Introduction
Indoleamine 2,3-dioxygenase (IDO) is the first and
rate-limiting metabolic enzyme catabolizing tryptophan (Trp), and
the kynurenine (Kyn) synthesis pathway (KP) is the metabolic
pathway responsible for the majority of Trp catabolism (1). Previous studies have suggested that
KP generates several bioactive catabolites with immunosuppressive
properties (2), such as Kyn,
3-hydroxykynurenine, kynurenic acid, 3-hydroxyanthranilic acid
(3-HAA), xanthurenic acid and quinolinic acid (QA), collectively
termed Kyns (3). Of note, IDO is
widely expressed at low levels under normal conditions and induced
mainly at the site of inflammation (4). Moreover, the inflammation-activated
Trp metabolism can cause changes in the systemic and intra- and
extracellular Kyn/Trp ratios, which in turn create an environment
of high local or systemic Kyn and low Trp content. The Kyn/Trp
ratio is an indicator of Trp degradation and reflects the activity
of IDO under different conditions (5). It can directly affect metabolic and
immune signaling pathways. The functions of adjacent cells (e.g., T
cells) are also changed by increasing local or systematic
environments in Kyn and decreasing in Trp (6). For example, Trp depletion enables the
production of anti-inflammatory cytokines by IDO-expressing
dendritic cells (DCs) and macrophages, promotes the recruitment of
regulatory T cells (Tregs), and prevents T cell activation and
proliferation. In addition, the increase of Kyn can cause the
differentiation of T cells toward Tregs and induce the apoptosis of
effector T cells (7).
Furthermore, numerous studies have demonstrated that
IDO is expressed in numerous cell types, including endothelial
cells, vascular smooth muscle cells, macrophages, leucocytes, DCs,
multiple types of cancer cells, and maternal-fetal interface cells
(8). Of note, DCs are known to
express the highest levels of IDO among immune cells (9). However, the ability of DCs to produce
IDO does not seem to be equally distributed among the different
subsets. For example, CD8α or CD103-positive DCs express more IDO
and establish immune tolerance compared to CD8α or CD103-negative
DCs. Similarly, plasmacytoid DCs (pDCs) have a strong ability to
produce IDO and mediate immunosuppression in specific settings
(9,10). Conversely, DCs expressing low
levels of IDO inhibit Treg development and T cell apoptosis
(11). In brief, DCs are
heterogeneous and exhibit both tolerogenicity and immunogenicity,
due to the difference in IDO expression in DC subsets (12). Furthermore, IDO expression induces
a stable, regulatory phenotype in DCs, which are designated as
tolerogenic DCs (tolDCs), promoting the spread of regulatory
functions to cells other than DCs (13).
Of note, the fate of the immunogenicity or DC
tolerance, which are demonstrated by their extracellular functions
are ultimately determined by their intracellular metabolism
(14). For example, active
oxidative phosphorylation in mitochondria is associated with
immature DCs or tolDCs, while increased pathogen-sensitive
glycolysis can promote the functions of immature DCs (15). As the rate-limiting metabolic
enzyme of Trp catabolism, IDO is preferentially expressed in DCs
that can mediate T(h) cell apoptosis for immune suppression
(9). However, the exact roles of
IDO in the immunological functions of DCs remain to be determined.
In the present study, the endogenous expression of IDO was
genetically altered in myeloid DCs to determine its variation on
the overall functions of DCs. To this end, IDO-overexpressing DCs
(IDOoeDCs) and IDO-knockdown DCs (IDOkdDCs)
were successfully constructed using the recombinant DNA technique.
It was further evaluated how IDO in DCs influenced Trp-Kyn
metabolism, phenotype, function and related regulatory mechanism to
provide a theoretical basis for the application of modified DC
vaccines to the treatment of autoimmune diseases.
Materials and methods
Mice and cells
A total of 18 healthy male C57BL/6 mice (age, 6-8
weeks; weight, 18±5 g) and a total of 6 healthy male Kunming mice
(age, 6-8 weeks; weight, 20±5 g) were purchased from BoYuan
Laboratory Animal Ltd (Hefei, China), bred and maintained in a
specific pathogen-free environment (temperature, 25±2˚C; humidity,
55±5%; 12 h light-dark cycles; and freely access to food and water)
in the animal facility of Anhui Normal University (Wuhu, China).
All animal health and behavior were monitored every 1 or 2 days. A
total of 24 healthy male mice (age, 8-10 weeks; weight, 20±5 g)
were anesthetized with pentobarbital sodium by intraperitoneal
injection at a dose of 40 mg/kg (0.036 g/ml), and then painlessly
sacrificed by cervical dislocation when they met the following
criteria: rapid drop in body temperature and difficulty breathing.
Absence of heartbeat and pupil dilation for 5 min was used to
confirm death. The total experimental period was about four months.
The experimental procedures were performed in accordance with the
conditions specified and approved by the Animal Experimentation
Ethics Committee of Anhui Normal University.
DC2.4 cells are an immature DC cell line derived
from C57BL/6 mouse bone marrow progenitor cells cultured under
GM-CSF conditions (16) and kindly
provided by Professor Dong Yongjun of Tsinghua University (Beijing,
China).
Cell cultures
The cells were cultured using standard methods as
previously described (17) with
slight modifications. Briefly, spleen cells were separated from
Kunming mice, and the immature murine DC line, DC2.4, and its
transduced derivatives were cultured in RPMI1640 medium
supplemented with 100 U/ml penicillin, 100 mg/l streptomycin, 2
mmol/l L-glutamine, 50 µM 2-ME and 10% FBS.
Generation of IDO-encoded
lentivirus
The complete mouse IDO sequence was cloned
into the EcoRI and NheI restriction sites of pLJM-enhanced green
fluorescent protein (EGFP) vector (pLJM1-EGFP#19319) (Fig. S1), whereas short hairpin knockdown
construct for IDO was synthesized and cloned into a lentiviral
vector pLKO.1 (pLKO.1-TRC cloning vector#10878) (Fig. S2). Following transformation into
E. coli cells, these two recombinant constructs were
amplified, and positive clones identified by reverse
transcription-quantitative PCR (RT-qPCR; Fig. S3A and B), before the correct DNA inserts were
confirmed again by sequencing (Fig.
S4A). Furthermore, the confirmed IDO-modifying
constructs and two packaging plasmids [psPAX2 (psPAX2#12260) and
pCMV-VSV-G (pCMV-VSV-G#8454)] were co-transfected into 70-80%
confluent 293FT human embryonic kidney cells in the presence of
Hieff Trans® Liposomal Transfection Reagent (cat. no.
40802ES03; Shanghai Yeasen Biotechnology Co., Ltd.) at 37˚C for 48
h to produce competent first-generation lentiviral particles, which
were harvested following transfection. Cell fragments in the
lentivirus containing supernatants were removed by centrifugation
(2,000 x g; 10 min; 4˚C) and filtered through a 0.45 mm cellulose
acetate filter for DC infection. The pLKO.1-TRC cloning
vector#10878, pLJM1-EGFP#19319, psPAX2#12260, and pCMV-VSV-G#8454
were kindly supplied by Professor Ye Shoudong from Anhui University
as gifts (Hefei, China). The shRNA sequences were synthesized by
Sangon Biotech Co., Ltd., as presented in Table I.
 | Table ISequences of shRNAs. |
Table I
Sequences of shRNAs.
| Gene | Sequence
(5'-3') |
|---|
| shRNA-IDO-F |
CCGGCCTCGCAATAGTAGATACTTACTCGAGTAAGTATCTACTATTGCGAGGTTTTTG |
| shRNA-IDO-R |
AATTCAAAAACCTCGCAATAGTAGATACTTACTCGAGTAAGTATCTACTATTGCGAGG |
|
shRNA-Scramble-F |
CCGGAATTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAATTTTTTTG |
|
shRNA-Scramble-R |
AATTCAAAAAAATTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAATT |
Lentiviral infection of DCs
For lentiviral transfection, 1x105 DC2.4
cells were cultured in 24-well plates. The next day, once cells had
reached 60-70% cell confluence, the first-generation lentiviral
solution encoding IDO or shIDO was added on to the cultured
DC2.4 at a multiplicity of infection (MOI) of 6 with lentiviral:
cell solution of 1:1, and co-continued at 37˚C (5% CO2)
for 24 h in the presence of polybrene (5 µg/ml, cat. no. HB-PB-05,
Hanbio Biotechnology Co., Ltd.), before the medium was replaced
with 2 ml fresh complete RPMI1640 medium. After 72 h, puromycin
[cat. no. 60209ES60; Yeasen Biotechnology (Shanghai) Co., Ltd.] was
added to screen the infected DC2.4 cells at 2 µg/ml the following
day. The overexpressed IDO or knockdown DC2.4 was assessed by
RT-qPCR for mRNA expression, and by flow cytometry to determine
intracellular protein expression.
