Introduction
During the pandemic of COVID-19, the test of virus
specific antibody and RNA is an effective way to control the
outbreak of the virus. The test not only helps physicians to
diagnose a patient with SARS-CoV-2 infection and to assess whether
the patient is cured, but also to evaluate the patient's immunity
status to the virus. Generally, two types virus specific antibodies
will be evaluated in a fast serology test of SARS-CoV-2:
Immunoglobulin G(IgG) and immunoglobulin M(IgM). A positive result
of IgG indicates that an individual has been exposed to the virus
or has undergone SARS-CoV-2 vaccination and acquired an immunity to
the virus. A positive result of IgM indicates an individual might
be infected recently and further tests including SARS-CoV-2 RNA
should been performed to verify the result.
Waldenström's Macroglobulinemia (WM) is a rare B
cell lymphoma, which accounts for <2% of all non-Hodgkin
lymphomas. The manifestations of WM include: Lymphadenopathy,
hepatosplenomegaly, anemia, neurologic symptoms and infiltration of
organs in some cases. Hyperglobulinemia of monoclonal IgM protein
is the hallmark of the disease. Recently, somatic mutation of the
MYD88 gene has been reported in the majority of patients with WM
(1). Notably, the response to
SARS-CoV-2 vaccine attenuates in patients with WM (2,3).
The present study reports a patient with duplicate
positive serology tests of SARS-CoV-2 which is hypothesized to be
due to monoclonal IgM caused by WM.
Case report
An 86-year-old Chinese woman was admitted to Taizhou
Central Hospital (Taizhou University Hospital) in December 2020 as
