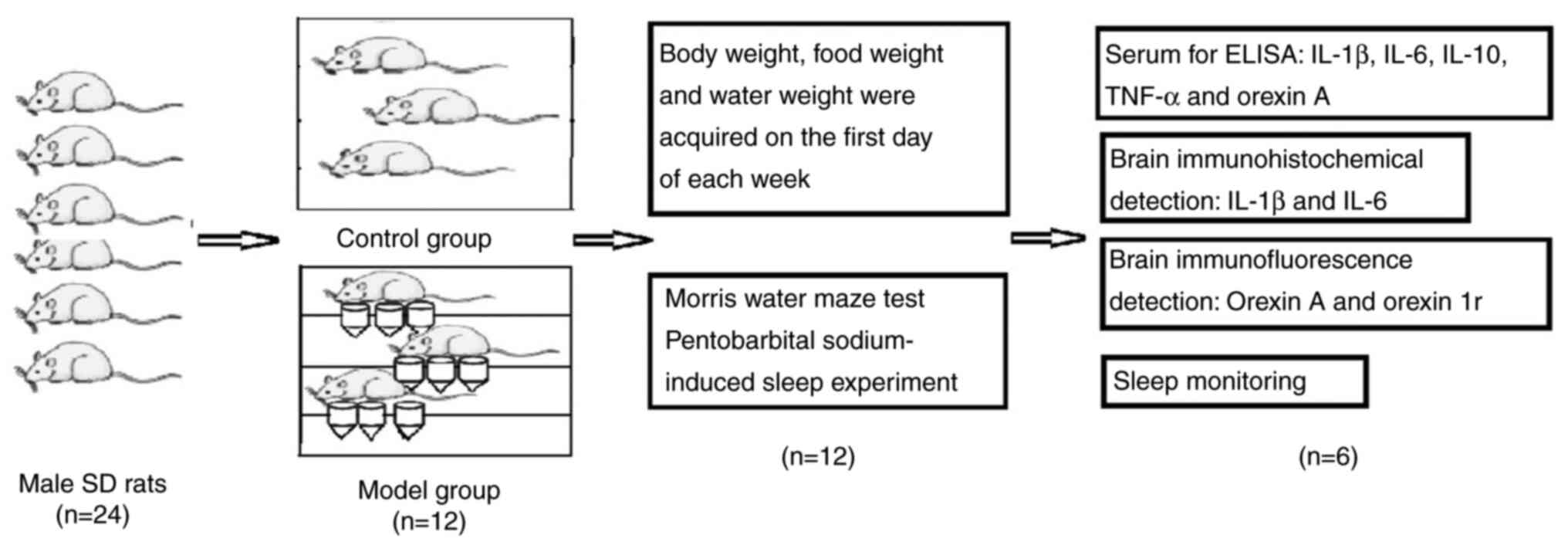
Introduction
Insomnia is a very common disease, and chronic sleep
fragmentation is a frequently occurring type of insomnia (1). According to the fifth edition of the
Diagnostic and Statistical Manual of Mental Disorders published by
the American Psychiatric Association (2) and the 3rd edition of the
International Classification of Sleep Disorders (3), chronic insomnia is defined as
symptoms of difficulty in the initiation of sleep, the maintenance
of sleep due to frequent arousal or difficulty in sleeping again
after arousal, or early waking, with daytime function impairment
occurring ≥3 times per week and lasting for ≥3 months with adequate
sleep opportunity (time in bed ≥7.5 h) (1). Chronic sleep difficulty is
essentially chronic sleep fragmentation caused by frequent arousal,
which affects normal rest, daytime physical activity and social
function in the same manner as any other type of insomnia (4,5). A
cross-sectional national online survey in Australia found that
41.5% of females and 35.3% of males suffered from chronic
difficulties in the initiation and maintenance of sleep, as well as
daytime symptoms (6). Chronic
insomnia with sleep fragmentation is also a social and financial
burden (1,4,5).
Therefore, the establishment of chronic insomnia models to
investigate this type of insomnia is of considerable
importance.
Modified multiple platforms surrounded by water have
been frequently been utilized to deprive rats or mice of rapid eye
movement (REM) sleep for 24-120 h (7-11).
This method is mainly suitable for the preparation of acute
insomnia models with 24 h sleep deprivation per day (12,13).
It is also suitable for the preparation of a chronic sleep
restriction model with 8-18 h sleep deprivation (13,14).
However, a ≥6-h rest may provide sufficient time for the rats to
sleep, which may result in failure to establish the insomnia model.
Since rats often begin to die following several days of 24 h/day
sleep deprivation, a functional 24 h/day rat model of chronic
insomnia using a modified multiple platform device for >10 days
has not yet been reported.
In the clinic, the body weights of patients with
chronic sleep fragmentation do not usually decrease significantly
and are often higher than those of normal subjects (15,16).
However, in animal experiments, the body weights of the rats used
to establish insomnia models have often been reported to be
decreased (17), which indicates
that the modeling methods used require reappraisal.
Clinically, sleep monitoring using polysomnography
is considered the gold standard for the measurement of sleep
duration. When the duration of sleep is <6 h (6-8 h is the
normal sleep duration) and daytime dysfunction presents, a
diagnosis of insomnia can be made (4,5). In
rat models, sleep is often monitored using electroencephalogram
(EEG)/electromyography (EMG) methods, particularly with a 2 EEG/1
EMG biosensor (18-21).
However, at present, no specific time standard has been established
for use when evaluating the success of REM sleep deprivation in
model rats. The method of comparing REM sleep time with that in the
control group is usually adopted.
Insomnia is accompanied by daytime dysfunction in
learning, memory, driving and communication, with sleepiness and
napping. The Morris water maze training and test experiment is a
method used to evaluate the learning and memory function of rats
(10). Pentobarbital
sodium-induced sleep is often used in the evaluation of animal
sleep (22-24).
It has been reported that the levels of inflammatory
cytokines, namely IL-1, IL-6, TNF-α and IL-10, are affected by
insomnia (25). The abnormal
levels of these cytokines further affect brain function, such as
learning and memory (26,27). Furthermore, orexin A and its
receptors play important roles in the regulation of food intake,
circadian rhythms, arousal and sleep (28-32).
In the current study, a rat model with sleep
fragmentation was established using multiple strings of unstable
platforms surrounded by shallow water. The performance of the model
was evaluated using the Morris water maze test and pentobarbital
sodium-induced sleep assessment. The duration of non-REM (NREM) and
REM sleep, frequency of arousal, frequency of daytime eating and
drinking, and body weight changes during the daytime and at night
were evaluated. The effects on the modeling process on the
expression levels of IL-1, IL-6, TNF-α, IL-10, orexin A and orexin
1 receptor (orexin 1r) were also investigated. The aim of the study
was to provide evidence for further research on insomnia. The
protocol of the experimental model used in the present study is
presented in Fig. 1.
Materials and methods
Reagents
Pentobarbital sodium (cat. no. P11011) was purchased
from Merck KGaA. The ELISA kits used for the detection of IL-1β
(cat. no. RAB0277), IL-6 (cat. no. RAB0311) and TNF-α (cat. no.
RAB0479) were purchased from Sigma-Aldrich (Merck KGaA). The rat
IL-10 ELISA kit (cat. no. ab214566) was purchased from Abcam and
the orexin A ELISA kit (cat. no. YX-E28754) was purchased from
Sinobestbio. The primary antibodies for IL-1β (cat. no. bs-0812R),
IL-6 (cat. no. bs-4539R), orexin A (cat. no. bs-15509R) and orexin
1r (cat. no. bs-18029R) and the secondary antibody kit (containing
H2O2, antigen blocking fluid, secondary
antibody, horseradish peroxidase; cat. no. SP-2003) were purchased
from BIOSS. The labeled antibody horseradish peroxidase-IgG (cat.
no. RCA015) and the dyes TYR-CY3 (red light; cat. no. RCA019) and
TCR-488 (green light; cat. no. RCF020) were purchased from
Recordbio. Anti-fluorescence attenuation sealing agent (cat. no.
s2100) was used in the immunofluorescence assay and was bought from
Beijing Solarbio Science & Technology Co., Ltd. Neutral balsam
(cat. no. G8590) were purchased from Beijing Solarbio Science &
Technology Co., Ltd. DAB (cat. no. ZLI-9018) was purchased from
Origene Technologies, Inc.
