Introduction
Meningitis is a life-threatening medical condition,
in which the inflammatory response plays a key role in the
pathogenesis of cerebral injury associated with different
aetiologies (1). Viral meningitis
(VM) is the most common form of the disease; it has a favourable
prognosis, only requires supportive treatment to alleviate the
symptoms, and resolves in 7-10 days (2,3). By
contrast, the course of bacterial meningitis (BM) is much more
severe. Despite marked improvements in intensive care therapy,
powerful antibiotic therapy and large-scale vaccination,
contracting BM is fatal in 5-10% of children and 20-30% of adults
(4,5). In addition, ~40% of survivors suffer
neurological sequelae (6,7). Classically, the diagnosis of
meningitis involves a lumbar puncture (LP), and analysis of the
collected cerebrospinal fluid (CSF) to determine its protein,
glucose and lactate levels, cell counts, Gram staining results,
cultures, supernatant colour and latex agglutination reactions, in
addition to polymerase chain reaction (PCR) assays (8). However, these methods may fail to
provide the required results in a timely manner. BM can be
challenging to differentiate from aseptic meningitis/encephalitis
as the clinical characteristics such as neck stiffness, fever and
altered mental status are common to both conditions but are
observed in only 41% of patients with BM (3,9). As
the delayed initiation of treatment of BM is strongly associated
with poor outcomes (10), empiric
antibiotic therapy and adjunctive corticosteroids are usually
started early (8,11), leading to frequent cases of
improper treatment with associated health and economic impacts
(12). Therefore, the
identification of early infection biomarkers to help discriminate
BM from non-BM would be highly important.
In recent years, increasing importance has been
ascribed to the cytokine/chemokine (CT/CK) levels of patients with
meningitis to identify particularities of the host immune response
that could represent a ‘host signature’ of infection (13-18).
CTs/CKs released during the host immune response have an important
role in the recruitment of innate and adaptive immune cells, but
they also promote inflammation, thus playing an essential role in
the pathogenesis of meningitis. Therefore, it may be hypothesised
that the invading bacteria influence CT/CK concentrations and
profiles, affecting disease severity and patient outcomes,
including long-term sequelae and survival (19). In meningitis, CTs/CKs have been
found to contribute to the loss of integrity of the blood-brain
barrier (BBB) (20) and favour
pleocytosis (21). They can even
induce neuronal cell death, either by increasing the secretion of
neurotoxic products by microglia (22) or by the direct activation of
apoptotic pathways (23-25).
In this context, CTs/CKs have been the subject of
numerous studies regarding the pathogenesis of meningitis. For
example, data from clinical observations and animal studies have
frequently demonstrated that CTs/CKs including tumour necrosis
factor-α (TNF-α), interleukin (IL)-1β, IL-6 and IL-8 are involved
in the inflammatory cascades in VM and BM, and mediate neuronal
damage (4,13,19).
Also, the intrathecal injection of TNF-α has been shown to
reproduce some of the pathological features of meningitis (26).
In one study, higher levels of IL-6, interferon-γ
(IFN-γ) and IL-10 were detected in the CSF of children with BM
compared with those without BM (15). In another study, it was found that
epithelial-neutrophil activating peptide-78 (ENA-78) was
undetectable in patients with aseptic meningitis or control
subjects but was present at high concentrations in patients with BM
(27). In an analysis of IL-1β,
IL-6, TNF-α, IFN-γ and IL-10 in CSF samples to distinguish between
various pathogens causing BM in patients of all ages (28), the authors found that only IFN-γ
was significantly higher in patients with Streptococcus
pneumoniae than in those with Neisseria meningitidis.
IL-6 has been demonstrated to be an important tool for the
diagnosis of BM (29,30). The authors of one study (31) suggested that the CSF/blood IL-6
ratio could be a promising biomarker for the discrimination of BM.
In addition, another study found that IL-1β and IL-18 levels in the
CSF of patients with BM were associated with systemic complications
and the survival rate (29).
Furthermore, in a study of BM in infants, the authors found that
IL-6 and IL-10 were valuable tools for planning the course of
treatment for culture-negative but antibiotic-pretreated subjects
(30).
Previous studies have shown that the analysis of
biomarkers in the CSF is promising for the discrimination of
patients with BM or VM, or without meningitis, while they have not
reached a consensus on what biomarkers are most appropriate
(27-29,31).
Therefore, reliable CT/CK tests for the discrimination of BM from
VM are not yet available to clinicians. In the present study, to
further explore the discriminating power of various CTs/CKs, the
concentrations of nine CTs/CKs relevant to the pathophysiology of
BM were assessed in CSF samples from patients with BM, VM and
meningism, and the correlations of defined CT/CK profiles with
other CSF parameters were investigated. Instead of focusing on
specific threshold values for specific CTs/CKs, the approach was to
evaluate whether a pattern of CTs/CKs could be robustly and
accurately powerful for discriminating between BM and VM. In this
respect, the study sought to predict BM using a machine learning
approach, namely the Random Forest (RF) algorithm.
Materials and methods
Study design
Residual CSF samples from the diagnostic LP of 145
patients of all ages who were admitted with a suspicion of
meningitis from October 2014 to July 2017 to the Clinical Hospital
for Infectious and Tropical Diseases ‘Dr Victor Babes’ were used in
the present study. The study followed the ethics policies on human
subject research of the Clinical Hospital for Infectious and
Tropical Diseases ‘Dr Victor Babes’, and was approved by the
Medical Ethics Committee of the hospital (approval no. 5105).
Written informed consent forms were signed by the patients or their
legal representatives before the samples requested by the attending
clinician were collected and analysed. Only the CSF specimens that
remained after the completion of routine analysis and diagnostic
procedures were used in the current research. Under no
circumstances were samples collected for any purpose other than
standard analysis for diagnosis. Data were gathered and kept in a
coded and securely stored electronic database.
The criteria for meningitis diagnosis at hospital
admission were the presence of two or more of the following
clinical signs: Fever, neck stiffness, meningeal irritation signs,
headache, altered consciousness and vomiting. The epidemiological
criteria were: Contact with other known cases, community origin
such as in an orphanage, nursery or army, recent travel to areas in
which meningitis is endemic (Africa and Asia) and VM epidemic.
According to the CSF cytochemical profile and
bacterial or viral detection results, patients were divided into
the BM group, VM group and non-meningitis control group (C group).
The CSF characteristics for the definition of BM according to the
clinical protocol were pleocytosis (100-5,000
leucocytes/mm3), the predominance of neutrophils, turbid
CSF, CSF glucose <50 mg/dl, CSF protein 0.5-2 g/l, positive
bacterial CSF culture or Gram stain, positive CSF latex
agglutination assay or PCR assay for a bacterial pathogen. The CSF
findings for the definition of VM were clear CSF, 50-100
leucocytes/mm3, CSF glucose >50 mg/dl, CSF protein
0.4-0.8 g/l and positive PCR, GenExpert or BioFire FilmArray
results.
For the patients considered in the study design, the
following inclusion and exclusion criteria were employed: Only
samples with positive culture or Gram stain, CSF latex
agglutination or PCR confirmation were included in the BM group;
for VM, the inclusion criteria comprised positive PCR, GenExpert or
BioFire FilmArray results; the C group included patients admitted
for meningitis who did not test positive in any of the
aforementioned tests. Exclusion criteria were as follows: Patients
with brain tumours, tuberculous meningitis, fungal meningitis,
skull fractures and human immunodeficiency virus infection.
Sample collection and detection
Samples were collected under full aseptic
precautions by LP at the time of admission to the hospital and were
analysed by the standard routine for the diagnosis of meningitis.
After undergoing routine analysis, the remaining CSF specimens were
centrifuged to remove cellular debris (715 x g for 20 min at 4˚C).