she had suffered lumbago for years. She denied contact with
SARS-CoV-2 patients or SARS-CoV-2 vaccination history or travel to
SARS-CoV-2 outbreak areas. Although not suffering from a fever or
cough, she was isolated in a separate ward in the infectious
diseases department after her SARS-CoV-2 spike protein and
nucleocapsid specific IgM proved to be positive. Her thoracic
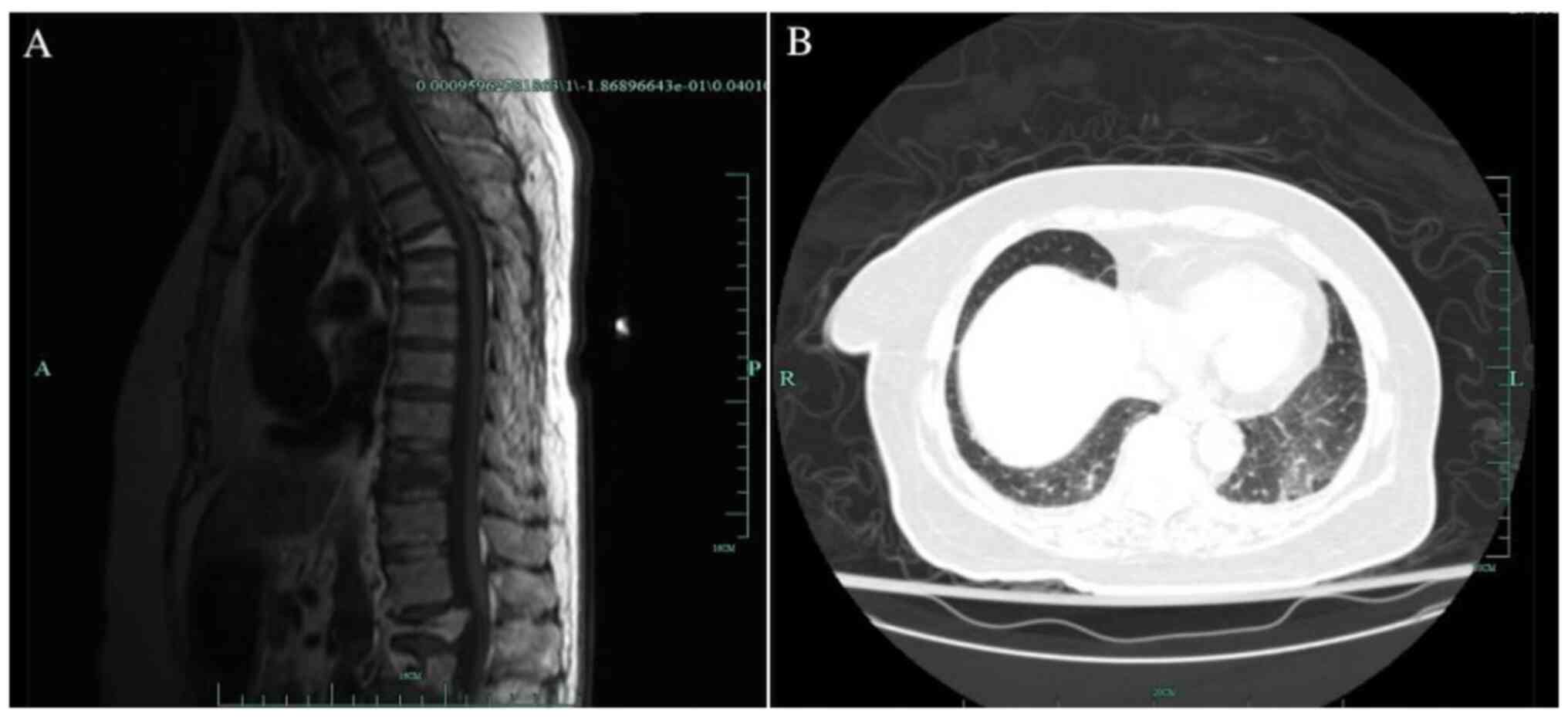
spinal MRI scan confirmed a fracture of 10th thoracic vertebra
which contributed to her lumbago. Her chest computerized tomography
(CT) scan showed mild exudation in the basal segment of bilateral
inferior lobes (Fig. 1). Repeated
serology and RNA tests for SARS-CoV-2 were performed. However, only
SARS-CoV-2 specific IgM was confirmed positive while SARS-CoV-2
specific IgG and RNA were negative (Table I).
 | Table ISerology and RNA tests for
SARS-CoV-2. |
Table I
Serology and RNA tests for
SARS-CoV-2.
| Days since
admission | 1 | 2 | 3 | 4 | 5 |
|---|
| IgM | + | + | + | + | + |
| IgG | - | - | - | - | - |
| RNA | - | - | - | - | - |
Further studies on her immunoglobulin showed a IgG
of 11.10 g/l, IgA of 0.81 g/l, IgM of 19.40 g/l, κ light chain of
18.70 g/l and λ light chain of 2.67 g/l. The light chain in her
urine was abnormal as well, with a κ light chain of 1,340.00 mg/l
and λ light chain <50.00 mg/l. Serum immunofixation
electrophoresis proved the existence of monoclonal IgM and κ light
chain. Tests for rheumatism including antinuclear antibody,
rheumatoid factor, anti-double stranded DNA antibody and anti SSA
and SSB antibodies were all negative. Serum tumor markers were also
negative.
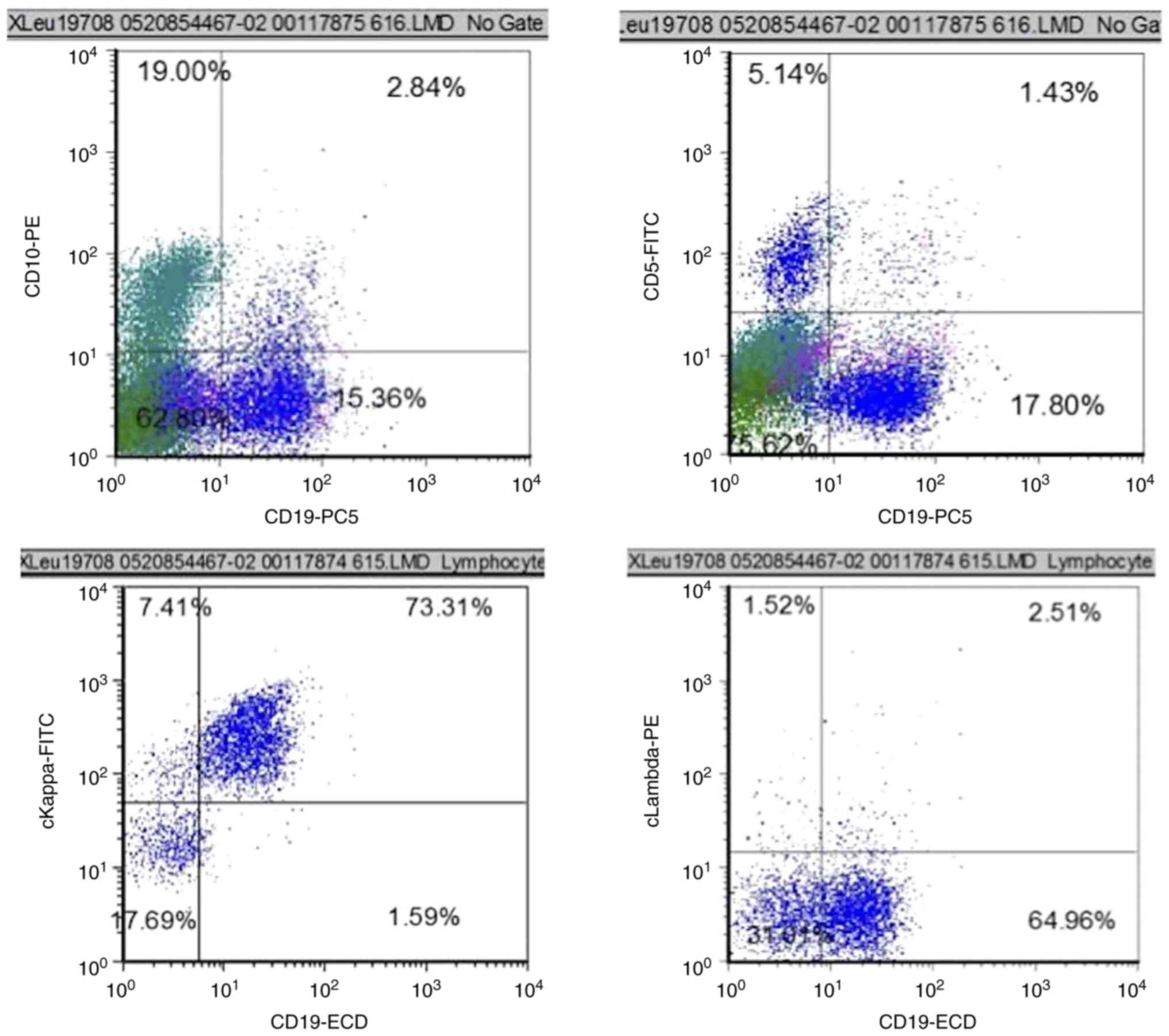