Instruments
The following instruments were used in the current
study: Morris water maze device (WMT-100; Chengdu Taimeng Science
and Technology Co., Ltd.), low-speed refrigerated centrifuge
(KDC-2044; Zonkia Scientific Instruments Co., Ltd.),
ultracentrifuge (Himac; Eppendorf), fluorescence microscope (DMi8;
Leica Microsystems, Inc.), microplate reader (Multiskan™
GO; Thermo Fisher Scientific, Inc.), stereotaxic instrument
(digital brain stereotaxic instrument company), sleep monitoring
instrument (2 EEG/1 EMG headmount; Pinnacle Technology, Inc.) and
infrared monitor (TP-LINK; TP-Link Technologies Co., Ltd.).
Immunohistochemical analysis software was also used (Image-Pro Plus
6.0; Media Cybernetics, Inc.).
Animals
A total of 24 male Sprague Dawley rats (body weight,
200±240 g; age, 8-9 weeks) were obtained from the Animal Experiment
Center of Xinjiang Medical University [animal certificate:
650007000940; production license: SYXK(X) 2016-0003]. The animals
were raised in the animal center situated in the experimental
building of the University. The room temperature was set to 23±2˚C
with 12-h light/dark cycles. The relative humidity was 40-60%. Food
and water were provided ad libitum. The animal experimental
protocol was approved by the Ethics Committee of Xinjiang Medical
University Animal Experiment Center (IACUC-20210115-07) for The
Graduate Innovation and Entrepreneurship Project of Xinjiang
Medical University (grant no. CXCY2021023) and The First Affiliated
Hospital of Xinjiang Medical University (IACUC-20150225-118) for
the project of National Natural Science Foundation of China (grant
no. 81560762). The protocol adhered to the guidelines of China
Experimental Animals Administration Legislation to minimize their
suffering.
Animal grouping and modeling
The rats were fed for 3 days to adapt to the
environment and were randomly distributed into control and model
groups (n=12/group). The rats were housed in a cage (length, 50 cm;
width, 35 cm; height, 20 cm) with 3 rats/cage. Each cage comprised
three strings of platforms surrounded by shallow water (depth, 1.5
cm). Each string of platforms comprised 3 or 4 bullet-shaped
platforms, which were composed of a 2-cm-high cylinder (diameter, 5
cm) with a hole parallel to the plane of the platform and a
1-cm-high cone touching the bottom of the cage. The platforms were
penetrated by a stainless-steel wire (diameter, 3 mm) to form a
string of platforms for a rat to sleep on. The steel wires were
fixed to the sidewalls of the cage in a manner that enabled the
platforms to rotate in a vertical plane perpendicular to the steel
wire when the rats caused an imbalance in the two sides of the
small platforms due to the loss of muscle tone during REM sleep.
This caused the rats fall into the water, thereby resulting in REM
sleep deprivation. The model rats were raised for 6 weeks in cages
with platforms surrounded by water, with the exception of the first
day of each week. The cages of the model group were washed and
fresh 25˚C water was added every morning and evening. The rats of
the control group were raised normally.
Changes in bodyweight and food and
water intake during the day and night
On the first day of each week, the model rats were
transferred to normal cages. The bodyweights of the rats and the
weights of available food and water were weighed at 6:00 and 18:00
on that day, and at 6:00 on the following day to estimate the body
weight changes of the rats and their food and water intake in the
daytime and at night, separately.
Learning and memory function
assessment using the Morris water maze
The Morris water maze training and test experiment
was carried out in the evening on days 37-41 according to a method
described in a previous study (33). The latency in approaching the
platform and the number of times the target was crossed were
recorded.
Pentobarbital sodium-induced sleep
experiment
The experiments were carried out on day 43 following
the intraperitoneal injection of 35 mg/kg pentobarbital sodium
dissolved in normal saline. The sleep latency and sleep time were
recorded as previously described (22-24).
Sleep monitoring
On day 44, 6 rats from each group were randomly
selected for sleep monitoring. Using the stereotaxic instrument,
the 2EEG/1 EMG head mounts were fixed to the parietal bones of the
heads (n=6) with four special screws and dental cement under
anesthesia. On day 46, the head mounts were connected to an
amplifier to monitor the sleep from 6:00 to 18:00 and from 18:00 to
6:00 on the following day. The monitoring was performed by the
2EEG/1 EMG mount with concomitant use of an infrared monitor. The
researchers were able to monitor the rats at any time using a
mobile phone. The frequency of waking up and sleep duration were
estimated from the polysomnography recordings by two independent
experts, who aided the monitoring process with infrared video
cameras. The duration of REM sleep being lower in the model group
than in the control group was regarded as the time standard for the
successful preparation of a REM sleep deprivation model in the
present study.
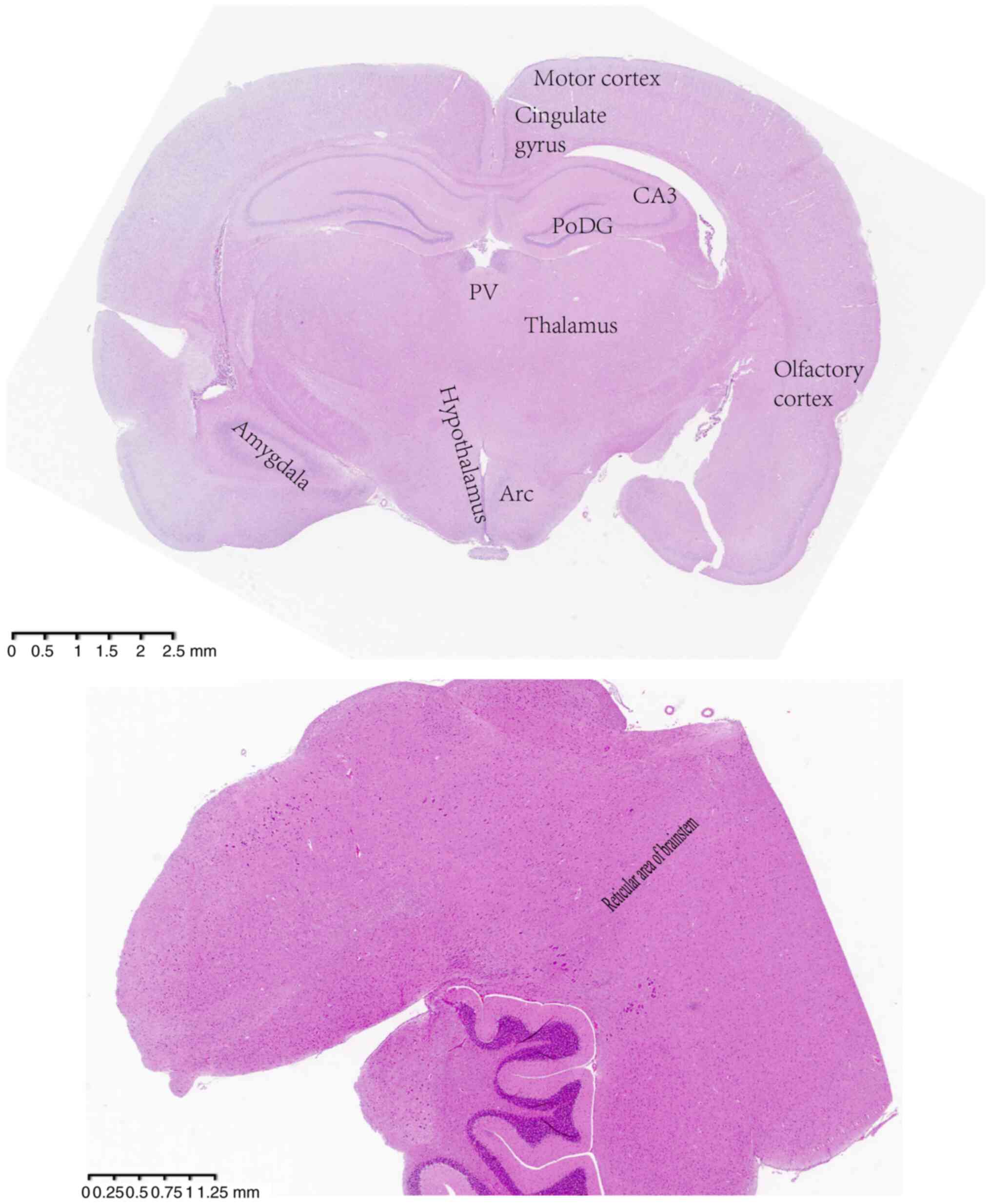
Sample collection
On the morning of day 44, the remaining 6
non-sleep-monitored rats (smallest body weight, 298 g) in each
group were anesthetized with 35 mg pentobarbital sodium, and 8 ml
blood was collected from the abdominal aorta. Following blood
collection, the rats were decapitated using tissue scissors while
still under anesthesia. Blood samples and brains of the
sleep-monitored rats were acquired in the same manner following
sleep monitoring. The serum was acquired following centrifugation
at 4˚C and 3,000 x g for 15 min. The coronal middle sections of the
brain ~0.4-cm thick were acquired and placed into 4%
paraformaldehyde in phosphate-buffered saline for fixation at room
temperature for 3 days. Slices 4-µm thick were made and stained
with hematoxylin and eosin at room temperature for 8 min, then
photographed under a light microscope (Eclipse-Ni-U; Nikon
Corporation). This staining was performed in order to identify the
areas of interest for comparison in the immunohistochemical and
immunofluorescence detection analyses (Fig. 2).
