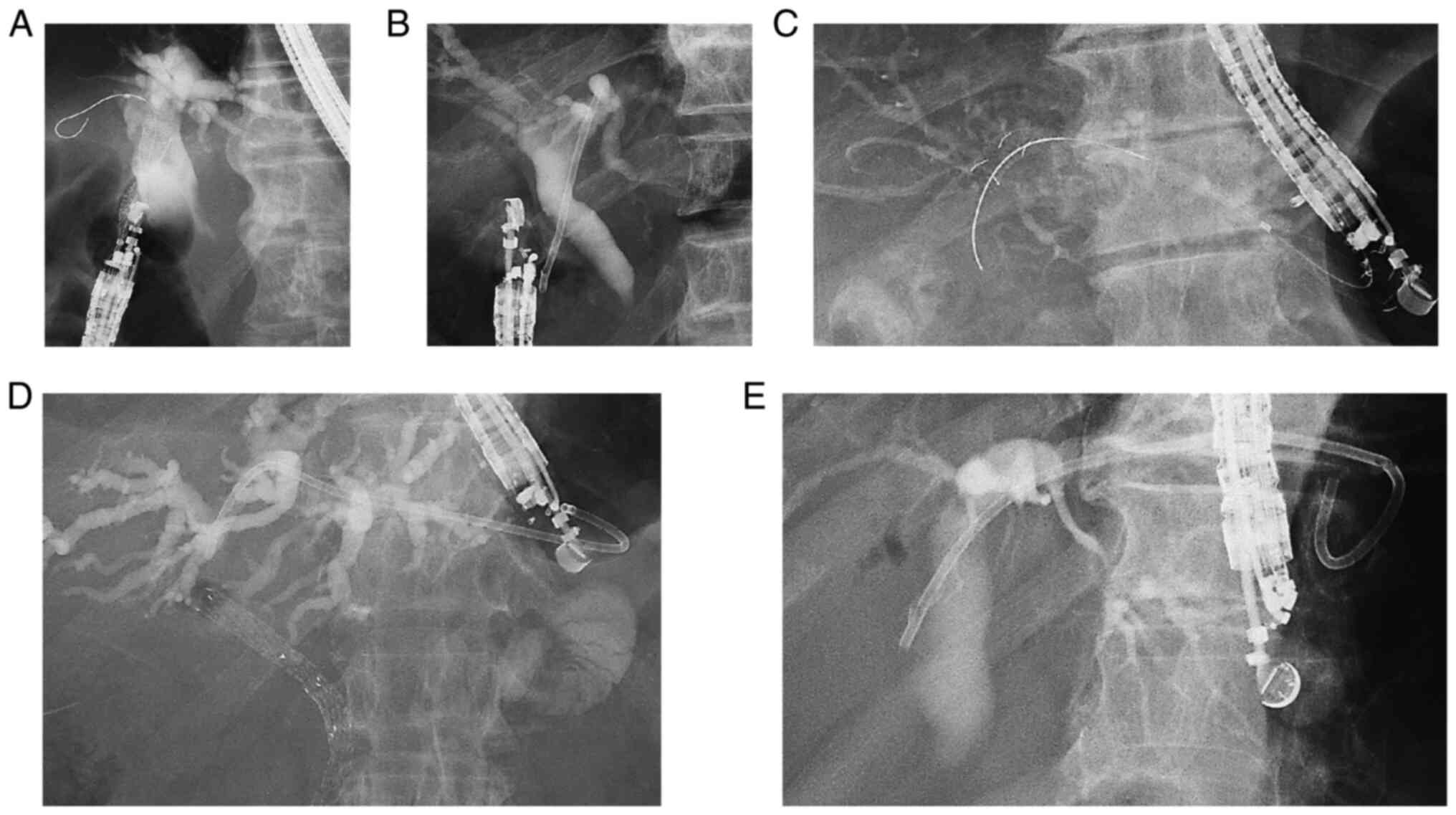
Introduction
For the treatment of biliary obstruction, endoscopic
transpapillary drainage is recommended first. However, endoscopic
transpapillary drainage is not always possible. When a patient has
a duodenal stricture or a history of upper gastrointestinal
surgery, endoscopic transpapillary drainage becomes difficult. In
these cases, endoscopic ultrasound-guided biliary drainage (EUS-BD)
may be an alternative treatment for biliary obstruction (1).
Metallic stents (MSs) and plastic stents (PSs) are
used for endoscopic biliary drainage. A benefit of using MSs
instead of PSs in transpapillary drainage is the expectation of a
longer time to recurrent biliary obstruction (TRBO) (2). For EUS-BD, covered self-expandable
metallic stents (CSEMSs) were originally used (3,4). A
drawback of using MSs is the greater risk. Adverse events of EUS-BD
using CSEMSs have been reported in 0-30% of cases (5), with severe events involving bile leak
sepsis and stent migration that occurred from shortening (6-8).
On the other hand, the benefits of using PSs include convenience
and safety, and a dedicated PS for EUS-BD that may be easily and
safely placed has been developed (3,9).
However, the shorter TRBO is a drawback of using PSs in
transpapillary drainage (2).
As described above, MSs are superior to PSs in terms
of TRBO in endoscopic transpapillary drainage (2). However, in EUS-BD, the stents do not
pass through the biliary stricture. Therefore, it has remained
elusive whether MSs are superior to PSs in EUS-BD. Comparing MSs
and PSs may provide information to aid decision-making regarding
which type of stent to use to achieve a longer TRBO. Thus, the
present study aimed to clarify whether PSs or MSs are more useful
for EUS-BD.
