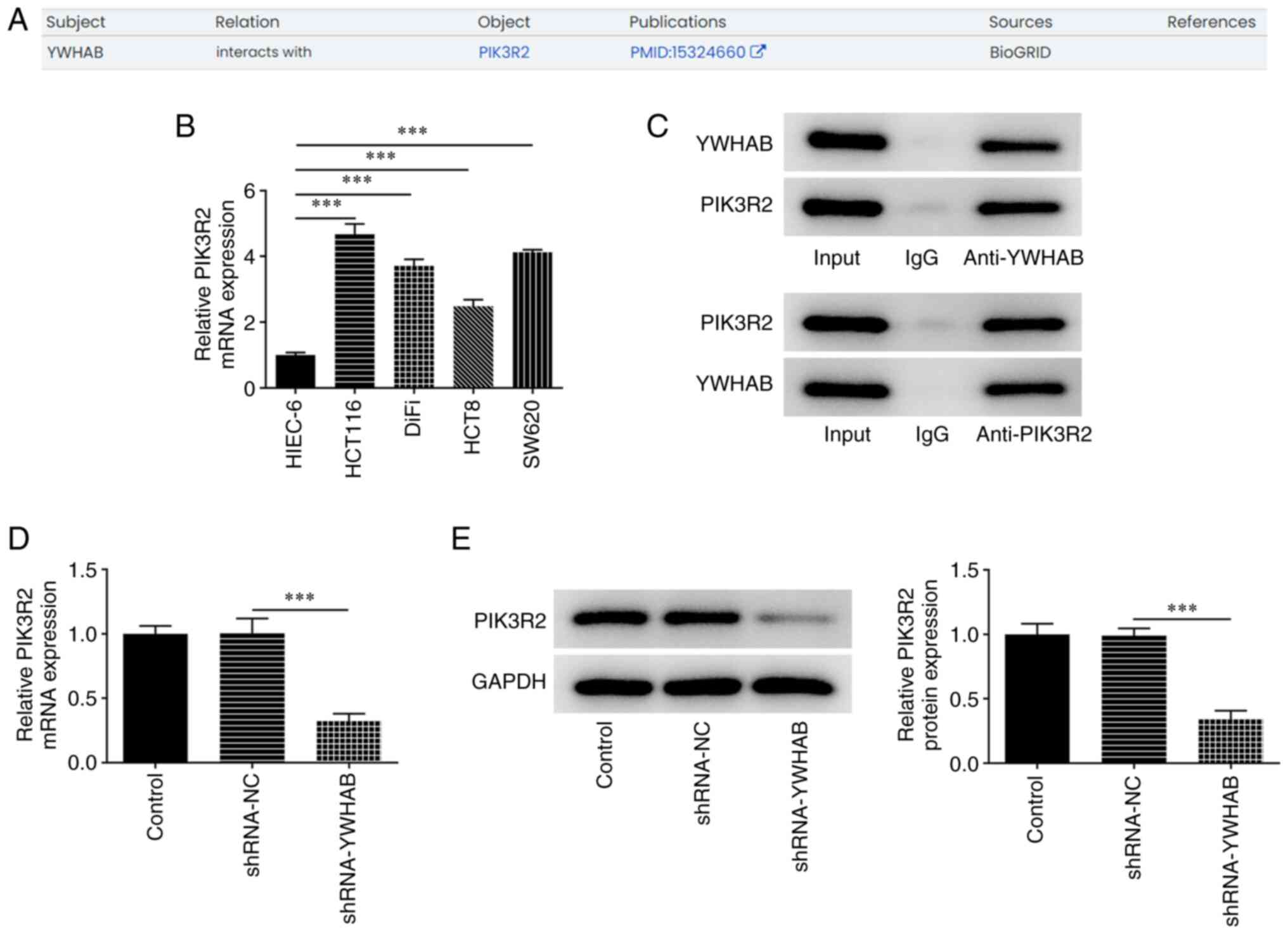
YWHAB can bind to PIK3R2 in colon
cancer cells
The Monarch Initiative database predicted that YWHAB
can interact with PIK3R2 (Fig.