ELISA
The serum levels of IL-1β, IL-6, IL-10, TNF-α and
orexin A were detected with the aforementioned ELISA kits according
to the manufacturer's instructions.
Immunohistochemical detection
The expression levels of IL-1β and IL-6 were
detected in the brain by immunohistochemical analysis according to
the instructions provided by the antibody manufacturer (BIOSS).
Incubation was performed with primary antibodies against IL-1β
(diluted at 1:100) and IL-6 (diluted at 1:100) at 4˚C overnight.
Finally, the stained tissues were sealed with neutral balsam. The
stained slices were imaged using a light microscope (Eclipse-Ni-U;
Nikon Corporation). The images were analyzed with Image Pro Plus
6.0 software to acquire the integrated optical density.
Immunofluorescence detection
Brain orexin A and orexin 1r were detected by
immunofluorescence analysis. Paraffin sections were put into xylene
I for 15 min, then into xylene II for 15 min to dewax the section,
and then into anhydrous ethanol I for 5 min, anhydrous ethanol II
for 5 min, 95% ethanol I for 5 min, 95% ethanol II for 5 min, 85%
ethanol I for 5 min, 85% ethanol II for 5 min and 70% ethanol for 5
min to remove the xylene, followed by rinsing under tap water for 5
min and under double distilled water for 5 min (3 times). The
hydrated sections were put into alkaline antigen repair solution
EDTA (pH 9.0) at 95˚C (water bath for 20 min) to repair the antigen
on the sections, then washed with pH 7.4 PBS for 5 min (3 times) on
a decoloring shaker. 3% hydrogen peroxide was added to the
sections, which were placed into a dark box to avoid the light for
15 min. The sections were then washed with PBS for 5 min (3 times)
on a decoloring shaker. A circle was drawn with an
immunohistochemistry pen around the tissues on the sections.
Antigen blocking fluid was dripped onto the tissues of the sections
to block the antigen for 30 min at room temperature. After the
blocking solution was shaken off, the primary antibody of orexin A
(1:200) was incubated with the sections at 4˚C overnight. The
sections were washed with PBS for 5 min (3 times) on a decoloring
shaker. The labeled antibody horseradish peroxidase-IgG was
incubated with the sections for 50 min in the dark at room
temperature. The sections were washed with PBS for 5 min (3 times)
on a decoloring shaker. TYR-CY3 was added in the dark for 10 min at
room temperature. The sections were washed again with PBS for 5 min
(3 times) on a decoloring shaker. The process from antigen repair
to TYR-CY3 addition (10 min) and PBS wash was repeated with the
primary antibody orexin 1r (1:200) to replace orexin A, and with
TCR-488 to replace TYR-550. The slices were sealed with
anti-fluorescence attenuation sealing agent. The stained tissues
were imaged using a fluorescence microscope and analyzed with Image
Pro Plus 6.0 software.
Statistical analysis
SPSS software (version 18.0; IBM Corp.) was used for
statistical analysis. One-way ANOVA was used to compare the indices
of the two groups at a P>0.05 following homogeneity of variance
and Shapiro-Wilk normality tests. P<0.05 was considered to
indicate a statistically significant difference. Data are shown as
the mean ± standard deviation.
Results
Changes in the general condition of
the model rats
The body weights of the model rats were comparable
with those in the control group (Fig.
3A). However, it was noted that the fur of the model rats
gradually became earth-yellow. The daytime activity of the model
rats, including their eating and drinking behavior was increased
and the night-time activity was decreased compared with that in the
control group as determined using an infrared monitor. These data
indicate that the rats are behaving similarly to insomnious
subjects with fragmented sleep who are exhausted and often nap in
the daytime and eat additional meals at night.
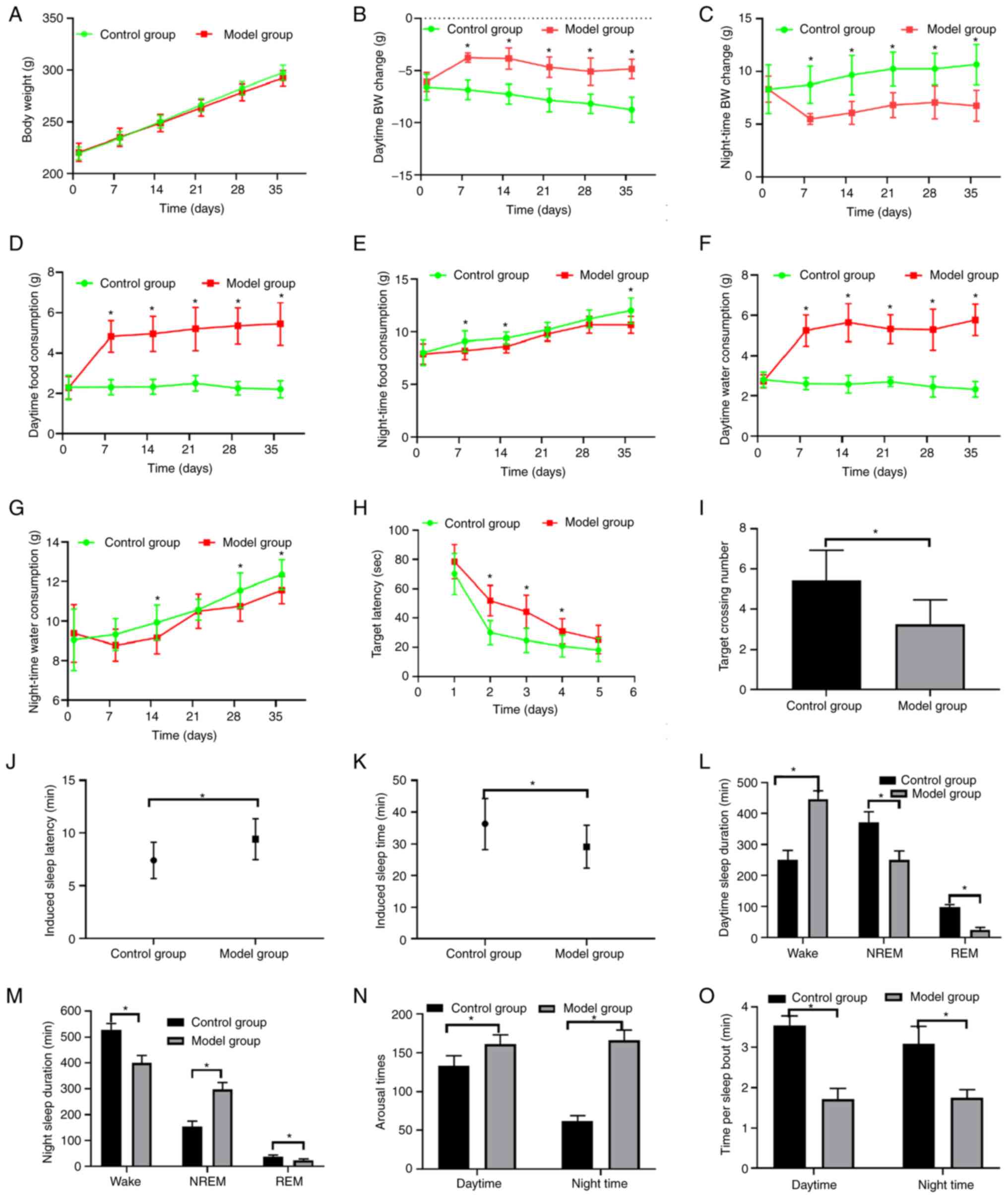 | Figure 3Comparison of body weights, food and
water intake, Morris water maze indices, pentobarbital-induced
sleep experiment and sleep monitoring results between the two
groups. (A) Body weights, (B) daytime body weight changes and (C)
night-time body weight changes. Comparison of (D) daytime and (E)
night-time food consumption and (F) daytime and (G) night-time
water consumption. (H) Target latency (escape latency) and (I) the
number of times the target was crossed in the Morris water maze
test. (J) Induced sleep latency and (K) induced sleep time in the
pentobarbital-induced sleep experiment. Comparison of (L) daytime
sleep duration, (M) night-time sleep duration, (N) numbers of
arousal episodes and (O) time per sleep bout in the model and
control groups. *P<0.05 compared with the control
group (A-K, n=12; L-O, n=6). BW, body weight; REM, rapid eye
movement; NREM, non-REM. |
Body weight changes of the model rats
are significantly decreased
The absolute values of the body weight changes of
the model rats were decreased compared with those of the control
group in the daytime and at night (Fig. 3B and C).
