Introduction
Oral cancer is the 16th most common type of cancer
worldwide, and oral squamous cell carcinoma (OSCC) accounts for
~90% of all oral cancer cases (1).
OSCC is a multifactorial disease that arises from the stratified
squamous epithelium in the oral mucosa (2,3).
Surgical resection of the primary tumor combined with radiotherapy
and chemotherapy is the most common treatment approach for advanced
OSCC (4). However,
cis-diamine-dichloroplatinum II (cisplatin, CDDP) chemoresistance
often results in treatment failure and a poor prognosis (5), with a 5-year survival rate of <50%
for patients with advanced OSCC (6). Therefore, developing effective
adjuvant treatment approaches to increase drug sensitivity to
complement current therapeutics and improve overall survival is of
great significance.
The ubiquitin-proteasome pathway is a major
intracellular pathway involved in protein degradation. Numerous
fundamental cellular processes depend on the ubiquitin-proteasome
pathway, including cell cycle, cell apoptosis, and cell
differentiation (7-9).
Additionally, the ubiquitin-proteasome pathway has been extensively
studied in cancer therapy (10,11).
A previous study showed that the proteasome inhibitor MG132
(carbobenzoxy-L-leucyl-L-leucyl-L-leucinal) could effectively
inhibit the proteolytic activity of the 26S proteasome complex
(12). It has been also reported
that MG132 exerts therapeutic effects in several types of cancer,
such as lung cancer, and hypopharyngeal cancer (13,14).
Furthermore, MG132 also increases the sensitivity of ovarian
carcinoma and esophageal squamous cell carcinoma cells to
chemotherapeutic drugs (15,16).
Although MG132 has been reported to affect CDDP sensitivity in oral
cancer, and the underlying molecular mechanism involved has been
partly explored (17-19),
further research is required to investigate the in-depth mechanism
underlying MG132 induced CDDP sensitivity in oral cancer.
In the current study, OSCC cells were co-treated
with MG132 and CDDP to evaluate the effect of MG132 on cell
viability, cell proliferation, apoptosis, and intracellular
reactive oxygen species (ROS) generation. Furthermore, the effect
of MG132 on the p53-mediated apoptotic signaling pathway in
CDDP-treated OSCC cells was determined.
Materials and methods
Cell culture and morphology
The human CAL27 OSCC cell line was a kind gift from
the Nanjing Stomatological Hospital. CAL27 cells were cultured in
high glucose DMEM (Wisent, Inc.) supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), 100 units/ml penicillin G, and 100
µg/ml streptomycin in a humidified incubator supplied with 5%
CO2 at 37˚C. When cells reached confluency, cells were
treated with trypsin to detach them and split or used as required.
The morphology of CAL27 cells treated with PBS (control cells), 0.2
µM MG132 (MedChemExpress), and/or 2 µM CDDP (MedChemExpress) for 48
h was directly observed using an Olympus inverted bright-field
light microscope CKX41 (Olympus Corporation; magnification,
x40).
Cell viability
Cells were seeded into 96-well plates at a density
of 5x103 cells/well and cultured overnight at 37˚C in a
5% CO2 incubator. Following treatment with CDDP, MG132,
or a combination of both for 48 h, each well of the 96-well plate
was treated with 10 µl Cell Counting Kit 8 (CCK-8) solution
(Beyotime Institute of Biotechnology), and cells were further
incubated for 4 h. The absorbance at a wavelength of 450 nm was
measured using a Varioskan LUX microplate reader (Thermo Fisher
Scientific, Inc.).
Measurement of ROS
The intracellular ROS levels were quantified using
an ROS assay kit (Beyotime Institute of Biotechnology). Following
treatment with CDDP and/or MG132, cells were harvested and
incubated with 10 µM DCFH-DA probe for 20 min at 37˚C. The labeled
cells were then washed twice with PBS and counted using a flow
cytometer (Beckman Coulter) at excitation and emission wavelengths
of 488 and 525 nm.
TUNEL assay
TUNEL assays were performed according to the
manufacturer's instructions. Briefly, cells were treated with PBS
(control), CDDP, MG132, or CDDP + MG132 for 48 h. Cells were then
fixed with 4% polyformaldehyde (Sinopharm Chemical Reagent Co.,
Ltd.) for 30 min at room temperature (RT), permeabilized with 0.3%
Triton X-100 (Sinopharm), and incubated with TUNEL detection
solution (Beyotime Institute of Biotechnology) for 1 h at 37˚C.
Following cell staining with Hoechst 33342 for 10 min at RT
(Beyotime Institute of Biotechnology), images were captured using a
Leica fluorescence microscope DMi8 (Leica Biosystems;
magnification, x200).
Colony formation assay
A colony formation assay was performed to evaluate
the proliferation potential of OSCC cells. Briefly, cells were
seeded into 6-well plates at a density of 1x103
cells/well and allowed to adhere overnight. Subsequently, cells
were treated with PBS, CDDP, MG132, or CDDP + MG132 and cultured
for up to 7 days. Following fixing with methanol for 30 min at RT,
the colonies were stained with 0.1% crystal violet (Sinopharm)
solution for 30 min at RT. Colonies consisting of >20 cells were
included in the analysis.
Ethynyl-2-deoxyuridine (EdU)
assay
Cell proliferation was assessed using an EdU
staining using the BeyoClick EdU kit (Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. Briefly,
cells were treated with PBS, CDDP, MG132, or CDDP + MG132 for 48 h
and were then incubated with 10 µM EdU at 37˚C for an additional 2
h. Following washing with PBS, cells were fixed with 4%
polyformaldehyde at RT, permeabilized with 0.3 Triton X-100
followed by incubation with Click additive solution (Beyotime
Institute of Biotechnology) at RT for 30 min in the dark. After
staining with EdU, cells were counterstained with Hoechst 33342 for
10 min at RT and observed under a Leica fluorescence microscope
DMi8 (Leica Biosystems; magnification, x200).