A bone marrow biopsy was then performed. The bone
marrow biopsy showed lymphocytes accounted for 32% of the total
nucleated cells and plasma cells 2%. Flow cytometric analysis
indicated a population of monoclonal B lymphocytes accounting for
17.4% of total nucleated cells in bone marrow. These B lymphocytes
were CD5 negative and CD10 dim in terms of immunophenotype
(Fig. 2). Droplet digital PCR
indicated a MYD88 L25P gene mutation with a mutation proportion of
17.9%. Taken together, she was diagnosed WM and transferred to
hematology department. She refused a vertebroplasty. Celecoxib was
given 200 mg twice a day to ameliorate her lumbago and alendronate
sodium 70 mg once a week to inhibit the bone destruction. Her
lumbago was alleviated after 2 weeks treatment and she was
discharged from hospital.
The present study was conducted according to the
guidelines of the Declaration of Helsinki and approved by the
ethics committee of Taizhou Central Hospital for publishing the
case details. Written informed consent was provided by the
participant in the study.
Discussion
Currently in China, it is a standard procedure to
perform SARS-CoV-2 screening before admitting patients into the
inpatient department. The screening strategy varies among different
hospitals in China. At Taizhou Central Hospital (Taizhou University
Hospital), it is mandatory to test SARS-CoV-2 specific IgG, IgM and
RNA simultaneously before a patient is hospitalized. Previous
studies reported that SARS-CoV-2 specific IgM could be detected in
patients at 1 week after onset. The level of the IgM peaks at the
second week, and begins to decline at the third or fourth week.