Food and water consumption of the
model rats is increased
The food and water consumption of the model group
demonstrated a marked and significant increase during the period
from 6:00 to 18:00 compared with that of the control group.
However, the food and water consumption from 18:00 to 6:00 the
following morning was similar to that of the control rats (Fig. 3D-G).
Development of learning and memory
dysfunction in the model rats
The results of the Morris water maze training and
testing experiments indicated that the model rats had longer
latency to approach the platform in the total course of the
training and test periods; they also crossed over the target
regions fewer times than the control rats, which indicated that
their learning and memory ability was significantly reduced
(Fig. 3H and I).
Model rats are resistant to sleep
induction
The pentobarbital sodium-induced sleep experiment
indicated that the model rats exhibited significantly longer sleep
latency and shorter sleep time than the control rats, which
indicated that the model rats were somewhat resistant to the
sleep-inducing drug (Fig. 3J and
K).
Model rats suffer from sleep
fragmentation insomnia
The model rats demonstrated a greater duration of
NREM at night, reduced NREM and REM sleep in the daytime, and
reduced REM sleep at night (Fig.
3L and M). The model rats were
awake more times during the day and at night (Fig. 3N). Therefore, they had a reduced
time period per bout of sleep (Fig.
3O).
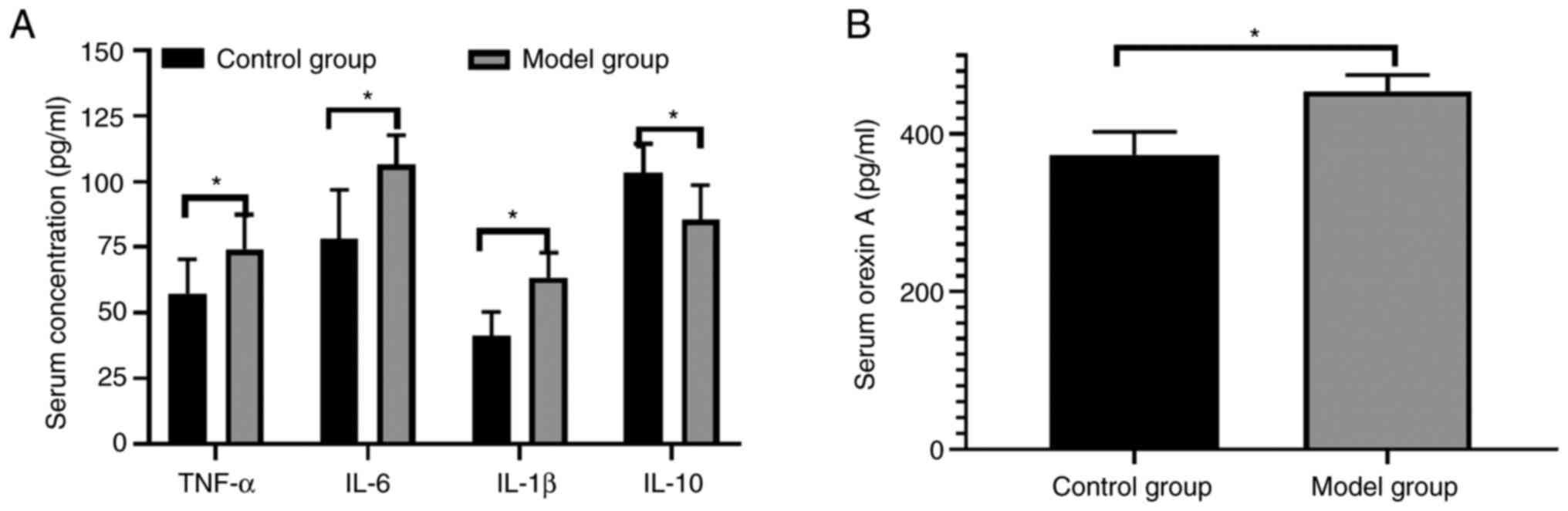
Immune and endocrine disorders occur
in model rats
The serum levels of IL-1β, IL-6, TNF-α and orexin A
were increased, whereas those of IL-10 were decreased in the model
rats compared with the controls (Fig.