Annexin-V apoptosis detection
assay
To assess cell apoptosis, cells were treated with
CDDP and/or MG132 for 48 h. Following trypsinization, cells were
incubated with an annexin V-FITC binding buffer containing annexin
V-FITC (Vazyme Biotechnology Co. Ltd.), and propidium iodide (PI)
at RT for 10 min. Following staining, cells were sorted using a
DxFLEX flow cytometer (Beckman Coulter, Inc.) with CytExpert For
DxFLEX software (version 2.0.0.283; Beckman Coulter, Inc.) and
analyzed using CytExpert For DxFLEX software (version 2.0.0.283;
Beckman Coulter, Inc.).
Cell cycle distribution analysis
Cell cycle distribution was determined by staining
DNA with PI (Beyotime Institute of Biotechnology). Briefly, cells
were treated with CDDP, MG132, or CDDP + MG132 for 48 h. After
treatment, cells were washed with PBS and fixed in 70% ethanol for
2 h at 4˚C. Cells were then washed again and incubated with PI
staining solution at 37˚C for 30 min. The percentage of cells in
the different phases of the cell cycle was measured using a DxFLEX
cytometer and analyzed using CytExpert For DxFLEX software. The
excitation and emission wavelengths were 488 and 617 nm.
Western blot analysis
Following treatment with CDDP and/or MG132, cells
were scraped, lysed and the protein extracts were finally
collected. Cell lysates were then resolved using 12% SDS-PAGE,
transferred to PVDF membranes, and blocked with 5% non-fat milk in
TBS-Tween 20. Subsequently, the membranes were incubated with
primary antibodies against Bcl-2 (1:1,000), p53 (1:1,000; both from
Abmart Pharmaceutical Technology Co., Ltd.), Bax (1:2,000; Abcepta,
Inc.), or GAPDH (1:1,000; Abmart Pharmaceutical Technology Co.,
Ltd.) at 4˚C overnight. Following incubation with the corresponding
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (1:5,000; Abmart Pharmaceutical Technology Co., Ltd.) at
room temperature for 1 h, the signals were visualized using an
enhanced chemiluminescence reagent (Beyotime Institute of
Biotechnology) and detected using the Odyssey Fc system (LI-COR
Biosciences).
Statistical analysis
Statistical analysis was performed using SPSS
version 19.0 (IBM Corp.). The results were analyzed using a one-way
ANOVA followed by a Tukey's post-hoc test. Data are expressed as
the mean ± SEM from at least three independent experiments. P≤0.05
was considered to indicate a statistically significant
difference.
Results
MG132 elevates the inhibitory effect
of CDDP on OSCC cell viability
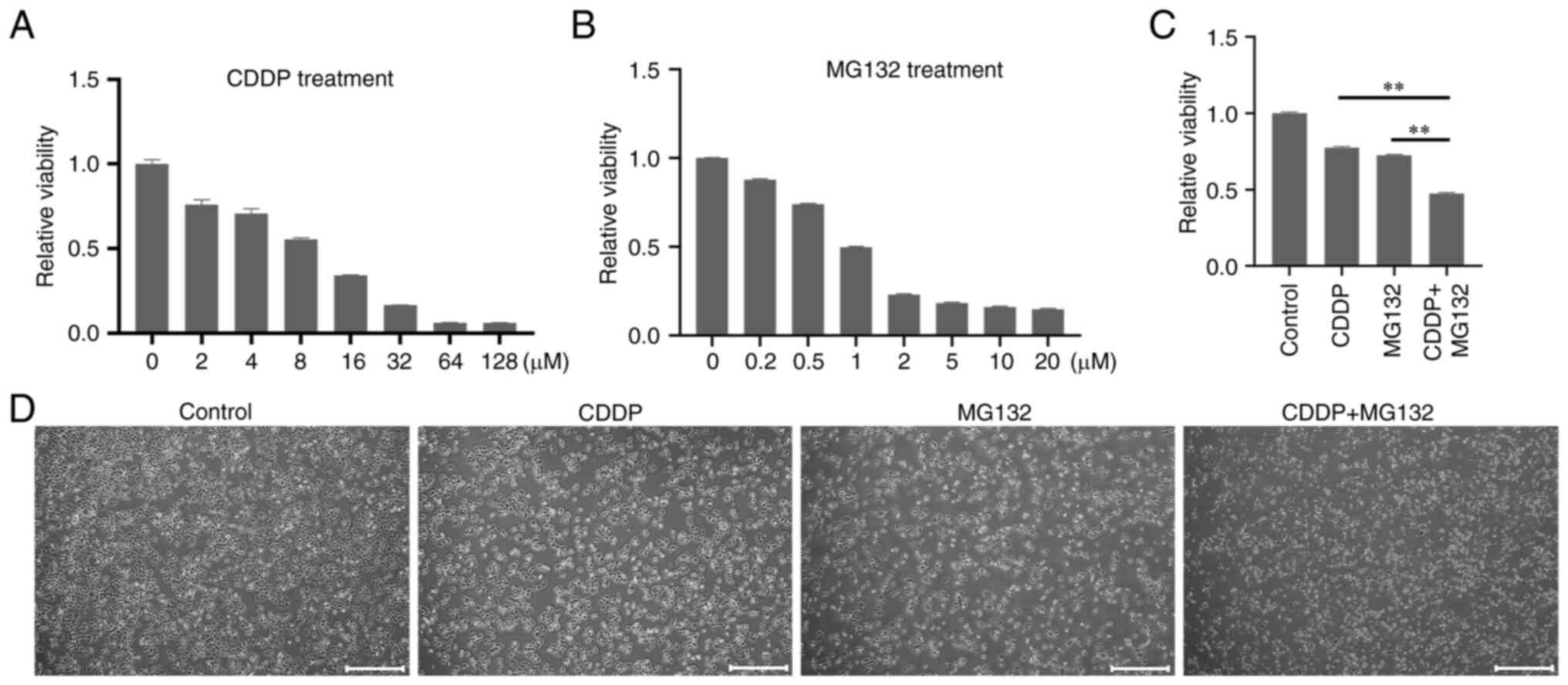
Τo determine whether the combination of MG132 and
CDDP had a synergistic anti-cancer effect, CAL27 cells were first
treated with increasing concentrations of MG132 and CDDP. The
results showed that cell treatment with 0.2 µM MG132 and 2 µM CDDP
could significantly reduce cell viability compared with untreated
control cells (Fig. 1A and
B). Furthermore, 0.2 µM MG132 and
2 µM of CDDP were the minimum concentrations required to induce a
significant decrease in cell viability. Therefore, these specific
concentrations were used for subsequent experiments. Next, CAL27
cells were treated with 0.2 µM MG132, 2 µM CDDP, or both for 48 h.
