Introduction
Thymomas are epithelial tumors originating from the
thymus gland and the most common tumors of the anterior mediastinum
(1). It is known that
approximately 2-5% of patients with thymoma develop pure red cell
aplasia (PRCA) (2,3), but the concurrent combination of
acquired amegakaryocytic thrombocytopenia (AAMT) is rarely
reported. The most common treatment used in PRCA complicated with
AAMT included cyclosporine, alone or in combination with
corticosteroid therapy (4-9).
The other salvage treatment strategies include antithymocyte
globulin, bone marrow transplantation, erythropoietin, iterative
transfusions, platelet transfusion and eltrombopag (4-7,9-11).
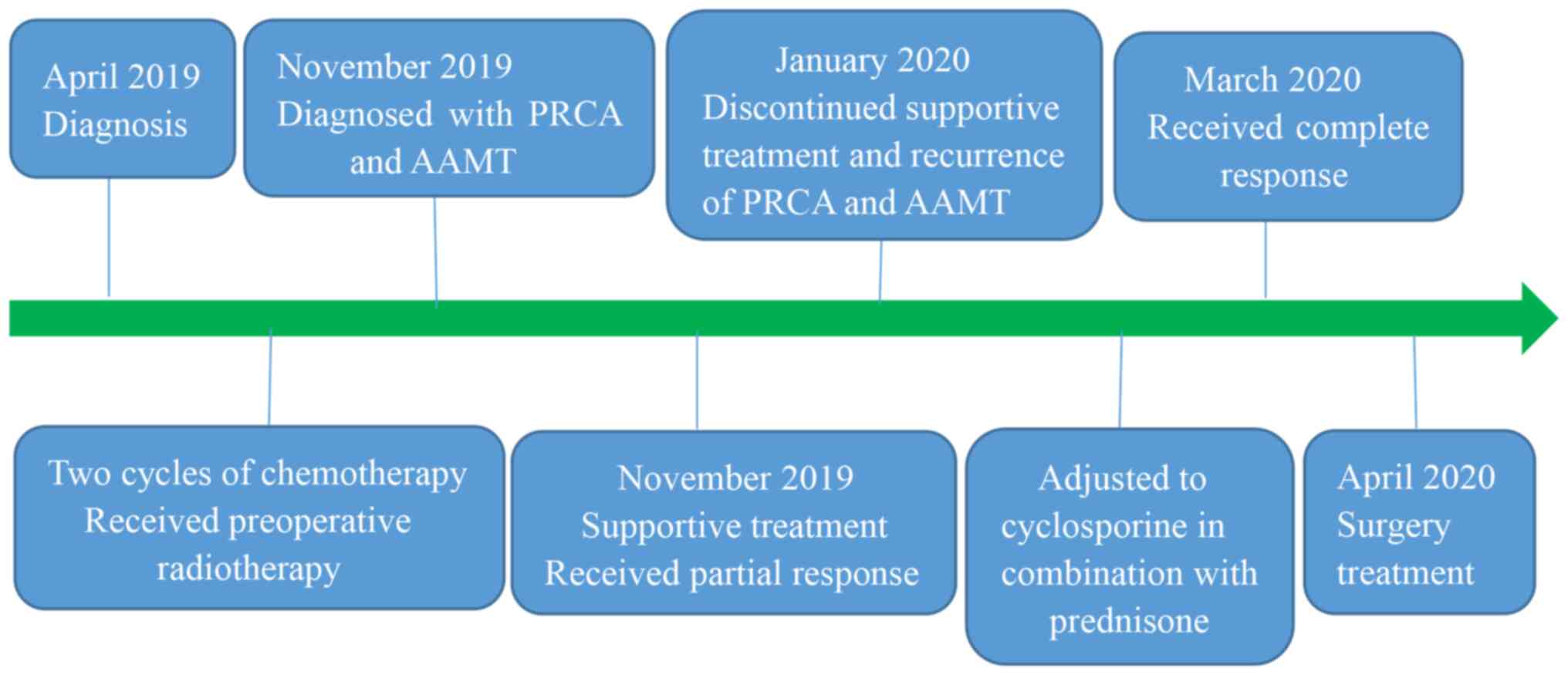
Here, this novel report details the course of our clinical
treatment of PRCA and AAMT induced by thymoma radiotherapy. The
patient underwent a complete resection of mediastinal tumor after
healing from AAMT and PRCA. Postoperative NGS testing revealed
mutation in the MSH3 variant, which was thought to enhance the
sensitivity of radiotherapy and promote apoptosis of tumor cells
and normal cells as one of the mechanisms leading to the
development of PRCA and AAMT, hitherto absent from prior
reports.
Case report
A 42-year-old female patient was diagnosed with
thymoma via mediastinal mass puncture biopsy on April 13, 2019, at
Shanghai Jiao Tong University Chest Hospital and received two
cycles of chemotherapy (paclitaxel and carboplatin). After
chemotherapy, a chest CT showed stable disease according to RECIST.