4A). The results in Fig. 4B
suggested that the mRNA expression levels of PIK3R2 were increased
in colon cancer cell lines compared with those in HIEC-6 cells. The
results of Co-IP assay demonstrated that PIK3R2 was particularly
enriched in lysate samples incubated with the anti-YWHAB antibody
(Fig. 4C). In addition, the
expression levels of PIK3R2 in HCT116 cells were significantly
decreased after transfection with shRNA YWHAB, compared with those
in the shRNA-NC group (Fig. 4D and
E), suggesting that YWHAB
positively regulated PIK3R2 expression in colon cancer.
YWHAB regulates cell proliferation and
cell cycle arrest in colon cancer cells by binding to PIK3R2
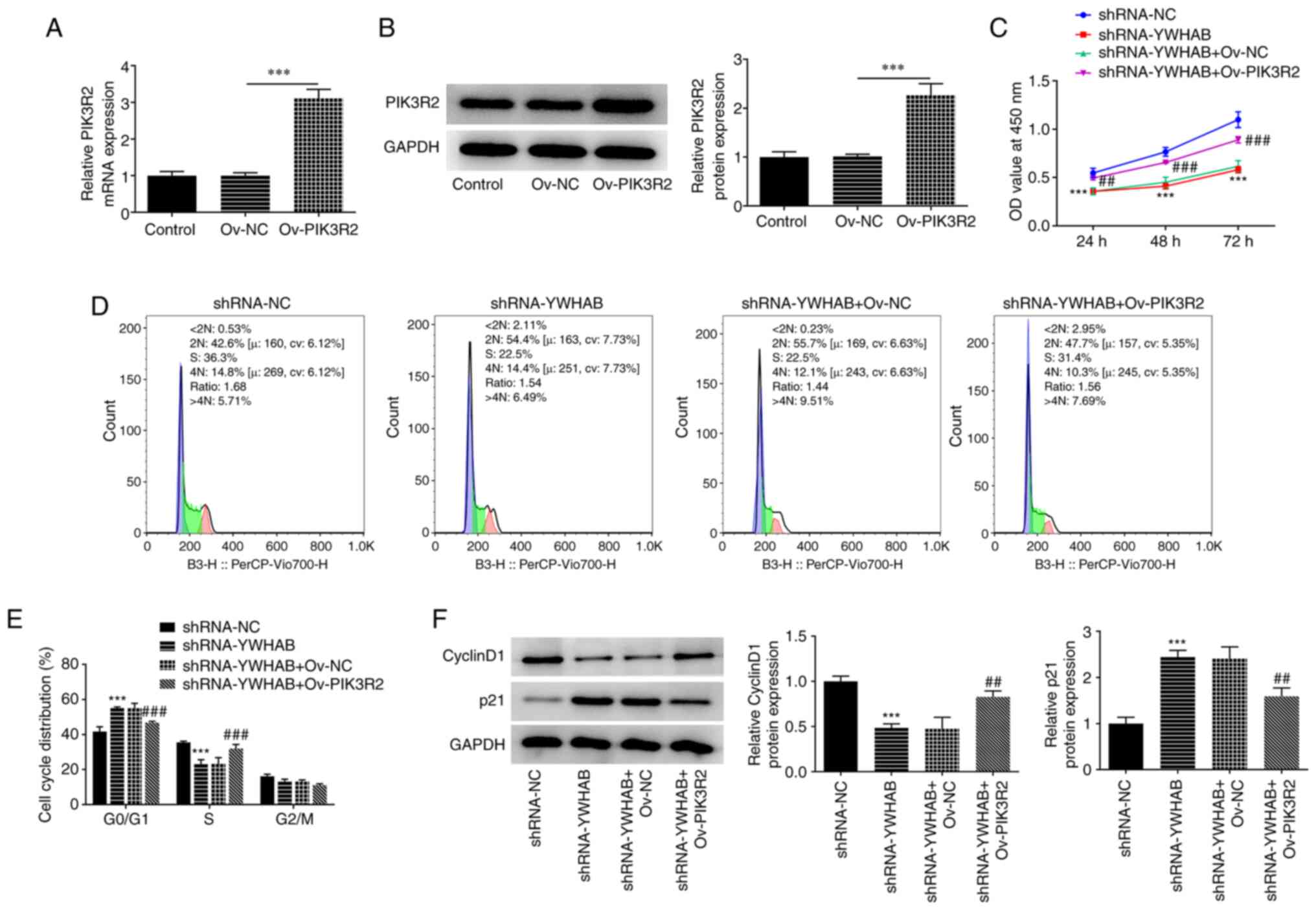
To detect overexpression efficiency, RT-qPCR and
western blotting were applied to examine the mRNA and protein
expression levels of PIK3R2. As shown in Fig. 5A and B, PIK3R2 expression at both mRNA and
protein levels was significantly enhanced after HCT116 cells were
transfected with the Ov-PIK3R2 plasmids compared with that in the
Ov-NC group. The reduction in the proliferation of YWHAB-silenced
HCT116 cells was significantly reversed by PIK3R2 overexpression,
suggesting that PIK3R2 overexpression exerted rescue effects on the
proliferation of YWHAB-depleted HCT116 cells (Fig. 5C). Compared with that in the