Materials and methods
Patients and inclusion and exclusion
criteria
The present study was an observational study
performed at Fukushima Medical University (Fukushima, Japan) and
Soma General Hospital (Soma, Japan) between May 2005 and July 2022.
The inclusion and exclusion criteria were as follows: Patients with
malignant biliary obstruction who underwent successful EUS-BD were
enrolled in the present study (n=39; 26 males and 13 females; mean
age, 70.8±9.6 years); patients who did not undergo successful
EUS-BD were excluded from the study. Successful EUS-BD was defined
as the procedure in which a biliary stent was placed from the
biliary tract to the gastrointestinal tract through the abdominal
cavity.
All patients underwent endoscopic transpapillary
biliary drainage prior to EUS-BD. Patients who underwent MS
insertion for EUS-BD were classified into the MS group, while
patients who underwent PS insertion for EUS-BD were classified into
the PS group. The requirement for informed consent was waived, as
the present study was a retrospective study using anonymized
clinical data. All patients agreed to undergo the clinical
examination and treatment by providing written informed consent.
This study was approved by the Institutional Review Board of
Fukushima Medical University (Fukushima, Japan; approval no.
2399).
Procedure
After all patients were sufficiently sedated with
midazolam, the echoendoscope was gently inserted. After visualizing
the hepatic duct or common bile duct (CBD) from the
gastrointestinal tract, the target biliary tract was determined.
Color Doppler mode was used to confirm the absence of blood flow in
the puncture route and the biliary tract was punctured by an
EUS-guided fine-needle aspiration (FNA) needle. The biliary tract
was visualized under X-ray imaging and a guidewire was successfully
inserted into the biliary tract. Fistula dilation was performed
through the guidewire and a PS or MS was finally placed from the
gastrointestinal tract to the target biliary duct.
X-ray images of EUS-BD are provided in Fig. 1. EUS-guided choledochoduodenostomy
(EUS-CDS) was performed for patients with a distal CBD stricture.
On the other hand, EUS-guided hepaticoenterostomy (EUS-HES) was
performed for patients with a hilar biliary obstruction, an
upstream or long CBD obstruction, a history of Billroth-II
reconstruction or Roux-en-Y reconstruction, or a duodenal stricture
that involved the duodenal bulb. If the duodenal stricture did not
reach the papilla of Vater and a guidewire was sufficiently
advanced to the duodenum, EUS-guided antegrade stenting (EUS-AGS)
was performed with EUS-HES.
The choice of a PS or an MS was made as follows:
When just a small amount of ascites was present or the punctured
biliary duct was distant from the gastrointestinal wall, a covered
MS was selected. When the lumen of the duodenal bulbs was narrow or
the end of the stent reached the hilar biliary duct, a PS was
selected.
The following devices were used: A UCT240-AL5 or
UCT260 echoendoscope (Olympus Medical Systems); an SSD-5500,
Prosound SSD α10 (Hitachi Aloka Medical), an EU-ME1 or EU-ME2
ultrasound system (Olympus Medical Systems); a 19 G EZ Shot 3 Plus
(Olympus Medical Systems), 22 G NA-11J-KB (Olympus Medical
Systems), 19 G SonoTip (Medi-Grobe), 19 G EchoTip Ultra (Cook
Medical), 19 or 22 G Expect (Boston Scientific Japan) FNA needle; a
0.018 Fielder 18 (Olympus Medical Systems), 0.025 VisiGlide, 0.025
VisiGlide 2 (Olympus Medical Systems), 0.025 Endoselector (Boston
Scientific Japan) and 0.025 or 0.035 Jagwire guidewire.
Regarding the dilators, a 6 Fr MTW ERCP catheter
taper (MTW Endoskopie), a 4 mm Hurricane RX Biliary Balloon
Dilation Catheter (Boston Scientific Japan), a 4 or 6 mm REN
biliary dilation catheter (Kaneka Corporation), a 6 Fr
Cysto-Gastro-Set (Endo-Flex GmbH) or an ES dilator (Zeon Medical
Co.) were used for fistula dilation. A 7 Fr double-pigtail (Cook
Medical), 7 Fr Flexima Plus or 7 Fr IT stent (Gadelius Medical,
Co., Ltd.) stent was used as the PS. A partially covered WallFlex
Biliary RX stent, 10 mm HANARO (Boston Scientific), partially
covered 10 mm Niti-S Comvi, or 8 mm partially CSEMS (Spring
Stopper; Taewoong Medical) stent was used as the MS.
Outcomes
The TRBO was the primary outcome of the present
study. Patient characteristics [age, sex, history of gastrectomy,
diseases, biliary stricture part (hilar or distal), indications for
EUS-BD, duodenal stent placement before dysfunction of EUS-BD
stent], factors related to the EUS-BD procedure and postprocedural
course were the secondary outcomes. As for the factors related to
the EUS-BD procedure, the period during which EUS-BD was performed
(earlier nine years: 2005-2013, later nine years: 2014-2021),
diameter of the target bile duct, puncture route distance, length
of the biliary obstruction, method of EUS-BD (CDS or HES, addition
of AGS), procedural time and adverse events were selected. As
factors of the postprocedural course, chemotherapy, death and
overall survival were selected.
The outcomes were defined according to the criteria
established by Isayama et al (10). TRBO was defined as the duration
between the first stent placement and RBO. RBO was defined as
recurrent jaundice, hepatic dysfunction or biliary tract dilation
on imaging, e.g., computed tomography or percutaneous
ultrasonography, which required additional endoscopic therapy.
