Introduction
Functional constipation (FC) is a common bowel
disorder, which affects 10-15% of the global population (1). In the past 30 years, the prevalence
of FC in China has increased two-fold (2). FC is characterized by persistently
difficult, incomplete or infrequent defecation without an
identifiable organic cause (3).
Among the effects of FC, the decreased health-associated quality of
life and increased levels of depression are particularly notable
and are associated with an elevated all-cause mortality rate
(4).
The causes of FC are multifactorial and include
diet, lifestyle, psychological factors, inflammation and oxidative
stress. Pathophysiologically, the aforementioned factors result in
dysfunctional motility of the colon, which is typically the primary
cause of FC (5). Neurotrophic
factors serve a vital role in affecting the enteric nervous system,
which regulates intestinal motility by neurotransmitters, including
excitatory transmitters such as motilin (MTL; a gastrointestinal
hormone) and inhibitory transmitters such as nitric oxide (NO)
(6,7). Furthermore, a previous study showed
that brain-derived neurotrophic factor (BDNF) is expressed in the
myenteric plexus (8). Exogenous
BDNF treatment significantly promotes contraction of longitudinal
muscle strips from the distal colon of mice (9). Interstitial cells of Cajal (ICC) that
specifically express receptor tyrosine kinase (C-Kit) are
mesenchymal cells that form a cellular network in the
gastrointestinal musculature (10). As a ligand of C-Kit, stem cell
factor (SCF) is key for maintaining the survival and development of
ICC and ICC networks (11). The
ICC are associated with the autonomic nerves, which primarily
promote intestinal motility (12).
Furthermore, a recent study reported that aquaporins (AQPs) are
expressed in the colon and regulate faecal water content (13). Due to increasing healthcare demands
with the concomitant complexity of FC, additional treatment methods
are required.
Dietary and lifestyle changes are current methods
for the management of FC in the majority of the population
(14). However, patients who
experience persistent FC are in need of pharmaceutical-based
therapies. Over-the-counter medications, including stimulants and
osmotic laxatives, are primarily used for FC treatment (15). However, chronic use of laxatives is
discouraged given high levels of dissatisfaction and the low
efficacy and safety (16). Thus,
it is worth exploring novel therapies for the management of FC.
Arctiin (Arc), extracted from the seeds of
Arctium lappa, is a lignan glycoside that is digested by
intestinal microbes and absorbed into the blood (17). Several studies have showed a wide
spectrum of pharmacological activity attributed to Arc including
neuroprotection, anti-inflammation and anti-oxidative stress
properties (18-20).
Xu et al (18) demonstrated
that Arc decreases neuronal injury and ameliorates depression in
mice. Furthermore, Arc inhibits inflammation in mice with colitis
(21). Additionally, the
anti-inflammatory effect of Arc is attributed to inhibiting NO
production by decreasing inducible NO synthase (iNOS) activity
(22). Extracted mixtures from the
roots of Arctium lappa are reported to have laxative
properties (23). However, the
effects of Arc on FC remain unclear.
Thus, the present study aimed to determine if Arc
influences FC. Changes in intestinal motility associated with
altered neurotransmitter expression levels and changes in ICC were
assessed using an FC mouse model established by administering
loperamide. The results of the present study provide a theoretical
mechanism through which Arc alleviates FC.
Materials and methods
Establishment of a loperamide-induced
FC mouse model and Arc treatment
A total of 84 male Institute of Cancer Research
(ICR) mice (age, 6 weeks, weighting 25-30 g) from the Laboratory
Animal Centre of the School of Basic Medical Sciences (Xi'an
Jiaotong University and Medical Laboratory Animal Centre of Air
Force Medical University, Xi'an, China) were used after
acclimatization to housing conditions (22±1˚C; 12/12-h light/dark
cycle; relative humidity 45-55%) and free access to normal mice
chow and water. The normal mice chow was purchased from Huanyu
Biotechnology Co., Ltd. and consisted of corn, soybean meal, flour,
fish meal, yeast powder, vegetable oil, amino acids, calcium
hydrophosphate, salt, vitamins and trace elements, which
corresponded to 18.0% crude protein, 4.0% crude fat, 5.0% crude
fibre, 8.0% ash, 1.0% calcium, 0.6% phosphorus and other macro- and
micronutrients. The mice were divided into four groups: Control;
FC; lactulose and Arc (n=6/group). The mice from the lactulose and
the Arc group received oral administration of 500 mg/kg lactulose
(Shanghai Aladdin Biochemical Technology Co., Ltd.) or 100 mg/kg
Arc (>98% purity; Shanghai Aladdin Biochemical Technology Co.,
Ltd.) daily for 14 days, respectively. An equal volume of pure
water was administered by oral gavage to mice in the control group
and the FC group. Induction of FC was performed as described
previously (24). The mice from
the FC, lactulose and Arc groups were administered loperamide (5
mg/kg; Shanghai Aladdin Biochemical Technology Co., Ltd.) orally by
gavage on days 12-14. Alterations in water consumption and food
intake were measured using a metabolic cage. Additionally, body
weight was measured daily on days 12-15 and faeces and distal colon
segments were collected on day 15. Mice were anesthetized with
inhalation of isoflurane at 3% induction and 2% maintenance. Blood
(1 ml) was collected from the postcava and left to stand for 30 min
at room temperature. Mice were sacrificed by exsanguination under
anesthesia. Serum was collected following centrifugation at 1,111 x
g at room temperature for 10 min. Other mice were euthanized by
intraperitoneal injection of 200 mg/kg sodium pentobarbital. In
this current study, the criteria for humane endpoint euthanasia
included inability to access food and water and obvious anxiety
resulting from FC and no mouse was sacrificed according to these.
All animal protocols were approved by the Institutional Animal Care
and Use Committee of the Shaanxi University of Chinese Medicine
(approval no. SUCMDL20190314001).
Measurement of intestinal transit in
FC mice
Determination of intestinal transit ratio (n=6 each
group) and transit time (n=6 each group) was performed as described
previously (25,26). Briefly, following 14 days of
treatment, mice fasted with free access to water for 12 h. For the
intestinal transit ratio analysis, the mice were orally
administered a meal containing 20% charcoal (Shanghai Aladdin
Biochemical Technology Co., Ltd.) and 10% gum arable (Shanghai
Aladdin Biochemical Technology Co., Ltd.) at a volume of 0.1 ml/10
g. Mice were euthanized by intraperitoneal injection of 200 mg/kg
sodium pentobarbital 30 min after meal ingestion and the intestine
from the pylorus to the rectum were collected immediately. The
lengths of the total intestinal tract and the distance the charcoal
meal had travelled from the pylorus were measured. The intestinal
transit ratio was calculated as follows: Intestinal transit ratio
(%)=length travelled by the charcoal meal/total length of intestine
x100. The transit time was evaluated in mice following gavage of a
0.1 ml volume of 5% Evans blue (MilliporeSigma) in 1.5%
methylcellulose (Shanghai Aladdin Biochemical Technology Co.,
Ltd.). Faeces were monitored at 10 min intervals for the presence
of Evans blue staining. The time to the excretion of the first blue
faeces was recorded.
Histopathological examination
Formalin (10%, at room temperature for 24 h)-fixed
distal colon segments were embedded in paraffin (at 60˚C for 2 h)
and sliced into serial 5-µm thick sections. Hematoxylin and eosin
(H&E) staining (2 g/l hematoxylin and 3.5 g/l eosin) at room
temperature for 5 min was used to assess the morphometric features.
To measure colonic mucosa thickness, colon sections were stained
with Alcian Blue Dye Solution (pH2.5) (Leagene Biotechnology) at
room temperature for 30 min. Images were acquired with a light
microscope (Olympus Corporation) at x200 magnification. Image-Pro
Plus software (Version 6.0.0.260; Media Cybernetics, Inc.) was used
to measure the thickness of muscle and mucosa.