control group (shRNA-NC-transfected HCT116 cells), YWHAB
downregulation increased the number of cells at the
G0/G1 phase but decreased the number of cells
in S phase, which was partially reversed after transfection with
the Ov-PIK3R2 plasmids compared with those in the shRNA-YWHAB +
Ov-NC group (Fig. 5D and E). Furthermore, YWHAB knockdown decreased
cyclin D1 expression and increased p21 expression, to both of which
PIK3R2 overexpression exerted significant oppositive effects
(Fig. 5F). Significantly increased
cyclin D1 expression and decreased p21 expression were observed in
the shRNA-YWHAB + Ov-PIK3R2 group compared with those in the
shRNA-YWHAB + Ov-NC group (Fig.
5F). These aforementioned results suggest that YWHAB regulates
cell proliferation and cell cycle arrest in colon cancer cells by
binding to PIK3R2.
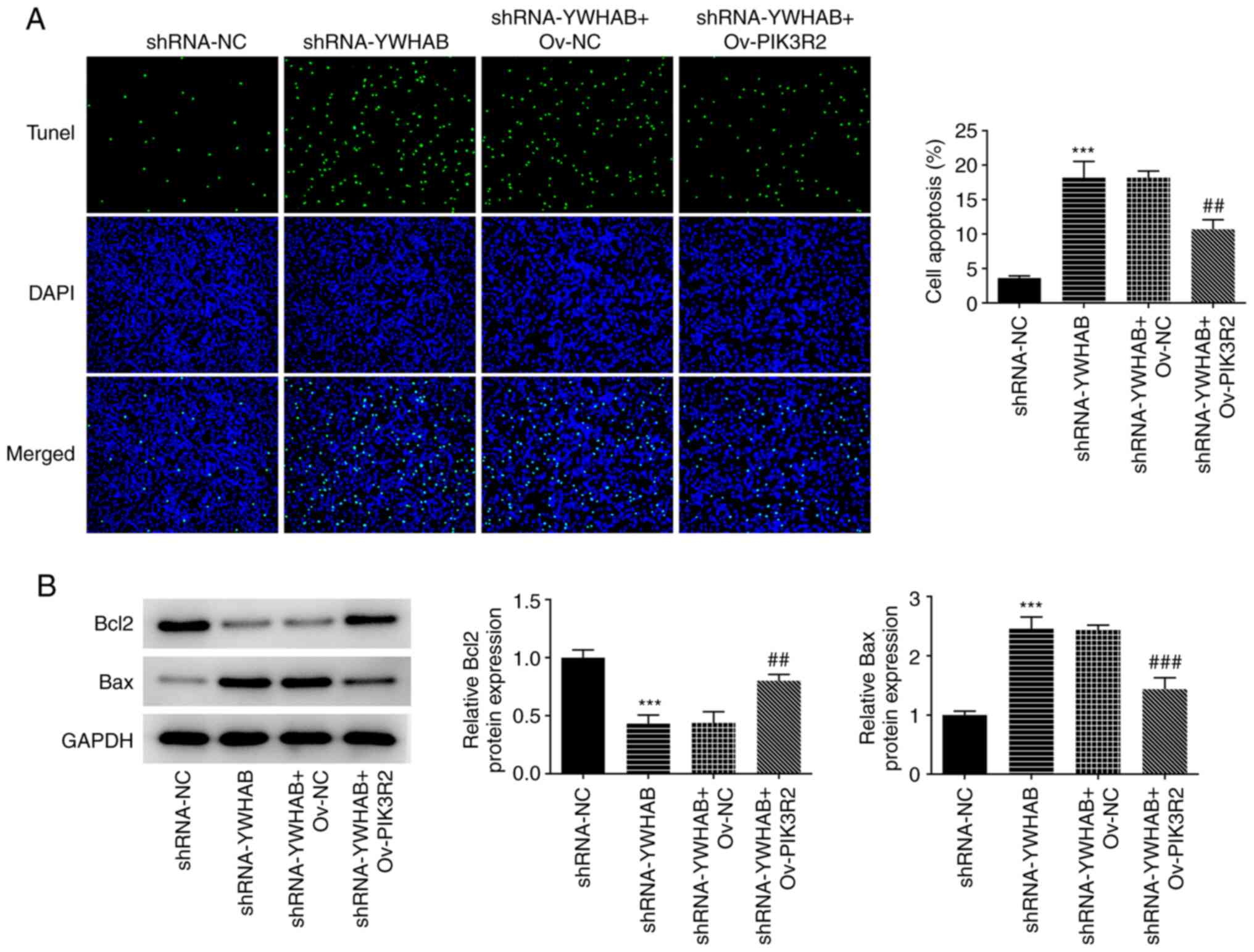
YWHAB regulates apoptosis in colon
cancer cells by binding to PIK3R2
Compared with that of cells in the control group
(shRNA-NC-transfected HCT116 cells), the apoptosis of HCT116 cells
was significantly promoted by YWHAB knockdown, which was then
partially but significantly reversed by the overexpression of
PIK3R2 (Fig. 6A). In addition,
YWHAB knockdown decreased Bcl2 expression but enhanced Bax
expression compared with those in the control group