Adverse events and the severity of adverse events were diagnosed
according to Cotton's criteria (11). Malignant biliary obstruction was
diagnosed by cytology (class IV or V), biopsy, or clinical course
and imaging findings. The causal diseases of biliary obstruction
were divided into pancreaticobiliary and metastatic according to
the report by Jang et al (12). The diameter of the punctured
biliary bile duct and puncture route distance were measured by EUS
imaging or cholangiography. The length of the biliary obstruction
was measured by endoscopic cholangiography or CT.
Statistical analysis
The TRBO was compared between groups using the
log-rank test. Continuous variables that followed a normal
distribution were compared by an unpaired Student's t-test.
Continuous variables that did not follow a normal distribution were
compared by the Mann-Whitney U-test. Nominal variables were
compared by Fisher's exact test. P<0.05 was considered to
indicate statistical significance. All statistical analyses were
performed using EZR version 1.40 (Saitama Medical Centre).
Results
Flow of patients who underwent
EUS-BD
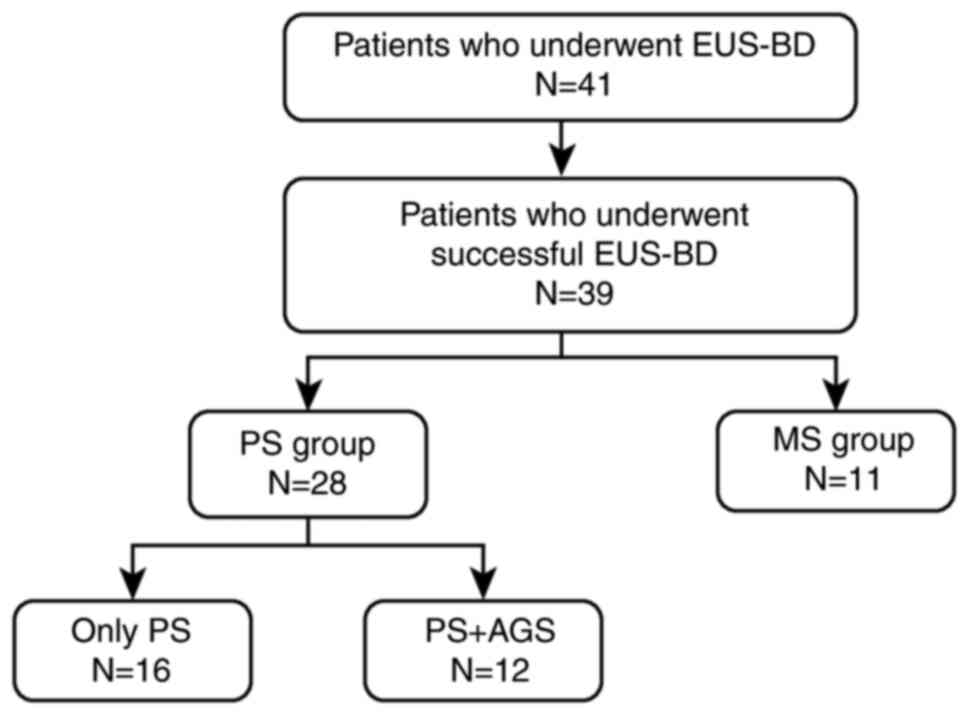
EUS-BD was performed in 41 patients, and the
procedure was successful in 39 patients (Fig. 2). Among them, 28 patients underwent
PS placement (PS group). In the PS group, 16 patients were treated
with a PS only and 12 patients were treated with a PS and underwent
AGS with an MS at the stricture. Furthermore, 11 patients underwent
placement of an MS only (MS group).
Comparison of patient
characteristics
The patient characteristics are compared in Table I. There were no significant
differences between the two groups in any of the parameters. All
five patients who had previously undergone gastrectomy were treated
with plastic stents. In addition, all five patients with metastatic
lesions were treated with plastic stents. However, the frequency of
history of gastrectomy and metastatic diseases were not
significantly different between the two groups.
 | Table IComparison of patient characteristics
between groups. |
Table I
Comparison of patient characteristics
between groups.
| Parameter | PS group (n=28) | MS group (n=11) | P-value |
|---|
| Age, years | 71.5±9.7 | 69.2±9.4 | 0.50 |
| Male sex | 18 (64.3) | 8 (72.7) | 0.72 |
| Antithrombotic
therapy | 4 (14.2) | 1 (9.1) | 1.00 |
| History of
gastrectomy | 5 (17.9) | 0 (0) | 0.30 |
|
Distal
gastrectomy with Billroth-I reconstruction | 1 | 0 | |
|
Distal
gastrectomy with Billroth-II reconstruction | 2 | 0 | |
|
Distal
gastrectomy with Roux-en-Y reconstruction | 1 | 0 | |
|
Total
gastrectomy with Roux-en-Y reconstruction | 1 | 0 | |
| Diagnosis | | | |
|
Pancreatic
cancer | 17 | 9 | |
|
Biliary
tract cancer | 5 | 2 | |
|
Duodenal
cancer | 1 | 0 | |
| Metastatic
diseases | 5 (17.9) | 0 (0) | 0.30 |
|
Gastric
cancer | 3 | 0 | |
|
Bladder
cancer | 1 | 0 | |
|
Urothelial
cancer | 1 | 0 | |
| Biliary stricture
part, hilar/distal | 1/27 | 1/10 | 0.49 |
| Reason for
EUS-BD | | | |
|
Duodenal
stricture | 14 | 7 | |
|
Difficult
biliary duct cannulation | 7 | 3 | |
|
Gastric
stricture | 2 | 0 | |
|
Difficult
biliary drainage | 2 | 1 | |
|
Difficult
endoscope insertion | 3 | 0 | |
| Duodenal stent
placement | 9 (32.1) | 6 (54.5) | 0.28 |
Comparison of EUS-BD procedure-related
factors and postprocedural course
The results of EUS-BD procedure-related factors and
postprocedural course comparison are presented in Table II. EUS-HES was performed
significantly more frequently in the PS group than in the MS group
[22 patients (78.6%) vs. 3 patients (27.3%), P<0.01]. However,
the period during which EUS-BD was performed, diameter of the
target bile duct, puncture route distance, length of biliary
obstruction, AGS operation, procedural time, adverse events,
chemotherapy, death and overall survival were not significantly
different between the two groups.