Transmission electron microscopy
Following fixation of distal colon samples in 1%
osmic acid at room temperature for 2 h, washing in phosphate buffer
and dehydrating in an ethanol series, the colon samples were
embedded in epoxy resin (SPI Supplies; Structure Probe, Inc.) at
37˚C overnight and polymerized at 60˚C for 48 h. The blocks were
cut into ultra-thin 70-nm thick sections and stained with 2% uranyl
acetate and 2.6% lead citrate at room temperature for 8 min. A
transmission electron microscope (H-7650, Hitachi) was used to
detect the morphology and structure of Cajal cells in the colon of
mice at x20,000 magnification. The images were acquired with an
accelerating voltage of 80 kV.
Assessment of MTL, BDNF and NO
Commercial ELISA kits for MTL (cat. no. CEA575Mu;
Cloud-Clone Corp.) and BDNF [cat. no. EK2127; Multisciences
(LIANKE) Biotech., Co., Ltd.] were used to measure their
concentration in the serum and colon tissues of mice, according to
the manufacturer's protocol. The levels of NO in the colon tissue
were assessed using an NO assay kit (cat. no. A013; Nanjing
Jiancheng Bioengineering Institute) according to the manufacturer's
instructions.
Immunoblotting
The colon tissue was homogenized in RIPA lysis
buffer (Beyotime Institute of Biotechnology). Following
quantification of protein concentrations using a BCA protein assay
kit (Beyotime Institute of Biotechnology), a total of 15 µg/lane
(C-Kit, SCF, AQP-3, and AQP-9) or 30 µg/lane (nNOS, eNOS, and iNOS)
protein was loaded on an 8% or 12% SDS-gel, resolved using SDS-PAGE
and transferred onto PVDF membranes (Thermo Fisher Scientific,
Inc.). The membranes were blocked with TBST containing 0.15%
Tween-20 and 5% BSA (BioFroxx, Inc.) and incubated with primary
antibodies against neuronal NOS (nNOS; 1:1,000; cat. no. A2649;
ABclonal Biotech Co., Ltd.), endothelial NOS (eNOS; 1:1,000; cat.
no. A15075; ABclonal Biotech Co., Ltd.), iNOS (1:1,000; cat. no.
A0312; ABclonal Biotech Co., Ltd.), C-Kit (1:1,000; cat. no.
AF6153; Affinity Biosciences), SCF (1:500; cat. no. 26582-1-AP;
ProteinTech Group, Inc.), AQP-3 (1:1,000; cat. no. A2838; ABclonal
Biotech Co., Ltd.), AQP-9 (1:1,000; cat. no. DF9225, Affinity
Biosciences) or β-actin (1:2,000; cat. no. 60008-1-Ig; ProteinTech
Group, Inc.) overnight at 4˚C. Subsequently, the membranes were
incubated with horseradish peroxidase (HRP)-conjugated secondary
antibodies (1:10,000; cat. nos. SA00001-1 and SA00001-2;
ProteinTech Group, Inc.) at 37˚C for 40 min. Protein bands were
visualized using ECL-Reagent (7 Sea Biotech) and imaged.
Gel-Pro-Analyzer software (Version 4.0, Media Cybernetics, Inc.)
was used for quantitative analysis of blots.
Immunohistochemistry and
immunofluorescence
Immunohistochemical analysis of the colon tissue was
performed on 5-µm sections. Briefly, antigen retrieval was
performed using a citric acid buffer and heating in an 800 W
microwave for 10 min, blocked with BSA (1%, Sangon Biotech Co.,
Ltd.) for 15 min at room temperature and incubated with primary
antibodies against C-Kit (1:100; cat. no. AF6153; Affinity
Biosciences), SCF (1:100; cat. no. 26582-1-AP; ProteinTech Group,
Inc.), AQP-3 (1:100; cat. no. A2838; ABclonal Biotech Co., Ltd.),
or AQP-9 (1:100; cat. no. DF9225, Affinity Biosciences) at 4˚C
overnight. For immunohistochemistry, following incubation with
horseradish peroxidase-conjugated secondary antibody (1:500; cat.
no. 31460; Thermo Fisher Scientific, Inc.) at 37˚C for 1 h,
sections were stained with DAB) detection kit (MXB Biotechnologies
Co., Ltd.) at room temperature for 3 min, examined by light
microscopy (Olympus Corporation) at x400 magnification equipped
with a digital camera (Olympus Corporation). For
immunofluorescence, the sections were incubated with Cy3-conjugated
secondary antibody (1:200; cat. no. A27039; Invitrogen; Thermo
Fisher Scientific, Inc.) at room temperature for 1 h. DAPI (100
ng/ml, Shanghai Aladdin Biochemical Technology Co., Ltd.) was used
for counterstaining at room temperature for 5 min. Images were
captured with a fluorescence microscope (Olympus Corporation) at
400x magnification. The evaluation of immunofluorescent and
immunohistochemical staining was performed using Image-Pro Plus
software (Version 6.0.0.260, Media Cybernetics, Inc.).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA in the distal colon tissues of mice was
extracted using TRIpure™ (BioTeke Corporation) and quantified using
a spectrophotometer (NanoDrop™ 2000; Thermo Fisher Scientific,
Inc.). Following cDNA synthesis with Moloney murine leukaemia virus
reverse transcriptase (BeyoRT™ II M-MLV, Beyotime Institute of
Biotechnology) according to the manufacturer's instructions,
RT-qPCR was performed on an Exicycler 96 PCR system (Bioneer
Corporation) with initial denaturation at 95˚C for 5 min followed
by 40 cycles of denaturation at 95˚C for 10 sec, annealing at 60˚C
for 10 s, and extension at 72˚C for 15 s. mRNA levels were
quantified using the 2-ΔΔCq method and normalized to the
internal reference gene β-actin (27). The primer sequences were as
follows: Tumor necrosis factor-α (TNF-α) forward,
5'-TCTCATTCCTGCTTGTGG-3' and reverse, 5'-CTTGGTGGTTTGCTACG-3';
interleukin (IL)-1β forward, 5'-CTCAACTGTGAAATGCCACC-3' and
reverse, 5'-GAGTGATACTGCCTGCCTGA-3'; IL-6 forward,
5'-TAACAGATAAGCTGGAGTC-3' and reverse, 5'-TAGGTTTGCCGAGTAGA-3' and
β-actin forward, 5'-CTGTGCCCATCTACGAGGGCTAT-3' and reverse,
5'-TTTGATGTCACGCACGATTTCC-3'.