Total RNA extraction and RT-qPCR
analysis
As previously described (18), total RNA was extracted from
gene-modified DCs and reverse-transcribed into complementary DNA
(NovoScript® Plus All-in-one 1st Strand cDNA Synthesis
SuperMix; Suzhou Novoprotein) according to the manufacturer's
instructions. RT-qPCR with SYBR Green detection
(NovoStart® SYBR qPCR SuperMix Plus; Suzhou Novoprotein)
was performed using a qPCR detection system (cat. no. CFX96;
Bio-Rad Laboratories, Inc. or LightCycler® 96; Roche
Diagnostics GmbH) to quantify RNA expression. All data are
expressed relative to β-actin, which served as the internal
control. Data analysis was performed using LightCycler®
96 SW 1.1 Software (Roche Diagnostics GmbH) or CFX Manager Software
(Bio-Rad Laboratories, Inc.). The 2-∆∆Cq method was used
to determine relative expression. The primers used are listed in
Table II.
 | Table IIPrimer sequences used for
quantitative PCR. |
Table II
Primer sequences used for
quantitative PCR.
| Gene product | Sequence
(5'-3') |
|---|
| IDO-forward |
GAGAGTACATGCCTCCAGCC |
| IDO-reverse |
CTCTTCCGACTTGTCGCCAT |
|
β-actin-forward |
TCATCACTATTGGCAACGAGC |
|
β-actin-reverse |
AACAGTCCGCCTAGAAGCAC |
Trp and Kyn levels
The total activity of IDO was evaluated by measuring
the levels of Trp (µmol/l; Shanghai Macklin Biochemical Co., Ltd.)
and kyn (µmol/l; Shanghai Macklin Biochemical Co., Ltd.), whose
concentrations in the supernatants of gene-modified DCs were
measured by high-performance liquid chromatography (HPLC; LC-20A
HPLC; Shimadzu Corporation), as previously described (19). The mobile phase was 15 mM acetic
acid-sodium acetate buffer (pH 4) containing 8% acetonitrile (v/v).
The UV monitoring wavelengths of Trp and Kyn were 280 and 360 nm,
respectively. Kyn/Trp was calculated by relating the concentrations
of Kyn to those of Trp, which allows for estimating IDO
activity.
Determination of cell viability by
annexin V/propidium iodide (PI) staining
As previously described (20), 1x106
vectorctrlDCs (control group) and IDOkdDCs
(IDO-knockdown DCs group) were treated with lipopolysaccharide
(LPS) overnight, trypsinized and washed with PBS three times. Next,
centrifugation was performed at 670 x g for 5 min. The cells were
suspended in 500 µl standard buffer and then stained with Annexin
V-FITC and PI (Annexin V-FITC/PI Apoptosis Detection Kit; 7Sea
Biotech Co., Ltd.) for 15 min at room temperature. The fluorescence
intensity of FITC and PI was measured quantitatively for every test
using a BD Canto II flow cytometer or BD FACS Melody flow cytometer
(BD Biosciences). Flow Jo software (Flow Jo version 10.6.2) was
used to analyze the data. Flow cytometry was performed to detect
the effect of IDO on the viability of DCs using four quadrants:
PI-positive and Annexin V-FITC-negative necrotic cells; PI and
Annexin V-FITC-positive late apoptotic cells; PI and Annexin
V-FITC-negative normal living cells; and Annexin V-FITC-positive
and PI-negative early apoptotic cells (21).
Assessment of DC migration in
vivo
As previously described (22), 10x106
vectorctrlDCs or IDOkdDCs were labelled with
carboxyfluorescein succinimidyl ester (CFSE) at 37˚C for 10 min and
treated with LPS overnight, before they were injected into the
footpads of 6 healthy male C57BL/6 mice (vectorctrlDCs,
age, 8-10 weeks, n=3; IDOkdDCs, age, 8-10 weeks, n=3).
After 24 h, mice were anesthetized with 40 mg/kg (0.036 g/ml)
sodium pentobarbital and then sacrificed. Single-cell suspensions
were prepared from popliteal lymph nodes, and CFSE-positive cells
of were detected by flow cytometry.
Immunophenotypic analysis by flow
cytometry
Flow cytometry of the DC immunophenotype and
intracellular expression of IDO was performed. As previously
described (23), gene-modified DCs
were stained with different combinations of monoclonal antibodies
against PD-L1 (cat. no. 10F.9G2-BV421; BioLegend), CD86 (cat. no.
GL1-FITC; eBioscience; Thermo Fisher Scientific, Inc.) and CD40
(cat. no. FGK45-PE; BioLegend) with the addition of PI (1 µg
ml-1) for phenotyping. Alternatively, these cells were
stained intracellularly using rat purified anti-mouse IDO antibody
(cat. no., mIDO-48; dilution, 1:200; BioLegend) and goat anti-rat
IgG (H+L) cross-adsorbed secondary antibody, Alexa Fluor™ 488 (cat.
no. A-11006; dilution, 1:500; Invitrogen; Thermo Fisher Scientific,
Inc.). Analysis of the expression levels of targeted proteins was
performed using a BD Canto II flow cytometer or BD FACS Melody flow
cytometer (BD Biosciences). The data was analyzed using Flow Jo
software (Flow Jo version 10.6.2).
Analysis of the uptake ability of
DCs
The uptake ability of DCs was examined as previously
described (22) with slight
modifications. Briefly, 0.2x106 gene modified DCs were
stimulated with LPS (1 µg/ml) and cultured with fluorescent chicken
ovalbumin (OVA-FITC; 50 µg/ml; Beijing Boshi Technology Co., Ltd.)
in 500 ml complete RPMI1640 medium for 4 h at 37˚C. Following
washing with phosphate-buffered saline, OVA-FITC by
vectorctrlDC or IDOkdDC phagocytosis was
detected by flow cytometry. DCs incubated with OVA-FITC at 4˚C were
used as the negative control. Flow Jo software (Flow Jo version
10.6.2) was used to analyze the data.
ELISA assay
As previously described (24), IL-12, IL-6, interferon gamma
(IFN-γ), and IL-10 levels in cell culture supernatant were
determined using ELISA kits (R&D Systems, Inc.) according to
the manufacturer's instructions. Briefly, vectorctrlDCs
or IDOkdDCs were stimulated with LPS (1 µg/ml) and
incubated at 37˚C for 24 h with CD4+T cells, before the
supernatants were collected, and their cytokine contents were
measured using the ELISA kits.
T cell proliferation assay
T-cell proliferation was performed using standard
methods as previously described (18) with slight modifications.
T-cell proliferation in situ (Ki67 assay):
Healthy male C57BL/6 mice were sensitized via an intraperitoneal
administration of 500 µg OVA protein once per week for 2
consecutive weeks. Three days after being last sensitized,
1x106 vectorctrlDCs, or IDOkdDCs
pulsed with OVA protein (100 µg/ml) and stimulated with LPS (1
µg/ml) were intravenously injected into OVA sensitized mice
(vectorctrlDC control group, 8-10-week-old, n=3;
IDOkdDC-treated group, 8-10-week-old, n=3). After 72 h,
mice were anesthetized with sodium pentobarbital at a dose of 40
mg/kg (0.036 g/ml), and then sacrificed. Splenic cells were
separated and incubated with FACS antibodies against CD3e (cat. no.
145-2C11-PE-Cy7; eBioscience; Thermo Fisher Scientific, Inc.), CD4
(cat. no. GK1.5-PE; Invitrogen; Thermo Fisher Scientific, Inc.) and
CD8 (cat. no. 1953-6-7-APC; BioLegend) for 30 min on ice. Next,
cells were fixed and permeabilized in commercial solutions (cat.
no. 51-2090KZ, BDBD Biosciences), and punched with Perm Buffer III
(cat. no. 558050, BD Biosciences). Next, cells were stained
intracellularly with Ki67 (cat. no. SoLA15-FITC; BioLegend) on ice
for 30 min. Cell analysis was performed using a flow cytometer.
CD4+T cell proliferation in
vitro (CFSE assay): The proliferation of T cells in
vitro was examined using standard methods, as previously
described (25) with slight
modifications. Briefly, male Kunming mice (age, 8-10 weeks; n=6)
were anesthetized using sodium pentobarbital at a dose of 40 mg/kg
(0.036 g/ml) and then sacrificed. Spleens of mice were extracted to
make a single cell suspension. T cells were stained with FACS
antibodies against CD3 and CD4 for 30 min on ice. Subsequently,
CD4+T cells >99% purity were FACS-sorted and labeled
with CFSE (cat. no. C34554; CellTrace™ CFSE Cell Proliferation Kit;
Invitrogen; Thermo Fisher Scientific, Inc.) for 10 min at 37˚C, and
washed three times. Gene-modified DCs of C57BL/6 origin were
treated with LPS for 16 h, followed by 10 g/ml mitomycin C (MMC)
for 1 h, before they were co-cultured with the CFSE stained
CD4+T cells at a ratio of 1:10 in a 96-well plate. Three
days later, the CFSE dilution of CD4+T cells was
examined using flow cytometry. The proliferated CD4+T
cells were calculated by the number of acquired CFSElow
CD4+T cells x added BD calibrate APC beads/acquired bead
number.
T cell differentiation assays in
vivo
T cell differentiation assays in vivo were
performed as previously as described (25,26)
with slight modifications. A total of 1x106 OVA-pulsed
LPS-treated gene-modified DCs were intravenously injected into 6
healthy male OVA-sensitized C57/BL mice, which were divided into
two groups: The vectorctrlDC-ctrl group (age, 8-10
weeks; n=3) and the IDOkdDC-treated group (age, 8-10
weeks; n=3). After 72 h, mice were anesthetized with sodium
pentobarbital at a dose of 40 mg/kg (0.036 g/ml), and then
sacrificed. Spleens of mice were extracted to make a single cell
suspension. The single cell suspension was obtained and cultured
for 4-6 h in the presence of phorbol 12-myristate 13-acetate (PMA),
ionomycin and brefeldin A. Next, cells were stained with CD3 and
CD4 followed by fixation and permeabilization, and stained
intracellularly with anti-mouse IL-17A (cat. no. TC11-18H10-APC; BD
Pharmingen; BD Biosciences), IL-10 (cat. no. JES5-16E3-APC; BD
Pharmingen; BD Biosciences), IL-4 (cat. no. 11B11-APC; BD
Pharmingen; BD Biosciences), and IFN-γ (cat. no. XMG1.2; BD
Pharmingen; BD Biosciences) mAb. Flow cytometry was performed using
a flow cytometer. Flow Jo software (Flow Jo version 10.6.2) was
used to analyze the data.