CCK-8 assays demonstrated that MG132 significantly enhanced the
CDDP-induced inhibition of cell viability (Fig. 1C). Consistently, light microscopy
also showed that the combined treatment synergistically enhanced
cell growth inhibition compared with cell treatment with each drug
alone (Fig. 1D). The above results
indicated that MG132 and CDDP exerted a synergistic anti-cancer
effect on OSCC.
MG132 promotes CDDP-induced ROS
production and DNA damage in OSCC cells
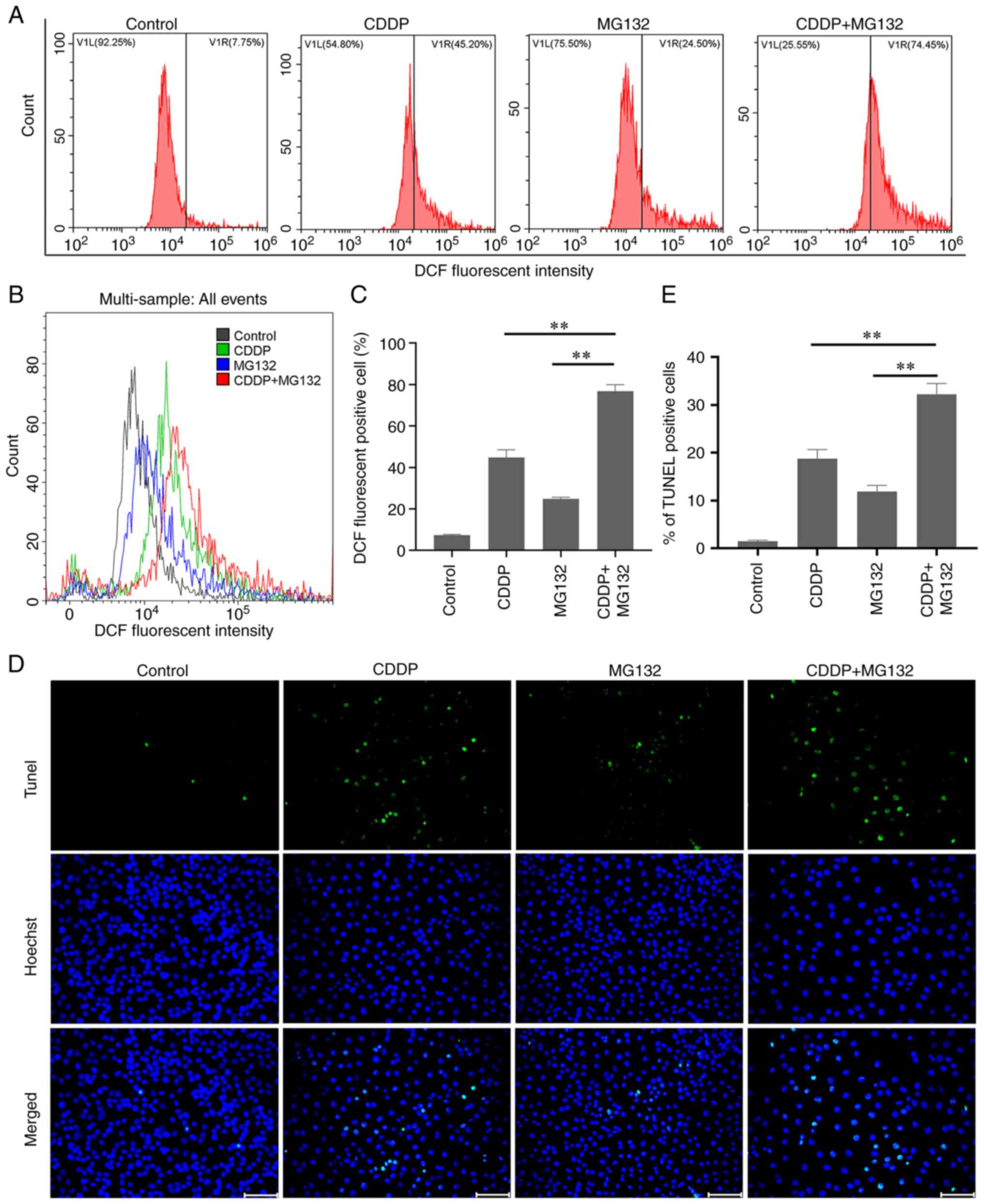
Subsequently, to investigate whether MG132 promoted
oxidative stress in OSCC cells, the intracellular ROS levels were
detected in CAL27 cells using DCFH staining followed by flow
cytometry. CAL27 cells were treated with MG132, CDDP, or both for
48 h and were then subjected to DCFH staining. The results showed
that the intracellular ROS levels were higher in cells co-treated
with CDDP and MG132 compared with cells treated with either CDDP or
MG132 alone (Fig. 2A-C).
Subsequently, DNA damage was assessed using a TUNEL assay. The
results showed that both treatment with MG132 or CDDP alone
enhanced DNA damage in OSCC cells. However, co-treatment of OSCC
cells with MG132 and CDDP further promoted DNA damage (Fig. 2D and E). Overall, the above findings suggested
that ROS may be involved in MG132-induced DNA damage. However, the
particular mechanisms remain unclear.