(shRNA-NC-transfected HCT116 cells) (Fig. 6B). However, the effects of YWHAB
knockdown on the expression of these apoptosis regulators were
significantly reversed by PIK3R2 overexpression. Significantly
increased Bcl2 expression and decreased Bax expression were both
observed in the shRNA-YWHAB + Ov-PIK3R2 group compared with those
in the shRNA-YWHAB + Ov-NC group (Fig.
6B). Therefore, these aforementioned results suggest that YWHAB
regulates apoptosis in colon cancer cells by binding to PIK3R2.
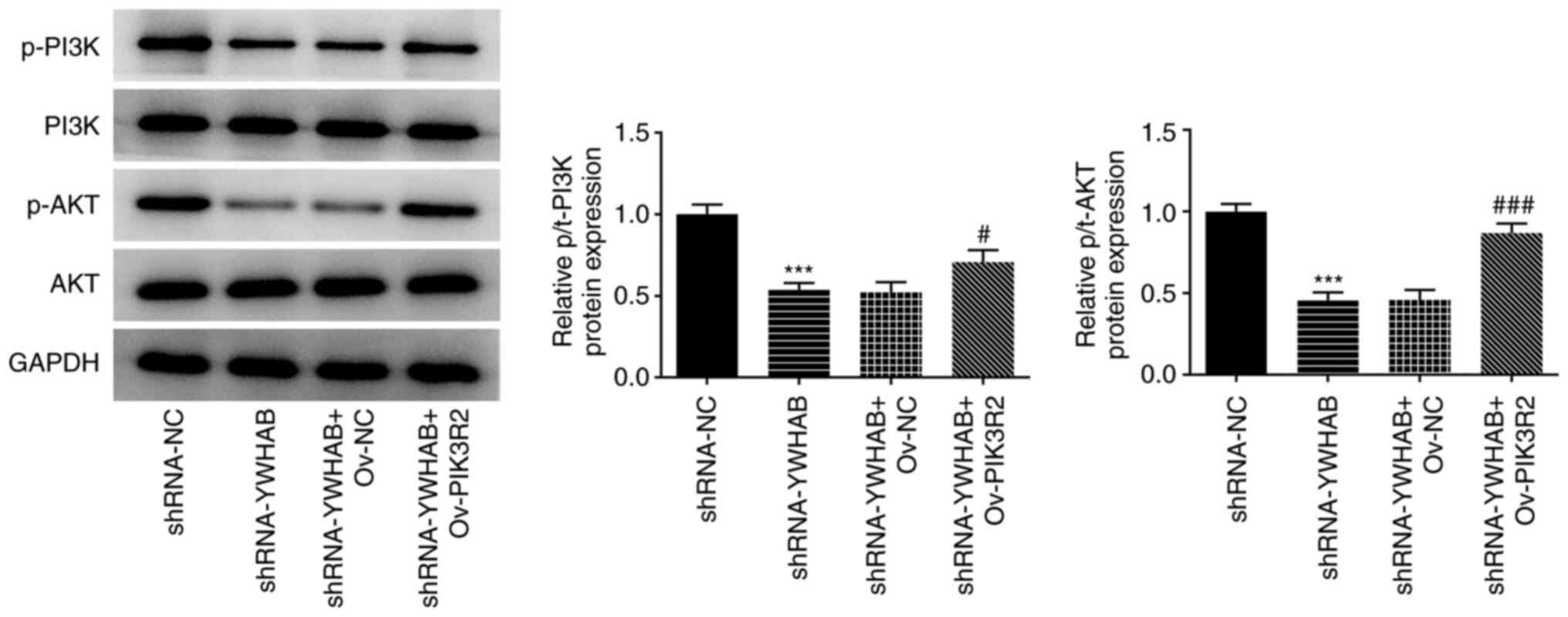
YWHAB regulates the PI3K/AKT signaling
pathway by binding to PIK3R2
As one of the PI3K p85 subunit family members,
PIK3R2 is a key gene in the PI3K/AKT signaling pathway (15-18).
To investigate the effect of YWHAB on the PI3K/AKT signaling
pathway, western blotting was performed to examine the protein
levels of PI3K/AKT signaling pathway markers p-PI3K, PI3K, p-AKT
and AKT. As shown in Fig. 7, YWHAB
knockdown significantly decreased the levels of p-PI3K and p-AKT
compared with those in the control group (shRNA-NC-transfected
HCT116 cells), whilst PIK3R2 overexpression significantly reversed
these aforementioned effects. Ov-PIK3R2 transfection significantly
increased the levels of p-PI3K and p-AKT in the shRNA-YWHAB + group
compared with those in the shRNA-YWHAB + Ov-NC group. These results
suggest that YWHAB can regulate the PI3K/AKT signaling pathway,
likely by targeting PIK3R2.
Discussion
In the present study, the Monarch Initiative
database analysis predicted that YWHAB could bind to PIK3R2. In the
present study, the cellular mechanism in colon cancer was examined,
revealing that YWHAB knockdown suppressed proliferation, induced
cell cycle arrest and promoted apoptosis in HCT116 cells.
Additionally, further experiments demonstrated that YWHAB regulated
cell proliferation, the cell cycle and apoptosis in colon cancer
and the PI3K/AKT signaling pathway, by binding to PIK3R2.
Colon cancer has attracted the attention of research
communities worldwide due to its high rates of mortality (21). Aberrant cell proliferation is a
typical feature of malignant tumors, such that inhibition of
proliferation has been found to suppress tumor development
(22). It has been previously
acknowledged that apoptosis forms an important part of the innate
tumor-suppression mechanism, which has been considered to be a
promising target for cancer therapy (23,24).
Cell cycle dysregulation is another frequently reported feature of
cancer in humans and several therapeutic strategies have been
investigated to target the cell division cycle in cancer (25,26).