The supernatants were divided into aliquots to optimise multiple
freeze-thaw cycles and stored at -80˚C until assayed. Each sample
was measured on the first thaw. The protein and glucose levels were
evaluated in the CSF samples, and the white blood cell (WBC) count
was determined. CT/CK contents in the CSF were measured
simultaneously using Human Multianalyte Profiling Base Kit A
(R&D Systems, Inc.). A panel of nine CTs/CKs, comprising
monocyte chemoattractant protein-1 (MCP-1), IL-8, IL-1β, IL-6,
macrophage inflammatory protein-1α (MIP-1α), ENA-78, IFN-γ, IL-10
and TNF-α, was selected and assessed according to the
manufacturer's protocol. Briefly, the standards and 4-fold diluted
samples were incubated with antibody-coated fluorescent
microspheres overnight at 4˚C. After washing, the samples were
incubated with biotinylated antibodies. Following another wash, a
streptavidin-phycoerythrin conjugate was added. Following the final
wash, the fluorescent microspheres were resuspended in the assay
buffer and analysed using a Luminex 200™ detection
platform (Luminex Corporation). Data were processed with Luminex
200 IS 2.3 Star Station software (Applied Cytometry). A 5-parameter
regression formula weighted with reciprocal y (1/y) was used to
calculate the sample concentrations from the standard curves.
Statistical analysis
Statistical analyses were performed using the R
programming language version 3.6.3 (https://www.r-project.org) and R Studio version
1.4.1106 (http://www.rstudio.com/). CSF
cytochemical parameters, including proteins, WBC count, glucose,
age and CT/CK levels were compared overall among groups using the
Kruskal-Wallis rank-sum test in R. P-values adjusted for multiple
pairwise comparisons were calculated using Dunn's test (dunn.test
version 1.3.5 in R) with Bonferroni corrections. Two-sided unpaired
two-sample Wilcoxon test in R was used to determine if
statistically significant differences existed between two groups
when the BM group was split into gram-positive and -negative
groups. Differences in sex distribution were assessed using
Pearson's Chi-square test in R. Samples with CT/CK levels <1
pg/ml were arbitrarily assigned as 1 pg/ml, and the CT/CK levels in
CSF specimens were compared after the log10
transformation of data. Categorical variables are presented as
proportions or percentages. Continuous variables are expressed as
the median and interquartile range (IQR). The ggplot2 R package
version 3.3.5 was used for data visualisation.
Correlation matrices were obtained using the
corrplot library in R (https://www.rdocumentation.org/packages/corrplot/versions/0.92),
and displayed as schematic correlograms. Spearman's correlation
coefficient (r) was used to evaluate the correlation between
specific variables. The package also provided P-values and
confidence intervals for the correlations.
Hierarchical clustering of the log-transformed data
matrix was performed to classify patient groups according to
different CT/CK types and the heatmap.2 library in R was used to
produce heatmaps (https://www.rdocumentation.org/packages/gplots/versions/2.3.0/topics/heatmap.2).
For all statistical tests, P<0.01 was considered to indicate a
statistically significant result.
Prediction of the BM cases was achieved with a
machine learning approach, via RF analysis, using the package
randomForest in R (https://www.rdocumentation.org/packages/randomForest/versions/4.7-1.1/topics/randomForest).
The method was applied to the dataset comprising all 119 BM and VM
samples from the 145 CSF samples. The samples were randomly split
into a training set (n=90) and a testing set (n=29). The samples
from the testing set were not used for training the prediction
model; they were only used to evaluate its performance. The
training set was used to build the model with the RF method
optimised using 10-fold cross-validation, repeated 10 times.
Results
Demographic, clinical and laboratory
findings
CSF samples were collected at the time of hospital
admission from 162 patients. These patients included 17 individuals
(10.5%) who had a clinical diagnosis of BM based on clinical
symptoms, CSF cytochemical findings and therapeutic response to the
antibiotic treatment but for whom no causative agent could be
identified. Therefore, these patients were not included in the
study. Of the remaining 145 patients, 85 (59%) were males. BM was
confirmed in 61 (42%) patients and VM in 58 (40%) patients. As the
collection of CSF is an invasive procedure that is only performed
in emergencies, it was not possible to include healthy controls.
The controls (C group) comprised 26 (18%) patients with symptoms
who tested negative for meningitis (Table I). The median (IQR) age of the
patients with BM was 40 (12-57) years as compared with 14 (9-36)
years for the patients with VM and 11 (5-29) years for the C group.
While the median age varied among the groups, the difference did
not reach statistical significance (P>0.01). Among the patients
with BM, 35 (57%) were males; the VM group comprised 35 (60%) males
and the C group comprised 15 (58%) males (Table I). The sex distribution was not
significantly different among the three groups (P>0.01).
 | Table ICharacteristics of patients included
in the study. |
Table I
Characteristics of patients included
in the study.
|
Characteristics | Overall cohort | Bacterial
meningitis | Viral
meningitis | Controls |
|---|
| Samples, n (%) | 145 | 61(42) | 58(40) | 26(18) |
| Adults, n (%) | 74(51) | 44(72) | 22(38) | 8(31) |
| Age, median (IQR),
years | 21 (8-47) | 40 (12-57) | 14 (9-36) | 11(5-29) |
| Male sex, n
(%) | 85(59) | 35(57) | 35(60) | 15(58) |
Of the 61 BM samples, gram-positive bacteria were
identified in 38 samples, which included Streptococcus
pneumoniae (n=31, 51%), Listeria monocytogenes (n=4,
7%), Streptococcus beta haemolyticus (n=1, 2%),
Staphylococcus haemolyticus (n=1, 2%) and Streptococcus
suis (n=1, 2%), whereas gram-negative bacteria were identified
in 23 samples, and included Neisseria meningitidis (n=16,
26%), Haemophylus influenzae (n=4, 7%) and Escherichia
coli (n=3, 5%).
CT/CK profiles of the BM, VM and C
groups
The levels of IL-1β, IL-6, IL-8, IL-10, TNF-α,
MIP-1α, MCP-1, IFN-γ and ENA-78 in the CSF samples were measured
using a multiplex CT assay. The matrix of
log10-transformed data of individual CT/CK
concentrations for each patient was used for hierarchical
clustering to examine if inflammatory response patterns could be
defined in the 145 patients. A heatmap (Fig. 1) was built using an agglomeration
algorithm to visualise the automatic grouping of samples based on
the CT/CK pattern determined in the CSF. In this figure, the
segregation of samples with the same clinical diagnostics can be
observed in clusters characterised by a particular CT/CK profile.
The hierarchical clustering of the 145 samples in the heatmap,
based on Euclidean distance in CT/CK secretion, showed moderate
grouping according to aetiology, with cases of BM mainly being
grouped in a separate branch. However, this analysis clustered
numerous BM samples with those of the VM and C groups.