Statistical analysis
The data are presented as the mean ± standard
deviation (n=6) and analyzed using one-way ANOVA followed by post
hoc Tukey's test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Arc attenuates loperamide-induced FC
in mice
FC was evaluated based on the changes in faecal
matter, including defecation frequency and faecal moisture
analysis. Loperamide was used to establish the FC model. Loperamide
resulted in a significant decrease in faecal numbers (4.00±0.89),
faecal weight (0.11±0.02 g) and water content (49.59±5.79%) in FC
mice compared with that in the control mice (20.50±3.45, 0.47±0.09
g and 67.18±6.38%, respectively; Table
I). Body weight and feeding behaviour did not vary between the
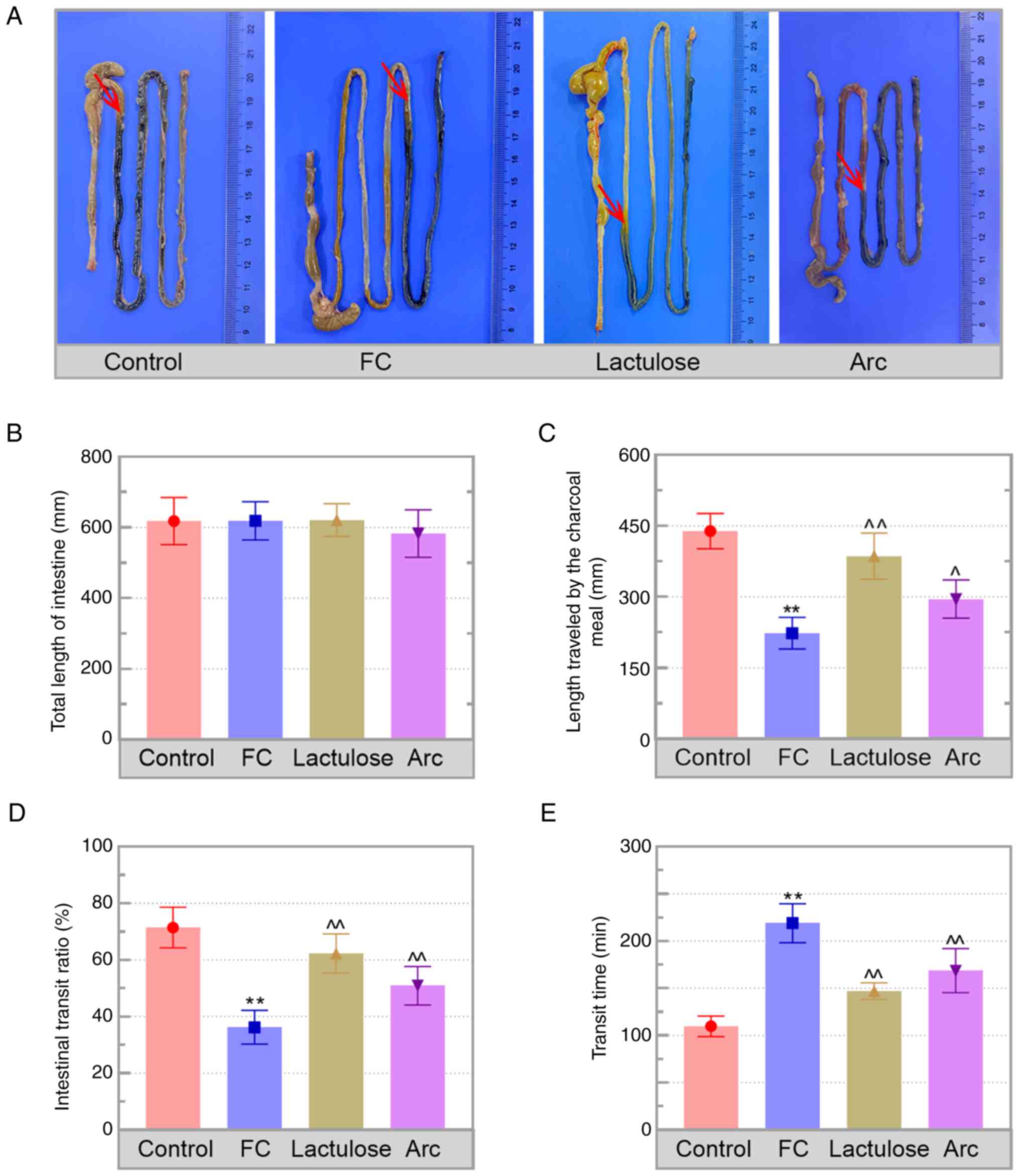
control and FC groups. Additionally, the length travelled by the
charcoal meal in the intestinal tract was shorter in
loperamide-treated mice compared with that in the control (red
arrows delineate the travelled location of the charcoal meal;
Fig. 1A). Furthermore, no
difference was observed in the total length of the intestine in
each group, but the length traveled by the charcoal was shorter in
FC mice than that of control (Fig.
1B and C). Measurement of
intestinal transit showed a decrease in intestinal transit ratio
and an increase in the transit time in the FC mice (Fig. 1D and E). The aforementioned results indicated
that the FC model was established successfully. Arc treatment not
only improved the faecal characteristics but also accelerated
intestinal transit in FC mice.
 | Table IDetection of body weight, feeding
behaviour and faeces secretion in FC mice. |
Table I
Detection of body weight, feeding
behaviour and faeces secretion in FC mice.
| Variable | | Control | FC | Lactulose | Arctiin |
|---|
| Body weight, g | Day 12 | 33.50±1.87 | 34.00±1.26 | 32.83±1.72 | 32.67±1.63 |
| | Day 13 | 33.83±1.47 | 34.17±1.17 | 33.17±2.04 | 33.00±1.41 |
| | Day 14 | 34.33±1.63 | 34.50±1.76 | 33.00±1.90 | 33.50±1.38 |
| | Day 15 | 34.33±1.51 | 35.17±1.47 | 33.33±1.97 | 34.00±1.41 |
| Feeding
behaviour | Water intake, ml/24
h | 6.17±1.47 | 6.50±1.52 | 7.17±0.98 | 6.83±0.75 |
| | Food intake, g/24
h | 5.17±1.72 | 4.83±0.75 | 5.33±1.21 | 5.00±0.63 |
| Faeces | Number, n | 20.50±3.45 |
4.00±0.89a |
10.17±2.14c |
7.83±1.47b |
| | Weight, g | 0.47±0.09 |
0.11±0.02a |
0.28±0.03c |
0.22±0.04c |
| | Water content,
% | 67.18±6.38 |
49.59±5.79a |
61.80±4.25c |
59.27±1.06b |
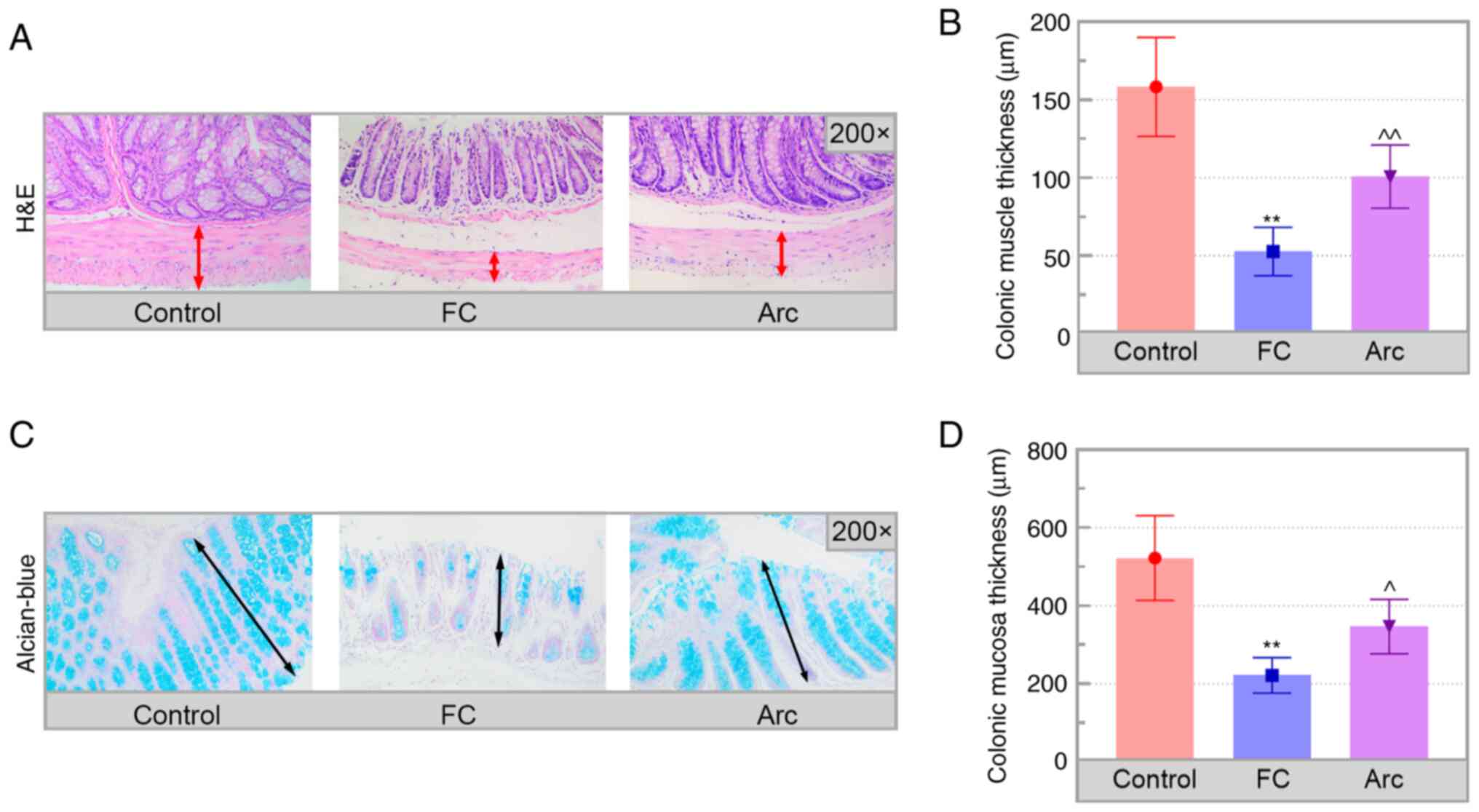
Arc decreases pathological injury to
the colon
Subsequently, changes in the colonic pathology in FC
mice were assessed. H&E staining of the colon sections showed
that the muscular layer was significantly thinner in the colon of
FC mice compared with that in the control mice (Fig. 2A and B; double-headed arrows delineate the
muscular layer). Furthermore, results of H&E staining could be
confirmed by alcian blue staining, which provided more evidence on
the protective effects of Arc on FC. The results of alcian blue
staining revealed that the thickness of the colonic mucosa was
significantly decreased in the FC group compared with the control
group (Fig. 2C and D; double-headed arrows delineate the
colonic mucosa). These changes were reversed in Arc-treated mice.