Furthermore, the availability of molecular markers greatly
facilitated the diagnosis and prognosis of colon cancer. In
particular, molecular targeted therapies are becoming important for
the treatment of colon cancer (27,28).
YWHAB is a member of the 14-3-3 protein family and has been
reported to regulate the physiology of numerous cancer types,
including lung cancer, prostate cancer and hepatocellular carcinoma
(29-31),
suggesting that YWHAB may serve a carcinogenic role in various
organs. Additionally, YWHAB expression was demonstrated to be
upregulated in colon cancer cells, where its upregulation was
associated with poorer prognosis in patients with this disease
(14). Consistent with these
findings, the mRNA and protein expression levels of YWHAB were
found to be markedly enhanced in colon cancer cells in the present
study. However, this upregulated YWHAB expression was decreased
after the cells were transfected with shRNA-YWHAB. In addition,
YWHAB knockdown induced cycle arrest in colon cancer cells at the
G0/G1 phase, as demonstrated by the increased
expression levels of p21. p21 is a tumor suppressor that has been
documented to be involved in the regulation of cycle arrest and
cell proliferation during G0/G1 phase
(32). In cell cycle progression,
p21 may inhibit the CDK activity to block the entry into S phase
when DNA damage occurs in G1 phase (33). Knocking down YWHAB expression also
promoted the apoptosis of colon cancer cells and increased the
expression levels Bax, a pro-apoptotic protein.
PIK3R2 encodes the p85β regulatory subunit of PI3K,
the expression of which has been frequently found to be increased
in cancer (34). PIK3R2 has been
reported to regulate the progression of numerous cancer types.
MicroRNA-126 has been found to exert suppressive effects on the
proliferation and metastasis of prostate cancer by targeting of
PIK3R2(35). In addition, ephrin
A4 was previously observed to promote proliferation and tumor
metastasis through PIK3R2 in hepatocellular carcinoma (36). In the present study, it was
revealed that PIK3R2 expression was markedly upregulated in colon
cancer cells. According to the Monarch Initiative database, YWHAB
could interact with PIK3R2, which was verified by Co-IP assay in
the present study. To further explore the mechanism of YWHAB in
colon cancer, additional functional experiments were performed.
YWHAB knockdown was found to inhibit cell proliferation, induce
cycle arrest and promote apoptosis in HCT116 cells. In addition,
all of the aforementioned effects induced by YWHAB knockdown were
reversed by PIK3R2 overexpression. This suggests that YWHAB can
regulate cell proliferation, cell cycle arrest and apoptosis in
colon cancer by binding to PIK3R2.
The aberrant activation of the PI3K/AKT signaling
pathway, which consists of PI3K and their downstream mediator AKTs,
is associated with cell proliferation (37,38).
Being a member of the PI3K p85 subunit family, PIK3R2 has been
demonstrated as a core regulator in the activation of the PI3K/AKT
signaling pathway (39). In the
present study, the protein levels of p-PI3K and p-AKT were
decreased by YWHAB knockdown compared with those in cells
transfected with shRNA-NC. By contrast, PIK3R2 overexpression
reversed these aforementioned effects, suggesting that YWHAB
regulated the PI3K/AKT signaling pathway by binding to PIK3R2.
In conclusion, the present study first revealed the
role of YWHAB in the malignant progression of colon cancer cells
and demonstrated that YWHAB regulated PI3K/AKT signaling by binding
to PIK3R2, which was the novelty of the present study. The present
findings could guide the future research and development of target
drugs. However, limitations exist in the present study. Flow
cytometry analysis was not performed for apoptosis analysis. In
addition, clinical tissue analysis, the role of YWHAB in other
colon cancer cells and an animal model of colon cancer were not
explored. Therefore, further studies are required.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TZ and XZ conceived and designed this study. XZ
conducted the experiments and AC analyzed the experimental data.
All authors have read and approved the final manuscript. TZ and XZ
confirmed the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Siegel RL, Miller KD, Goding Sauer A,
Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal
A: Colorectal cancer statistics, 2020. CA Cancer J Clin.