MG132 enhances the inhibitory effects
of CDDP on OSCC cell proliferation
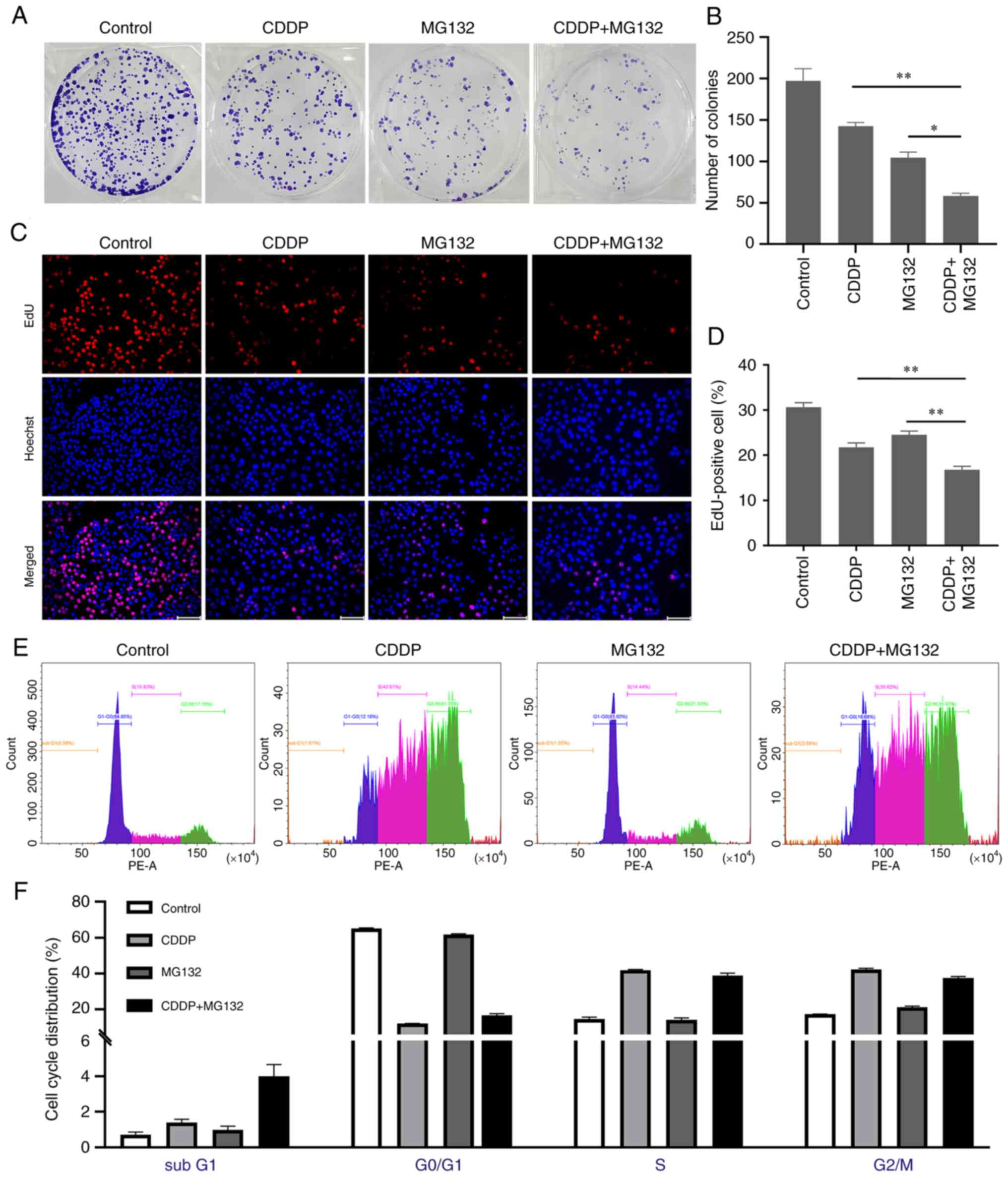
It has been reported that excessive accumulation of
ROS can inhibit tumor growth by inhibiting cancer cell
proliferation (20). Herein, to
uncover the effects of MG132 and/or CDDP on OSCC cell
proliferation, a colony formation assay was performed on CAL27
cells treated with 0.2 µM MG132, 2 µM CDDP, or both for 48 h. The
data demonstrated that the combination of MG132 and CDDP
significantly decreased the colony formation ability of CAL27 cells
compared with cells treated with either MG132 or CDDP alone
(Fig. 3A and B). Furthermore, an EdU proliferation
assay was performed to evaluate the proliferation ability of OSCC
cells. As shown in Fig. 3C and
D, MG132 combined with CDDP
markedly reduced EdU staining compared with the MG132 and CDDP
groups. To elucidate the mechanism of growth inhibition, cell cycle
distribution experiments were performed. Compared with the
untreated control cells, CDDP significantly increased the
proportion of cells at sub-G1, S, and G2/M phases (P<0.01),
whereas the proportion of cells in the G0/G1 phase was reduced
(P<0.01), suggesting that CDDP induced cell cycle arrest in
sub-G1, S, and G2/M phases in CAL27 cells. Conversely, MG132
significantly increased the proportion of cells in the G2/M phase
(P<0.01) and reduced the proportion of cells in the G0/G1 phase
(P<0.01), indicating that MG132 could induce G2/M arrest
(Fig. 3E and F).
MG132 enhances CDDP-induced apoptosis
in OSCC cells
ROS production and DNA damage are considered key
mechanisms involved in cell death (21). To examine whether MG132 and CDDP
exerted a synergistic effect on promoting cancer cell apoptosis,
double staining with V-FITC/PI was performed. Cell apoptosis was
then assessed by flow cytometry. The results showed that cell
treatment with CDDP or MG132 alone notably elevated cell apoptosis
rate, which was further enhanced in cells co-treated with CDDP and
MG132 (Fig. 4A and B). It has been reported that p53 plays a
significant role in cancer cell apoptosis and regulates downstream
mitochondrial apoptosis-related pathways (22). Therefore, in the current study, the
expression levels of p53 and those of its downstream
apoptosis-related signal pathways were determined. MG132
upregulated p53 expression and further enhanced the CDDP-induced
p53 expression levels in OSCC cells (Fig. 4C). To uncover the underlying
downstream pro-apoptotic mechanisms induced by p53, the expression
levels of the Bcl-2 family members were detected. As shown in
Fig. 4C, Bax was markedly
upregulated and Bcl-2 was significantly downregulated in cells
co-treated with CDDP and MG132 compared with those treated with
MG132 or CDDP alone (Fig. 4C),
thus indicating that p53 and its downstream apoptosis-related genes
could play a key role in MG132-induced OSCC apoptosis.