Thus, Arc mitigated colonic damage in the FC mice.
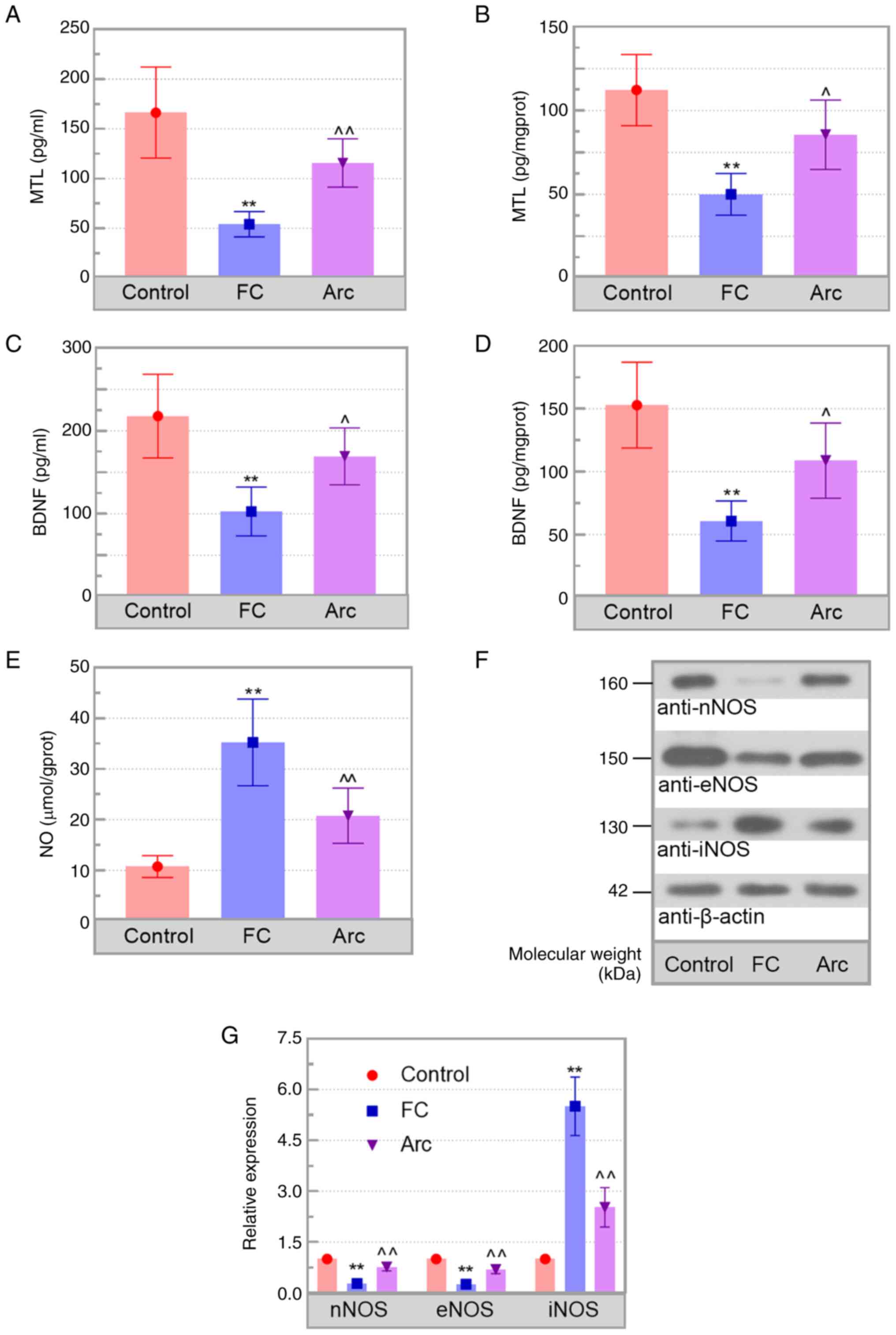
Levels of gastrointestinal
motility-associated neurotransmitters and inflammation in colon of
FC mice are regulated by Arc
To investigate the underlying mechanism by which Arc
attenuated FC, levels of excitatory (MTL) and inhibitory
neurotransmitters (NO), as well as BDNF, were determined in the
serum and colon tissue of mice. Arc ameliorated the decrease in MTL
and BDNF levels, as well as the increase in NO observed in
loperamide-treated mice compared with those in the FC mice
(Fig. 3A-E). Western blot analysis
showed higher expression of nNOS and eNOS and lower expression of
iNOS in the Arc group compared with the FC group (Fig. 3F and G). Moreover, the mRNA levels of TNF-α,
IL-1β and IL-6 were increased in FC mice compared with those in the
control. Additionally, the inflammatory induction was significantly
inhibited by Arc (Fig. S1A-C).
These findings showed that Arc regulated levels of
neurotransmitters and inflammation in FC mice.