70:145–164. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mojtahedi Z, Mohmedi M, Rahimifar S,
Erfani N, Hosseini SV and Ghaderi A: Programmed death-1 gene
polymorphism (PD-1.5 C/T) is associated with colon cancer. Gene.
508:229–232. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhang Y, Wu Y, Gong ZY, Ye HD, Zhao XK, Li
JY, Zhang XM, Li S, Zhu W, Wang M, et al: Distinguishing rectal
cancer from colon cancer based on the support vector machine method
and RNA-sequencing data. Curr Med Sci. 41:368–374. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Labianca R, Beretta GD, Kildani B, Milesi
L, Merlin F, Mosconi S, Pessi MA, Prochilo T, Quadri A, Gatta G, et
al: Colon cancer. Crit Rev Oncol Hematol. 74:106–133.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rosen AW, Degett TH and Gögenur I:
Individualized treatment of colon cancer. Ugeskr Laeger.
178(V11150916)2016.PubMed/NCBI(In Danish).
|
|
9
|
Bellizzi A, Sebastian S, Ceglia P,
Centonze M, Divella R, Manzillo EF, Azzariti A, Silvestris N,
Montemurro S, Caliandro C, et al: Co-expression of CD133(+)/CD44(+)
in human colon cancer and liver metastasis. J Cell Physiol.
228:408–415. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
van Hemert MJ, Steensma HY and van Heusden
GP: 14-3-3 proteins: Key regulators of cell division, signalling
and apoptosis. Bioessays. 23:936–946. 2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mackintosh C: Dynamic interactions between
14-3-3 proteins and phosphoproteins regulate diverse cellular
processes. Biochem J. 381:329–342. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hua Y, Wang H, Wang H, Wu X, Yang L, Wang
C, Li X, Jin Y, Li M, Wang L, et al: Circular RNA Circ_0006282
promotes cell proliferation and metastasis in gastric cancer by
regulating microRNA-144-5p/tyrosine 3-monooxygenase/tryptophan
5-monooxygenase activation protein β axis. Cancer Manag Res.
13:815–827. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang X, Zhang Q, Zhang K, Wang F, Qiao X
and Cui J: Circ SMARCA5 inhibited tumor metastasis by interacting
with SND1 and downregulating the YWHAB gene in cervical cancer.
Cell Transplant. 30(963689720983786)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ahluwalia P, Mondal AK, Bloomer C, Fulzele
S, Jones K, Ananth S, Gahlay GK, Heneidi S, Rojiani AM, Kota V and
Kolhe R: Identification and clinical validation of a novel 4
gene-signature with prognostic utility in colorectal cancer. Int J
Mol Sci. 20(3818)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gao J, Zhou XL, Kong RN, Ji LM, He LL and
Zhao DB: microRNA-126 targeting PIK3R2 promotes rheumatoid
arthritis synovial fibro-blasts proliferation and resistance to
apoptosis by regulating PI3K/AKT pathway. Exp Mol Pathol.
100:192–198. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xiao J, Lin HY, Zhu YY, Zhu YP and Chen
LW: MiR-126 regulates proliferation and invasion in the bladder
cancer BLS cell line by targeting the PIK3R2-mediated PI3K/Akt
signaling pathway. Onco Targets Ther. 9:5181–5193. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Xi T, Jin F, Zhu Y, Wang J, Tang L, Wang
Y, Liebeskind DS and He Z: MicroRNA-126-3p attenuates blood-brain
barrier disruption, cerebral edema and neuronal injury following
intracerebral hemorrhage by regulating PIK3R2 and Akt. Biochem
Biophys Res Commun. 494:144–151. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dornan GL and Burke JE: Molecular
mechanisms of human disease mediated by oncogenic and primary
immunodeficiency mutations in class IA phosphoinositide 3-kinases.
Front Immunol. 9(575)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cortés I, Sánchez-Ruíz J, Zuluaga S,
Calvanese V, Marqués M, Hernández C, Rivera T, Kremer L,
González-García A and Carrera AC: p85β phosphoinositide 3-kinase
subunit regulates tumor progression. Proc Natl Acad Sci USA.