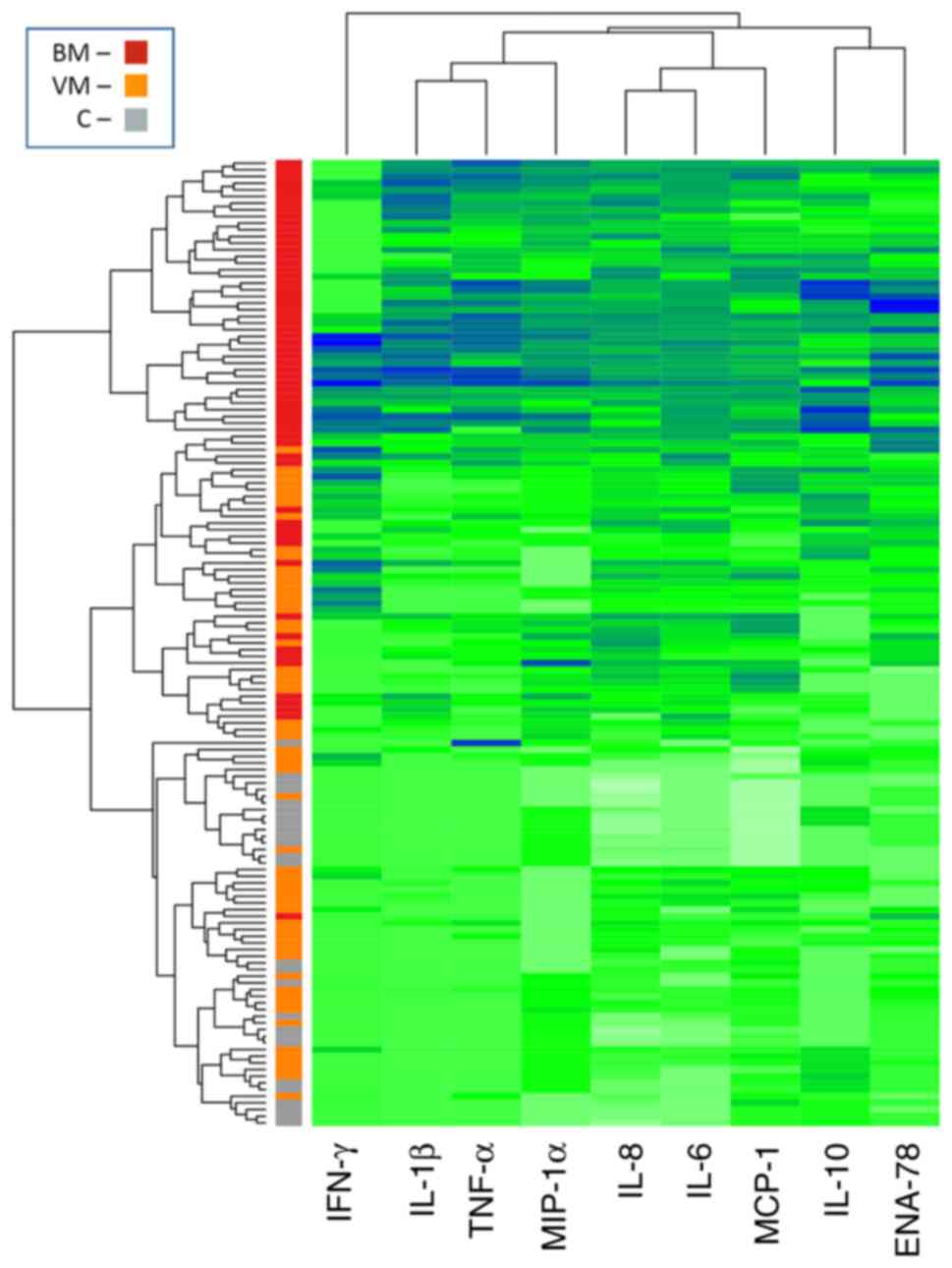 | Figure 1Hierarchical clustering of 145
cerebrospinal fluid samples based on CT/CK levels. The spectrum
from white to green to blue corresponds to the increasing gradient
of log10-transformed CT/CK concentrations. Each patient group is
reported as a colour code on the left column, where red represents
the BM group, orange represents the VM group and grey represents
the C group. CT, cytokine; CK, chemokine; BM, bacterial meningitis;
VM, viral meningitis; C, control; IFN-γ, interferon-γ; IL,
interleukin; TNF-α, tumor necrosis factor-α; MIP-1α, macrophage
inflammatory protein-1α; MCP-1, monocyte chemoattractant protein-1;
ENA-78, epithelial-neutrophil activating peptide-78. |
To better illustrate the differences between groups,
the median levels and IQR of the CSF cytochemical parameters WBC
count, glucose and proteins (Table
II) and the nine measured CTs/CKs (Table III) were compared between the BM
and C groups, VM and C groups and BM and VM groups. The comparison
revealed that the levels of all CTs/CKs were significantly higher
in the BM group compared with the C group (P<0.01). As compared
with CT/CK levels in the VM group, those in the BM group were
significantly higher (P<0.01), except for IFN-γ (P=0.192). While
the CT/CK levels were generally higher in the VM group than in the
C group, the differences did not reach statistical significance for
IL-1β, MIP-1α, ENA-78, IL-10 and TNF-α. However, significantly
higher levels of MCP-1, IL-8, IL-6 and IFN-γ were measured in
samples from the VM group compared with those from the C group
(Table III, Fig. 2).
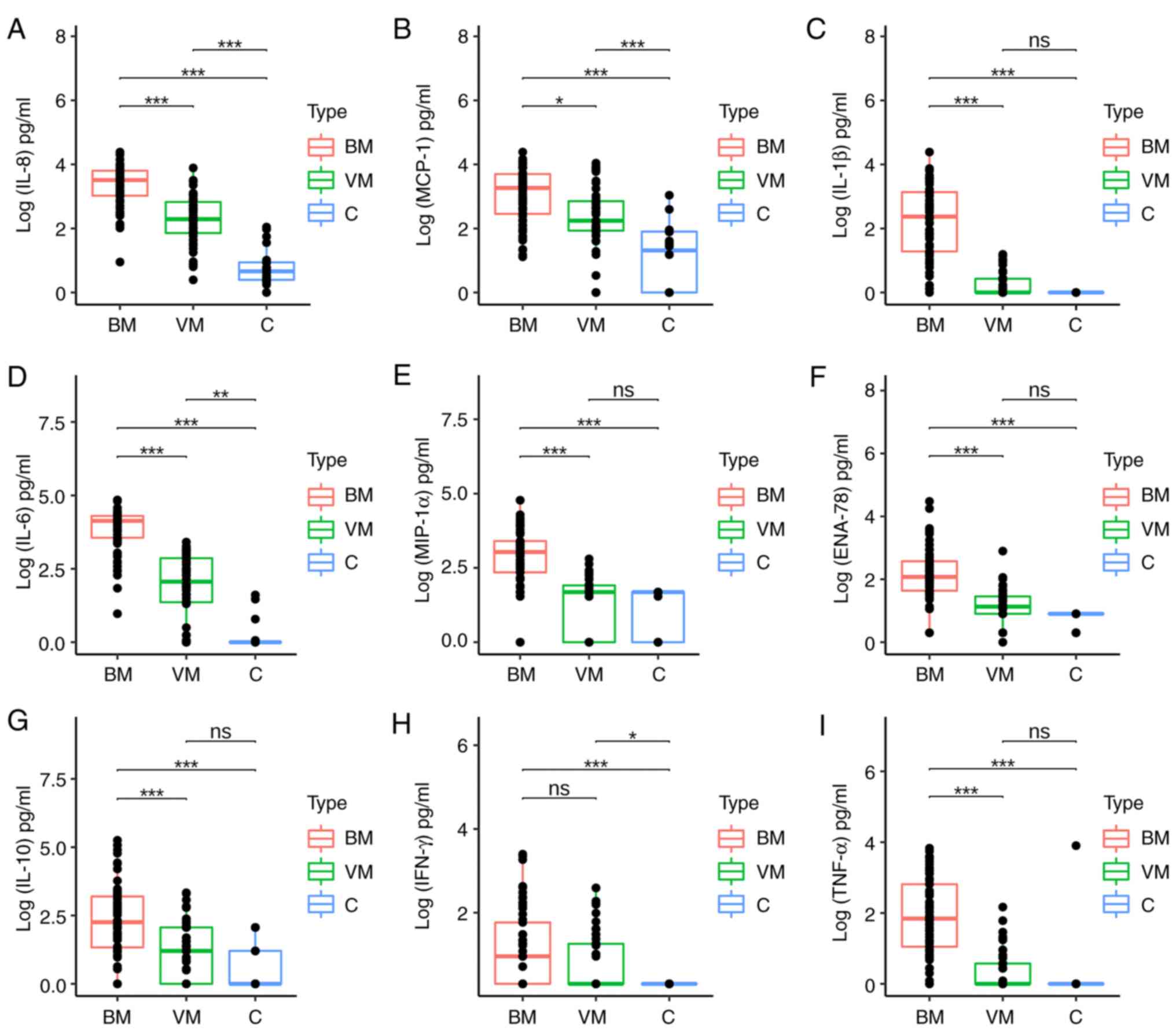 | Figure 2Comparison of
log10-transformed CT/CK concentrations among the study
groups, BM, VM and C. Concentrations of (A) IL-8, (B) MCP-1, (C)
IL-1β, (D) IL-6, (E) MIP-1α, (F) ENA-78, (G) IL-10, (H) IFN-γ and
(I) TNF-α. The boxes represent the IQR, the horizontal lines inside
each box represent the median, and the dots represent individual
measurements. Significance was calculated using Dunn's test with
Bonferroni post hoc tests, following Kruskal-Wallis testing.