Statistical analysis
Data are expressed as the mean ± SEM; all
experiments were repeated at least 3 times with similar results.
Statistical differences were performed using an unpaired Student's
t-test. Prism 6.01 (GraphPad Software, Inc.) was used for the
statistical analysis of all data. P<0.05 was considered to
indicate a statistically significant difference.
Results
Successful construction of stable DC
lines with altered IDO expression and Trp-Kyn metabolism
To identify the role of IDO in DC functions, the
intracellular expression of this rate-limiting enzyme was
genetically altered in the Kyn pathway of DCs by both gain- and
reduction-of-function approaches. The CD region of the ido
gene was amplified and cloned into an overexpressing lentiviral
vector, pLJM-EGFP. Similarly, a short hairpin knockdown construct
for IDO was created by synthesizing shIDO and cloning it
into a lentiviral vector, pLKO.1. The transfection efficiency of
the EGFP-containing construct was evaluated under a fluorescence
microscope (Fig. S4B). Finally,
stable cell lines of IDOoeDCs and IDOkdDCs
were established by infecting the DCs with the aforementioned
competent lentiviral particles. Finally, their expression of the
IDO gene, protein and IDO enzyme activity were examined by RT-qPCR,
flow cytometry and HPLC, respectively.
First, it was demonstrated that IDOoeDCs
were successfully established via a lentiviral infection of DCs
with recombinant pLJM-IDO plasmid, expressing more IDO transcripts
than that of mock transduced DCs with control vector,
VectorCtrlDCs (Fig.
1A). In addition, the successful overexpression of the
ido gene in the IDOoeDCs was further verified in
protein expression (Fig. 1B).
Conversely, following infection of lentivirus containing
recombinant pLKO.1-shIDO plasmid that can knock-down ido
gene expression in the target cells, IDOkdDCs exhibited
a significantly compromised expression of the IDO transcript and
protein than the DCs infected with virus containing control vector
(vectorctrlDCs; Fig. 1C
and D). Furthermore, the
activities of the IDO enzyme were also examined in the genetic
variants by calculating the ratio of Kyn/Trp in the culture medium
samples of DCs. HPLC detection demonstrated that Trp concentration
was significantly lower in IDOoeDCs than in
VectorCtrlDCs. By contrast, the Kyn concentration and
Kyn/Trp ratio were significantly higher in the IDOoeDC
group, as compared with VectorCtrlDCs (Figs. 1E and S5A). Of note, Trp concentration was
significantly higher while Kyn concentration and Kyn/Trp ratio in
IDOkdDCs was significantly lower than that of
vectorctrlDCs (Figs. 1F
and S5B), indicating a
compromised activity in the Kyn pathway with a reduced IDO
expression, which was not caused by the viability of DCs transduced
with different vectors as the modified DCs had a similar survival
rate (Fig. S6A). In combination,
these data suggested that IDO expression was not only successfully
modified, but its activity was also altered in terms of Trp-Kyn
metabolism in DCs.
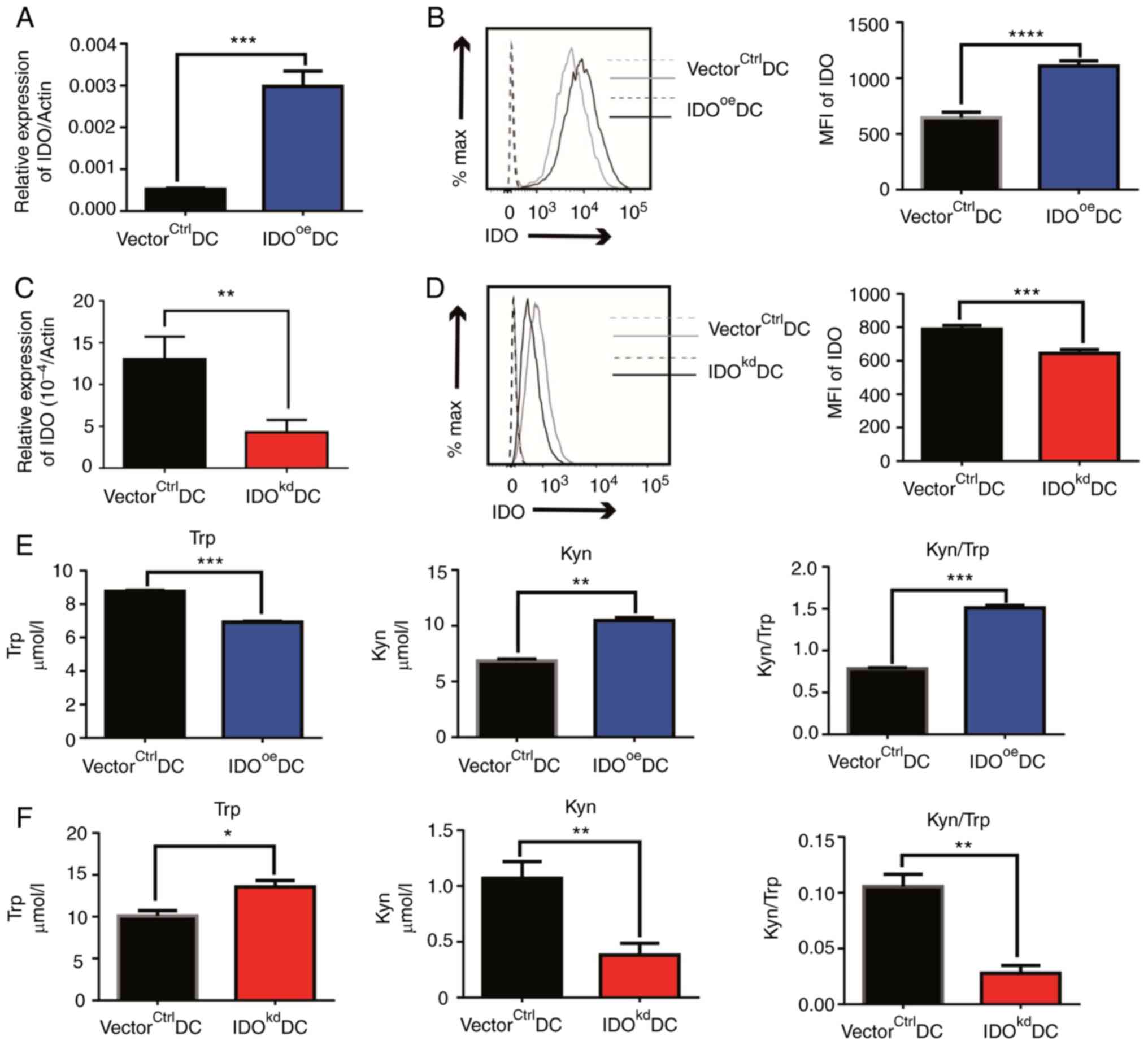 | Figure 1Effects of IDO on the Trp-Kyn
metabolism of DCs in vitro. (A) DCs were infected with
lentivirus containing pLJM1 or pLJM1-IDO plasmids to obtain
VectorCtrlDCs or IDOoeDCs, respectively. IDO
expression of VectorCtrlDCs and IDOoeDCs was
detected by qPCR. (B) IDO protein expression on the two types of
DCs in (A) was detected by flow cytometry (dotted line, unstained
control; solid line, stained group). Representative histograms
(left) and statistics (right) are shown. (C) DCs were infected with
lentivirus containing pLKO1 or pLKO1-shIDO plasmids to obtain
vectorctrlDCs and IDOkdDCs. IDO expression
was detected by qPCR. (D) IDO protein expression on the two types
of DCs in (C) was detected by flow cytometry. Representative
histograms (left) and statistics (right) are shown. (E) Detection
of Trp (left) and Kyn (center) concentrations, and the Kyn to Trp
ratio (right) in the culture medium samples of
VectorCtrlDCs and IDOoeDCs cultured for 48 h
by HPLC. (F) Detection of Trp (left) and Kyn (center)
concentrations, and the Kyn to Trp ratio (right) in the culture
medium samples of vectorctrlDCs and IDOkdDCs
cultured for 48 h by HPLC. The results are presented as the mean ±
SEM (n=3). *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. Each graph is
representative of 2-3 independent experiments. DC, dendritic cell;
HPLC, high-performance liquid chromatography; IDO, indoleamine
2,3-dioxygenase; Kyn, kynurenine; MFI, mean fluorescence intensity;
qPCR, quantitative PCR; Trp, tryptophan; VectorCtrlDCs,
DCs infected with control vector of pLJM1-EGFP;
IDOoeDCs, IDO-overexpressing DCs;
vectorctrlDCs, DCs infected with control vector of
pLKO.1; IDOkdDCs, IDO-knockdown DCs. |
DC-derived IDO inhibits T cell
proliferation both in vitro and in vivo
As professional APCs, the prime function of DCs is
to stimulate T cells to activate adaptive immunity. We asked
whether the proliferation of T cells was affected by the altered
IDO-related Trp-Kyn metabolism in DCs. To this end, OVA protein
(500 µg/mouse) was intraperitoneally injected into C57BL/6 mice
once a week for 2 consecutive weeks. Next, 1x106
gene-modified DCs were pulsed with 100 µg/ml OVA protein, stimulated
with 1 µg/ml LPS and injected intravenously into the OVA-sensitized
mice. Their trafficking of injected DCs was then examined, and the
expression of Ki-67, a marker of proliferation, on T cells in
vivo was detected by flow cytometry. While the migration of the
DCs in vivo was not affected by IDO knockdown (Fig. S6B), it was found that the adoptive
transfer of OVA-pulsed IDO-sufficient DCs
(vectorctrlDCs) can effectively stimulate the
proliferation of CD4+T and CD8+T cells in the
OVA-primed mice, demonstrating a successful establishment of the
antigen-specific activation of T cells in vivo by DCs
(Fig. 2A and B). However, when IDOkdDCs with
limited IDO amounts and activity in this working animal model was
injected, the proportion and number of proliferating
CD4+Ki67+T and
CD8+Ki67+T cells in mice were significantly
enhanced (Fig. 2A and B). To exclude the possibility of
environmental interference from other cell lineages in vivo,
CD4+T cell proliferation was assessed using a CFSE
dilution in vitro. IDOkdDCs were co-cultured with
CFSE-stained allogeneic CD4+T cells sorted for 3 days
using a flow sorting apparatus, it was then found that the
proliferation of CD4+T cells co-cultured with
IDOkdDCs was higher than that of T cells co-cultured
with vectorctrlDCs, regardless of LPS stimulation, as
measured by the percentage of CFSE low population that entered the
cell cycle (Fig. 2C). In
combination, these data suggested that IDO deficiency with
compromised enzymatic activity for cellular Trp-Kyn metabolism
exerted great effects on the T cell stimulating function of DCs,
both in vivo and in vitro.