109:11318–11323. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yaghoubi A, Khazaei M, Avan A, Hasanian SM
and Soleimanpour S: The bacterial instrument as a promising therapy
for colon cancer. Int J Colorectal Dis. 35:595–606. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gao F, Xu T, Wang X, Zhong S, Chen S,
Zhang M, Zhang X, Shen Y, Wang X, Xu C and Shen Z: CIP2A mediates
fibronectin-induced bladder cancer cell proliferation by
stabilizing beta-catenin. J Exp Clin Cancer Res.
36(70)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kaczanowski S: Apoptosis: its origin,
history, maintenance and the medical implications for cancer and
aging. Phys Biol. 13(031001)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ahmad I, Fakhri S, Khan H, Jeandet P,
Aschner M and Yu ZL: Targeting cell cycle by beta-carboline
alkaloids in vitro: Novel therapeutic prospects for the treatment
of cancer. Chem Biol Interact. 330(109229)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Manchado E, Guillamot M and Malumbres M:
Killing cells by targeting mitosis. Cell Death Differ. 19:369–377.
2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
de Castro Sant' Anna C, Junior AGF, Soares
P, Tuji F, Paschoal E, Chaves LC and Burbano RR: Molecular biology
as a tool for the treatment of cancer. Clin Exp Med. 18:457–464.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Herzig DO and Tsikitis VL: Molecular
markers for colon diagnosis, prognosis and targeted therapy. J Surg
Oncol. 111:96–102. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xu C, Du Z, Ren S, Liang X and Li H:
MiR-129-5p sensitization of lung cancer cells to etoposide-induced
apoptosis by reducing YWHAB. J Cancer. 11:858–866. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Singh AN and Sharma N: Quantitative
SWATH-based proteomic profiling for identification of
mechanism-driven diagnostic biomarkers conferring in the
progression of metastatic prostate cancer. Front Oncol.
10(493)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu Y, Qin Z, Cai L, Zou L, Zhao J and
Zhong F: Selection of internal references for qRT-PCR assays of
human hepatocellular carcinoma cell lines. Biosci Rep.
37:2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huang KC, Huang TW, Chuang PY, Yang TY and
Chang SF: Zoledronate induces cell cycle arrest and differentiation
by upregulating p21 in mouse MC3T3-E1 preosteoblasts. Int J Med
Sci. 16:751–756. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mansilla SF, de la Vega MB, Calzetta NL,
Siri SO and Gottifredi V: CDK-independent and PCNA-dependent
functions of p21 in DNA replication. Genes (Basel).
11(593)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rao L, Mak VCY, Zhou Y, Zhang D, Li X,
Fung CCY, Sharma R, Gu C, Lu Y, Tipoe GL, et al: p85β regulates
autophagic degradation of AXL to activate oncogenic signaling. Nat
Commun. 11(2291)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Song L, Xie X, Yu S, Peng F and Peng L:
MicroRNA126 inhibits proliferation and metastasis by targeting
pik3r2 in prostate cancer. Mol Med Rep. 13:1204–1210.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lin J, Zeng C, Zhang J, Song Z, Qi N, Liu
X, Zhang Z, Li A and Chen F: EFNA4 promotes cell proliferation and
tumor metastasis in hepatocellular carcinoma through a
PIK3R2/GSK3β/β-catenin positive feedback loop. Mol Ther Nucleic
Acids. 25:328–341. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Noorolyai S, Shajari N, Baghbani E,
Sadreddini S and Baradaran B: The relation between PI3K/AKT
signalling pathway and cancer. Gene. 698:120–128. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yu JS and Cui W: Proliferation, survival
and metabolism: The role of PI3K/AKT/mTOR signalling in
pluripotency and cell fate determination. Development.
143:3050–3060. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang J, Zhang Z, Zhang DY, Zhu J, Zhang T
and Wang C: microRNA 126 inhibits the transition of endothelial
progenitor cells to mesenchymal cells via the PIK3R2-PI3K/Akt
signalling pathway. PLoS One. 8(e83294)2013.PubMed/NCBI View Article : Google Scholar
|