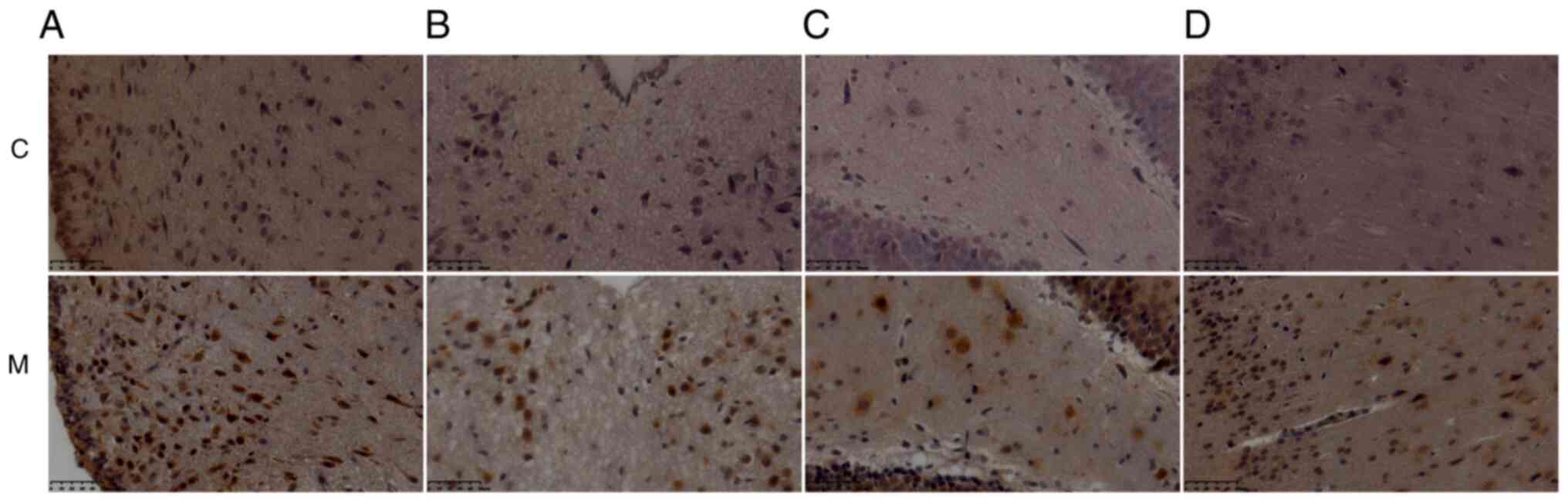
4). The expression levels of IL-1β were increased in certain
brain regions of the model rats, including the arcuate nucleus of
the hypothalamus, the paraventricular nucleus of the thalamus, the
dentate gyrus of the hippocampus, and the cingulate gyrus of the
cerebral cortex (Fig. 5 and
Table I). IL-6 expression also
increased in various brain regions of the model rats, including the
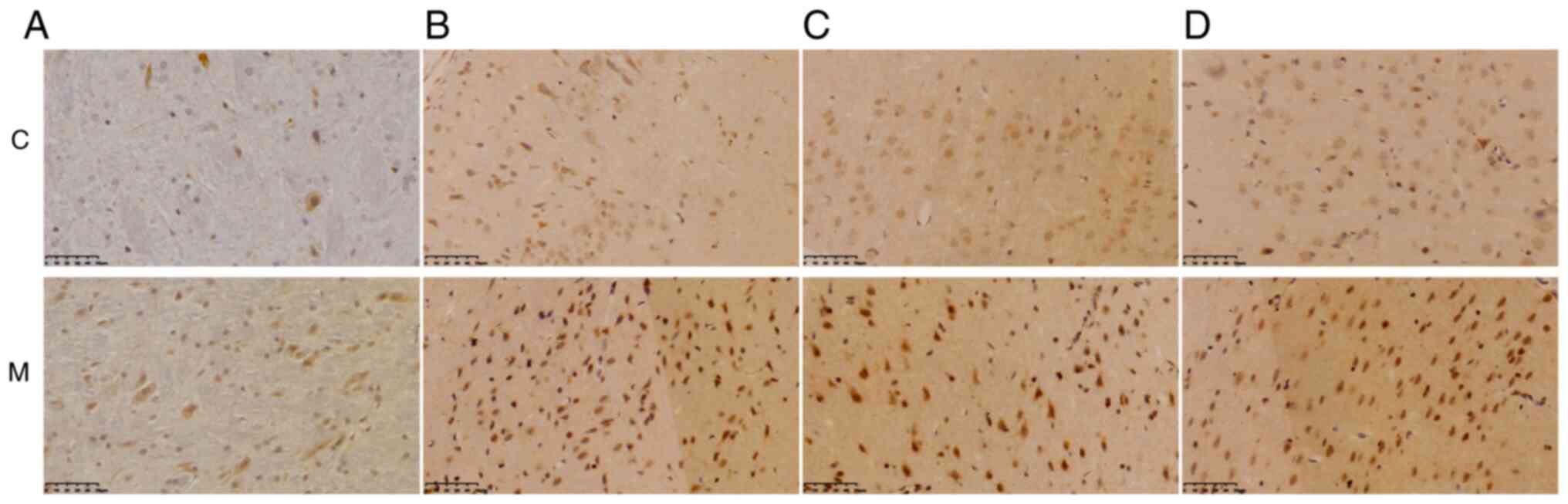
brain stem, amygdala, motor cortex and olfactory cortex (Fig. 6 and Table II).
 | Table IComparison of IL-1β expression in
different brain regions. |
Table I
Comparison of IL-1β expression in
different brain regions.
| | Integrated optical
density (x106) |
|---|
| Group (n=6) | Hypothalamus | Thalamus | Hippocampus | Cerebral
cortex |
|---|
| Control | 3.55±1.05 | 4.14±0.78 | 2.33±0.47 | 2.95±0.90 |
| Model |
5.74±1.31a |
5.82±1.30a |
3.70±0.84a |
8.81±0.78a |
 | Table IIComparison of IL-6 expression in
different brain regions. |
Table II
Comparison of IL-6 expression in
different brain regions.
| | Integrated optical
density (x106) |
|---|
| Group (n=6) | Brain stem | Amygdala | Motor cortex | Olfactory
cortex |
|---|
| Control | 0.94±0.33 | 4.91±0.45 | 0.94±0.33 | 2.34±0.29 |
| Model |
2.92±0.46a |
6.05±0.36a |
2.92±0.46a |
3.79±0.47a |
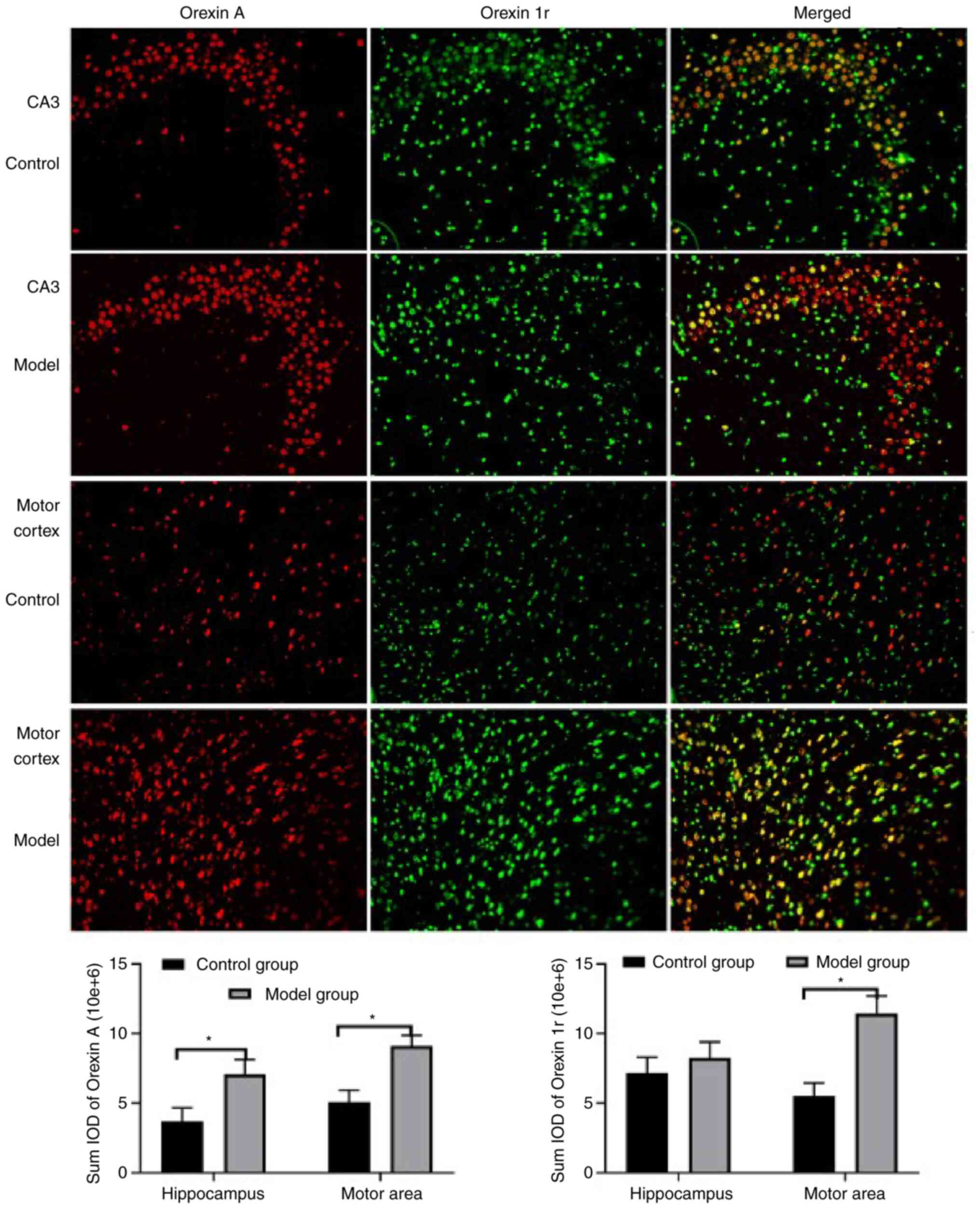
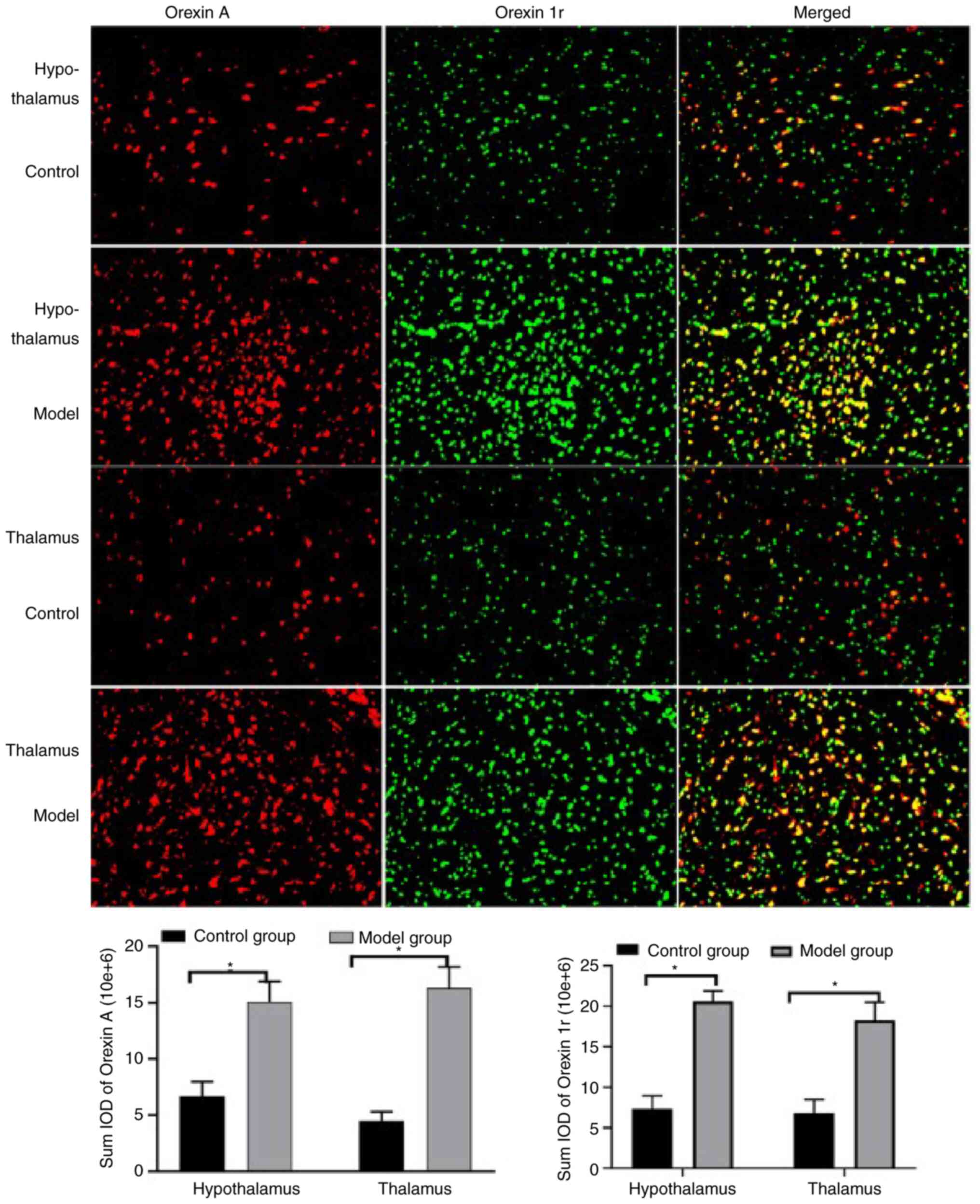
Expression levels of orexin A in serum
and in the brain tissues are increased in model rats
The serum levels of orexin A were detected by ELISA
(Fig. 4B) and the expression
levels of this peptide in the brain were also detected (Figs. 7 and 8). Specifically, orexin A levels were
determined by immunofluorescence in the CA3 region of the
hippocampus (Fig. 7), the motor
area of the cortex (Fig. 7), the
hypothalamus (Fig. 8) and the
thalamus (Fig. 8); the quantified
results indicate that the orexin A levels in all four regions were
significantly increased in the model group compared with the
control group.
Orexin 1r levels of the model rats are
increased in the majority of the brain areas
The results reveal a significant increase in the
levels of orexin 1r in the model group in the motor area of the
cortex (Fig. 7), the hypothalamus
(Fig. 8) and the thalamus
(Fig. 8). However, no significant
difference was noted in the expression levels of this marker in the
hippocampus between the model and control groups (Fig. 7).
Discussion
In the clinic, numerous subjects suffer from chronic
insomnia. In order to investigate this condition, modified multiple
separate platforms surrounded by water have been used to prepare
acute sleep deprivation or chronic sleep restriction models in
various studies (7-11).
However, to the best of our knowledge, chronic sleep deprivation
models involving modified multiple platforms have not previously
been reported. In the present study, self-made multiple strings of
unstable platforms were used. This novel device has several
advantages. Firstly, the low-strung (3 cm high) platforms are very
convenient for the rats to stand up, walk or sleep on. The model
rats can easily lie down on their backs on the short-strung
platforms in order to sleep or change their positions. Therefore,