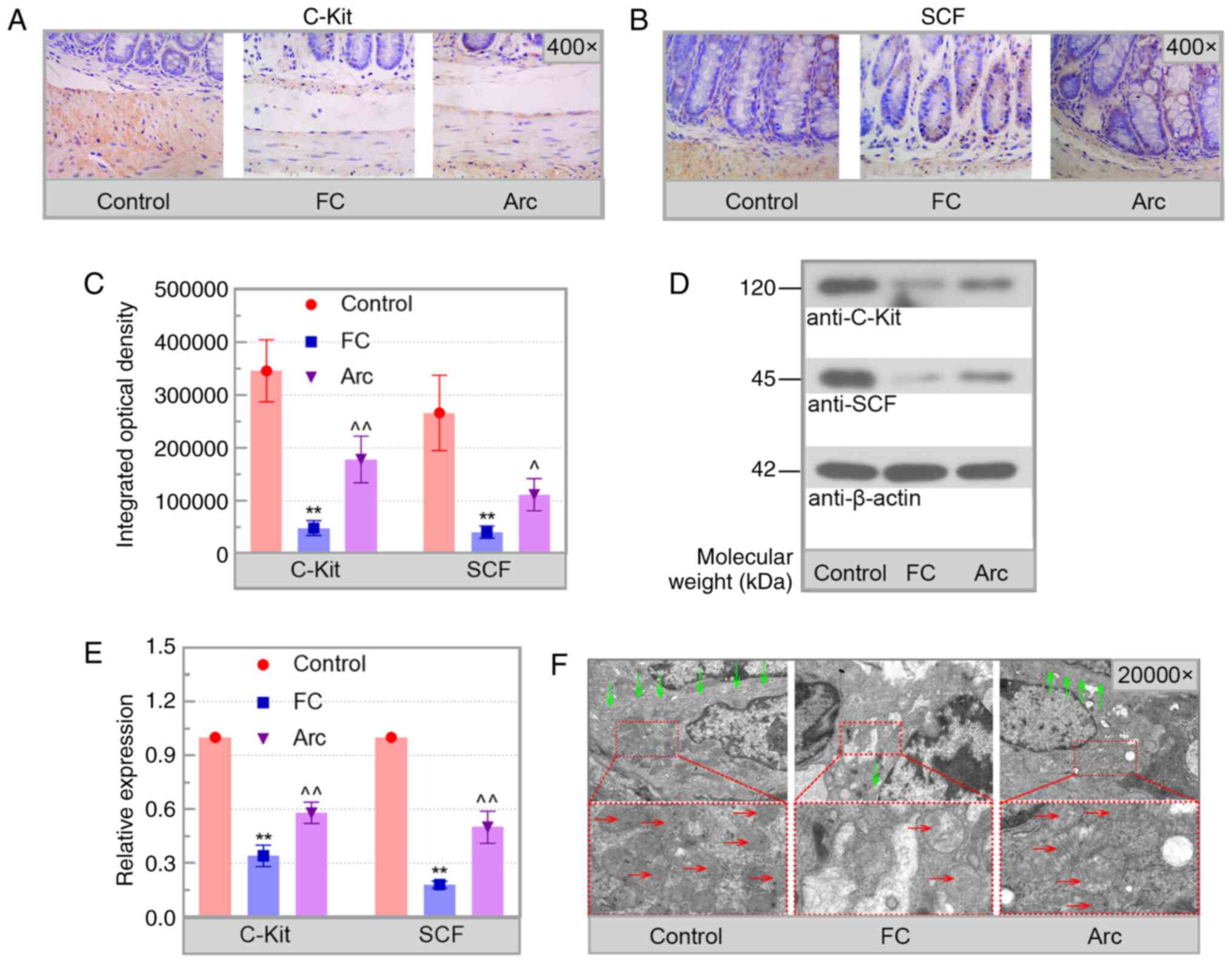
Arc decreases ICC injury
The levels of C-Kit and SCF and the condition of
mitochondria/ER were used to evaluate ICC injury.
Immunohistochemical analysis showed that C-Kit and SCF were
primarily present in the submucosa and muscle layers (Fig. 4A-C). Arc reversed the decrease in
C-Kit and SCF levels in the colon of FC mice, which was confirmed
by western blot analysis (Fig. 4D
and E). Electron microscopy
results showed that the ICC in the colon of control mice was rich
in cell organelles, including endoplasmic reticulum and
mitochondria. Injured ICC in the FC group were characterized by
swollen mitochondria with disrupted cristae and partial cytoplasmic
depletion. By contrast, the damage to the ICC in the Arc group was
notably decreased (Fig. 4F). These
results showed that Arc reduced ICC damage in the colon of FC
mice.
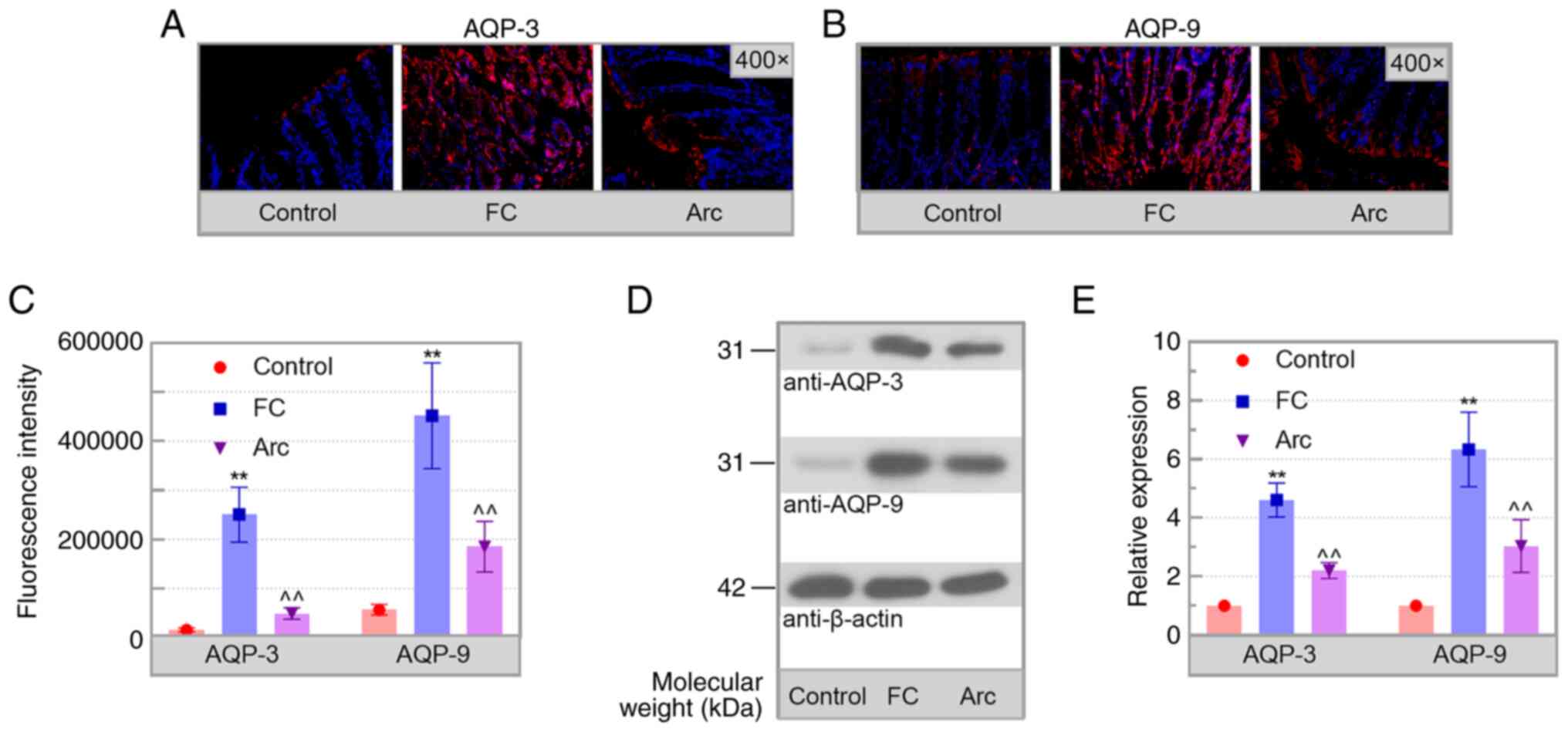
Arc decreases the levels of AQPs
Based on the involvement of AQPs in FC progression
(28), the expression levels of
AQPs in the colon of mice were detected. Increased expression of
AQP-3 and AQP-9 was observed in the colon of FC mice and this was
significantly reduced by Arc treatment (Fig. 5A-E). Therefore, Arc downregulated
the levels of AQPs in the colon of mice with FC.
Discussion
FC is a common gastrointestinal disorder that
affects the quality of life of individuals worldwide (29). The present study aimed to evaluate
the therapeutic effects of Arc on FC. In vivo experiments
were used to investigate ICR mouse intestinal motility and changes
in the ICC following induction of FC. To the best of our knowledge,
the present study is the first to show that Arc alleviated
loperamide-induced FC by decreasing pathological damage and
inflammation, regulating levels of gastrointestinal
motility-related neurotransmitters and attenuating ICC injury in
the colon of FC mice. It was also demonstrated that Arc could
reverse the elevated levels of AQPs in the colon of mice with FC.