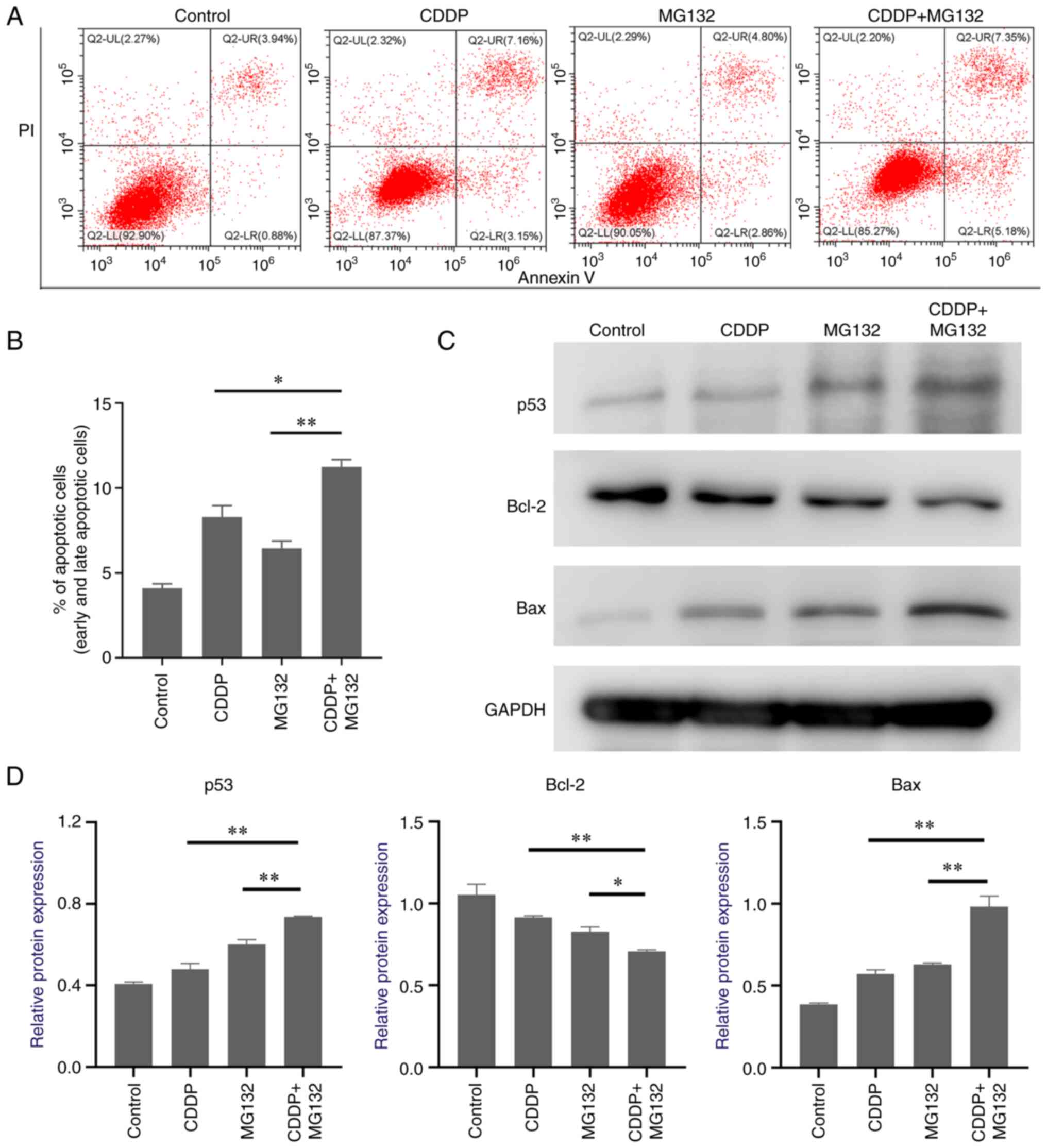 | Figure 4CDDP-induced apoptosis of oral
squamous cell carcinoma cells is enhanced by MG132. (A)
Representative image of flow cytometry analysis of CAL27 cells
treated with CDDP, MG132, or CDDP + MG132. Cell apoptosis was
assessed using an Annexin V/PI kit. Annexin V-/PI- staining
indicates viable cells, Annexin V+/PI- early
apoptotic cells, Annexin V+/PI+ late
apoptotic cells, and Annexin V-/PI+ necrotic
cells. (B) Quantitative analysis of total apoptotic cells (early
and late apoptosis) is shown. Data are expressed as the mean ± SEM
of three independent experiments. (C) Representative images of p53,
Bcl-2, and Bax protein expression levels detected by western blot.
(D) Densitometry analysis of the p53, Bcl-2, and Bax protein
expression levels relative to GAPDH. *P<0.05 and
**P<0.01. CDDP, cis-diamine-dichloroplatinum II; PI,
propidium iodide. |
Discussion
In the majority of patients with OSCC,
chemotherapeutic drugs are recommended as adjuvant therapy after
surgery. CDDP is widely used as a chemotherapeutic agent for the
treatment of several solid tumors. In 1978, CDDP became the first
FDA-approved platinum-based compound for cancer treatment (23). Currently, CDDP, as a first-line
treatment, is the most commonly used chemotherapeutic drug for
OSCC. However, the development of CDDP resistance limits its
application and effectiveness. Therefore, combination therapies of
CDDP with other anti-cancer drugs have been applied as novel
therapeutic strategies for treating several types of cancer. The
current study aimed to investigate whether combination therapy with
MG132 and CDDP could reduce OSCC progression.
As a natural triterpene proteasome inhibitor derived
from Chinese medicinal plants, MG132 can inhibit the proteolytic
activity of the 26S proteasome complex (22,24).
It has been reported that MG132 can be used to treat several types
of cancer. Previous studies showed that MG132 could enhance ROS
generation (25), inhibit cell
proliferation (16), and promote
cell apoptosis in different types of cancer (22). Additionally, the role of MG132 has
been largely investigated in OSCC. Chen et al (26) demonstrated that MG132 could induce
cell apoptosis via regulating glucose-regulated protein 78 and
caspase 12 in the OSCC cell line Tca-8113. Additionally, Tsunoda
et al (27) showed that
MG132 could upregulate c-Jun in the OSCC cell lines Ca9-22 and
HSC3, which in turn could form homologous dimers to enhance IL-8
expression. Other studies revealed that connective tissue growth
factor upregulation (28) and
deubiquitinating protein 3 downregulation (29) could both promote OSCC cell
apoptosis. However, the above effect could be abolished by MG132
treatment. It has been reported that the programmed cell death
(PD)-1/PD-ligand 1 (PD-L1) signaling pathway is involved in a type
of tumor immune evasion strategy. Wu et al (30) showed that MG132 could inhibit the
degradation of PD-L1 by deubiquitinating and stabilizing its
protein expression in the OSCC cell lines HN4 and HN30.