NREM can be retained to a certain degree by the rats adapting
flexible postures, rather than the rats only being allowed to sit
on the platforms. Secondly, unlike the deep-water design used for
multi-platform devices in previous studies (34), the shallow water allows the rats to
walk freely and to communicate or play with each other without
getting most of their body wet, which can protect them from
developing depression or soaking-associated skin diseases. Thirdly,
although the platforms allow the rats to sleep lightly, they may
rotate around the steel wire when the balance between the two sides
of the platform is distorted during REM sleep. This is due to loss
of muscle tone, the dreaming activities of the animals and the very
low level of consciousness in REM sleep, and may result in the rats
entering the water. Furthermore, in the present study, three
strings of platforms were present and three rats were housed in
each cage, which allowed them to influence each other's sleep.
Abnormalities were noted in the model rats in the
daytime and at night with regard to body weight, food and water
intake, response to the Morris water maze training and testing
experiment, pentobarbital sodium-induced sleep experiment, infrared
monitoring and 2 EEG/1 EMG polysomnography.
Normally, the body weight of rats decreases in the
daytime, while it increases at night. This process is regulated by
orexin/orexin receptors (30-32).
In the present study, the body weight of the model rats decreased
by a lower extent than that of the controls during the daytime,
which can be explained by their higher food and water intake during
the day. Secondly, the monitoring indicated that the model rats
spent more time eating and drinking when the control rats were
sleeping. Furthermore, molecular analysis indicated that orexin A
levels were increased in the serum and brain tissues, whereas
orexin 1r was increased in the brain in the morning, which may be
an internal mechanism to which the food intake increase in the
daytime period can be attributed. Since the sleep deprived rats
spent more time awake, they consumed more energy. The body weights
of the model rats were basically normal, as they were comparable to
those in the control group. These results differ from those
reported in a previous study (17).
In contrast to human subjects, rats sleep more time
during the day and carry out more activities during the night. In
the present study, the rats became highly alert and influenced each
other's sleep. They also spent more time eating and drinking, and
fell into the water several times. Therefore, their sleep in the
daytime became shorter and the NREM sleep at night became longer as
compensation. However, the duration of REM sleep was shorter in the
model rats in the daytime and at night due to the REM sleep
deprivation associated with the platforms surrounded by water
(28). The average duration of
each bout of sleep was also decreased due to the reduced time spent
sleeping and a higher number of arousals. In order to compensate
for the lost and non-restorative sleep, the rats had to reduce
their night-time activities in order to sleep due to physical
exhaustion.