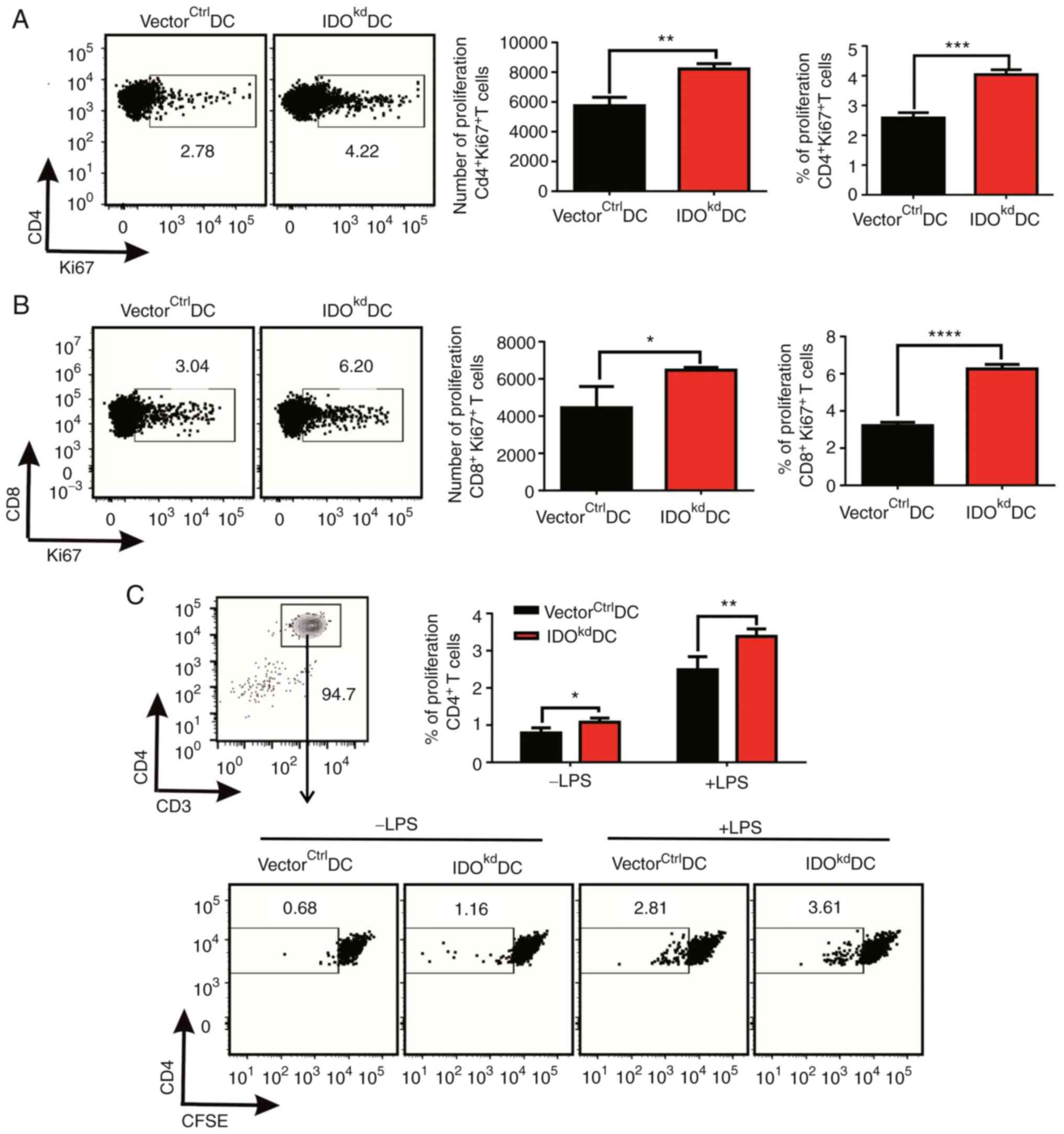 | Figure 2Effects of IDO on DC-stimulated T
cell proliferation in vivo and in vitro. A total of
1x106 OVA-pulsed vectorctrlDCs or
IDOkdDCs were intravenously injected into OVA-sensitized
mice (n=3 mice per group). After 72 h, spleens of mice were
extracted to make a single cell suspension, and flow cytometry was
conducted to analyze the percentage and number of (A)
CD4+Ki67+T cells and (B)
CD8+Ki67+T cells. Statistics are shown on the
right. (C) Effects of IDO on DC-stimulated CD4+T cell
proliferation in mixed lymphocyte reaction. (C) Single cell
suspensions from the spleens of Kunming mice were made and stained
with Anti-PE-cy7-CD3 and Anti-PE-CD4 monoclonal antibodies, before
CD3+CD4+T cells were sorted using a flow
sorting apparatus (left panel). DCs of C57/B6 mice origin were
treated with LPS overnight, before treatment with mitomycin C at a
final concentration of 10 µg/ml for 1 h. Afterwards, the DCs were
co-cultured with CFSE-stained allogeneic CD4+T cells
sorted by flow cytometry at a ratio of 1:10 for 3 days, and flow
cytometry was employed to analyze the percentage of
CFSE+ cells. (C) Representative scatter plot (bottom)
with CD3+CD4+T cells gated to show the
expression levels of CFSE and statistical analysis of multiple
experiments (right). The results are presented as the mean ± SEM
(n=3). *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. CFSE,
carboxyfluorescein diacetate succinimidyl ester; DC, dendritic
cell; IDO, indoleamine 2,3-dioxygenase; LPS, lipopolysaccharides;
OVA, ovalbumin; vectorctrlDCs, DCs infected with control
vector of pLKO.1; IDOkdDCs, IDO-knockdown DCs; PE,
phycoerythrin. |
IDO inhibits phagocytosis,
downregulates CD86 and upregulates PD-L1 in DCs
The suppressive effect of DC-derived IDO on T cell
proliferation prompted us to investigate the mechanism underlying
the intracellular Trp metabolic abnormality due to the IDO
variation. Since DCs stimulate proliferation by presenting
phagocytosed antigen on their surface, in the presence of a series
of co-receptors with either stimulatory or inhibitory functions, we
wanted to examine the impact of IDO on both antigen phagocytosis
and co-receptor expression in DCs. To this end, both
vectorctrlDCs and IDOkdDCs were co-cultured
with OVA-FITC for 4 h, before the OVA uptake capacity by the DCs
was analyzed using flow cytometry. It was found that
IDOkdDCs had a stronger uptake capacity of OVA antigen
compared with vectorctrlDCs, implying that suppressed
Trp metabolism due to reduced IDO inhibit the phagocytosis of DCs.
The MFI of this is shown in a bar chart (Fig. 3A). Next, we studied the impact of
altered IDO expression on the surface molecules of DCs. The
co-stimulatory molecules of IDOoeDCs and
IDOkdDCs were detected by flow cytometry, and it was
found that, compared with VectorCtrlDCs, CD40 was
unchanged, CD86 was downregulated and PD-L1 was upregulated in
IDOoeDCs. The MFI of this is shown in a bar chart
(Fig. 3B). Conversely, the
expression of CD86 on the surface of IDOkdDCs was
higher, PD-L1 was lower and no change was observed in CD40, when
compared with vectorctrlDCs (Fig. 3C). Collectively, these data
suggested that IDO in DCs suppressed their antigen uptake, and
inhibited stimulatory but promoted co-inhibitory molecules on DCs,
which ultimately resulted in diminished T cell proliferation.