P-values adjusted for multiple comparisons are indicated above each
plot. ns, P>0.01 (not significant), *P<0.01,
**P<0.001, ***P<0.0001. CT, cytokine;
CK, chemokine; BM, bacterial meningitis; VM, viral meningitis; C,
control; IL, interleukin; MCP-1, monocyte chemoattractant
protein-1; MIP-1α, macrophage inflammatory protein-1α; ENA-78,
epithelial-neutrophil activating peptide-78; IFN-γ, interferon-γ;
TNF-α, tumor necrosis factor-α. |
 | Table IILaboratory findings for the CSF
samples. |
Table II
Laboratory findings for the CSF
samples.
| | Aetiological
group | bP-value |
|---|
| CSF variable | BM, median
(IQR) | VM, median
(IQR) | C, median
(IQR) | aP-value | BM vs. VM | BM vs. C | VM vs. C |
|---|
| WBC,
cells/mm3 | 3,750
(1,360-12,985) | 156 (74-261) | 2 (1-4) | <0.01 | <0.01 | <0.01 | <0.01 |
| Glucose, mg/dl | 25 (24-39) | 61 (51-77) | 66 (53-76) | <0.01 | <0.01 | <0.01 | 1 |
| Protein, mg/dl | 260 (158-401) | 57 (33-87) | 21 (15-32) | <0.01 | <0.01 | <0.01 | 0.015 |
 | Table IIICT/CK levels in the cerebrospinal
fluid samples of all studied patients. |
Table III
CT/CK levels in the cerebrospinal
fluid samples of all studied patients.
| | Aetiological
group | bP-value |
|---|
| CT/CK | BM, median (IQR),
pg/ml | VM, median (IQR),
pg/ml | C, median (IQR),
pg/ml | aP-value | BM vs. VM | BM vs. C | VM vs. C |
|---|
| MCP-1 | 1,840
(286-5,007) | 174 (86-714) | 21.6 (1-80) | <0.01 | <0.01 | <0.01 | <0.01 |
| IL-8 | 3,233
(1,055-6,365) | 196 (72-674) | 4.6 (2.4-8.7) | <0.01 | <0.01 | <0.01 | <0.01 |
| IL-1β | 235 (19-1,364) | 1 (1-2.7) | 1 (1-1) | <0.01 | <0.01 | <0.01 | 0.074 |
| IL-6 | 13,585
(3,622-20,000) | 117 (23-728) | 1 (1-1) | <0.01 | <0.01 | <0.01 | <0.01 |
| MIP-1α | 1,079
(224-2,531) | 48 (91-82) | 48 (1-48) | <0.01 | <0.01 | <0.01 | 0.717 |
| ENA-78 | 119 (44-374) | 14 (8-29) | 8 (8-8) | <0.01 | <0.01 | <0.01 | 0.017 |
| IFN-γ | 9 (2-58) | 2 (2-18) | 2 (2-2) | <0.01 | 0.192 | <0.01 | <0.01 |
| IL-10 | 180 (22-1,579) | 16 (1-116) | 1 (1-16) | <0.01 | <0.01 | <0.01 | 0.094 |
| TNF-α | 70 (11-650) | 1 (1-3.7) | 1 (1-1) | <0.01 | <0.01 | <0.01 | 0.145 |
When the BM group was split into gram-positive and
-negative samples, the comparison showed no significant difference
between the two categories, except for IFN-γ levels (Table SI), which were higher in the
patients with pneumococcal meningitis (P=0.01).
Data for WBC, protein and glucose values in CSF were
available for 54 BM cases, 27 VM cases and 26 controls. The median
CSF WBC count and protein levels were significantly elevated and
the median CSF glucose level was significantly decreased in the
group with confirmed BM compared with the other two groups
(P<0.01; Table II).
Correlation between CT/CK
concentrations and CSF parameters
The correlations between different CSF CT/CK levels
and between CTs/CKs and CSF WBC counts, protein and glucose levels
were examined. All the correlations were performed using Spearman's
correlation analysis. The correlation matrices are displayed as
schematic correlograms and only the correlations with a statistical
significance of P<0.01 are shown (Fig. 3). Correlations between CSF
cytochemical markers and CT/CK values in the BM group showed that
glucose negatively correlated with the proteins ENA-78, IL-10, IL-6
and TNF- α while IL-1β positively correlated with the WBC count. In
the VM group, a negative correlation was detected between glucose
and protein levels and a positive correlation was identified
between WBC counts and IL-6. However, as data for CSF cytochemical
markers were not available for all the patients in the study, only
the correlations among CTs/CKs are shown.
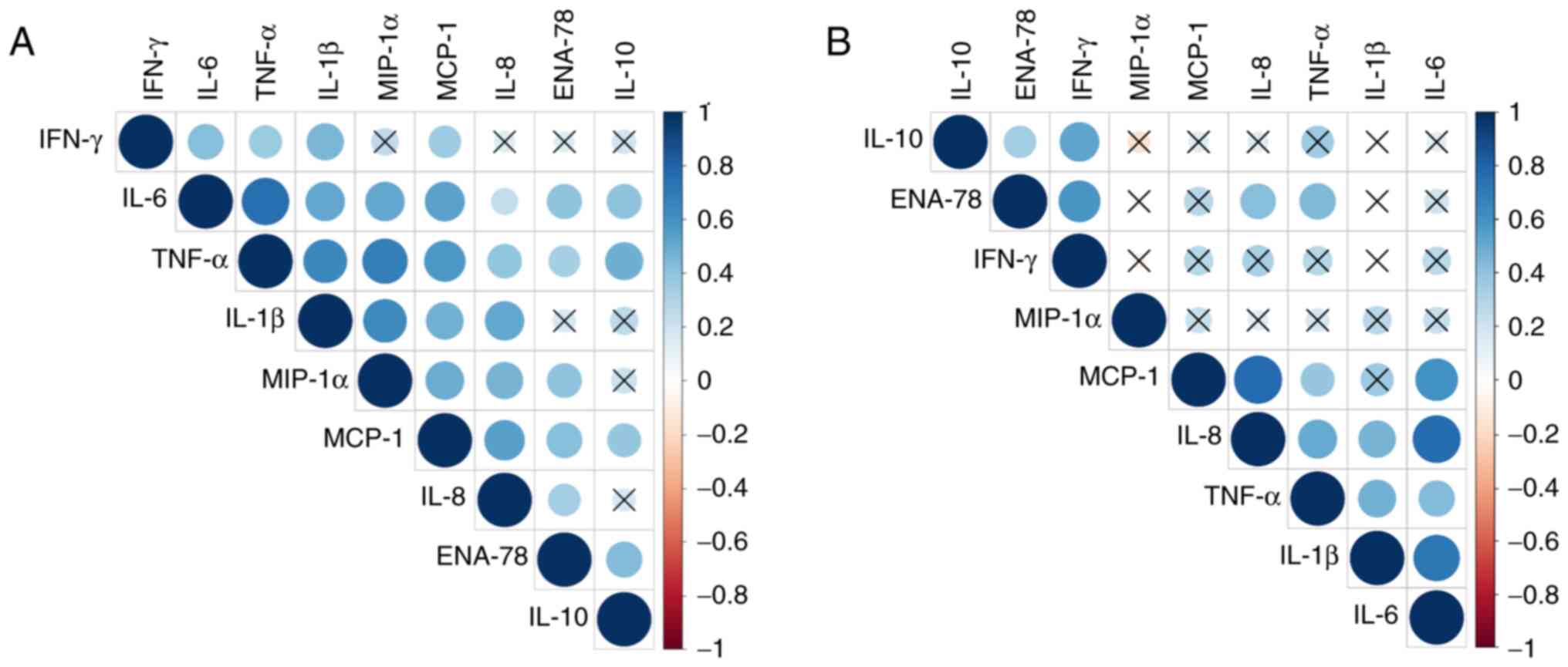 | Figure 3Correlograms representing the
correlations among the CT/CK concentrations in patients with BM and
VM. Correlograms for (A) BM and (B) VM are presented. The dark blue
colour represents the highest positive correlation between the
concentrations of two parameters (r=1), and the darkest red colour
represents the strongest negative correlation (r=-1). Correlations
with a significance level >0.01 are marked with an ‘X’. (A) In
the BM group 28 significant correlations among the CTs/CKs analysed
were detected and (B) in the VM group, 14 significant correlations
were detected. CT, cytokine; CK, chemokine; BM, bacterial
meningitis; VM, viral meningitis; IFN-γ, interferon-γ; IL,
interleukin; TNF-α, tumor necrosis factor-α; MIP-1α, macrophage
inflammatory protein-1α; MCP-1, monocyte chemoattractant protein-1;
ENA-78, epithelial-neutrophil activating peptide-78. |
Regarding the correlations in the BM and VM groups,
distinct patterns can be observed for the two aetiologies. In the
BM group, 28 significant correlations were identified, with the
strongest positive correlations occurring among TNF-α, IL-6, IL-1β
and MIP-1α (Fig. 3A, Table SIIA). Fewer correlations with
statistical significance were found in the VM group (Fig. 3B). However, the strength of
correlation was high for MCP-1 with IL-8 and IL-6, and IL-6 was
also correlated with IL-1β (Fig.