Furthermore, He et al (31)
demonstrated that co-treatment of cells with metformin and 4SC-202
exerted an anti-OSCC effect by suppressing the proliferation and
promoting the intrinsic apoptosis of OSCC cells both in
vitro and in vivo. This study also suggested that MG132
could block the synergistic action of metformin and 4SC-202 by
inhibiting the degradation of ΔNp63.
In addition to the above findings, it has also been
reported that MG132 can cooperate with several genes to treat OSCC
(32-34).
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand
(TRAIL) can induce apoptosis in several types of tumor cells
(35). However, resistance to
TRAIL response has been verified in different types of cancer,
including OSCC (34). A previous
study demonstrated that MG132 enhanced TRAIL action to increase
apoptosis of the OSCC cell lines HSC-2 and HSC-3(34). In addition, MG132 could effectively
cooperate with TRAIL agonists to promote OSCC cell apoptosis by
stabilizing truncated Bid and Bik (33). Additionally, MG132 could synergize
with short hairpin RNA against X-box-binding protein 1 to activate
the inositol-requiring enzyme 1a/TNF receptor-associated
factor-2/apoptosis signal-regulating kinase-1/Jun kinase pathway to
promote the apoptosis of the OSCC cells, TCA8113(32).
The present study showed that MG132 reduced OSCC
cell viability in a dose-dependent manner, thus supporting its
direct anti-cancer effect on OSCC. Previous studies also
demonstrated that chemotherapeutic drugs combined with proteasome
inhibitors improves chemotherapy sensitivity (15,16,36).
However, the combination of MG132 and CDDP on OSCC has not been
previously assessed. The results revealed that MG132 combined with
CDDP markedly reduced cell viability compared with either MG132 or
CDDP alone, thus suggesting that MG132 exhibited a synergistic
effect with CDDP on OSCC cells. Previously, it has been shown that
MG132 promoted neural stem cell death (37), and Bax et al (9) also found that MG132 negatively
regulates pluripotent stem cell survival and motor neuron
differentiation. The aforementioned findings indicate that the
future clinical applications of MG132 may be limited. However,
further studies are required to determine the toxicity and side
effects of MG132 prior to clinical use.
ROS plays a crucial role in several biological
processes. Maintaining ROS homeostasis is essential for the
maintenance of a physiological state (9,38).
Excessive ROS production often leads to oxidative stress. Under
conditions of oxidative stress, excessive ROS attacks nitrogenous
bases and the sugar-phosphate backbone of DNA, eventually leading
to single- and double-stranded DNA breaks (39). Previous studies demonstrated that
both CDDP (38) and MG132(40) could promote ROS generation
individually. The results of the present study also revealed that
both CDDP and MG132 could increase ROS levels, as well as DNA
damage in OSCC cells. Additionally, a synergistic effect between
CDDP and MG132 on promoting ROS generation and DNA damage was
observed, thus indicating that MG132 exerted an anti-cancer effect
by inducing DNA damage via ROS.
Previously, the mechanisms underlying the effects of
MG132 on inducing intracellular ROS generation have not been
investigated. Park et al (41) demonstrated that the MG132-induced
GSH downregulation was involved in ROS generation. In addition, the
levels of O2−, primarily originating from the
mitochondria, were shown to be increased in MG132-treated human
pulmonary fibroblast cells (42).
Therefore, the particular mechanisms involved in the effects of
MG132 on inducing ROS production should be addressed in future
studies.
It has been reported that numerous basic biological
processes, including cellular proliferation, require moderate to
low ROS levels (38).
Additionally, a previous study revealed that excessive ROS
production overwhelms antioxidant systems, thus leading to cell
cycle arrest as well as inhibition of cell proliferation (37). The current study demonstrated that
the combined use of MG132 and CDDP could significantly reduce the
proliferative ability of OSCC cells, as evidenced by the decreased
number of cell colonies and reduced cell proliferation ratio. These
findings suggested that MG132 could promote the CDDP-induced
inhibition of OSCC cell proliferation by enhancing ROS
production.
When ROS induces oxidative DNA damage, p53 is
activated by ROS via DNA damage checkpoint pathways (23). p53 is an essential tumor suppressor
factor, encoded by the tumor suppressor gene TP53. Activation of
p53 is considered an attractive anti-cancer therapy approach. p53
can induce tumor cell apoptosis via regulation of its downstream
mitochondrial apoptotic signaling pathway (22). Bax and Bcl-2 are both members of
the Bcl-2 family. Bax promotes cell apoptosis and it is
transcriptionally regulated by p53. Therefore, its expression is
positively associated with p53 expression (43). By contrast, the anti-apoptotic
protein Bcl-2 plays a crucial role in cell survival. A p53-negative
response element has been identified in the promoter region of
Bcl-2. Therefore, it has been reported that Bcl-2 is negatively
regulated by p53(44). Here, p53
and its downstream apoptotic signaling pathways were shown to be
activated in the MG132 treatment group, indicating that the
MG132-induced cell apoptosis was mediated by the ROS/DNA damage/p53
axis.
In addition to the ROS/DNA damage/p53 signaling
pathway, it has been also reported that MG132 can affect CDDP
sensitivity in OSCC through different signaling pathways. A
previous study demonstrated that MG132 inhibited the ubiquitination
of phosphoglycerate kinase 1, enhance its expression, and activate
the Akt/mTOR signaling pathway, eventually leading to CDDP
resistance in OSCC cells (17).
Additionally, another study revealed the synergistic effect of
MG132 and CDDP on the SCC-25 OSCC cell line via regulation of the
E-cadherin/β-catenin complex signaling pathway (18). Furthermore, minichromosome
maintenance deficient 5 (MCM5) promoted DNA repair in OSCC cells
via interacting with long non-coding RNA POP1-1, thus resulting in
the onset of resistance in OSCC cells. MG132 could also
significantly downregulate MCM5 in OSCC cells, thus suggesting that
MG132 could inhibit CDDP resistance (19).