 | Table IIComparison of EUS-BD
procedure-related factors and postprocedural course. |
Table II
Comparison of EUS-BD
procedure-related factors and postprocedural course.
| Parameter | PS group
(n=28) | MS group
(n=11) | P-value |
|---|
| Later nine years
(2014-2021) | 22 (78.6) | 6 (54.5) | 0.23 |
| Diameter of target
bile duct, mm | 7.9 (2.0-20.0) | 10.0
(5.0-20.0) | 0.10 |
| Distance of
puncture route, mm | 15.3±6.8 | 13.9±4.5 | 0.52 |
| Length of biliary
obstruction, mm | 30.6±13.6 | 33.2±15.1 | 0.62 |
| Method of
EUS-BD | | | <0.01 |
|
CDS | 6 (21.4) | 8 (72.7) | |
|
HES | 22 (78.6) | 3 (27.3) | |
| AGS operation | 12 (42.9) | 1 (9.1) | 0.06 |
| Procedural time,
min | 48.0
(30.0-157.0) | 60.0
(24.0-131.0) | 0.34 |
| Adverse events | 2 (7.1) | 2 (18.2) | 0.56 |
| Dislocation | 1 | 2 | |
| Pancreatitis | 1 | | |
| Chemotherapy | 16 (57.1) | 4 (36.4) | 0.30 |
| Death | 19 (67.9) | 10 (90.9) | 0.23 |
| Overall survival,
days | 128 (9-874) | 75 (18-740) | 0.45 |
Comparison of patient characteristics
without AGS
When those patients who had undergone EUS-AGS were
removed from the analysis, the patient characteristics were not
significantly different between the PS group and the MS group
(Table III).
 | Table IIIComparison of patient characteristics
(excluding patients with antegrade stenting). |
Table III
Comparison of patient characteristics
(excluding patients with antegrade stenting).
| Parameter | PS group
(n=16) | MS group
(n=10) | P-value |
|---|
| Age, years | 71.5±11.3 | 70.1±9.3 | 0.75 |
| Male sex | 12 (75.0) | 7 (70.0) | 1.00 |
| Antithrombotic
therapy | 3 (18.8) | 1 (10.0) | 1.00 |
| History of
gastrectomy | 2 (12.5) | 0 (0) | 0.51 |
|
Distal
gastrectomy with Billroth-I reconstruction | 1 | 0 | |
|
Distal
gastrectomy with Billroth-II reconstruction | 1 | 0 | |
| Diagnosis | | | |
|
Pancreatic
cancer | 10 | 8 | |
|
Biliary
tract cancer | 5 | 2 | |
|
Metastatic
disease | 1 (6.3) | 0 (0) | 1.00 |
|
Urothelial
cancer | 1 | 0 | |
| Biliary stricture
location, hilar/distal | 1/15 | 1/9 | 1.00 |
| Reason for
EUS-BD | | | |
|
Duodenal
stricture | 6 | 6 | |
|
Difficult
biliary duct cannulation | 7 | 3 | |
|
Difficult
biliary drainage | 2 | 1 | |
|
Difficult
endoscope insertion | 1 | 0 | |
| Duodenal stent
placement | 4 (25.0) | 5 (50.0) | 0.23 |
Comparison of EUS-BD procedure-related
factors and postprocedural course without AGS
When those patients who had undergone EUS-AGS were
removed from the analysis, EUS-BD procedure-related factors and
postprocedural course were not significantly different between the
PS group and the MS group (Table
IV).
 | Table IVComparison of EUS-BD
procedure-related factors and postprocedural course (excluding
patients with antegrade stenting). |
Table IV
Comparison of EUS-BD
procedure-related factors and postprocedural course (excluding
patients with antegrade stenting).