3B, Table SIIB).
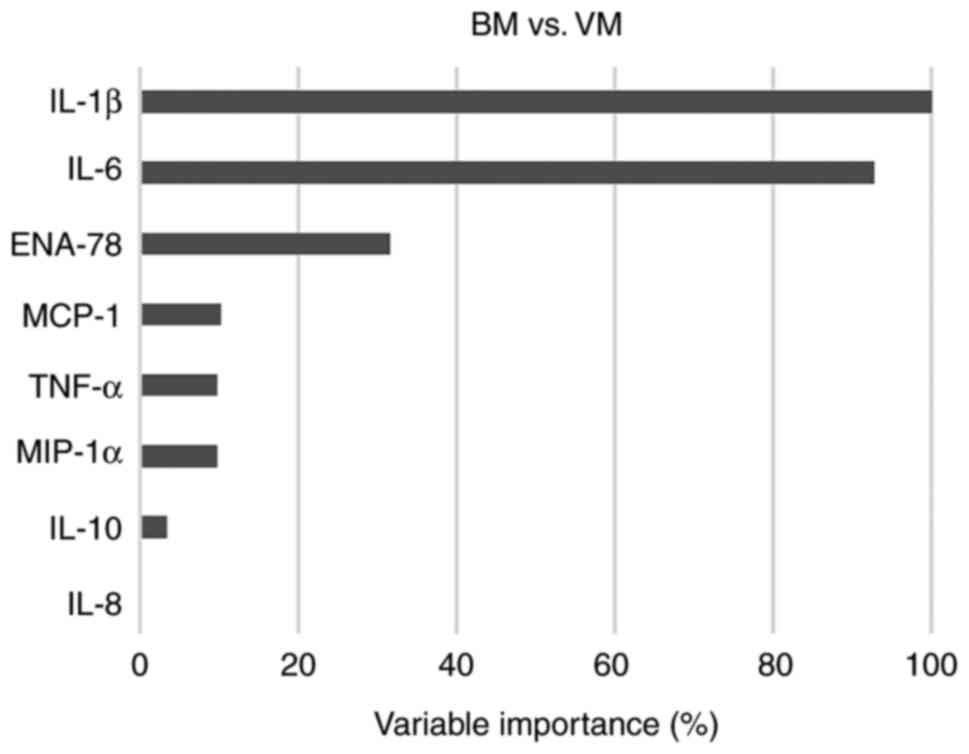
RF variable importance analysis
The hierarchical clustering (Fig. 1) grouped numerous BM samples with
VM and C samples. Therefore, to identify the CT/CK patterns that
best predict and classify BM and VM, a dataset comprising the CT/CK
concentrations of 119 BM and VM patient samples (90 for training
and 29 for testing) was used as input for a machine learning
algorithm. A variable importance plot was thereby generated to rank
the utility of each CT/CK as a predictor (Fig. 4). The predictors with the highest
importance were IL-1β and IL-6 (Fig.
4, Table SIIIA). Due to IFN-γ
inter-individual variability and the lack of significant
differences in the levels of this CT between BM and VM (P=0.154),
IFN-γ was removed from the dataset for further analysis.
Of the 29 testing samples (15 BM and 14 VM), the RF
algorithm correctly classified 28, with 93% specificity and 92%
sensitivity. When used for classifying BM vs. C (Fig. S1, Table SIIIB), the sensitivity and
specificity were both 100%, while the comparison of VM vs. C had
90% specificity and a lower sensitivity of 77% (Fig. S2, Table SIIIC).
Discussion
The present study investigated nine CT/CK levels in
the CSF samples of 145 patients with BM, VM and meningism. The
results demonstrated that the patients with BM had significantly
higher CSF levels of IL-1β, IL-6, ENA-78, MCP-1, TNF-α, MIP-1α,
IL-10, and IL-8 than the patients with VM or the controls. Using RF
analysis, the usefulness of these parameters in predicting BM was
assessed. This analysis identified a CT/CK signature that was able
to differentiate BM from VM with 93% specificity and 92%
sensitivity. IL-1β and IL-6 were identified as the variables with
the highest importance score, followed by ENA-78.
The host inflammatory reaction caused by pathogens
in the CSF includes the increased intrathecal production of soluble
mediators, such as CTs/CKs. Pathogen-associated molecular patterns
(PAMPs) are evolutionary conserved pathogen components, including
the lipopolysaccharides (LPS) of gram-negative bacteria,
peptidoglycans, lipoteichoic acid from gram-positive bacteria, and
the DNA and RNA of bacteria and viruses. They are detected by a
wide array of innate sensors, using pattern recognition receptors
(PRRs), such as the Toll-like receptor, Nod-like receptor and
RIG-like receptor families (32).
The detection of PRRs by PAMPs triggers the activation of I-κB
kinase NF-κB pathways and mitogen-activated protein kinase
pathways, resulting in the secretion of signalling molecules that
interact to orchestrate the early host response to infection and
later adaptive immunity (33).
Among these signalling molecules, CTs/CKs are the driving force in
shaping a plethora of host responses, with CT signalling being
indispensable to disease resolution but also responsible for
numerous deleterious effects of dysregulated inflammatory
responses.
Perivascular macrophages and resident cells in the
central nervous system react to LPS, peptidoglycans and nucleic
acids released by bacteria, producing the early response
inflammatory CKs TNF-α, IL-1β and IL-6. Although they are generally
considered to be necessary for active protection against BM
(34), TNF-α and IL-1β also
initiate meningeal inflammation (26) and stimulate the recruitment of
monocytes and neutrophils to infection sites and activate them.
Several studies have demonstrated that TNF-α, IL-1β and IL-6 are
upregulated in BM, and demonstrate good sensitivity and specificity
for the diagnosis of this disease (4,13,19).
The data in the present study reveal three orders of magnitude
difference in the IL-1β and IL-6 concentrations of patients with BM
compared with those with VM, which supports these findings. In
previous studies, higher levels of TNF-α and IL-1β CSF were found
to be associated with complications and unfavourable disease
outcomes when the relationship between inflammatory mediators and
disease outcome was investigated (29,35).
Also, the administration of TNF-α into the CSF was shown to result
in pathophysiological changes characteristic of BM (26), while the blocking of IL-1β in a
murine model of BM led to a reduction in disease severity (29). In the present study, the median
TNF-α was significantly higher in patients with BM compared with
patients with VM and the controls. However, in the present
research, there were patients with BM who had low TNF-α levels,
which might be explained by the timing of the LP. A previous study
of patients with BM observed that TNF-α levels were significantly
elevated in the CSF when the LP was performed ≤48 h from the onset
of symptoms as compared with >48h, and the elevated TNF-α levels
were not maintained because of the rapid decline of TNF-α during BM
(28). Furthermore, in an animal
model, the kinetics of TNF-α release showed that TNF-α reached its
maximal level 1-2 h after LPS injection and was not detectable
after 24 h (36).