Based on these findings, the protective effects of Arc on FC were
shown and these results highlight the potential value of Arc in the
treatment of FC (Fig. 6).
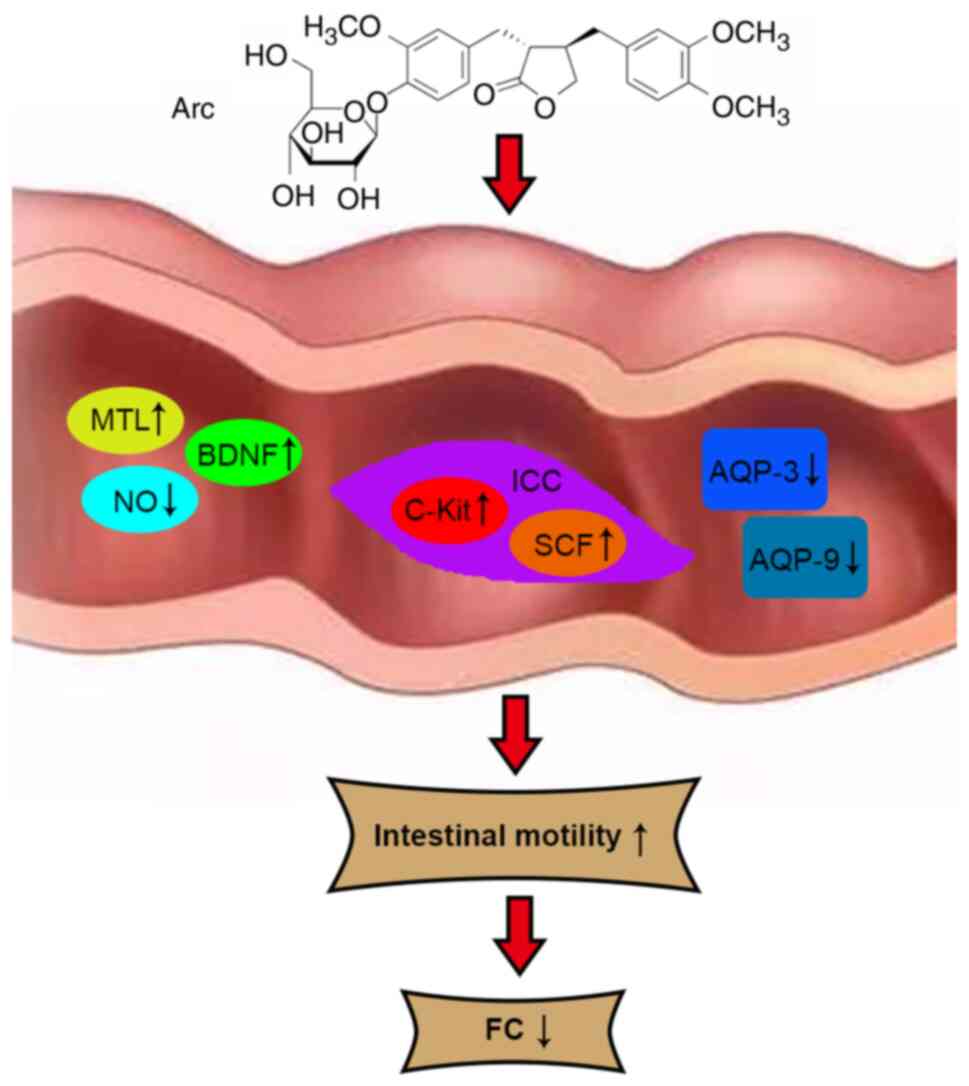 | Figure 6Hypothetical model of Arc-attenuated
FC. Arc regulates levels of gastrointestinal motility-associated
neurotransmitters and decreases ICC injury and AQP expression,
alleviating FC. Arc, Arctiin; FC, functional constipation; ICC,
interstitial cells of Cajal; SCF, stem cell factor; MTL, motilin;
BDNF, brain-derived neurotrophic factor; NO, nitric oxide; AQP,
aquaporin; C-Kit, receptor tyrosine kinase. |
FC is a common gastrointestinal disorder that is
associated with intestinal motility (30). Delayed gastrointestinal transit is
associated with abnormal gut muscular movement, which may be caused
by insufficient inputs from the enteric neural circuitry (31). Therefore, neuromodulators
regulating survival and development of neurons in the enteric
nervous system regulate intestinal function (32). As one of the neurotrophin ligands,
BDNF dose-dependently accelerates colon motility by varying the
intestinal innervation structure (33). Conditional knockout of BDNF in the
gut induces dopaminergic neuronal loss, motor dysfunction and
constipation (34). Moreover, MTL
enhances gastrointestinal motility via direct action on smooth
muscle cells (35). By contrast,
NO is the primary inhibitory neurotransmitter and levels of NO in
the myenteric plexus serve a role in inhibiting the colonic motor
complex (36). Synthesis of NO is
catalyzed by NOS enzymes, including eNOS, nNOS and iNOS. The nNOS
isoform is abundant in neural tissues and participates in
pathological and physiological processes, such as nerve
transmission and muscle contraction in the intestine (37). Under normal physiological
conditions, NO is primarily produced from eNOS, which is abundant
in endothelial cells where it regulates vascular homeostasis
(38). Nevertheless, iNOS levels
associated with excessive production of NO are increased in the
colon of FC mice (39). Hence,
regulating the levels of NOS can reduce NO content and mitigate FC.
In the present study, the number and the water content of faeces
were higher and the intestinal transit time was shorter in the Arc
treatment group compared with the FC group. In addition, Arc
increased the levels of BDNF, MTL, nNOS and eNOS while decreasing
the levels of iNOS and NO in the colon of FC mice. Therefore, Arc
treatment may relieve FC by regulating neuromodulators that enhance
neural function, leading to elevated colonic contractility.
ICC, as mesenchymal cells, are pacemakers for the
peristaltic reflex by which propulsive contraction in the
gastrointestinal tract is achieved (40). ICC are rich in multiple organelles,
including numerous mitochondria and abundant endoplasmic reticula
(41). With a highly branched
morphology and cellular network, ICC are an important component of
the gastrointestinal tract (42).
Additional evidence has shown that ICC contribute to intestinal
motility by generating spontaneous electrical depolarization and
increasing smooth muscle excitability (43). Furthermore, ICC differentiation and
development rely on signals from C-Kit and its ligand SCF (44). Previous studies have ICC injury in
the colons of patients and rats with FC (12,45).
The present results revealed that C-Kit and SCF were mainly present
in the submucosa and muscle layers, which is consistent with
previous studies (46,47). Furthermore, a novel finding of the
present study was that Arc prevented the increase in damage and
increased the density of ICC in the colon of FC mice. Arc may
promote intestinal motility by protecting against ICC injury and
subsequently alleviating FC.
AQPs are key membrane proteins that primarily
transport water across the plasma membranes (48). At present, 13 isoforms of AQP have
been identified in mammals and these are divided into three groups.
Water-selective AQPs, including AQP0, AQP1, AQP2, AQP4, AQP5, AQP6
and AQP8, are selectively permeable to water. Aquaglyceroporins,
including AQP3, AQP7, AQP9 and AQP10, are permeable to water,
glycerol and urea. Finally, super-aquaporins, including AQP11 and
AQP12, are highly different from the aforementioned AQPs with low
homology (49). AQPs are
associated with transepithelial water movement in the intestinal
tract and are key for maintaining body water homeostasis (50). Upregulation of AQP3 and AQP9
results in severe constipation by decreasing mucus secretion and
faecal water content, as well as decreasing bowel peristalsis in
rats (51). Multiple studies have
showed that AQP-3 and AQP-9 are localized in the mucosal layer,
including epithelial and goblet cells (52-55).
Of note, suppression of AQP3 function in the colon results in
diarrhoea (52). FC mice show
increased levels of AQP3 and AQP9 compared with those in controls,
which is consistent with the present results (56). Here, loperamide-induced elevation
in AQP3 and AQP9 were decreased by Arc treatment. Therefore, Arc
treatment may decrease AQP expression, thus relieving abnormal
water transport and inhibiting the development of FC.