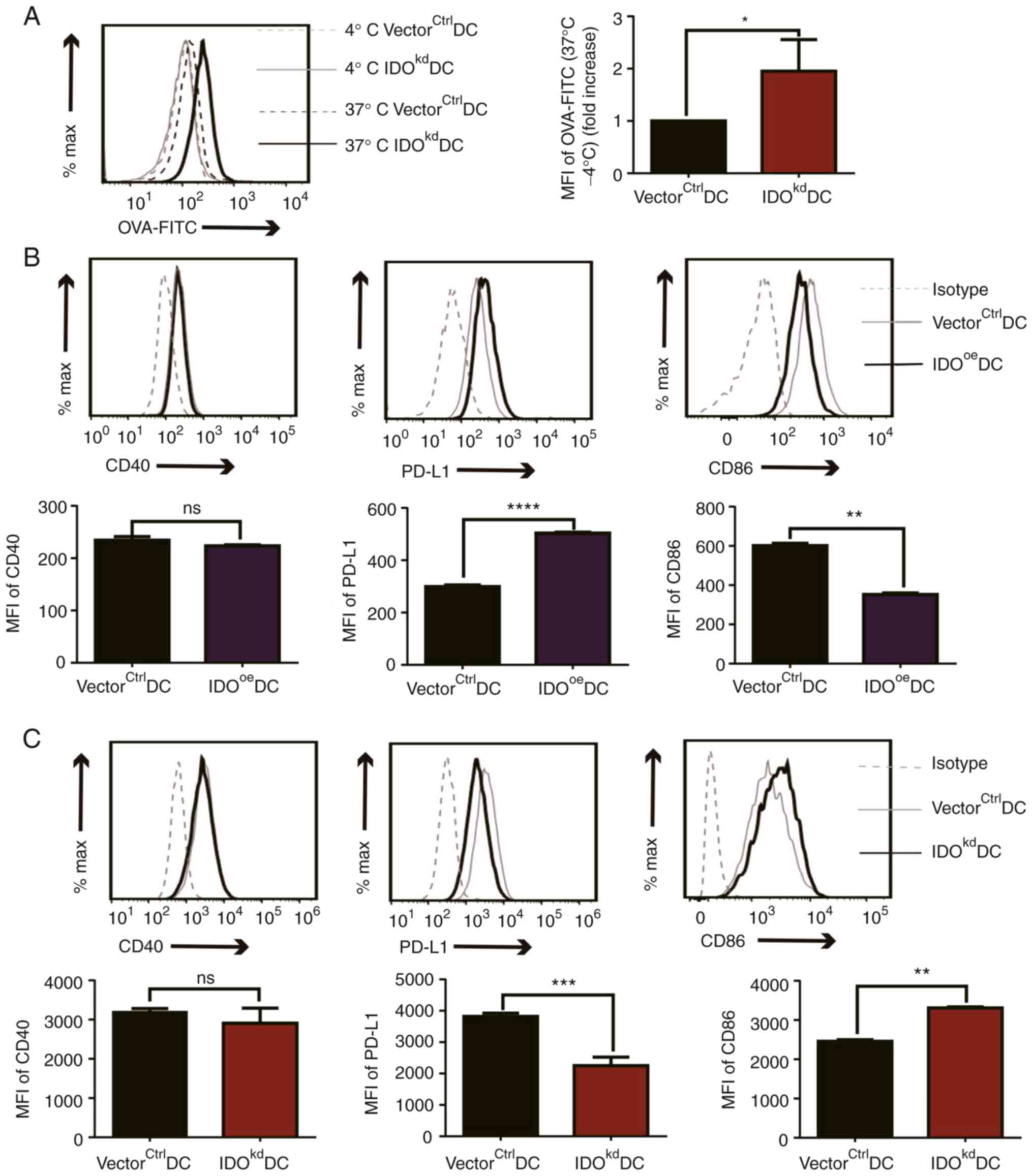 | Figure 3Effects of IDO on the phagocytosis
and phenotype of DCs. (A) Phagocytosis of OVA-FITC by the
genetically modified DCs was analyzed by FACS after co-c ulture at
37˚C for 4 h. The culture environment at 4˚C was used as a control.
(B) Both VectorCtrlDCs and IDOoeDCs were
treated with LPS overnight, and the MFI of CD40, CD86 and PD-L1 on
the surface of IDOoeDCs was detected by flow cytometry.
(C) Both vectorctrlDCs and IDOkdDCs were
treated with LPS overnight, and the MFI of CD40, CD86 and PD-L1 on
the surface of IDOkdDCs was detected by flow cytometry.
The results are presented as the mean ± SEM (n=3).
*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. DC, dendritic
cell; IDO, indoleamine 2,3-dioxygenase; LPS, lipopolysaccharides;
MFI, mean fluorescence intensity; ns, not significant (P>0.05);
OVA-FITC, fluorescein isothiocyanate-labeled ovalbumin; PD-L1,
programmed cell death ligand 1; VectorCtrlDCs, DCs
infected with control vector of pLJM1-EGFP; IDOoeDCs,
IDO-overexpressing DCs; vectorctrlDCs, DCs infected with
control vector of pLKO.1; IDOkdDCs, IDO-knockdown
DCs. |
Diminished Kyn metabolism in
IDOkdDCs skews T cell differentiation toward
proinflammatory phenotypes
The upregulation of PD-L1 on DCs by IDO suggests
their tolerogenic potential. Consistently, as the first and
rate-limiting enzyme in Trp metabolism, IDO plays an important role
in immune tolerance (27,28), and Trp metabolism-generated
metabolite Kyn promotes Treg differentiation, which may also affect
Th17 and Th1/Th2 cell generation from T cells (29). Therefore, it was studied whether,
in addition to T cell proliferation, T cell differentiation was
also affected by IDO-related Trp-Kyn metabolism in DCs. To check
this, OVA-pulsed vectorctrlDCs or IDOkdDCs
were intravenously injected into C57BL/6 mice sensitized by an
intraperitoneal administration of OVA antigen twice. After 72 h,
the spleens of mice were extracted to make a single cell
suspension, treated with PMA/ionomycin, and analyzed by flow
cytometry for intracellular IFN-γ, IL-17A, IL-4, and IL-10, as
indicators of Th1, Th17, Th2, and Treg subsets, respectively. It
was found that, as compared to vectorctrlDCs, the
administration of IDOkdDCs significantly up-regulated
CD4+IFN-γ+Th1 cells, but down-regulated
CD4+IL-4+Th2 and
CD4+IL-10+Treg cells, whereas
CD4+IL-17A+Th17 cells remained unchanged
(Fig. 4A and B). Consistent with the above data in
vivo, it was also confirmed that IDO had a similar effect on
DC-stimulated T cell differentiation in vitro, as
supernatants from the co-culture of IDOkdDCs and
CD4+T cells expressed more IFN-γ and less IL-10 compared
with the vectorctrlDCs in vitro (Fig. 4C). In combination, these data
suggested that the absence of IDO may impair the tolerogenic
activities of DCs and consequently skew T-cell differentiation
toward Th1 cells away from Tregs, possibly via the IDO-related
Trp-Kyn metabolism.