In the present study, the early response CKs
significantly correlated with each other (P<0.01): TNF-α/IL-6
(r=0.75), TNF-α/IL-1β (r=0.64) and IL-1β/IL-6 (r=0.52). The high
levels of proinflammatory CKs in BM stimulate the secretion of CKs
such as IL-8, ENA-78, MCP-1 and MIP-1α. CKs play an essential role
in the recruitment and trafficking of leukocytes. The strong
inflammatory response contributes to the loss of integrity of the
BBB due to the recruitment of leukocytes, alteration of the
meningeal vasculature and upregulation of various adhesion
molecules on the endothelial cells, including selectins,
intercellular adhesion molecules and vascular endothelial adhesion
molecules. In parallel, proteins, complement factors and
immunoglobulins leak into the CSF (37). While MCP-1 and MIP-1α are the major
chemoattractants for monocytes during inflammatory responses, IL-8
and ENA-78 are the major chemoattractants for neutrophils that pass
between the activated endothelial cells entering the subarachnoid
space. It has been reported that IL-8 plays an important role in
the pathogenesis of pneumococcal disease (19), pneumolysin is a factor influencing
IL-8 levels (38) and the
neuraminidase expressed by S. pneumoniae, NanA, mediates
changes in IL-8 release (39).
ENA-78, also known as C-X-C motif chemokine 5 is a
small CK secreted by immune and vascular endothelial cells which,
like other CKs, facilitates chemotaxis and leucocyte recruitment
and is involved in BBB dysfunction (40-42).
ENA-78 is considered an important biomarker of neuroinflammation
and neurodegeneration. Its role is currently being studied in
Alzheimer's disease (42,43), multiple sclerosis relapse (40) and other neuroinflammatory diseases,
such as HTLV-1-associated myelopathy (37), juvenile gangliosidoses diseases
(44) and primary progressive
aphasia (45). In a study using a
machine learning approach, this CK and three other markers showed
high discriminatory performance between patients with Alzheimer's
disease and controls (42).
However, studies on the expression of this CK in BM cases are
scarce. The only study on this topic (27), to the best of our knowledge, showed
that ENA-78 and IL-8 had profoundly elevated concentrations in the
CSF of patients with BM and were not detectable in patients with
VM. Confirming these results, the present study also found
significantly higher levels of ENA-78 and IL-8 in patients with BM
compared with patients with VM and the controls.
MIP-1α and MCP-1 are the other two important
mediators of chemoattraction evaluated in the present study that
were upregulated in the BM group compared with the VM and/or C
groups. In line with these results, MIP-1α and MCP-1 levels have
been observed to be elevated in the CSF of patients with
pneumococcal and meningococcal meningitis in previous studies
(28,35), with MCP-1 significantly higher in
cases of pneumococcal meningitis compared with meningococcal
meningitis (35). In the present
study, MIP-1α and MCP-1 correlated with proinflammatory CKs but not
with the WBC. Other authors have shown similar results, where no
correlation was found between IL-8, MIP-1α and MCP-1 levels and the
WBC in the CSF during BM (28).
Inflammatory markers in the CSF that have previously
been reported to help diagnose BM include the WBC count, glucose
and protein levels (13,30). To better understand the
pathophysiology of meningitis, these parameters were analysed in
the present study alongside CSF CTs/CKs. Confirming the findings of
other studies (13,28,30),
the CSF protein levels and WBC count in the present study were
significantly higher in the BM group compared with the VM and C
groups, whereas the glucose level was significantly lower. Other
authors considered these parameters as having only modest
sensitivity and specificity for meningitis detection (30,46).
In the present study, the protein and glucose levels
and WBC count were not found to correlate well with the other
parameters. Furthermore, as the data for CSF protein levels,
glucose levels and WBC count were not available for all 145
patients, these correlations are not shown.
IFN-γ was the only CK with similar concentrations in
the three studied groups. However, when the BM group was split into
gram-positive and -negative samples, IFN-γ was the only CK to
differentiate between the two groups, with a higher concentration
in patients with gram-positive BM, confirming the results of a
previous study (28).
Following the above analysis of how the expression
of CSF biomarkers differs in various conditions, the effectiveness
of this information for distinguishing the three groups (BM, VM and
C) to help facilitate early-stage diagnosis requires consideration.
Although all the studied CTs/CKs, with the exception of IFN-γ, were
expressed at significantly higher levels in the BM group than in
the other two groups, the goal of the study was to identify CT/CK
patterns that best predict and classify BM and VM. Combining the
expression of a number of the CTs/CKs would be expected to lead to
more accurate and robust predictions. Other studies have mainly
explored the expression dynamics of specific CKs in different forms
of meningitis (1,4,16,31),
as well as how they correlate with parameters such as CSF glucose,
protein and WBC count (13,30).
Attempts have been made to combine such results for early
diagnostic purposes with promising results (15,34,46).
In the present study, the RF state-of-the-art machine learning
algorithm was employ for this purpose. This algorithm has been
demonstrated to be useful, for instance, in the differentiation of
the CK signatures of SARS-CoV-2 and influenza (47), classification of the risk of
coronary artery disease using plasma CKs (48), and identification of CK profiles
for distinguishing children with Plasmodium falciparum
malaria from those with bacterial bloodstream infections (49). RF is considered to outperform
numerous other known techniques for this type of data and
predictions (48,50).
As an ensemble method, RF is based on the concept
that a group of weak learners can work together and perform as one
strong learner. In this case, the weak learners are decision trees,
which, taken individually, are prone to overfitting and bias.
However, RF uses bagging, also known as bootstrap aggregation,
which entails choosing random samples from the training dataset
(with replacement) and generating independent decision trees from
these samples. A ‘forest’ of decision trees generated via this
method is used to classify the data. Each tree votes for a class,
and the majority decides. As an important detail, when generating a
decision tree, in the present study, only two-thirds of the data
samples were used for building the classification model, while
one-third were set aside as ‘out-of-bag’ samples for data
evaluation. To avoid the issue of correlated features (predictors)
in the dataset, RF uses random subsets of features in each node to
guide the node split. This results in uncorrelated decision trees
and, together with the bagging technique, ensures a more robust
classification method with a reduced risk of overfitting or bias
(48,51,52).
The RF method is also optimised using k-fold cross-validation, and
in the present study, 10-fold cross-validation was used.
Due to the stochastic character of the process of
growing decision trees, it can be challenging to interpret the
results of the RF algorithm. Therefore, the RF algorithm evaluates
the so-called variable importance of each predictor in the dataset.
To that end, the increase in the mean squared error of the
prediction is evaluated when a feature/predictor is removed from
the model, allowing the importance of the variable to be estimated.
In the present study, the most important predictors for
discriminating BM and VM identified by the RF algorithm were IL-1β
and IL-6. The patients with BM were correctly classified in 14 out
of 15 cases, with very good specificity (93%) and sensitivity
(92%), making RF a promising tool in the differentiation of BM from
VM.
Regarding the strengths of the present study, using
the sophisticated RF machine-learning algorithm, eight measured
CT/CK levels were employed for classifying patients in the BM, VM
and C groups. To assess classification strength, 29 random and
completely independent out-of-bag test samples were used to
establish the classification model. However, a limitation of the
study was that CSF cytochemical marker data were not available for
all the patients included in the study. Therefore, it was decided
to conduct the classification with only CTs/CKs. This enabled the
number of patients to be kept reasonably high. Despite using only
eight CTs/CKs, only one of the 29 test samples was misclassified
when distinguishing BM from VM. Another limitation was that the CSF
samples were collected during hospital admission. While this is the
clinically relevant time point, it may be a different moment in the
disease for each patient, and the level of early inflammatory
markers may have already declined for some patients.
In conclusion, in the present study, the
concentrations of nine relevant CTs/CKs from CSF samples were
assessed in the pathophysiology of BM, VM and meningism, and the
correlation of defined CT/CK profiles with other CSF parameters was
further investigated. Finally, whether a pattern of CTs/CKs has
good discriminating power between BM and VM was studied using the
RF machine learning algorithm. With 28 of 29 test samples correctly
classified, the results suggest that CTs/CKs measured in the CSF,
directly sampled when meningitis is suspected, can accurately
classify BM and VM. Furthermore, based on these results, it is
likely that improved patient care could be obtained in the future
with a rapid test developed to enable the prompt measurement of
these biomarkers.