However, SARS-CoV-2 specific IgG emerges later than IgM. The medium
time of SARS-CoV-2 specific IgG to be detected is 12 days to 14
days. The level of the IgG rises fast and peaks at the third or
fourth week (4-9).
In general, IgG antibody could maintain in peripheral blood for a
long time. However, Long et al (10) reported that the level of SARS-CoV-2
specific IgG declined by 70% at the second month after SARS-CoV-2
infection in >90% infected patients, which is different to the
change of SARS specific IgG. This indicates that SARS-CoV-2
specific IgG might not protect patients against the second
infection.
The present study adopted the gold
immunochromatography assay (GICA) to test SARS-CoV-2 specific IgG
and IgM. The sensitivity and specificity of the assay for
anti-SARS-CoV-2 IgG are 0.85 and 0.99 respectively, and 0.74, 0.99
for IgM respectively. Pan et al (11) used GICA to analyze 86 samples of 67
SARS-CoV-2 patients who were confirm by reverse transcription (RT)
PCR and found that the IgM positive rate increased from 11.1% in
early stage (1-7 days after onset) to 78.6 and 74.2% in
intermediate stage (8-14 days after onset) and late stage (>15
days), respectively. The IgG positive rate increased from 3.6% in
early stage, 57.1% in intermediate stage to 96.8% in late stage,
respectively. Notably, IgM and IgG combinatorial detection
significantly increased the sensitivity of GICA, especially at the
intermediate stage. Nevertheless, several studies and case reports
showed that the result of GICA could be interfered with by
auto-immune diseases, Kawasaki disease, pregnancy or other
conditions. A high level of rheumatoid factor brings a marked false
positiveness of SARS-CoV-2 specific antibodies (12-15).
According to European Hematology Association's consensus statement
on the management of WM patients during the SARS-CoV-2 pandemic,
serological lab tests for SARS-CoV-2 specific IgM should not be
affected by the total IgM or paraprotein levels (16). However, in the present case, the
patient had no history of contacting any SARS-CoV-2 patients or
traveling to a SARS-CoV-2 outbreak area. Neither had she any signs
of rheumatoid diseases or malignancies. In addition, the CT scan
and the dynamic assays of her SARS-CoV-2 specific IgM, IgG and RNA
did not fulfill the criteria of SARS-CoV-2 pneumonia. As a result,
her SARS-CoV-2 specific IgM is considered to be false positive due
to an immune cross-reactivity between SARS-CoV-2 and WM
paraprotein. It should be noted that the patient's titer of the IgM
was not measured.
Several conditions could attribute to false positive
result of SARS-CoV-2 specific antibodies with GICA. The GICA result
should be interpreted in the context of patients' clinical
manifestation and RT-PCR test, together with a consistent
radiological picture. In spite of the European Hematology
Association's consensus statement, SARS-CoV-2 specific IgM test by
GICA could be affected by monoclonal IgM. Further study is
warranted.
Acknowledgements
The authors thank Professor Sai Chen from the
Hematology Department of Taizhou Central Hospital (Taizhou
University Hospital) for providing general support to the present
study.
Funding
Funding: The present study was funded by grant from Taizhou
Science and Technology Bureau (grant no. 20ywa31).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YQZ and HRS conceived the present study. LZL and XYY
were responsible for the methodology. LLX, NNL and HRS were
responsible for the investigation. LZL and BYS checked assays
results, the raw data and validated the authenticity of the data.
LLX and NNL wrote the original draft. HRS and NNL were responsible
for review and editing. LLX was responsible for funding
acquisition. LZL and BYS confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki and approved by the
ethics committee of Taizhou Central Hospital for publishing the
case details. Written informed consent was provided by the
participant in the study.