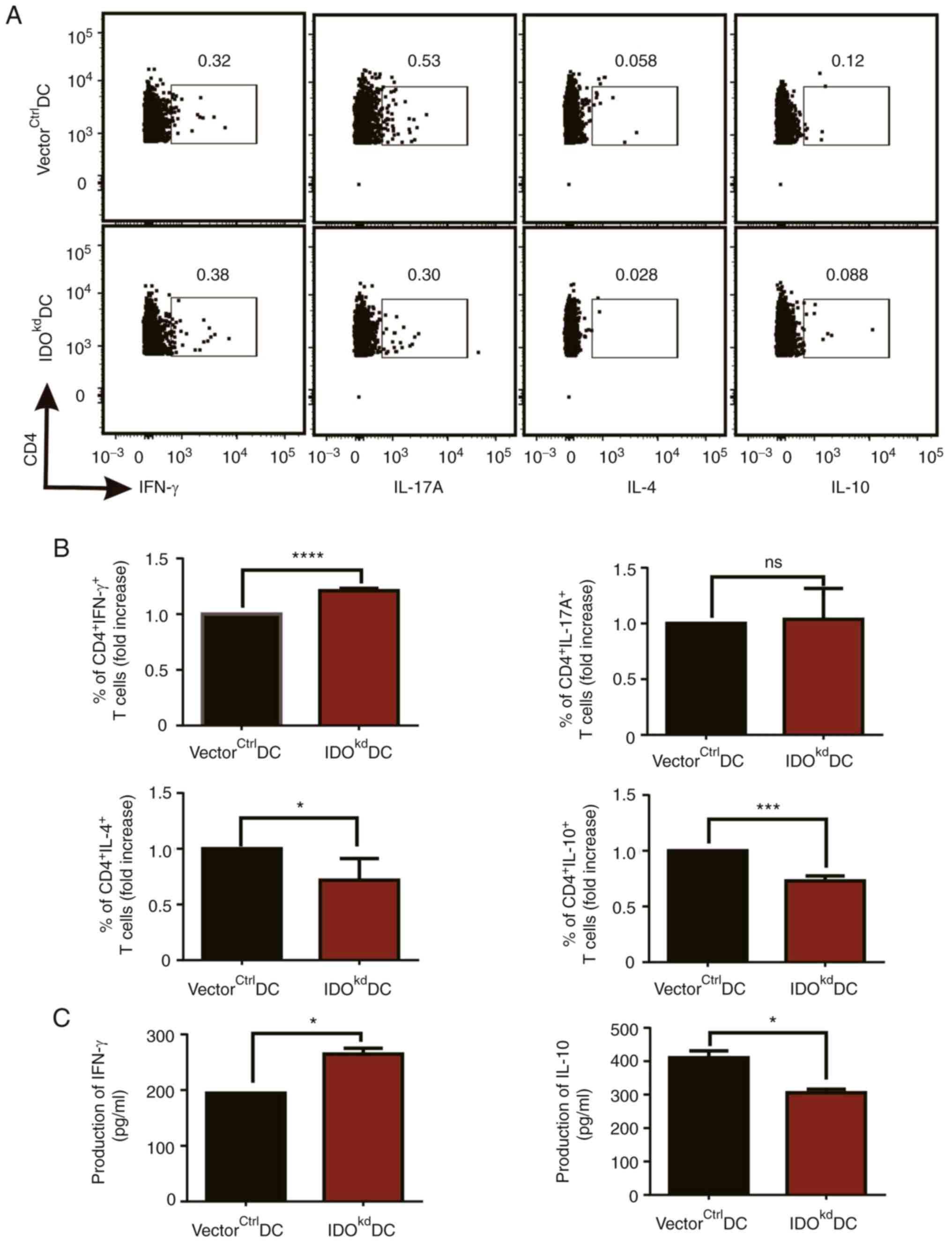 | Figure 4Effects of IDO on DC-stimulated T
cell differentiation in vivo and in vitro. A total of
1x106 OVA-pulsed vectorctrlDCs and
IDOkdDCs were intravenously injected into OVA-sensitized
mice (n=3 mice per group). After 72 h, the spleens of the mice were
extracted to make a single cell suspension. The secretion of T
cytokines was detected by flow cytometry. (A) Representative
scatter plots and (B) statistics are shown. (C) Effects of IDO on
DC-stimulated T cell differentiation in vitro. Sorted
CD3+CD4+T cells from the spleens of Kunming
mice were co-cultured with vectorctrlDCs or
IDOkdDCs. The supernatants of CD4+T cells
co-cultured with vectorctrlDCs or IDOkdDCs
were collected 3 days later, and the secretion of T cytokines was
detected by ELISA. The results are presented as the mean ± SEM
(n=3). *P<0.05, ***P<0.001,
****P<0.0001. DC, dendritic cell; IDO, indoleamine
2,3-dioxygenase; ns, not significant (P>0.05); OVA, ovalbumin;
vectorctrlDCs, DCs infected with control vector of
pLKO.1; IDOkdDCs, IDO-knockdown DCs. |
IDO inhibits IL-12 but promotes IL-10
production from DCs
Since DC-mediated T cell differentiation is driven
by a number of polarizing cytokines secreted by them, the impact of
IDO on the production of these cytokines in DCs was investigated.
VectorctrlDCs or IDOkdDCs were treated with
LPS (1 µg/ml) and incubated at 37˚C for 24 h; their cytokine
production in the supernatants was then quantified using ELISA. It
was found that IDOkdDCs secreted more Th1 polarizing
IL-12p70 (Fig. 5A) but less Treg
polarizing IL-10 (Fig. 5B) than
that of vectorctrlDCs, whereas the production of Th17
polarizing cytokine IL-6 was similar between the two
lentiviral-infected DCs (Fig. 5A).
The differential impacts of IDO reduction on the production of the
three cytokines suggested that IDO specifically affects
Th1-polarizing IL-12 and Treg-polarizing IL-10 secretion from DCs,
which fit in well with their downstream T cell differentiation
profiles. These data demonstrated molecular evidence to account for
the tolerogenic regulation of DC-mediated T cell differentiation by
IDO.
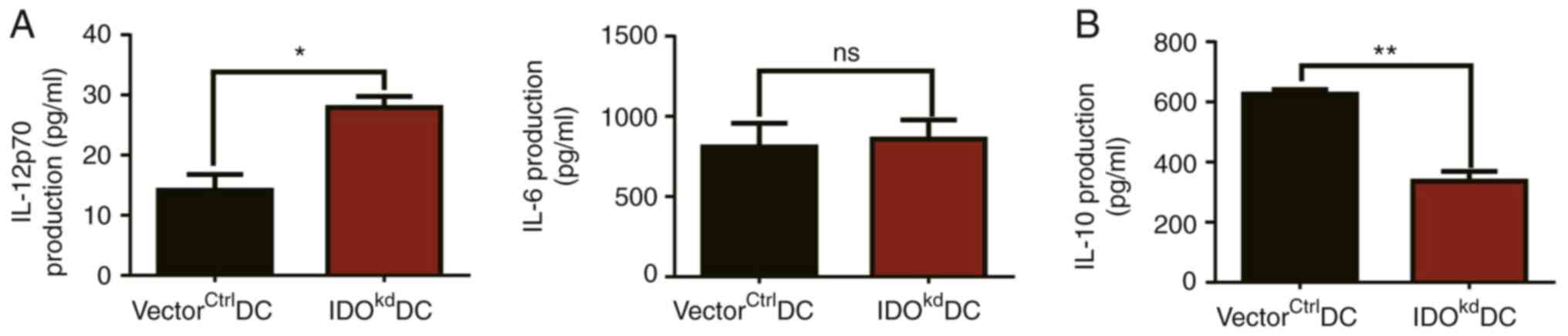 | Figure 5Effect of IDO on T cell polarization
factors secreted by DCs. Both vectorctrlDCs and
IDOkdDCs were incubated at 37˚C for 24 h, and the
supernatants of vectorctrlDCs and IDOkdDCs
medium were collected. Subsequently, the levels of (A) IL-12p70,
IL-6 and (B) IL-10 were examined in these supernatants by ELISA.
The results are presented as the mean ± SEM (n=3).
*P<0.05, **P<0.01. DC, dendritic cell;
IDO, indoleamine 2,3-dioxygenase; ns, not significant (P>0.05);
vectorctrlDCs, DCs infected with control vector of
pLKO.1; IDOkdDCs, IDO-knockdown DCs. |
Discussion
Several studies have shown that, as the
rate-limiting enzyme in cell metabolism, IDO plays key roles in DC
biology (9,30). For example, Hwu et al
(31) found that activated DCs can
express IDO using Northern blot analysis of IDO mRNA and HPLC to
detect production of functionally active IDO. Subsequently, Terness
et al (32) proposed the
term ‘IDO-expressing dendritic cells’. Likewise, many scholars also
used the term ‘IDO-expressing dendritic cells’ to study the role of
IDO-expressing DCs in immune related diseases (33,34).
IDO-expressing DCs could be characterized phenotypically by
co-expression of CD123 and CCR6, expression of major
histocompatibility complex class II and costimulatory molecules,
functionally by suppressing the T-cell response and promoting
immune tolerance (35). In the
present study, the immunomodulatory effect of IDO on the functions
of DCs both in vitro and in vivo was systematically
studied by successfully constructing stable DC lines using both
gain-of-function and reduction-of-function methods for IDO with
lentiviral infection. The present study provided evidence that IDO
is a key molecule that induces DC tolerance and inhibits T cell
immunity by altering intracellular Trp metabolism to regulate
surface molecule and cytokine expression in DCs. It was shown that
IDO modulated Trp-Kyn metabolism in DCs, which led to the
downregulation of CD86, upregulation of PD-L1, and suppression of
antigen uptake to promote the transformation of DCs into tolDCs s;
this ultimately resulted in a decrease in T cell proliferation. In
addition, it was also identified that IDO inhibited the secretion
of IL-12, while promoting the secretion of IL-10 from DCs for Treg
differentiation. Collectively, these findings uncovered a key
mechanism of IDO in DC tolerance by altering Trp metabolism, thus
opening a door for the use of immunotherapy in autoimmune diseases
with IDO-expressing DCs as tolerance-inducting vaccines.
Cellular metabolism has been identified as a key
component in determining the fate of DC immunogenicity or tolerance
(36). The significant finding in
this study lies in the identification that IDO is primarily
responsible for inducing DC tolerance via Trp metabolism. To date,
several specific candidate markers of tolDCs have been identified.
For example, co-stimulatory molecules such as CD80 and CD86 are
considered to be representative indicators of tolDCs (12,37).
Inhibitory molecules, such as programmed cell death ligand 1
(PD-L1), PD-L2, CD83, and C-C Motif Chemokine Ligand 22 are also
considered to be markers of tolDCs (38,39).