| Parameter | PS group
(n=16) | MS group
(n=10) | P-value |
|---|
| Later nine years
(2014-2021) | 10 (62.5) | 6 (60.0) | 1.00 |
| Diameter of target
bile duct, mm | 9.7±5.3 | 12.5±5.9 | 0.24 |
| Distance of
puncture route, mm | 13.9±7.4 | 13.5±4.6 | 0.87 |
| Length of biliary
obstruction, mm | 31.9±10.3 | 31.6±14.9 | 0.95 |
| Method of
EUS-BD | | | 0.051 |
|
CDS | 6 (37.5) | 8 (80.0) | |
|
HES | 10 (62.5) | 2 (20.0) | |
| Procedural time,
min | 38.5
(30.0-157.0) | 55.0
(24.0-131.0) | 0.27 |
| Adverse events | 1 (6.3) | 2 (20.0) | 0.54 |
| Dislocation | 1 | 2 | |
| Chemotherapy | 8 (50.0) | 4 (40.0) | 0.70 |
| Death | 12 (75.0) | 9 (90.0) | 0.62 |
| Overall survival,
days | 122 (33-874) | 91 (26-74) | 0.87 |
Comparison of the TRBO
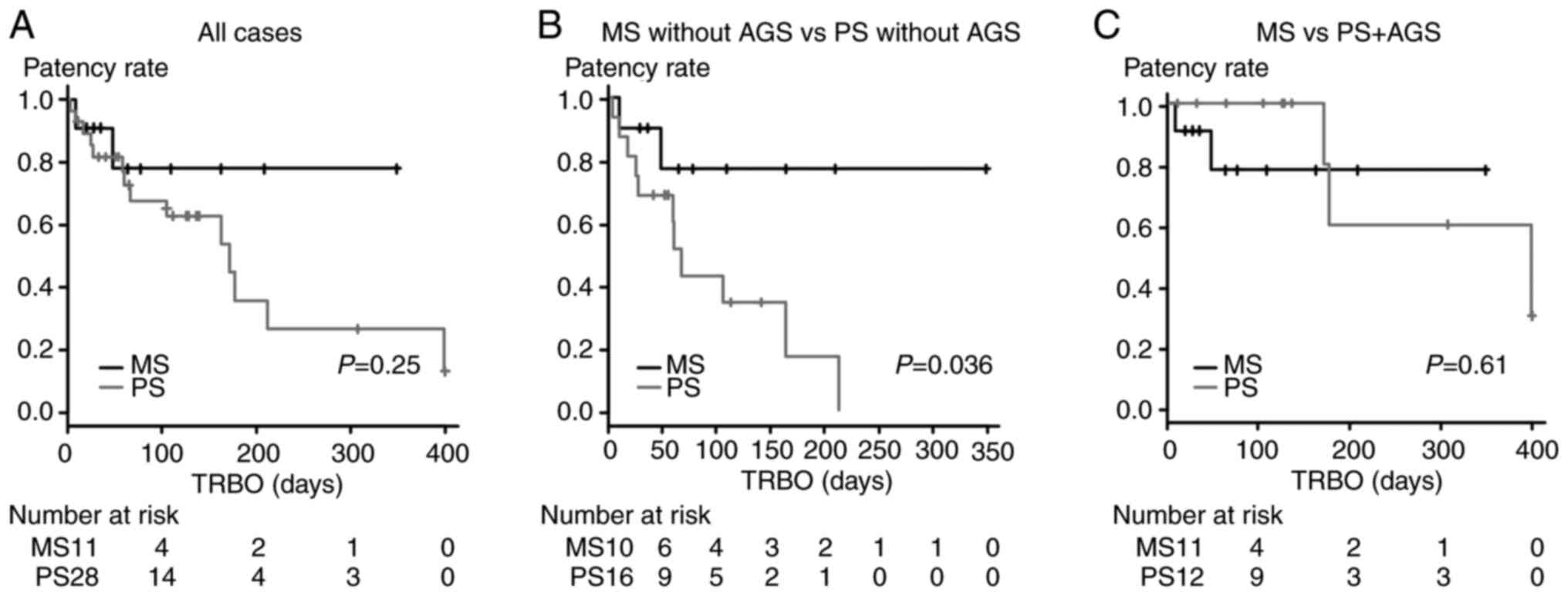
The results of the TRBO comparison between MSs and
PSs are provided in Fig. 3. The
TRBO was not significantly different between the PS group and the
MS group (P=0.25, Fig. 3A). When
the analysis was limited to patients without AGS, the TRBO was
significantly longer in the MS group than in the PS group (P=0.036,
Fig. 3B). When the patients in the
PS group were limited to those patients who had undergone PS
placement and AGS, the patency period was not significantly
different between the groups (P=0.61, Fig. 3C).