The Morris water maze test can be used to study
dysfunction of learning and memory ability (35-40).
Increased expression levels of pro-inflammatory factors contribute
to dysfunction of learning and memory ability (26,27).
In the present study, the night-time Morris water maze training and
test experiment indicated that the memory dysfunction in the model
rats was accompanied by a longer escape latency and reduced number
of times crossing the target regions. The night-time period was
selected because rats tend to sleep more in the daytime and to be
more active at night. The results of serum analysis indicated an
increase in the expression levels of certain pro-inflammatory
factors, namely TNF-α, IL-1β and IL-6, and a reduction in the
expression levels of the anti-inflammatory factor IL-10(41), which may be a contributing factor
to the impairment of learning and memory ability.
Induction of sleep by pentobarbital sodium is often
used in the evaluation of the sleep function of model rats
(23,24,42).
In the present study, the pentobarbital sodium-induced sleep
experiment demonstrated sleep resistance of the model rats due to a
longer sleep latency and shorter time spent sleeping. However, the
mechanism of action for this is as yet unknown.
Insomnia promotes pathophysiological changes of an
immunological nature, and the expression levels of TNF-α, IL-1β,
IL-6 and IL-10 in serum or in tissues reflect the functional state
of immunity (41). In the present
study, IL-1β and IL-6 levels were increased in serum and brain
tissues, whereas the serum levels of TNF-α were increased and those
of IL-10 were decreased in the model rats. These findings are
consistent with those reported in previous clinical studies
(43-45),
indicating the presence of immune disorders in the model rats.
Insomnia promotes an increase in the expression
levels of feeding and arousal regulators. Orexin is a
hormone/neurotransmitter synthesized and secreted by the
hypothalamus, which can stabilize REM sleep (46,47).
Since REM sleep is reduced when rats are kept on platforms
surrounded by water, orexin expression increases as a compensatory
mechanism. However, the increase in orexin A levels promotes
arousal and feeding through orexin 1r (48). In the present study, serum orexin A
levels were increased, and the expression levels of orexin and
orexin 1r in the brain tissues were increased in the model rats,
which is consistent with the results reported in previous studies
(48,49).
In conclusion, the present study established a
chronic insomnia rat model with sleep fragmentation using self-made
multiple strings of platforms surrounded by water. The changes
induced in the model rats with regard to sleep duration,
sleep-inducing drug resistance, diurnal and nocturnal body weight
changes, and cognitive function were evaluated. Furthermore, the
expression levels of inflammatory factors and neurohumoral
regulators involved in feeding behavior, arousal and sleep were
assessed. The results indicate that the present chronic insomnia
rat model is suitable for use in studying the mechanism of action
of chronic insomnia with sleep fragmentation.
However, the present study has several limitations.
Firstly, the sample size was very small, which should be expanded
in future studies. Secondly, a positive control group was not
included for confirmatory purposes. Thirdly, quantitative
histopathology for specific analysis is lacking, which requires
further investigation. Furthermore, several mechanisms involved in
changes of the parameters measured in the model rats are not clear
and merit investigation in future studies.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant nos. 82160873, 81960837 and
81560762), Xinjiang Uygur Autonomous Region Key Discipline in
Traditional Chinese Medicine (grant no. 1005) and a graduate
innovation and entrepreneurship project of Xinjiang Medical
University (grant no. CXCY2021023).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DQY designed the study and wrote the manuscript.
DQY, ND, WHZ, TL, QWY, ZPT, KKW, GYW and ZTL performed experiments
and analyzed the data. XPZ designed the study and performed
experiments. XPZ and DQY confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were conducted in
accordance with China Experimental Animals Administration
Legislation and were approved by the Ethics Committee of Xinjiang
Medical University (approval no. IACUC-20150225-118 Urumqi,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Qaseemg A, Kansagara D, Forciea MA, Cooke
M and Denberg TD: Clinical Guidelines Committee of the American
College of Physicians. Management of chronic insomnia disorder in
adults: A clinical practice guideline from the American college of
physicians. Ann Intern Med. 165:125–133. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
American Psychiatric Association:
Diagnostic and statistical manual of mental disorders, 5th edition.
Bejing University Publishing House, Beijing, pp151, 2019.
|
|
3
|
American Academy of Sleep Medicine:
International classification of sleep disorders (3rd edition).
American Academy of Sleep Medicine, Darien, IL, pp1387, 2014.
|
|
4
|
Zhang P, Li YP, Wu HJ and Zhao ZX: Chinese
guidelines for the diagnosis and treatment of insomnia in adults
(2017 edition). Chin J Neurol. 51:324–335. 2018.
|
|
5
|
China Sleep Research Association. Chinese
guidelines for the diagnosis and treatment of insomnia. Chin J Med.
97:1844–1856. 2017.(In Chinese).
|
|
6
|
Appleton SL, Reynolds AC, Gill TK, Melaku
YA and Adams RJ: Insomnia prevalence varies with symptom criteria
used with implications for epidemiological studies: Role of
anthropometrics, sleep habit, and comorbidities. Nat Sci Sleep.
14:775–790. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Javad-Moosavi BZ, Nasehi M, Vaseghi S,
Jamaldini SH and Zarrindast MR: Activation and inactivation of
nicotinic receptnors in the dorsal hippocampal region restored
negative effects of total (TSD) and REM sleep deprivation (RSD) on
memory acquisition, locomotor activity and pain perception.
Neuroscience. 433:200–211. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dai D, Zheng B, Yu Z, Lin S, Tang Y, Chen
M, Ke P, Zheng C, Chen Y and Wu X: Right stellate ganglion block
improves learning and memory dysfunction and hippocampal injury in
rats with sleep deprivation. BMC Anesthesiol.
21(272)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cao Y, Li Q, Yang Y, Ke Z, Chen S, Li M,
Fan W, Wu H, Yuan J, Wang Z and Wu X: Cardioprotective effect of
stem-leaf saponins from panax notoginseng on mice with sleep
derivation by inhibiting abnormal autophagy through PI3K/Akt/mTOR
pathway. Front Cardiovasc Med. 8(694219)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bao LD, Si Lg, Wang Yh, Wu YG and Bo A:
Effect of two GABA-ergic drugs on the cognitive functions of rapid
eye movement in sleep-deprived and recovered rats. Exp Ther Med.
12:1075–1084. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
López-Armas G, Flores-Soto ME,
Chaparro-Huerta V, Jave-Suarez LF, Soto-Rodríguez S, Rusanova I,
Acuña-Castroviejo D, González-Perez O and González-Castañeda RE:
Prophylactic role of oral melatonin administration on neurogenesis
in adult Balb/C mice during REM sleep deprivation. Oxid Med Cell
Longev. 2016(2136902)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Suchecki D and Tufik S: Social stability
attenuates the stress in the modified multiple platform method for
paradoxical sleep deprivation in the rat. Physiol Behav.
68:309–316. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zager A, Andersen ML, Ruiz FS, Antunes IB
and Tufik S: Effects of acute and chronic sleep loss on immune
modulation of rats. Am J Physiol Regul Integr Comp Physiol.
293:R504–R509. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Fahmawi A, Khalifeh MS, Alzoubi KH and
Rababa'h AM: The effects of acute and chronic sleep deprivation on
the immune profile in the rat. Curr Mol Pharmacol. 16:101–108.
2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen DR, Truong KD and Tsai MJ: Prevalence
of poor sleep quality and its relationship with body mass index
among teenagers: Evidence from Taiwan. J Sch Health. 83:582–588.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Klink ME, Dodge R and Quan SF: The
relation of sleep complaints to respiratory symptoms in a general
population. Chest. 105:151–154. 1994.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rodrigues NC, da Cruz NS, de Paula
Nascimento C, da Conceição RR, da Silva AC, Olivares EL and Marassi
MP: Sleep deprivation alters thyroid hormone economy in rats. Exp
Physiol. 100:193–202. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hargis K, Buechel HM, Popovic J and
Blalock EM: Acute psychosocial stress in mid-aged male rats causes
hyperthermia, cognitive decline, and increased deep sleep power,
but does not alter deep sleep duration. Neurobiol Aging. 70:78–85.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Miladinović Đ, Muheim C, Bauer S, Spinnler
A, Noain D, Bandarabadi M, Gallusser B, Krummenacher G, Baumann C,
Adamantidis A, et al: SPINDLE: End-to-end learning from EEG/EMG to
extrapolate animal sleep scoring across experimental settings, labs
and species. PLoS Comput Biol. 15(e1006968)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang C, Guerriero LE, Huffman DM, Ajwad
AA, Brooks TC, Sunderam S, Seifert AW and O'Hara BF: A comparative
study of sleep and diurnal patterns in house mouse (Mus musculus)
and Spiny mouse (Acomys cahirinus). Sci Rep.