The present study found that IDO in IDOoeDCs inhibited
phagocytosis and CD86 expression and promoted PD-L1 expression,
leading to the induction of tolDCs. A previous report showed
that IDO exerted its effect on cell proliferation. For example, IDO
in macrophages affects T cell proliferation (40). Of note, Trp starvation limited T
cell proliferation by disrupting the T cell cycle mechanism
(31), and Trp catabolites Kyn or
3-HAA inhibited T cell proliferation (41). Coincidentally, it was found in the
present study that IDO deficiency was accompanied by a reduction in
Trp consumption and Kyn production in IDOkdDCs, which
promoted T cell proliferation both in vitro and in
vivo, suggesting that IDO likely inhibits T cell proliferation
through a Trp metabolism-mediated regulation of surface molecules
CD86 and PD-L1 on DCs. In addition, the examination of cytokines is
important when examining the immunomodulatory activities of DCs
(42). For example,
anti-inflammatory cytokines, including IL-4 and IL-10, are also
regarded as markers of tolDCs (38). Furthermore, tolDCs or semimature
DCs at an early stage of maturation usually exhibit a decreased
expression of IL-12 and increased expression of tolerogenic
cytokines IL-10, IL-27 and TGF-β (43). These DC-derived cytokines, which
are an essential link between innate and acquired immunity, are
associated with T cell differentiation and other immune regulatory
roles (44). For example,
DC-secreted IL-10 promoted Treg development, while IL-12 induced
the differentiation of CD4+T cells into Th1 cells
(45,46). In addition, TGF-β played a negative
role on the function of several cells, as was reported in the
cancer patients associated with natural killer (NK) cell inhibition
(47). In the present study, it
was demonstrated that IDO in IDOkdDCs inhibited the
secretion of IL-10 but promoted that of IL-12 in DCs, leading to
the inhibition of naive T-cell differentiation toward Th1 cells and
away from Tregs, suggesting that the absence of IDO impairs DC
tolerance and consequently promotes T cell immunity. It should be
noted that active Trp metabolism in DCs also affects the
microenvironment and T cell responses. For example, Trp depletion
resulted in preferential apoptosis in Th1 cells over Th2 cells due
to the increased sensitivity of Th1 cells to Kyn metabolites
(48). Trp metabolites bound to
AHR to induce forkhead box P3 expression and promoted the
generation and differentiation of Tregs involved in
immunosuppression (49). In
addition, Kyn promoted the differentiation of naive
CD4+T-cells into Tregs instead of Th17 cells (50). Based on these data, we assumed that
IDOkdDCs skews T cell differentiation toward Th1 cells
rather than Treg cells by Trp metabolism and cytokine regulation.
Overall, considering the formidable immunomodulatory effects of IDO
on DCs and T cells, we propose that Trp metabolism-mediated
regulation of surface molecule and cytokine expression in DCs is
the important pathway through which IDO induces DC tolerance to
inhibit T cell immunity.
In conclusion, the data presented in the present
study demonstrated that IDO is a key molecule that induces the
transformation of DCs into tolDCs and inhibits T cell immunity by
altering Trp metabolism, regulating surface molecules and cytokines
in DCs. This conclusion can provide a theoretical basis of
therapeutic drugs for the prevention and treatment of autoimmune
diseases. To date, only a few clinical trials on inflammatory and
autoimmune disorders have been performed. Therefore, as tolDCs of
metabolic regulation, the genetic modification of IDO in DCs, such
as the IDOoeDCs in the present study, could be used for
vaccine inoculation in disease models in the future, offering more
possibilities for the application of tolerogenic vaccine in
clinical trials.
Supplementary Material
Map of pLJM1-EGFP vector. EGFP,
enhanced green fluorescent protein.
Map of pLKO.1-TRC cloning vector. TRC,
The RNAi Consortium.
Lentivirus containing IDO-modifying
plasmids was successfully constructed and transfected into 293FT
cells. The IDO (A) overexpression or (B) knockdown recombinant
plasmids were transformed into active Escherichia coli and
amplified. The positive colonies were identified by bacterial PCR.
Lanes 1-4 were all PCR products from individual colonies of
Escherichia coli transformed with IDO-overexpressing
recombinant plasmid (1,224 bp). Lanes 5-8 were the PCR products
from individual colonies of Escherichia coli transformed
with IDO-knockdown recombinant plasmid (258 bp). IDO, indoleamine
2,3-dioxygenase; sh, short hairpin RNA.
Sequence alignment and transfection
293FT cells. (A) Sequences inserted into vector were confirmed by
comparing with the theoretical sequence of shIDO. (B) In the
presence of liposome transfection reagent, the confirmed EGFP-IDO
overexpression construct and two packaging plasmids were
co-transfected into 293FT cells to produce effective lentivirus
particles. The transfection efficiency in 293FT cells was observed
under a fluorescence microscope (magnification, x10; scale bar,
230.5 μm). EGFP, enhanced green fluorescent protein; IDO,
indoleamine 2,3-dioxygenase; shRNA/sh, short hairpin RNA.
Chromatogram demonstration of Trp and
Kyn in genetically modified DCs. Detection of Trp and Kyn
concentrations in the culture medium samples of (A)
IDOoeDCs or (B) IDOkdDCs with their controls
and standard was performed by high-performance liquid
chromatography. DC, dendritic cell; IDO, indoleamine
2,3-dioxygenase; Kyn, kynurenine; Trp, tryptophan;
VectorCtrlDCs, DCs infected with control vector of
pLJM1-EGFP; IDOoeDCs, IDO-overexpressing DCs;
vectorctrlDCs, DCs infected with control vector of
pLKO.1; IDOkdDCs, IDO-knockdown DCs.
Effects of IDO on the survival and
migration of DCs. (A) Flow cytometric analyses of viable cells
stained with FITC-Annexin V and PI to detect the effect of IDO on
the viability of DCs. The cells negative for both Annexin V and PI
were considered alive. Representative scatter plots (left) and
statistics (right) are shown. (B) After CFSE staining (37˚C for 10
min), both 10x106 vectorctrlDCs and
IDOkdDCs were subcutaneously injected into mice (n=3
mice per group). After 24 h, the popliteal lymph nodes of mice were
extracted to make a single cell suspension. The CFSE+DCs
were detected by flow cytometry. Representative scatter plots
(left) and statistics (right) are shown. The results are presented
as the mean ± SEM (n=3). DC, dendritic cell; CFSE,
carboxyfluorescein diacetate succinimidyl ester; IDO, indoleamine
2,3-dioxygenase; ns, not significant; FSC-A, forward scatter area;
vectorctrlDCs, DCs infected with control vector of
pLKO.1; IDOkdDCs, IDO-knockdown DCs.
Acknowledgements
Not applicable.
Funding
Funding: The present study was financially supported by National
Natural Science Foundation of China Major Research Plan Project
(grant no. 91742101), Anhui International Science and Technology
Collaborative Project, China (grant no. 1604b0602017), Natural
Science Foundation of Anhui Province, China (grant no.
1608085MH160), Molecular Enzymology and Molecular Detection
Excellent Innovation Team of Universities in Anhui Province (grant
no. 2022AH010012), Outstanding Innovative Research Team for
Molecular Enzymology and Detection in Anhui Provincial Universities
(grant no. 2022AH010012), and The Undergraduate Research Fund of
Wannan Medical College (grant no. 18103010160).
Availability of data and materials
All the data generated or analyzed during this
study are included in this published article.
Authors' contributions
FW and LL confirm the authenticity of all the raw
data. FW and LL conducted the experiments and analyzed the data. FW
wrote the first draft of the manuscript. LZ, JW, ML, WZ and CZ
helped perform the experiments. YX designed the study and wrote the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by
Anhui Normal University Animal Experimentation Ethics Committee
(approval number AHNU-ET2022015; Wuhu, China). All animal
experiments were carried out in accordance with the guidelines for
the care and use of laboratory animals. All laboratory procedures
were used to reduce the pain of the mice.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sudar-Milovanovic E, Gluvic Z, Obradovic
M, Zaric B and Isenovic ER: Tryptophan metabolism in
atherosclerosis and diabetes. Curr Med Chem. 29:99–113.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sadok I and Staniszewska M:
Electrochemical determination of kynurenine pathway
metabolites-challenges and perspectives. Sensors (Basel).
21:2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ramprasath T, Han YM, Zhang D, Yu CJ and
Zou MH: Tryptophan catabolism and inflammation: A novel therapeutic
target for aortic diseases. Front Immunol.
12(731701)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Silvano A, Seravalli V, Strambi N, Cecchi
M, Tartarotti E, Parenti A and Tommaso MD: Tryptophan metabolism
and immune regulation in the human placenta. J Reprod Immunol.
147(103361)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kudo Y, Boyd CA, Sargent IL and Redman CW:
Decreased tryptophan catabolism by placental indoleamine
2,3-dioxygenase in preeclampsia. Am J Obstet Gynecol. 188:719–726.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sorgdrager FJH, Naude PJW, Kema IP, Nollen
EA and Deyn PP: Tryptophan metabolism in inflammaging: From
biomarker to therapeutic target. Front Immunol.
10(2565)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Favre D, Mold J, Hunt PW, Kanwar B, Loke
P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F, et al:
Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the
balance of TH17 to regulatory T cells in HIV disease. Sci Transl
Med. 2(32ra36)2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhai L, Ladomersky E, Lenzen A, Nguyen B,
Patel R, Lauing KL, Wu M and Wainwright DA: IDO1 in cancer: A
Gemini of immune checkpoints. Cell Mol Immunol. 15:447–457.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fallarino F, Vacca C, Orabona C,
Belladonna ML, Bianchi R, Marshall B, Keskin DB, Mellor AL,
Fioretti MC, Grohmann U, et al: Functional expression of
indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells.
Int Immunol. 14:65–68. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Alahdal M, Zhang H, Huang R, Sun W, Deng
Z, Duan L, Ouyang H and Wang D: Potential efficacy of dendritic
cell immunomodulation in the treatment of osteoarthritis.
Rheumatology (Oxford). 60:507–517. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen D, Koropatnick J, Jiang N, Zheng X,
Zhang X, Wang H, Yuan K, Siu KS, Shunnar A, Way C, et al: Targeted
siRNA silencing of indoleamine 2,3-dioxygenase in
antigen-presenting cells using mannose-conjugated liposomes: A
novel strategy for treatment of melanoma. J Immunother. 37:123–134.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nam JH, Lee JH, Choi SY, Jung NC, Song JY,
Seo HG and Lim DS: Functional ambivalence of dendritic cells:
Tolerogenicity and immunogenicity. Int J Mol Sci.