Supplementary Material
Variable importance plot of each
cytokine or chemokine in the discrimination of BM from C as
determined by the Random Forest algorithm. BM, bacterial
meningitis; C, control; IL, interleukin; TNF-α, tumor necrosis
factor-α; ENA-78, epithelial-neutrophil activating peptide-78;
MCP-1, monocyte chemoattractant protein-1; MIP-1α, macrophage
inflammatory protein-1α.
Variable importance plot of each
cytokine or chemokine in the discrimination of VM from C determined
by the Random Forest algorithm. VM, viral meningitis; C, control;
IL, interleukin; ENA-78, epithelial-neutrophil activating
Peptide-78; MIP-1α, macrophage inflammatory protein-1α; TNF-α,
tumor necrosis factor-α; MCP-1, monocyte chemoattractant
protein-1.
CT/CK levelsin the gram-negative and
-positivecerebrospinal fluid samples of patients with bacterial
meningitis.
Correlations among the CT/CK
concentrationsin the cerebrospinal fluid of patients with BM and
VM.
Variable importance scores of each
CT/CK for discriminating the BM, VM and C groups as determined by
the Random Forest algorithm.
Acknowledgements
The authors would like to thank Dr Aurora Sălăgeanu,
Immunology Laboratory, ‘Cantacuzino’ National Institute for
Medico-Military Research and Development (Bucharest, Romania) for
reviewing the manuscript and Professor Stefan Andersson-Engels,
Head of Biophotonics, Tyndall National Institute (Cork, Ireland)
for very helpful suggestions.
Funding
Funding: The project was financially supported by the Ministry
of Education and Research in Romania, with project number
35/2014_PN-II-PT-PCCA-2013-4-2836. This funding covered the cost of
the research only.
Availability of data and materials
The data used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The study was designed by IC, CT and VL. Clinical
diagnosis, sample collection and management, and clinical data
collection were performed by SAF and DSL. Data were measured by IC,
CT and RC, while data analysis was performed by RC and CT. RC wrote
the manuscript, and RC, CT, IC, VL, SAF and DSL reviewed the
manuscript. VL and IC supervised the study. RC, IC and CT confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of the Clinical Hospital for Infectious and Tropical
Diseases ‘Dr Victor Babes’ (approval no. 5105). All samples were
collected with written informed consent from all participants or
parents/guardians in the case of children under 18 years old and
was conducted based on the principles expressed in the 1975
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
García-Hernández P, Prieto B,
Martínez-Morillo E, Rodríguez V and Álvarez FV: Interleukin-6 in
cerebrospinal fluid as a biomarker of acute meningitis. Ann Clin
Biochem. 53:155–163. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ai J, Xie Z, Liu G, Chen Z, Yang Y, Li Y,
Chen J, Zheng G and Shen K: Etiology and prognosis of acute viral
encephalitis and meningitis in Chinese children: A multicentre
prospective study. BMC Infect Dis. 17(494)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mount HR and Boyle SD: Aseptic and
bacterial meningitis: Evaluation, treatment, and prevention. Am Fam
Physician. 96:314–322. 2017.PubMed/NCBI
|
|
4
|
Prasad R, Kapoor R, Srivastava R, Mishra
OP and Singh TB: Cerebrospinal fluid TNF-α, IL-6, and IL-8 in
children with bacterial meningitis. Pediatr Neurol. 50:60–65.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sharew A, Bodilsen J, Hansen BR, Nielsen H
and Brandt CT: The cause of death in bacterial meningitis. BMC
Infect Dis. 20(182)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hsu MH, Hsu JF, Kuo HC, Lai MY, Chiang MC,
Lin YJ, Huang HR, Chu SM and Tsai MH: Neurological complications in
young infants with acute bacterial meningitis. Front Neurol.
9(903)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zainel A, Mitchell H and Sadarangani M:
Bacterial meningitis in children: Neurological complications,
associated risk factors, and prevention. Microorganisms.
9(535)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
van de Beek D, Cabellos C, Dzupova O,
Esposito S, Klein M, Kloek AT, Leib SL, Mourvillier B, Ostergaard
C, Pagliano P, et al: ESCMID guideline: Diagnosis and treatment of
acute bacterial meningitis. Clin Microbiol Infect. 22 (Suppl
3):S37–S62. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
van de Beek D, Brouwer M, Hasbun R, Koedel
U, Whitney CG and Wijdicks E: Community-acquired bacterial
meningitis. Nat Rev Dis Primer. 2(16074)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Torres SD, Kim CY, Das M, Ankam JV, Luche
N, Harmon M, Schorr EM, Glassberg B, Morse SS, Weiss D, et al:
Delays in diagnosis and treatment of bacterial meningitis in NYC:
Retrospective cohort analysis. Neurohospitalist. 12:268–272.
2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Brouwer MC, McIntyre P, Prasad K and van
de Beek D: Corticosteroids for acute bacterial meningitis. Cochrane
Database Syst Rev. 2015(CD004405)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Salman O, Procter SR, McGregor C, Paul P,
Hutubessy R, Lawn JE and Jit M: Systematic review on the acute
cost-of-illness of sepsis and meningitis in neonates and infants.
Pediatr Infect Dis J. 39:35–40. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Q, Gao Y, Zhang B, Sun F, Yang Q, Liu
Y, Wu J, Chen K, Weng X, Zhang W, et al: Cytokine profiles in
cerebrospinal fluid of patients with meningitis at a tertiary
general hospital in China. J Microbiol Immunol Infect. 53:216–224.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Xu J, Jiang J, Zhang Y and Li W: Cytokine
characteristic of cerebrospinal fluid from children with
enteroviral meningitis compared to bacterial meningitis. J Clin Lab
Anal. 34(e23198)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Jafari M, Mohammadzadeh Jahani P,
Choopanizadeh M, Jamalidoost M, Pourabbas B, Pouladfar G and Kalani
M: Investigating the role of T helper related cytokines in
cerebrospinal fluid for the differential diagnosis of bacterial
meningitis in pre-treated paediatric patients. Biomarkers.
25:171–178. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Srinivasan L, Kilpatrick L, Shah SS,
Abbasi S and Harris MC: Elevations of novel cytokines in bacterial
meningitis in infants. PLoS One. 13(e0181449)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lepennetier G, Hracsko Z, Unger M, Van
Griensven M, Grummel V, Krumbholz M, Berthele A, Hemmer B and
Kowarik MC: Cytokine and immune cell profiling in the cerebrospinal
fluid of patients with neuro-inflammatory diseases. J
Neuroinflammation. 16(219)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kul G, Sencan I, Kul H, Korkmaz N and
Altunay E: The role of cerebrospinal fluid biomarkers in the
diagnosis of post-neurosurgical meningitis. Turk Neurosurg.
30:513–519. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Müller A, Schramm DB, Kleynhans J, de
Gouveia L, Meiring S, Ramette A, von Gottberg A and Hathaway LJ:
Cytokine response in cerebrospinal fluid of meningitis patients and
outcome associated with pneumococcal serotype. Sci Rep.
11(19920)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Le Guennec L, Coureuil M, Nassif X and
Bourdoulous S: Strategies used by bacterial pathogens to cross the
blood-brain barrier. Cell Microbiol. 22(e13132)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee KY, Seol JH, Yi CH and Lee WH:
Cerebrospinal fluid type I interferon and cytokine profiles in
enteroviral meningitis according to the presence or absence of
pleocytosis. Pediatr Neonatol. 62:305–311. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Perdomo-Celis F, Torres MA, Ostos H,
Gutierrez-Achury J, Molano V, Durán LF, González G and Narváez CF:
Patterns of local and systemic cytokines in bacterial meningitis
and its relation with severity and long-term sequelae. Biomark
Insights. 10:125–131. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Too LK, Hunt N and Simunovic MP: The role
of inflammation and infection in age-related neurodegenerative
diseases: Lessons from bacterial meningitis applied to alzheimer
disease and age-related macular degeneration. Front Cell Neurosci.