The present study has limitations. The symptoms of
depression, which may be associated with constipation severity
(57), were not investigated in
the current FC model. Constipated mice show notable increases in
immobility time of the forced swimming and tail suspension test
(58). In addition, the specific
effect of Arc on BDNF, MTL and NOS remains unclear. Further
studies, including analyses of the symptoms of depression in FC
mice and the mechanism of Arc on BDNF, MTL and NOS, should be
performed to improve understanding of the function of Arc in the
management of FC.
In conclusion, the present study showed that Arc
promoted colonic contractility and decreased intestinal transit
time by relieving colonic damage and ICC injury while
downregulating inflammation induction and AQP expression levels in
the colon to mitigate FC.
Supplementary Material
Arc inhibits loperamide-induced
inflammation. Reverse transcription-quantitative PCR was used to
measure the mRNA levels of (A) TNF-α, (B) IL-1β and (C) IL-6.
**P<0.01 vs. Control. ^^P<0.01 vs. FC.
FC, functional constipation; Arc, Arctiin.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 81973865).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW designed the study and wrote the manuscript. HJ
designed the study and revised the manuscript. YW, LW, HG, and WL
performed the experiments. YW, XX, LH, and ZL analyzed the data.
YW, WL and ZL confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All animal protocols were approved by the
Institutional Animal Care and Use Committee of the Shaanxi
University of Chinese Medicine (approval no.
SUCMDL20190314001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barberio B, Judge C, Savarino EV and Ford
AC: Global prevalence of functional constipation according to the
Rome criteria: A systematic review and meta-analysis. Lancet
Gastroenterol Hepatol. 6:638–648. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen Z, Peng Y, Shi Q, Chen Y, Cao L, Jia
J, Liu C and Zhang J: Prevalence and risk factors of functional
constipation according to the Rome Criteria in China: A systematic
review and meta-analysis. Front Med (Lausanne).
9(815156)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vilanova-Sanchez A and Levitt MA: Surgical
interventions for functional constipation: An update. Eur J Pediatr
Surg. 30:413–419. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Koloski NA, Jones M, Wai R, Gill RS, Byles
J and Talley NJ: Impact of persistent constipation on
health-related quality of life and mortality in older
community-dwelling women. Am J Gastroenterol. 108:1152–1158.
2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bharucha AE and Lacy BE: Mechanisms,
evaluation, and management of chronic constipation.
Gastroenterology. 158:1232–1249 e3. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mori H, Verbeure W, Schol J, Carbone F and
Tack J: Gastrointestinal hormones and regulation of gastric
emptying. Curr Opin Endocrinol Diabetes Obes. 29:191–199.
2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yip JLK, Balasuriya GK, Spencer SJ and
Hill-Yardin EL: Examining enteric nervous system function in rat
and mouse: An inter-species comparison of gastrointestinal
motility. Am J Physiol Gastrointest Liver Physiol. 323:G477–G487.
2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lommatzsch M, Braun A, Mannsfeldt A,
Botchkarev VA, Botchkareva NV, Paus R, Fischer A, Lewin GR and Renz
H: Abundant production of brain-derived neurotrophic factor by
adult visceral epithelia. Implications for paracrine and
target-derived Neurotrophic functions. Am J Pathol. 155:1183–1193.
1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen FX, Yu YB, Yuan XM, Zuo XL and Li YQ:
Brain-derived neurotrophic factor enhances the contraction of
intestinal muscle strips induced by SP and CGRP in mice. Regul
Pept. 178:86–94. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yagasaki R, Shikaya Y, Kawachi T, Inaba M,
Takase Y and Takahashi Y: Newly raised anti-c-Kit antibody
visualizes morphology of interstitial cells of Cajal in the
developing gut of chicken embryos. Dev Growth Differ. 64:446–454.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Iino S, Horiguchi K and Horiguchi S:
c-Kit-stem cell factor signal-independent development of
interstitial cells of Cajal in murine small intestine. Cell Tissue
Res. 379:121–129. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Huizinga JD, Hussain A and Chen JH:
Interstitial cells of Cajal and human colon motility in health and
disease. Am J Physiol Gastrointest Liver Physiol. 321:G552–G575.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Na JR, Kim E, Na CS and Kim S: Citric
acid-enriched extract of ripe prunus mume (Siebold) Siebold &
Zucc. Induces laxative effects by regulating the expression of
aquaporin 3 and prostaglandin E2 in rats with loperamide-induced
constipation. J Med Food. 25:12–23. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rao SSC, Lacy BE, Emmanuel A,
Müller-Lissner S, Pohl D, Quigley EMM and Whorwell P: Recognizing
and defining occasional constipation: Expert consensus
recommendations. Am J Gastroenterol. 117:1753–1758. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Camilleri M, Ford AC, Mawe GM, Dinning PG,
Rao SS, Chey WD, Simrén M, Lembo A, Young-Fadok TM and Chang L:
Chronic constipation. Nat Rev Dis Primers. 3(17095)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Schiller LR: Chronic constipation: New
insights, better outcomes? Lancet Gastroenterol Hepatol. 4:873–882.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lu Z, He B, Chen J, Wu LJ, Chen XB, Ye SQ,
Yang WH, Shao ZY, Jin EG, Wang SJ, et al: Optimisation of the
Conversion and Extraction of Arctigenin From Fructus arctii Into
Arctiin Using Fungi. Front Microbiol. 12(663116)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xu X, Zeng XY, Cui YX, Li YB, Cheng JH,
Zhao XD, Xu GH, Ma J, Piao HN, Jin X and Piao LX: Antidepressive
effect of arctiin by attenuating neuroinflammation via HMGB1/TLR4-
and TNF-α/TNFR1-Mediated NF-κB activation. ACS Chem Neurosci.
11:2214–2230. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu X, Wang J, Dou P, Zhang X, Ran X, Liu
L and Dou D: The ameliorative effects of arctiin and arctigenin on
the oxidative injury of lung induced by silica via
TLR-4/NLRP3/TGF-β signaling pathway. Oxid Med Cell Longev.
2021(5598980)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fierascu RC, Georgiev MI, Fierascu I,
Ungureanu C, Avramescu SM, Ortan A, Georgescu MI, Sutan AN,
Zanfirescu A, Dinu-Pirvu CE, et al: Mitodepressive, antioxidant,
antifungal and anti-inflammatory effects of wild-growing Romanian
native Arctium lappa L. (Asteraceae) and Veronica persica Poiret
(Plantaginaceae). Food Chem Toxicol. 111:44–52. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hyam SR, Lee IA, Gu W, Kim KA, Jeong JJ,
Jang SE, Han MJ and Kim DH: Arctigenin ameliorates inflammation in
vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing
M1 macrophages to M2-like macrophages. Eur J Pharmacol. 708:21–29.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gao Q, Yang M and Zuo Z: Overview of the
anti-inflammatory effects, pharmacokinetic properties and clinical
efficacies of arctigenin and arctiin from Arctium lappa L. Acta
Pharmacol Sin. 39:787–801. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li K, Zhu L, Li H, Zhu Y, Pan C, Gao X and
Liu W: Structural characterization and rheological properties of a
pectin with anti-constipation activity from the roots of Arctium
lappa L. Carbohydr Polym. 215:119–129. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hayeeawaema F, Wichienchot S and Khuituan
P: Amelioration of gut dysbiosis and gastrointestinal motility by
konjac oligo-glucomannan on loperamide-induced constipation in
mice. Nutrition. 73(110715)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yan S, Yue YZ, Wang XP, Dong HL, Zhen SG,
Wu BS and Qian HH: Aqueous extracts of herba cistanche promoted
intestinal motility in loperamide-induced constipation rats by
ameliorating the interstitial cells of cajal. Evid Based Complement
Alternat Med. 2017(6236904)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ge X, Ding C, Zhao W, Xu L, Tian H, Gong
J, Zhu M, Li J and Li N: Antibiotics-induced depletion of mice
microbiota induces changes in host serotonin biosynthesis and
intestinal motility. J Transl Med. 15(13)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ikarashi N, Kon R and Sugiyama K:
Aquaporins in the colon as a new therapeutic target in diarrhea and
constipation. Int J Mol Sci. 17(1172)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Belsey J, Greenfield S, Candy D and
Geraint M: Systematic review: Impact of constipation on quality of
life in adults and children. Aliment Pharmacol Ther. 31:938–949.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Singh R, Zogg H, Ghoshal UC and Ro S:
Current treatment options and therapeutic insights for
gastrointestinal dysmotility and functional gastrointestinal
disorders. Front Pharmacol. 13(808195)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sanders KM, Koh SD, Ro S and Ward SM:
Regulation of gastrointestinal motility-insights from smooth muscle
biology. Nat Rev Gastroenterol Hepatol. 9:633–645. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Spencer NJ and Hu H: Enteric nervous
system: Sensory transduction, neural circuits and gastrointestinal
motility. Nat Rev Gastroenterol Hepatol. 17:338–351.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen F, Yu Y, Wang P, Dong Y, Wang T, Zuo
X and Li Y: Brain-derived neurotrophic factor accelerates gut
motility in slow-transit constipation. Acta Physiol (Oxf).