In conclusion, the present study demonstrated that
MG132 affected the behavior of OSCC cells by inhibiting cell growth
and triggering cell apoptosis. In addition to the inhibitory effect
of MG132 alone, a synergistic effect was also revealed in cells
co-treated with CDDP and MG132 via the ROS/DNA damage/p53 axis. The
above findings supported the clinical application of MG132 as an
effective adjuvant with CDDP in the management of OSCC.
Acknowledgements
The authors would like to thank Professor Zhiyong
Wang (Nanjing Stomatological Hospital, Nanjing, China) for
providing the CAL27 OSCC cell line.
Funding
Funding: The current work was supported by funding from Nantong
Science and Technology Bureau (Nantong, China; grant no.
MS12020032).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ wrote the first draft of the manuscript and
performed the experiments. XW designed the study and analyzed data.
DC designed the study, revised the manuscript, and obtained the
funding. All authors have read and approved the final manuscript,
and confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Warnakulasuriya S and Kerr AR: Oral cancer
screening: Past, present, and future. J Dent Res. 100:1313–1320.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Papa F, Siciliano RA, Inchingolo F, Mazzeo
MF, Scacco S and Lippolis R: Proteomics pattern associated with
gingival oral squamous cell carcinoma and epulis: A case analysis.
Oral Sci Int. 15:41–47. 2018.
|
|
3
|
Mohapatra P, Shriwas O, Mohanty S, Ghosh
A, Smita S, Kaushik SR, Arya R, Rath R, Das Majumdar SK, Muduly DK,
et al: CMTM6 drives cisplatin resistance by regulating Wnt
signaling through the ENO-1/AKT/GSK3β axis. JCI Insight.
6(e143643)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huang SH and O'Sullivan B: Oral cancer:
Current role of radiotherapy and chemotherapy. Med Oral Patol Oral
Cir Bucal. 18:e233–e240. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kulkarni B, Gondaliya P, Kirave P, Rawal
R, Jain A, Garg R and Kalia K: Exosome-mediated delivery of miR-30a
sensitize cisplatin-resistant variant of oral squamous carcinoma
cells via modulating Beclin1 and Bcl2. Oncotarget. 11:1832–1845.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yuan Y, Xie X, Jiang Y, Wei Z, Wang P,
Chen F, Li X, Sun C, Zhao H, Zeng X, et al: LRP6 is identified as a
potential prognostic marker for oral squamous cell carcinoma via
MALDI-IMS. Cell Death Dis. 8(e3035)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bassermann F, Eichner R and Pagano M: The
ubiquitin proteasome system-implications for cell cycle control and
the targeted treatment of cancer. Biochim Biophys Acta.
1843:150–162. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gupta I, Singh K, Varshney NK and Khan S:
Delineating crosstalk mechanisms of the ubiquitin proteasome system
that regulate apoptosis. Front Cell Dev Biol. 6(11)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bax M, McKenna J, Do-Ha D, Stevens CH,
Higginbottom S, Balez R, Cabral-da-Silva MEC, Farrawell NE, Engel
M, Poronnik P, et al: The ubiquitin proteasome system is a key
regulator of pluripotent stem cell survival and motor neuron
differentiation. Cells. 8(581)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Narayanan S, Cai CY, Assaraf YG, Guo HQ,
Cui Q, Wei L, Huang JJ, Ashby CR Jr and Chen ZS: Targeting the
ubiquitin-proteasome pathway to overcome anti-cancer drug
resistance. Drug Resist Updat. 48(100663)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang H, Chen X, Li K, Cheaito H, Yang Q,
Wu G, Liu J and Dou QP: Repurposing old drugs as new inhibitors of
the ubiquitin-proteasome pathway for cancer treatment. Semin Cancer
Biol. 68:105–122. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Guo N and Peng Z: MG132, a proteasome
inhibitor, induces apoptosis in tumor cells. Asia Pac J Clin Oncol.
9:6–11. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhu W, Liu J, Nie J, Sheng W, Cao H, Shen
W, Dong A, Zhou J, Jiao Y, Zhang S and Cao J: MG132 enhances the
radiosensitivity of lung cancer cells in vitro and in
vivo. Oncol Rep. 34:2083–2089. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Qiang W, Sui F, Ma J, Li X, Ren X, Shao Y,
Liu J, Guan H, Shi B and Hou P: Proteasome inhibitor MG132 induces
thyroid cancer cell apoptosis by modulating the activity of
transcription factor FOXO3a. Endocrine. 56:98–108. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Guo N, Peng Z and Zhang J: Proteasome
inhibitor MG132 enhances sensitivity to cisplatin on ovarian
carcinoma cells in vitro and in vivo. Int J Gynecol Cancer.
26:839–844. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dang L, Wen F, Yang Y, Liu D, Wu K, Qi Y,
Li X, Zhao J, Zhu D, Zhang C and Zhao S: Proteasome inhibitor MG132
inhibits the proliferation and promotes the cisplatin-induced
apoptosis of human esophageal squamous cell carcinoma cells. Int J
Mol Med. 33:1083–1088. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jiang Q, Wang Z, Qi Q, Li J, Xin Y and Qiu
J: lncRNA SNHG26 promoted the growth, metastasis, and cisplatin
resistance of tongue squamous cell carcinoma through PGK1/Akt/mTOR
signal pathway. Mol Ther Oncolytics. 24:355–370. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lü L, Liu X, Wang C, Hu F, Wang J and
Huang H: Dissociation of E-cadherin/β-catenin complex by MG132 and
bortezomib enhances CDDP induced cell death in oral cancer SCC-25
cells. Toxicol In Vitro. 29:1965–1976. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jiang Y, Guo H, Tong T, Xie F, Qin X, Wang
X, Chen W and Zhang J: lncRNA lnc-POP1-1 upregulated by VN1R5
promotes cisplatin resistance in head and neck squamous cell
carcinoma through interaction with MCM5. Mol Ther. 30:448–467.
2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang R, Chen H, Liang J, Li Y, Yang J,
Luo C, Tang Y, Ding Y, Liu X, Yuan Q, et al: Dual role of reactive
oxygen species and their application in cancer therapy. J Cancer.