15(635486)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Farmen K, Tofiño-Vian M and Iovino F:
Neuronal damage and neuroinflammation, a bridge between bacterial
meningitis and neurodegenerative diseases. Front Cell Neurosci.
15(680858)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Parthasarathy G and Philipp MT: Review:
Apoptotic mechanisms in bacterial infections of the central nervous
system. Front Immunol. 3(306)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ramilo O, Sáez-Llorens X, Mertsola J,
Jafari H, Olsen KD, Hansen EJ, Yoshinaga M, Ohkawara S, Nariuchi H
and McCracken GH Jr: Tumor necrosis factor alpha/cachectin and
interleukin 1 beta initiate meningeal inflammation. J Exp Med.
172:497–507. 1990.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zwijnenburg PJG, de Bie HMA, Roord JJ, van
der Poll T and van Furth AM: Chemotactic activity of CXCL5 in
cerebrospinal fluid of children with bacterial meningitis. J
Neuroimmunol. 145:148–153. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Coutinho LG, Grandgirard D, Leib SL and
Agnez-Lima LF: Cerebrospinal-fluid cytokine and chemokine profile
in patients with pneumococcal and meningococcal meningitis. BMC
Infect Dis. 13(326)2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Geldhoff M, Mook-Kanamori BB, Brouwer MC,
Troost D, Leemans JC, Flavell RA, Van der Ende A, Van der Poll T
and Van de Beek D: Inflammasome activation mediates inflammation
and outcome in humans and mice with pneumococcal meningitis. BMC
Infect Dis. 13(358)2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Srinivasan L, Kilpatrick L, Shah SS,
Abbasi S and Harris MC: Cerebrospinal fluid cytokines in the
diagnosis of bacterial meningitis in infants. Pediatr Res.
80:566–572. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ye Q, Shao WX, Shang SQ, Shen HQ, Chen XJ,
Tang YM, Yu YL and Mao JH: Clinical value of assessing cytokine
levels for the differential diagnosis of bacterial meningitis in a
pediatric population. Medicine (Baltimore).
95(e3222)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zheng K, He FB, Liu H and He Q: Genetic
variations of toll-like receptors: Impact on susceptibility,
severity and prognosis of bacterial meningitis. Infect Genet Evol.
93(104984)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Doran KS, Fulde M, Gratz N, Kim BJ, Nau R,
Prasadarao N, Schubert-Unkmeir A, Tuomanen EI and Valentin-Weigand
P: Host-pathogen interactions in bacterial meningitis. Acta
Neuropathol. 131:185–209. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Belogurov AA Jr, Ivanova OM, Lomakin YA,
Ziganshin RH, Vaskina MI, Knorre VD, Klimova EA, Gabibov AG, Ivanov
VT and Govorun VM: Mediators and biomarkers of inflammation in
meningitis: Cytokine and peptidome profiling of cerebrospinal
fluid. Biochemistry (Mosc). 81:1293–1302. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Grandgirard D, Gäumann R, Coulibaly B,
Dangy JP, Sie A, Junghanss T, Schudel H, Pluschke G and Leib SL:
The causative pathogen determines the inflammatory profile in
cerebrospinal fluid and outcome in patients with bacterial
meningitis. Mediators Inflamm. 2013(312476)2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
van Deuren M, Netea MG, Hijmans A,
Demacker PN, Neeleman C, Sauerwein RW, Bartelink AK and van der
Meer JW: Posttranscriptional down-regulation of tumor necrosis
factor-alpha and interleukin-1beta production in acute
meningococcal infections. J Infect Dis. 177:1401–1405.
1998.PubMed/NCBI View
Article : Google Scholar
|
|
37
|
Souza FDS, Freitas NL, Gomes YCP, Torres
RC, Echevarria-Lima J, da Silva-Filho IL, Leite ACCB, de Lima MASD,
da Silva MTT, Araújo AQC and Espíndola OM: Following the clues:
Usefulness of biomarkers of neuroinflammation and neurodegeneration
in the investigation of HTLV-1-associated myelopathy progression.
Front Immunol. 12(737941)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Baumgartner D, Aebi S, Grandgirard D, Leib
SL, Draeger A, Babiychuk E and Hathaway LJ: Clinical Streptococcus
pneumoniae isolates induce differing CXCL8 responses from human
nasopharyngeal epithelial cells which are reduced by liposomes. BMC
Microbiol. 16(154)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chang YC, Uchiyama S, Varki A and Nizet V:
Leukocyte inflammatory responses provoked by pneumococcal
sialidase. mBio. 3:e00220–11. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Blackmore S, Hernandez J, Juda M, Ryder E,
Freund GG, Johnson RW and Steelman AJ: Influenza infection triggers
disease in a genetic model of experimental autoimmune
encephalomyelitis. Proc Natl Acad Sci USA. 114:E6107–E6116.
2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Haarmann A, Schuhmann MK, Silwedel C,
Monoranu CM, Stoll G and Buttmann M: Human brain endothelial CXCR2
is inflammation-inducible and mediates CXCL5- and CXCL8-triggered
paraendothelial barrier breakdown. Int J Mol Sci.
20(602)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gaetani L, Bellomo G, Parnetti L, Blennow
K, Zetterberg H and Di Filippo M: Neuroinflammation and Alzheimer's
disease: A machine learning approach to CSF proteomics. Cells.
10(1930)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Whelan CD, Mattsson N, Nagle MW,
Vijayaraghavan S, Hyde C, Janelidze S, Stomrud E, Lee J, Fitz L,
Samad TA, et al: Multiplex proteomics identifies novel CSF and
plasma biomarkers of early Alzheimer's disease. Acta Neuropathol
Commun. 7(169)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Utz JRJ, Crutcher T, Schneider J, Sorgen P
and Whitley CB: Biomarkers of central nervous system inflammation
in infantile and juvenile gangliosidoses. Mol Genet Metab.
114:274–280. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sogorb-Esteve A, Swift IJ, Woollacott IOC,
Warren JD, Zetterberg H and Rohrer JD: Differential chemokine
alteration in the variants of primary progressive aphasia-a role
for neuroinflammation. J Neuroinflammation. 18(224)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liu ZH, Tu PH, Chen NY, Yip PK, Bowes AL,
Lee CC, Chan SH, Kung CC, Wang AY, Wu CT and Lee ST: Raised
proinflammatory cytokine production within cerebrospinal fluid
precedes fever onset in patients with neurosurgery-associated
bacterial meningitis. Crit Care Med. 43:2416–2428. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Karaba AH, Zhou W, Hsieh LL, Figueroa A,
Massaccesi G, Rothman RE, Fenstermacher KZJ, Sauer L, Shaw-Saliba
K, Blair PW, et al: Differential cytokine signatures of severe
acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza
infection highlight key differences in pathobiology. Clin Infect
Dis. 74:254–262. 2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Saharan SS, Nagar P, Creasy KT, Stock EO,
Feng J, Malloy MJ and Kane JP: Machine learning and statistical
approaches for classification of risk of coronary artery disease
using plasma cytokines. BioData Min. 14(26)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Struck NS, Zimmermann M, Krumkamp R,
Lorenz E, Jacobs T, Rieger T, Wurr S, Günther S, Gyau Boahen K,
Marks F, et al: Cytokine profile distinguishes children with
Plasmodium falciparum malaria from those with bacterial blood
stream infections. J Infect Dis. 221:1098–1106. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Goyal M, Khanna D, Rana PS, Khaibullin T,
Martynova E, Rizvanov AA, Khaiboullina SF and Baranwal M:
Computational intelligence technique for prediction of multiple
sclerosis based on serum cytokines. Front Neurol.
10(781)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Cai F, Zhao Y, Chen Q, Hu Y, Su S and Lu
Y: Serum cytokine analysis reveals predictors of progression from
chronic hepatitis B to liver cirrhosis. Folia Biol (Praha).
67:28–36. 2021.PubMed/NCBI
|
|
52
|
Speiser JL, Miller ME, Tooze J and Ip E: A
comparison of random forest variable selection methods for
classification prediction modeling. Expert Syst Appl. 134:93–101.
2019.PubMed/NCBI View Article : Google Scholar
|