22:2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Belladonna ML, Orabona C, Grohmann U and
Puccetti P: TGF-beta and kynurenines as the key to infectious
tolerance. Trends Mol Med. 15:41–49. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
O'Neill LAJ and Pearce EJ:
Immunometabolism governs dendritic cell and macrophage function. J
Exp Med. 213:15–23. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wculek SK, Khouili SC, Priego E,
Heras-Murillo I and Sancho D: Metabolic control of dendritic cell
functions: Digesting information. Front Immunol.
10(775)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shen Z, Reznikoff G, Dranoff G and Rock
KL: Cloned dendritic cells can present exogenous antigens on both
MHC class I and class II molecules. J Immunol. 158:2723–2730.
1997.PubMed/NCBI
|
|
17
|
Chen S, Li X, Zhang W, Zi M and Xu Y:
Inflammatory compound lipopolysaccharide promotes the survival of
GM-CSF cultured dendritic cell via PI3 kinase-dependent
upregulation of Bcl-x. Immunol Cell Biol. 96:912–921.
2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sun L, Zhang W, Zhao L, Zhao Y, Wang F,
Lew AM and Xu Y: Self-tolerance of vascular tissues is broken down
by vascular dendritic cells in response to systemic inflammation to
initiate regional autoinflammation. Front Immunol.
13(823853)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cole JE, Astola N, Cribbs AP, Goddard ME,
Park I, Green P, Davies AD, Williams OR, Feldmann M and Monaco C:
Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and
its metabolites provide new opportunities for drug development.
Proc Natl Acad Sci USA. 112:13033–13038. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yang HL, Liu HW, Shrestha S, Thiyagarajan
V, Huang HC and Hseu YC: Antrodia salmonea induces apoptosis
and enhances cytoprotective autophagy in colon cancer cells. Aging
(Albany NY). 13:15964–15989. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jurisic V, Srdic-Rajic T, Konjevic G,
Bogdanovic G and Colic M: TNF- α induced apoptosis is accompanied
with rapid CD30 and slower CD45 shedding from K-562 cells. J Membr
Biol. 239:115–122. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang W, Zi M, Sun L, Wang F, Chen S, Zhao
Y, Liang S, Hu J, Liu S, Liu L, et al: Cystatin C regulates major
histocompatibility complex-II-peptide presentation and
extracellular signal-regulated kinase-dependent polarizing cytokine
production by bone marrow-derived dendritic cells. Immunol Cell
Biol. 97:916–930. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sun L, Rautela J, Delconte RB,
Souza-Fonseca-Guimaraes F, Carrington EM, Schenk RL, Herold MJ,
Huntington ND, Lew AM, Xu Y and Zhan Y: GM-CSF quantity has a
selective effect on granulocytic vs. monocytic myeloid development
and function. Front Immunol. 9(1922)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhan Y, Vega-Ramos J, Carrington EM,
Villadangos JA, Lew AM and Xu Y: The inflammatory cytokine, GM-CSF,
alters the developmental outcome of murine dendritic cells. Eur J
Immunol. 42:2889–2900. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhang W, Ding Y, Sun L, Hong Q, Sun Y, Han
L, Zi M and Xu Y: Bone marrow-derived inflammatory and steady state
DCs are different in both functions and survival. Cell Immunol.
331:100–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomics. 17:663–674.
2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mellor AL, Lemos H and Huang L:
Indoleamine 2,3-dioxygenase and tolerance: Where are we now? Front
Immunol. 8(1360)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mellor AL and Munn DH: IDO expression by
dendritic cells: Tolerance and tryptophan catabolism. Nat Rev
Immunol. 4:762–774. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nguyen NT, Kimura A, Nakahama T, Chinen I,
Masuda K, Nohara K, Fujii-Kuriyama Y and Kishimoto T: Aryl
hydrocarbon receptor negatively regulates dendritic cell
immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad
Sci USA. 107:19961–19966. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pallotta MT, Orabona C, Volpi C, Vacca C,
Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M,
Bicciato S, et al: Indoleamine 2,3-dioxygenase is a signaling
protein in long-term tolerance by dendritic cells. Nat Immunol.
12:870–878. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hwu P, Du MX, Lapointe R, Do M, Taylor MW
and Young HA: Indoleamine 2,3-dioxygenase production by human
dendritic cells results in the inhibition of T cell proliferation.
J Immunol. 164:3596–3599. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Terness P, Bauer TM, Rose L, Dufter C,
Watzlik A, Simon H and Opelz G: Inhibition of allogeneic T cell
proliferation by indoleamine 2,3-dioxygenase-expressing dendritic
cells: Mediation of suppression by tryptophan metabolites. J Exp
Med. 196:447–457. 2002.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fallarino F, Gizzi S, Mosci P, Grohmann U
and Puccetti P: Tryptophan catabolism in IDO+ plasmacytoid
dendritic cells. Curr Drug Metab. 8:209–216. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Park MJ, Min SY, Park KS, Cho YG, Cho ML,
Jung YO, Park HS, Chang SH, Cho SG, Min JK, et al: Indoleamine
2,3-dioxygenase-expressing dendritic cells are involved in the
generation of CD4+CD25+ regulatory T cells in
Peyer's patches in an orally tolerized, collagen-induced arthritis
mouse model. Arthritis Res Ther. 10(R11)2008.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Munn DH, Sharma MD, Lee JR, Jhaver KG,
Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess
R, et al: Potential regulatory function of human dendritic cells
expressing indoleamine 2,3-dioxygenase. Science. 297:1867–1870.
2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sim WJ, Ahl PJ and Connolly JE: Metabolism
is central to tolerogenic dendritic cell function. Mediators
Inflamm. 2016(2636701)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bolandi N, Derakhshani A, Hemmat N,
Baghbanzadeh A, Asadzadeh Z, Nour MA, Brunetti O, Bernardini R,
Silvestris N and Baradaran B: The positive and negative
immunoregulatory role of B7 family: Promising novel targets in
gastric cancer treatment. Int J Mol Sci. 22(10719)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Castenmiller C, Keumatio-Doungtsop BC, van
Ree R, de Jong EC and van Kooyk Y: Tolerogenic immunotherapy:
Targeting DC surface receptors to induce antigen-specific
tolerance. Front Immunol. 12(643240)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ge W, Arp J, Lian D, Liu W, Baroja ML,
Jiang J, Ramcharran S, Eldeen FZ, Zinser E, Steinkasserer A, et al:
Immunosuppression involving soluble CD83 induces tolerogenic
dendritic cells that prevent cardiac allograft rejection.
Transplantation. 90:1145–1156. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Frumento G, Rotondo R, Tonetti M, Damonte
G, Benatti U and Ferrara GB: Tryptophan-derived catabolites are
responsible for inhibition of T and natural killer cell
proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med.
196:459–468. 2002.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Siska PJ, Jiao J, Matos C, Singer K,
Berger RS, Dettmer K, Oefner PJ, Cully MD, Wang Z, Quinn III WJ, et
al: Kynurenine induces T cell fat catabolism and has limited
suppressive effects in vivo. EBioMedicine.
74(103734)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Dzopalic T, Kostic M, Kostic M, Marjanović
G, Guzina J, Jurišić V and Nedeljković BB: Effects of galectin-1 on
immunomodulatory properties of human monocyte-derived dendritic
cells. Growth Factors. 38:235–246. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Takenaka MC and Quintana FJ: Tolerogenic
dendritic cells. Semin Immunopathol. 39:113–120. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Geginat J, Sallusto F and Lanzavecchia A:
Cytokine-driven proliferation and differentiation of human naive,
central memory and effector memory CD4+ T cells. Pathol
Biol (Paris). 51:64–66. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Macatonia SE, Hosken NA, Litton M, Vieira
P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM and
O'Garra A: Dendritic cells produce IL-12 and direct the development
of Th1 cells from naive CD4+ T cells. J Immunol.
154:5071–5079. 1995.PubMed/NCBI
|
|
46
|
Xu H, Jia Y, Li Y, Wei C, Wang W, Guo R,
Jia J, Wu Y, Li Z, Wei Z, et al: IL-10 dampens the Th1 and Tc
activation through modulating DC functions in BCG vaccination.
Mediators Inflamm. 2019(8616154)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Martinović KM, Vuletić A, Mališić E,
Srdić-Rajić T, Miletić NT, Babović N and Jurišić V: Increased
circulating TGF-β1 is associated with impairment in NK cell
effector functions in metastatic melanoma patients. Growth Factors.
40:231–239. 2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Van der Leek AP, Yanishevsky Y and
Kozyrskyj AL: The kynurenine pathway as a novel link between
allergy and the gut microbiome. Front Immunol.
8(1374)2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kawasaki H, Chang HW, Tseng HC, Hsu SC,
Yang SJ, Hung CH, Zhou Y and Huang SK: A tryptophan metabolite,
kynurenine, promotes mast cell activation through aryl hydrocarbon
receptor. Allergy. 69:445–452. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Mezrich JD, Fechner JH, Zhang X, Johnson
BP, Burlingham WJ and Bradfield CA: An interaction between
kynurenine and the aryl hydrocarbon receptor can generate
regulatory T cells. J Immunol. 185:3190–3198. 2010.PubMed/NCBI View Article : Google Scholar
|



