12:5543–5561. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Srinivas US, Tan BWQ, Vellayappan BA and
Jeyasekharan AD: ROS and the DNA damage response in cancer. Redox
Biol. 25(101084)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Aubrey BJ, Kelly GL, Janic A, Herold MJ
and Strasser A: How does p53 induce apoptosis and how does this
relate to p53-mediated tumour suppression? Cell Death Differ.
25:104–113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Baruah A, Chang H, Hall M, Yuan J, Gordon
S, Johnson E, Shtessel LL, Yee C, Hekimi S, Derry WB and Lee SS:
CEP-1, the Caenorhabditis elegans p53 homolog, mediates opposing
longevity outcomes in mitochondrial electron transport chain
mutants. PLoS Genet. 10(e1004097)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gonzalez-Campora R, Davalos-Casanova G,
Beato-Moreno A, Garcia-Escudero A, Pareja Megia MJ, Montironi R and
Lopez-Beltran A: BCL-2, TP53 and BAX protein expression in
superficial urothelial bladder carcinoma. Cancer Lett. 250:292–299.
2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Han YH, Moon HJ, You BR and Park WH: The
effect of MG132, a proteasome inhibitor on HeLa cells in relation
to cell growth, reactive oxygen species and GSH. Oncol Rep.
22:215–221. 2009.PubMed/NCBI
|
|
26
|
Chen SF, Chen HY, Liu XB, Zhang YX, Liu W,
Wang WH, Zhang B and Wang LX: Apoptotic effect of MG-132 on human
tongue squamous cell carcinoma. Biomed Pharmacother. 65:322–327.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tsunoda M, Fukasawa M, Nishihara A, Takada
L and Asano M: JunB can enhance the transcription of IL-8 in oral
squamous cell carcinoma. J Cell Physiol. 236:309–317.
2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lai WT, Li YJ, Wu SB, Yang CN, Wu TS, Wei
YH and Deng YT: Connective tissue growth factor decreases
mitochondrial metabolism through ubiquitin-mediated degradation of
mitochondrial transcription factor A in oral squamous cell
carcinoma. J Formos Med Assoc. 117:212–219. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Luo F, Zhou Z, Cai J and Du W: DUB3
facilitates growth and inhibits apoptosis through enhancing
expression of EZH2 in oral squamous cell carcinoma. Onco Targets
Ther. 13:1447–1460. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wu J, Guo W, Wen D, Hou G, Zhou A and Wu
W: Deubiquitination and stabilization of programmed cell death
ligand 1 by ubiquitin-specific peptidase 9, X-linked in oral
squamous cell carcinoma. Cancer Med. 7:4004–4011. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
He Y, Tai S, Deng M, Fan Z, Ping F, He L,
Zhang C, Huang Y and Cheng B: Metformin and 4SC-202 synergistically
promote intrinsic cell apoptosis by accelerating ΔNp63
ubiquitination and degradation in oral squamous cell carcinoma.
Cancer Med. 8:3479–3490. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chen H, Yang H, Pan L, Wang W, Liu X, Ren
X, Liu Y, Liu W, Zhang Y, Jiang L, et al: The molecular mechanisms
of XBP-1 gene silencing on IRE1α-TRAF2-ASK1-JNK pathways in oral
squamous cell carcinoma under endoplasmic reticulum stress. Biomed
Pharmacother. 77:108–113. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sung ES, Park KJ, Choi HJ, Kim CH and Kim
YS: The proteasome inhibitor MG132 potentiates TRAIL receptor
agonist-induced apoptosis by stabilizing tBid and Bik in human head
and neck squamous cell carcinoma cells. Exp Cell Res.
318:1564–1576. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yoshiba S, Iwase M, Kurihara S, Uchida M,
Kurihara Y, Watanabe H and Shintani S: Proteasome inhibitor
sensitizes oral squamous cell carcinoma cells to TRAIL-mediated
apoptosis. Oncol Rep. 25:645–652. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Deng D and Shah K: TRAIL of hope meeting
resistance in cancer. Trends Cancer. 6:989–1001. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang Y, Yang B, Zhao J, Li X, Zhang L and
Zhai Z: Proteasome inhibitor
Carbobenzoxy-L-Leucyl-L-Leucyl-L-Leucinal (MG132) enhances
therapeutic effect of paclitaxel on breast cancer by inhibiting
nuclear factor (NF)-κB signaling. Med Sci Monit. 24:294–304.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kim YM and Kim HJ: Proteasome inhibitor
MG132 is toxic and inhibits the proliferation of rat neural stem
cells but increases BDNF expression to protect neurons.
Biomolecules. 10(1507)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kleih M, Böpple K, Dong M, Gaißler A,
Heine S, Olayioye MA, Aulitzky WE and Essmann F: Direct impact of
cisplatin on mitochondria induces ROS production that dictates cell
fate of ovarian cancer cells. Cell Death Dis.
10(851)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Han YH, Kim SZ, Kim SH and Park WH:
Reactive oxygen species and glutathione level changes by a
proteasome inhibitor, MG132, partially affect calf pulmonary
arterial endothelial cell death. Drug Chem Toxicol. 33:403–409.
2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Han YH, Moon HJ, You BR and Park WH: The
attenuation of MG132, a proteasome inhibitor, induced A549 lung
cancer cell death by p38 inhibitor in ROS-independent manner. Oncol
Res. 18:315–322. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Park S, Park JA, Yoo H, Park HB and Lee Y:
Proteasome inhibitor-induced cleavage of HSP90 is mediated by ROS
generation and caspase 10-activation in human leukemic cells. Redox
Biol. 13:470–476. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Park WH and Kim SH: MG132, a proteasome
inhibitor, induces human pulmonary fibroblast cell death via
increasing ROS levels and GSH depletion. Oncol Rep. 27:1284–1291.
2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Miyashita T, Krajewski S, Krajewska M,
Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC: Tumor
suppressor p53 is a regulator of bcl-2 and bax gene expression in
vitro and in vivo. Oncogene. 9:1799–1805. 1994.PubMed/NCBI
|
|
44
|
Zhang Y, Zhang Y, Zhong C and Xiao F:
Cr(VI) induces premature senescence through ROS-mediated p53
pathway in L-02 hepatocytes. Sci Rep. 6(34578)2016.PubMed/NCBI View Article : Google Scholar
|