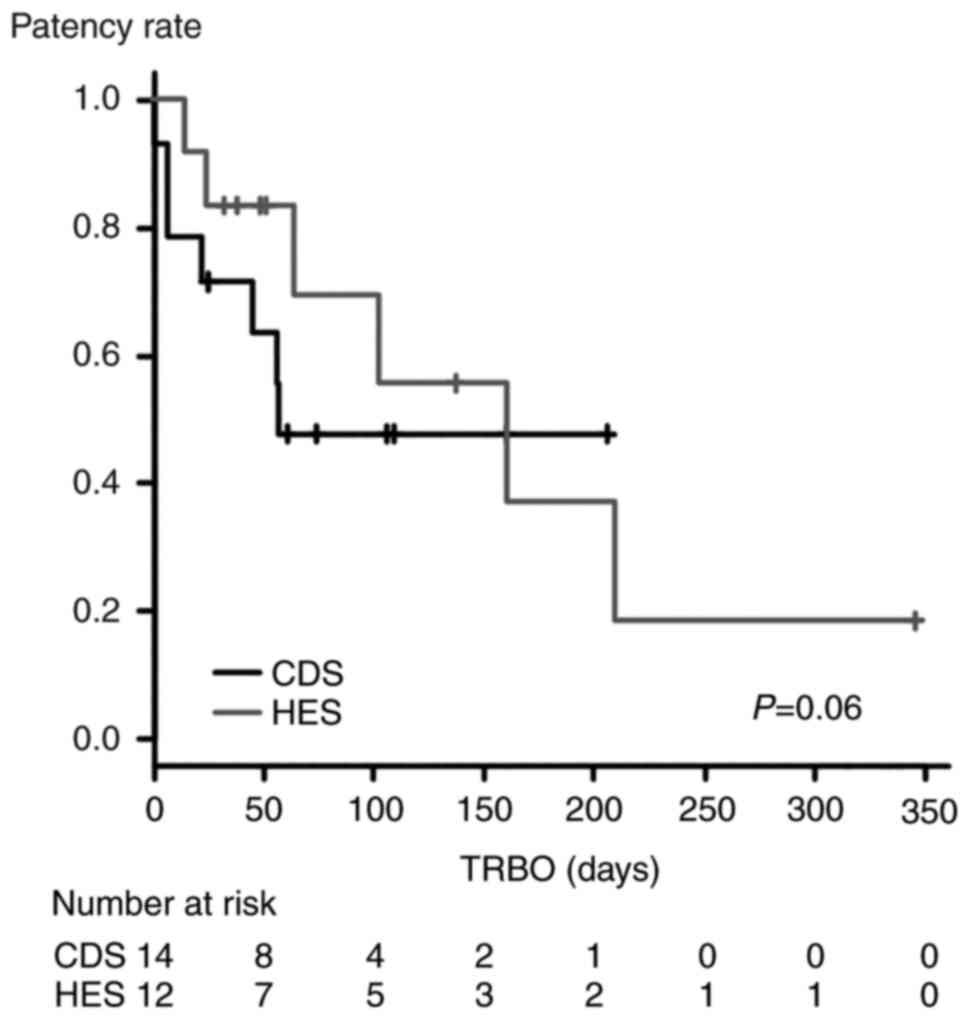
The results of the TRBO comparison between EUS-CDS
and EUS-HES are presented in Fig.
4. The TRBO was not significantly different between the two
EUS-BD methods (P=0.06). In addition, the TRBO was not
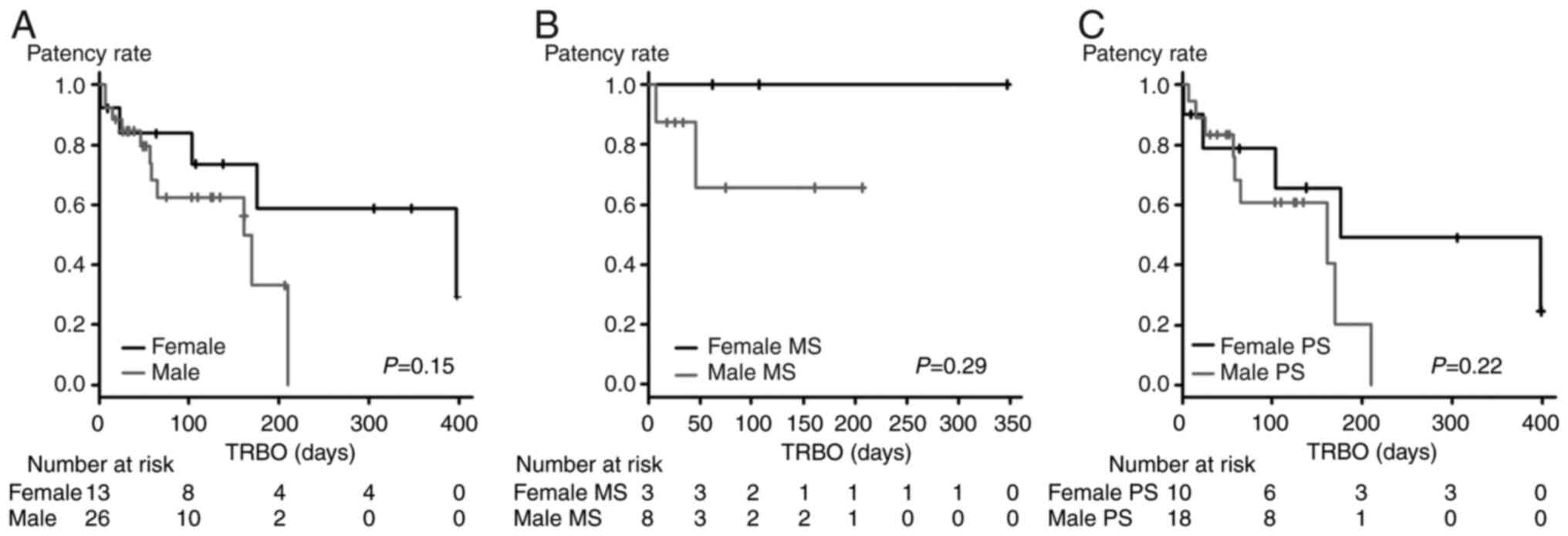
significantly different between males and females (female vs. male,
P=0.15, Fig. 5A; female MS vs.
male MS, P=0.29, Fig. 5B; female
PS vs. male PS, P=0.22, Fig.
5C).
Discussion
EUS-BD is an alternative option for cases in which
endoscopic transpapillary biliary drainage is difficult. In
endoscopic transpapillary biliary drainage, a longer TRBO is
expected with SEMSs as compared to PSs (13). However, the stent does not pass
through the biliary stricture in EUS-CDS and HES. Therefore,
whether an MS or PS should be used for EUS-BD has remained elusive.
The present study indicated that the TRBO was longer with an MS
than with a PS in EUS-BD.
The present results indicated that TRBO was not
significantly different between the MS and PS groups. Although the
EUS-BD method (CDS or HES) did not influence TRBO, the EUS-BD
method was significantly different between the PS group and the MS
group. Therefore, comparisons that excluded patients with AGS were
performed. The results indicated that patient characteristics,
EUS-BD procedure-related factors, and postprocedural course were
not significantly different between the PS group and the MS group.