10(10944)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mountney A, Blaze J, Wang Z, Umali M,
Flerlage WJ, Dougherty J, Ge Y, Shear D and Haghighi F: Penetrating
ballistic brain injury produces acute alterations in sleep and
circadian-related genes in the rodent cortex: A preliminary study.
Front Neurol. 12(745330)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hu Y, Wang YN, Zhang GQ, Dong XZ, Liu WW
and Liu P: Gan-Dan-Liang-Yi-Tang alleviates
p-chlorophenylalanine-induced insomnia through modification of the
serotonergic and immune system. Exp Ther Med. 12:3087–3092.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Dong YJ, Jiang NH, Zhan LH, Teng X, Fang
X, Lin MQ, Xie ZY, Luo R, Li LZ, Li B, et al: Soporific effect of
modified Suanzaoren Decoction on mice models of insomnia by
regulating Orexin-A and HPA axis homeostasis. Biomed Pharmacother.
143(112141)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kim S, Jo K, Hong KB, Han SH and Suh HJ:
GABA and l-theanine mixture decreases sleep latency and improves
NREM sleep. Pharm Biol. 57:65–73. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bo A, Si L, Wang Y, Bao L and Yuan H:
Mechanism of Mongolian medical warm acupuncture in treating
insomnia by regulating miR-101a in rats with insomnia. Exp Ther
Med. 14:289–297. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Alhowail A, Alsikhan R, Alsaud M,
Aldubayan M and Rabbani SI: Protective effects of pioglitazone on
cognitive impairment and the underlying mechanisms: A review of
literature. Drug Des Devel Ther. 16:2919–2931. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ullah A, Al Kury LT, Althobaiti YS, Ali T
and Shah FA: Benzimidazole derivatives as new potential NLRP3
inflammasome inhibitors that provide neuroprotection in a rodent
model of neurodegeneration and memory impairment. J Inflamm Res.
15:3873–3890. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Willie JT, Takahira H, Shibahara M, Hara
J, Nomiyama M, Yanagisawa M and Sakurai T: Ectopic overexpression
of orexin alters sleep/wakefulness states and muscle tone
regulation during REM sleep in mice. J Mol Neurosci. 43:155–161.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hatori M, Vollmers C, Zarrinpar A,
DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M,
Fitzpatrick JA, et al: Time-restricted feeding without reducing
caloric intake prevents metabolic diseases in mice fed a high-fat
diet. Cell Metab. 15:848–860. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xiao X, Yeghiazaryan G, Hess S, Klemm P,
Sieben A, Kleinridders A, Morgan DA, Wunderlich FT, Rahmouni K,
Kong D, et al: Orexin receptors 1 and 2 in serotonergic neurons
differentially regulate peripheral glucose metabolism in obesity.
Nat Commun. 12(5249)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Michael NJ and Elmquist JK: Coordination
of metabolism, arousal, and reward by orexin/hypocretin neurons. J
Clin Invest. 130:4540–4542. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Saper CB, Scammell TE and Lu J:
Hypothalamic regulation of sleep and circadian rhythms. Nature.
437:1257–1263. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tripathi S and Jha SK: REM sleep
deprivation alters learning-induced cell proliferation and
generation of newborn young neurons in the dentate gyrus of the
dorsal hippocampus. ACS Chem Neurosci. 13:194–206. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ren XJ, Wang QQ, Zhang XP, Wang GY, Liu T,
Deng N and Yan DQ: Establishment of a rat model with ageing
insomnia induced by D-galactosef and para-chlorophenylalanine. Exp
Ther Med. 20:3228–3236. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
McKenna JT, Gamble MC, Anderson-Chernishof
MB, Shah SR, McCoy JG and Strecker RE: A rodent cage change
insomnia model disrupts memory consolidation. J Sleep Res.
28(e12792)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Furukawa T, Nikaido Y, Shimoyama S,
Masuyama N, Notoya A and Ueno S: Impaired cognitive function and
hippocampal changes following chronic diazepam treatment in
middle-aged mice. Front Aging Neurosci. 13(777404)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gamble MC, Katsuki F, McCoy JG, Strecker
RE and McKenna JT: The dual orexinergic receptor antagonist DORA-22
improves the sleep disruption and memory impairment produced by a
rodent insomnia model. Sleep. 43(zsz241)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chen Y, Kartsonaki C, Clarke R, Guo Y, Yu
C, Bian Z, Jiang Q, Li S, Chen J, Li L, et al: Characteristics and
correlates of sleep duration, daytime napping, snoring and insomnia
symptoms among 0.5 million Chinese men and women. Sleep Med.
44:67–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Van Erum J, Van Dam D, Sheorajpanday R and
De Deyn PP: Sleep architecture changes in the APP23 mouse model
manifest at onset of cognitive deficits. Behav Brain Res.
373(112089)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Su H, Zhang C, Zou X, Lu F, Zeng Y, Guan
H, Ren Y, Yuan F, Xu L, Zhang M and Dong H: Jiao-tai-wan inhibits
inflammation of the gut-brain-axis and attenuates cognitive
impairment in insomnic rats. J Ethnopharmacol.
250(112478)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Al-Sharif FM and El-Kader SMA:
Inflammatory cytokines and sleep parameters response to life style
intervention in subjects with obese chronic insomnia syndrome. Afr
Health Sci. 21:1223–1229. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bian Z, Zhang W, Tang J, Fei Q, Hu M, Chen
X, Su L, Fei C, Ji D, Mao C, et al: Mechanisms underlying the
action of Ziziphi spinosae semen in the treatment of insomnia: A
study involving network pharmacology and experimental validation.
Front Pharmacol. 12(752211)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Al-Jiffri OH and Abd El-Kader SM: Aerobic
versus resistance exercises on systemic inflammation and sleep
parameters in obese subjects with chronic insomnia syndrome. Afr
Health Sci. 21:1214–1222. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Xia L, Zhang P, Niu JW, Ge W, Chen JT,
Yang S, Su AX, Feng YZ, Wang F, Chen G and Chen GH: Relationships
between a range of inflammatory biomarkers and subjective sleep
quality in chronic insomnia patients: A clinical study. Nat Sci
Sleep. 13:1419–1428. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ren CY, Rao JX, Zhang XX, Zhang M, Xia L
and Chen GH: Changed signals of blood adenosine and cytokines are
associated with parameters of sleep and/or cognition in the
patients with chronic insomnia disorder. Sleep Med. 81:42–51.
2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Feng H, Wen SY, Qiao QC, Pang YJ, Wang SY,
Li HY, Cai J, Zhang KX, Chen J, Hu ZA, et al: Orexin signaling
modulates synchronized excitation in the sublaterodorsal tegmental
nucleus to stabilize REM sleep. Nat Commun. 11(3661)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Mignot E, Zeitzer J, Pizza F and Plazzi G:
Sleep problems in narcolepsy and the role of hypocretin/orexin
deficiency. Front Neurol Neurosci. 45:103–116. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Parks GS, Warrier DR, Dittrich L, Schwartz
MD, Palmerston JB, Neylan TC, Morairty SR and Kilduff TS: The dual
hypocretin receptor antagonist almorexant is permissive for
activation of wake-promoting systems. Neuropsychopharmacology.
41:1144–1155. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Barson JR and Leibowitz SF:
Orexin/hypocretin system: Role in food and drug overconsumption.
Int Rev Neurobiol. 136:199–237. 2017.PubMed/NCBI View Article : Google Scholar
|