212:226–238. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ahn EH, Kang SS, Liu X, Cao X, Choi SY,
Musazzi L, Mehlen P and Ye K: BDNF and Netrin-1 repression by
C/EBPβ in the gut triggers Parkinson's disease pathologies,
associated with constipation and motor dysfunctions. Prog
Neurobiol. 198(101905)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Verbeure W, van Goor H, Mori H, van Beek
AP, Tack J and van Dijk PR: The role of gasotransmitters in gut
peptide actions. Front Pharmacol. 12(720703)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Balasuriya GK, Nugapitiya SS, Hill-Yardin
EL and Bornstein JC: Nitric oxide regulates estrus cycle dependent
colonic motility in mice. Front Neurosci. 15(647555)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bulbul A, Bulbul T, Sevimli A and Yilmaz
O: The effect of dietary supplementation of nitric oxide donor and
inhibitor on nNOS expression in and motility of the small intestine
of broilers. Biotech Histochem. 88:258–266. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Mattila JT and Thomas AC: Nitric oxide
synthase: Non-canonical expression patterns. Front Immunol.
5(478)2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhou X, Chen Y, Ma X, Yu Y, Yu X, Chen X
and Suo H: Efficacy of Bacillus coagulans BC01 on loperamide
hydrochloride-induced constipation model in Kunming mice. Front
Nutr. 9(964257)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Koh SD, Drumm BT, Lu H, Kim HJ, Ryoo SB,
Kim HU, Lee JY, Rhee PL, Wang Q, Gould TW, et al: Propulsive
colonic contractions are mediated by inhibition-driven poststimulus
responses that originate in interstitial cells of Cajal. Proc Natl
Acad Sci USA. 119(e2123020119)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Means SA and Cheng LK: Mitochondrial
calcium handling within the interstitial cells of Cajal. Am J
Physiol Gastrointest Liver Physiol. 307:G107–G121. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Drumm BT, Cobine CA and Baker SA: Insights
on gastrointestinal motility through the use of optogenetic sensors
and actuators. J Physiol. 600:3031–3052. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Drumm BT, Rembetski BE, Huynh K, Nizar A,
Baker SA and Sanders KM: Excitatory cholinergic responses in mouse
colon intramuscular interstitial cells of Cajal are due to enhanced
Ca2+ release via M3 receptor activation.
FASEB J. 34:10073–10095. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tan YY, Ji ZL, Zhao G, Jiang JR, Wang D
and Wang JM: Decreased SCF/c-kit signaling pathway contributes to
loss of interstitial cells of Cajal in gallstone disease. Int J
Clin Exp Med. 7:4099–4106. 2014.PubMed/NCBI
|
|
45
|
Lee SM, Kim N, Jo HJ, Park JH, Nam RH, Lee
HS, Kim HJ, Lee MY, Kim YS and Lee DH: Comparison of changes in the
interstitial cells of cajal and neuronal nitric oxide
synthase-positive neuronal cells with aging between the ascending
and descending colon of F344 Rats. J Neurogastroenterol Motil.
23:592–605. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen LL, Zhu J, Schumacher J, Wei C,
Ramdas L, Prieto VG, Jimenez A, Velasco MA, Tripp SR, Andtbacka
RHI, et al: SCF-KIT signaling induces endothelin-3 synthesis and
secretion: Thereby activates and regulates endothelin-B-receptor
for generating temporally- and spatially-precise nitric oxide to
modulate SCF- and or KIT-expressing cell functions. PLoS One.
12(e0184154)2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu YA, Chung YC, Shen MY, Pan ST, Kuo CW,
Peng SJ, Pasricha PJ and Tang SC: Perivascular interstitial cells
of cajal in human colon. Cell Mol Gastroenterol Hepatol. 1:102–119.
2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yde J, Keely SJ and Moeller HB:
Expression, regulation and function of Aquaporin-3 in colonic
epithelial cells. Biochim Biophys Acta Biomembr.
1863(183619)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ishibashi K, Tanaka Y and Morishita Y: The
role of mammalian superaquaporins inside the cell: An update.
Biochim Biophys Acta Biomembr. 1863(183617)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhu C, Chen Z and Jiang Z: Expression,
distribution and role of aquaporin water channels in human and
animal stomach and intestines. Int J Mol Sci.
17(1399)2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kon R, Ikarashi N, Hayakawa A, Haga Y,
Fueki A, Kusunoki Y, Tajima M, Ochiai W, Machida Y and Sugiyama K:
Morphine-Induced constipation develops with increased aquaporin-3
expression in the colon via increased serotonin secretion. Toxicol
Sci. 145:337–347. 2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ikarashi N, Kon R, Iizasa T, Suzuki N,
Hiruma R, Suenaga K, Toda T, Ishii M, Hoshino M, Ochiai W and
Sugiyama K: Inhibition of aquaporin-3 water channel in the colon
induces diarrhea. Biol Pharm Bull. 35:957–962. 2012.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ikarashi N, Ushiki T, Mochizuki T, Toda T,
Kudo T, Baba K, Ishii M, Ito K, Ochiai W and Sugiyama K: Effects of
magnesium sulphate administration on aquaporin 3 in rat
gastrointestinal tract. Biol Pharm Bull. 34:238–242.
2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Ren Z, Zhang X, Fan H, Yu Y, Yao S, Wang
Y, Dong Y, Deng H, Zuo Z, Deng Y, et al: Effects of different
dietary protein levels on intestinal aquaporins in weaned piglets.
J Anim Physiol Anim Nutr (Berl): May 9, 2022 (Epub ahead of
print).
|
|
55
|
Okada S, Misaka T, Matsumoto I, Watanabe H
and Abe K: Aquaporin-9 is expressed in a mucus-secreting goblet
cell subset in the small intestine. FEBS Lett. 540:157–162.
2003.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Meng Y, Li X, Wang X, Zhang L and Guan J:
Network pharmacological prediction and molecular docking analysis
of the combination of Atractylodes macrocephala Koidz and Paeonia
lactiflora. Pall. in the treatment of functional constipation and
its verification. Animal Model Exp Med. 5:120–132. 2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Albiani JJ, Hart SL, Katz L, Berian J, Del
Rosario A, Lee J and Varma M: Impact of depression and anxiety on
the quality of life of constipated patients. J Clin Psychol Med
Settings. 20:123–132. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Xu N, Fan W, Zhou X, Liu Y, Ma P, Qi S and
Gu B: Probiotics decrease depressive behaviors induced by
constipation via activating the AKT signaling pathway. Metab Brain
Dis. 33:1625–1633. 2018.PubMed/NCBI View Article : Google Scholar
|