Patient consent for publication
The patient provided the consent for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Varettoni M, Zibellini S, Defrancesco I,
Ferretti VV, Rizzo E, Malcovati L, Gallì A, Porta MGD, Boveri E,
Arcaini L, et al: Pattern of somatic mutations in patients with
Waldenström macroglobulinemia or IgM monoclonal gammopathy of
undetermined significance. Haematologica. 102:2077–2085.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gavriatopoulou M, Terpos E,
Ntanasis-Stathopoulos I, Briasoulis A, Gumeni S, Malandrakis P,
Fotiou D, Migkou M, Theodorakakou F, Eleutherakis-Papaiakovou E, et
al: Poor neutralizing antibody responses in 106 patients with WM
after vaccination against SARS-CoV-2: a prospective study. Blood
Adv. 5:4398–4405. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Konishi Y, Sklavenitis-Pistofidis R, Yue
H, Ferrari F, Redd RA, Lightbody ED, Russo M, Perry J, Horowitz E,
Justis AV, et al: Attenuated response to SARS-CoV-2 vaccine in
patients with asymptomatic precursor stages of multiple myeloma and
Waldenstrom macroglobulinemia. Cancer Cell. 40:6–8. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhao HT, Peng ZB, Yang XK, Li ZL, Ren MR,
Qin Y, Sun XJ, Yu JX, An ZJ, Mao NY, et al: Progress in research of
specific antibody dynamic characteristics in patients with
COVID-19. Zhonghua Liu Xing Bing Xue Za Zhi. 42:39–43.
2021.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
5
|
Guo L, Ren L, Yang S, Xiao M, Chang D,
Yang F, Dela Cruz CS, Wang Y, Wu C, Xiao Y, et al: Profiling early
humoral response to diagnose novel coronavirus disease (COVID-19).
Clin Infect Dis. 71:778–785. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hou H, Wang T, Zhang B, Luo Y, Mao L, Wang
F, Wu S and Sun Z: Detection of IgM and IgG antibodies in patients
with coronavirus disease 2019. Clin Transl Immunology.
9(e01136)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lou B, Li TD, Zheng SF, Su YY, Li ZY, Liu
W, Yu F, Ge SX, Zou QD, Yuan Q, et al: Serology characteristics of
SARS-CoV-2 infection after exposure and post-symptom onset. Eur
Respir J. 56(2000763)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xiao AT, Gao C and Zhang S: Profile of
specific antibodies to SARS-CoV-2: The first report. J Infect.
81:147–178. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang G, Nie S and Zhang Z and Zhang Z:
Longitudinal change of severe acute respiratory syndrome
coronavirus 2 antibodies in patients with coronavirus disease 2019.
J Infect Dis. 222:183–188. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Long QX, Tang XJ, Shi QL, Li Q, Deng HJ,
Yuan J, Hu JL, Xu W, Zhang Y, Lv FJ, et al: Clinical and
immunological assessment of asymptomatic SARS-CoV-2 infections. Nat
Med. 26:1200–1204. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pan Y, Li X, Yang G, Fan J, Tang Y, Zhao
J, Long X, Guo S, Zhao Z, Liu Y, et al: Serological
immunochromatographic approach in diagnosis with SARS-CoV-2
infected COVID-19 patients. J Infect. 81:e28–e32. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
To KK, Chua GT, Kwok KL, Wong JS, Au DCY,
Lam YY, Wong WH, Ho MH, Chan GC, Chui CS, et al: False-positive
SARS-CoV-2 serology in 3 children with Kawasaki disease. Diagn
Microbiol Infect Dis. 98(115141)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tzouvelekis A, Karampitsakos T, Krompa A,
Markozannes E and Bouros D: False positive COVID-19 antibody test
in a case of granulomatosis with polyangiitis. Front Med
(Lausanne). 7(399)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang Q, Du Q, Guo B, Mu D, Lu X, Ma Q, Guo
Y, Fang L, Zhang B, Zhang G and Guo X: A method to prevent
SARS-CoV-2 IgM false positives in gold immunochromatography and
enzyme-linked immunosorbent assays. J Clin Microbiol. 58:e00375–20.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xiao X, Duan J, Wu S, Yang B, Zhu J and
Cui L: False-positive results of gold immunochromatography assay
for IgM and IgG antibodies to 2019-nCoV. Chin J Lab Med.
43:1080–1085. 2020.
|
|
16
|
Talaulikar D, Advani RH, Branagan AR,
Buske C, Dimopoulos MA, D'Sa S, Kersten MJ, Leblond V, Minnema MC,
Owen RG, et al: Consensus statement on the management of
waldenström macroglobulinemia patients during the COVID-19
pandemic. Hemasphere. 4(e433)2020.PubMed/NCBI View Article : Google Scholar
|