When the extraneous factors were matched in this way, the TRBO in
the MS group was significantly longer than that in the PS
group.
As described in the introduction, CSEMSs have been
used in EUS-BD (3,4), but several adverse events have been
reported, including bleeding, bacteremia, bile leakage and bile
peritonitis, stent migration, pneumoperitoneum and biloma.
Furthermore, adverse events may at times become severe and fatal
(6-8,14).
Among these adverse events, bleeding may be decreased by using an
ultratapered mechanical dilator called the ES dilator (Zeon Medical
Co., Ltd.) for fistula dilation (15,16).
In addition, a single-pigtail PS with two flanges on the distal end
and proximal end called the IT stent (Gadelius Medical Co., Ltd.)
has been reported to address stent migration (9) and be easy to place. However, in the
present study, an MS was more appropriate for a longer TRBO than a
PS. In a previous study, the combination of EUS-HES with PS
placement and EUS-AGS using an MS was expected to result in a long
TRBO (17). In the present study,
the combination of EUS-HES with PS placement and EUS-AGS using an
MS was not inferior to EUS-BD using an MS in terms of the TRBO for
patients with malignant biliary strictures. Therefore, the
disadvantages of EUS-BD using a PS may be overcome by performing
EUS-AGS with an MS at the same time.
On the other hand, MSs for EUS-BD have been
improved. Lumen-apposing metal stents (LAMSs) have been used for
EUS-CDS (18,19). The LAMS delivery system (Hot AXIOS;
Boston Scientific) may also be used as an electrocautery dilator.
Therefore, the puncture, fistula dilation and stent placement
processes may be achieved in one step. In addition, the
dumbbell-like shape of the LAMS prevents stent migration. The
function of the LAMS as an electrocautery dilator may lead to the
possibility of bleeding. However, only a small number of studies
have reported bleeding with the use of an LAMS in EUS-CDS (20,21).
Of note, the use of LAMSs for EUS-BD is not covered by the National
Health Insurance of Japan. Therefore, LAMSs were not used in the
present study.
For EUS-HES, the use of a dedicated partially
covered SEMS (Spring Stopper; Taewoong Medical) has been reported
(22). The distal end of the SEMS
is uncovered and the proximal end resembles an umbrella. This shape
prevents stent migration to the abdominal cavity. In addition, the
delivery system of the stent matches a 0.025 guidewire. Therefore,
sufficient trackability to the guidewire and insertion into the
bile duct are expected. Improvement in SEMS performance may help to
overcome the risk of migration and contribute to achieving a long
TRBO in patients treated with EUS-BD.
There are certain limitations to the present study.
First, the study was retrospective, performed at a single
institution and had a small sample size. Furthermore, different
stents were used in each group. In the future, multicenter
prospective studies that use a PS or an MS are required to validate
these results. In addition, a large proportion of the patients
(n=26/39) were male. However, there was no significant difference
in patient sex between the MS group and the PS group.
In conclusion, a longer TRBO is expected in EUS-BD
with MSs than with PSs. Although MSs had sufficient patency,
critical adverse events were reported with EUS-BD using MSs. On the
other hand, PSs are easy to place. The use of PSs may help to
overcome the disadvantage of a short TRBO if combined with EUS-AGS
using an MS. In the future, improvements to MSs may contribute to
the advancement of EUS-BD.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author upon
reasonable request.
Authors' contributions
MS wrote the manuscript, contributed to the study
design and performed the research. TT contributed to the study
design and supervised the research. RS, YW, NK, HA, YS, HI, JN, MT,
MH, TK, RK, TY and TH performed analyses and interpretation of
data. HO supervised the study, performed analyses and
interpretation of data, and wrote the manuscript. All authors have
read and approved the final manuscript. TT and HI confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Fukushima Medical University (Fukushima, Japan; approval
no. 2399).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mukai S, Itoi T, Baron TH, Takada T,
Strasberg SM, Pitt HA, Ukai T, Shikata S, Teoh AYB, Kim MH, et al:
Indications and techniques of biliary drainage for acute
cholangitis in updated Tokyo Guidelines 2018. J Hepatobiliary
Pancreat Sci. 24:537–549. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Lam R and Muniraj T: Fully covered metal
biliary stents: A review of the literature. World J Gastroenterol.
27:6357–6373. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Matsunami Y, Itoi T, Sofuni A, Tsuchiya T,
Ishii K, Tanaka R, Tonozuka R, Honjo M, Mukai S, Nagai K, et al:
EUS-guided hepaticoenterostomy with using a dedicated plastic stent
for the benign pancreaticobiliary diseases: A single-center study
of a large case series. Endosc Ultrasound. 10:294–304.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Harai S, Hijioka S, Nagashio Y, Ohba A,
Maruki Y, Sone M, Saito Y, Okusaka T, Fukasawa M and Enomoto N:
Usefulness of the laser-cut, fully covered, self-expandable
metallic stent for endoscopic ultrasound-guided
hepaticogastrostomy. J Hepatobiliary Pancreat Sci. 29:1035–1043.
2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bishay K, Boyne D, Yaghoobi M, Khashab MA,
Shorr R, Ichkhanian Y and Forbes N: Endoscopic ultrasound-guided
transmural approach versus ERCP-guided transpapillary approach for
primary decompression of malignant biliary obstruction: A
meta-analysis. Endoscopy. 51:950–960. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Martins FP, Rossini LG and Ferrari AP:
Migration of a covered metallic stent following endoscopic
ultrasound-guided hepaticogastrostomy: Fatal complication.
Endoscopy. 42 (Suppl 2):E126–E127. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ramírez-Luna MA, Téllez-Ávila FI,
Giovannini M, Valdovinos-Andraca F, Guerrero-Hernández I and
Herrera-Esquivel J: Endoscopic ultrasound-guided biliodigestive
drainage is a good alternative in patients with unresectable
cancer. Endoscopy. 43:826–830. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Paik WH and Park DH: Outcomes and
limitations: EUS-guided hepaticogastrostomy. Endosc Ultrasound.
8:S44–S49. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Umeda J, Itoi T, Tsuchiya T, Sofuni A,
Itokawa F, Ishii K, Tsuji S, Ikeuchi N, Kamada K, Tanaka R, et al:
A newly designed plastic stent for EUS-guided hepaticogastrostomy:
A prospective preliminary feasibility study (with videos).
Gastrointest Endosc. 82:390–396 e392. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Isayama H, Hamada T, Yasuda I, Itoi T,
Ryozawa S, Nakai Y, Kogure H and Koike K: TOKYO criteria 2014 for
transpapillary biliary stenting. Dig Endosc. 27:259–264.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cotton PB, Eisen GM, Aabakken L, Baron TH,
Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT,
Petrini JL, et al: A lexicon for endoscopic adverse events: report
of an ASGE workshop. Gastrointest Endosc. 71:446–454.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jang S, Stevens T, Parsi M, Lopez R,
Zuccaro G, Dumot J and Vargo JJ: Association of covered metallic
stents with cholecystitis and stent migration in malignant biliary
stricture. Gastrointest Endosc. 87:1061–1070. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Scatimburgo M, Ribeiro IB, de Moura DTH,
Sagae VMT, Hirsch BS, Boghossian MB, McCarty TR, Dos Santos MEL,
Franzini TAP, Bernardo WM and de Moura EGH: Biliary drainage in
inoperable malignant biliary distal obstruction: A systematic
review and meta-analysis. World J Gastrointest Surg. 13:493–506.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hayat U, Bakker C, Dirweesh A, Khan MY,
Adler DG, Okut H, Leul N, Bilal M and Siddiqui AA: EUS-guided
versus percutaneous transhepatic cholangiography biliary drainage
for obstructed distal malignant biliary strictures in patients who
have failed endoscopic retrograde cholangiopancreatography: A
systematic review and meta-analysis. Endosc Ultrasound. 11:4–16.
2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Honjo M, Itoi T, Tsuchiya T, Tanaka R,
Tonozuka R, Mukai S, Sofuni A, Nagakawa Y, Iwasaki H and Kanai T:
Safety and efficacy of ultratapered mechanical dilator for
EUS-guided hepaticogastrostomy and pancreatic duct drainage
compared with electrocautery dilator (with video). Endosc
Ultrasound. 7:376–382. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kanno Y, Ito K, Koshita S, Ogawa T, Masu
K, Masaki Y and Noda Y: Efficacy of a newly developed dilator for
endoscopic ultrasound-guided biliary drainage. World J Gastrointest
Endosc. 9:304–309. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamamoto K, Itoi T, Tsuchiya T, Tanaka R,
Tonozuka R, Honjo M, Mukai S, Fujita M, Asai Y, Matsunami Y, et al:
EUS-guided antegrade metal stenting with hepaticoenterostomy using
a dedicated plastic stent with a review of the literature (with
video). Endosc Ultrasound. 7:404–412. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Venkatachalapathy SV, James MW, Huggett
MT, Paranandi B, Pereira SP, Johnson G, Aravinthan AD and Aithal
GP: Utility of palliative EUS-guided biliary drainage using
lumen-apposing metal stents: A prospective multicenter feasibility
study (with video). Gastrointest Endosc. 94:321–328.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Garcia-Sumalla A, Loras C, Sanchiz V, Sanz
RP, Vazquez-Sequeiros E, Aparicio JR, de la Serna-Higuera C,
Luna-Rodriguez D, Andujar X, Capilla M, et al: Multicenter study of
lumen-apposing metal stents with or without pigtail in endoscopic
ultrasound-guided biliary drainage for malignant obstruction-BAMPI
TRIAL: An open-label, randomized controlled trial protocol. Trials.
23(181)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Paccard JR, Lambin T, Rivory J, Rostain F,
Thivolet A, Lafeuille P and Pioche M: Aneurysm rupture after
choledochoduodenostomy with lumen-apposing metal stent: Endoscopic
ultrasound-guided stenting of the bile duct in an endoscopically
blind situation due to massive bleeding. Endoscopy. 54:E322–E323.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Garcia-Alonso FJ, Sanchez-Ocana R,
Peñas-Herrero I, Law R, Sevilla-Ribota S, Torres-Yuste R, Gil-Simón
P, de la Serna Higuera C and Perez-Miranda M: Cumulative risks of
stent migration and gastrointestinal bleeding in patients with
lumen-apposing metal stents. Endoscopy. 50:386–395. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yamamura M, Ogura T, Ueno S, Okuda A,
Nishioka N, Yamada M, Ueshima K, Matsuno J, Yamamoto Y and Higuchi
K: Partially covered self-expandable metal stent with antimigratory
single flange plays important role during EUS-guided
hepaticogastrostomy. Endosc Int Open. 10:E209–E214. 2022.PubMed/NCBI View Article : Google Scholar
|