For further treatment, the patient sent pathological sections from
this hospital to ours (West China Hospital of Sichuan University in
Chengdu) for consultation and was also diagnosed with thymoma on
August 2, 2019. The patient's pre-radiotherapy CT chest enhancement
suggested a soft tissue mass in the left anterior mediastinum, with
a size of approximately 7.4x8.2 cm, and the mass was poorly
demarcated from the left border of the pericardium and adjacent
large vessels. In order to improve the success rate of complete
surgical resection, preoperative neoadjuvant radiotherapy was used
to shrink the size of the mass to achieve better radical surgical
resection. Prior to chest radiotherapy, her complete blood count
(CBC) test was normal. Her hemoglobin was 133 g/l (range, 115-150
g/l), platelet count 261x109 cells/l (range,
100-300x109 cells/l, and white blood cell (WBC) count
7.59x109 cells/l (range, 3.5-9.5x109
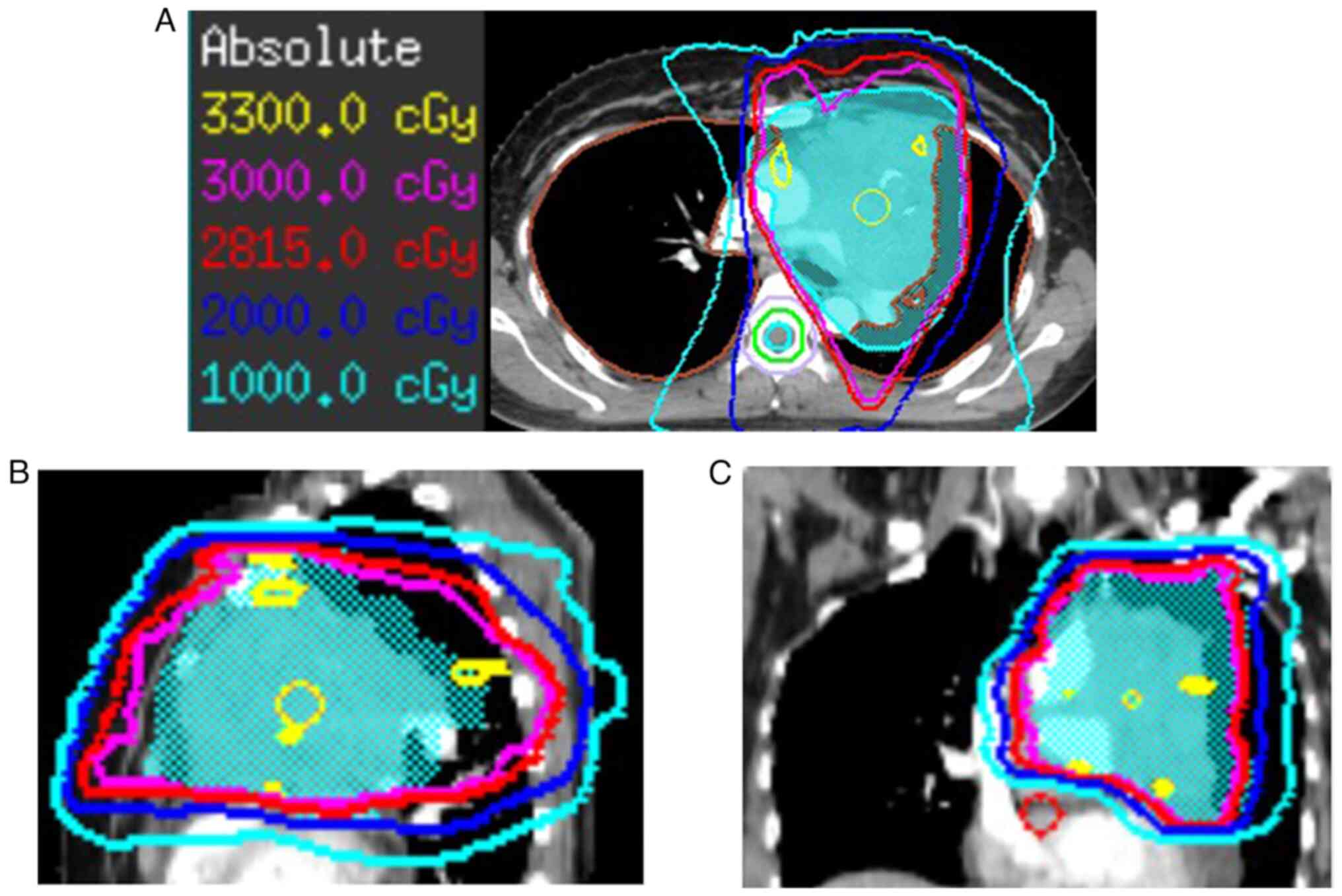
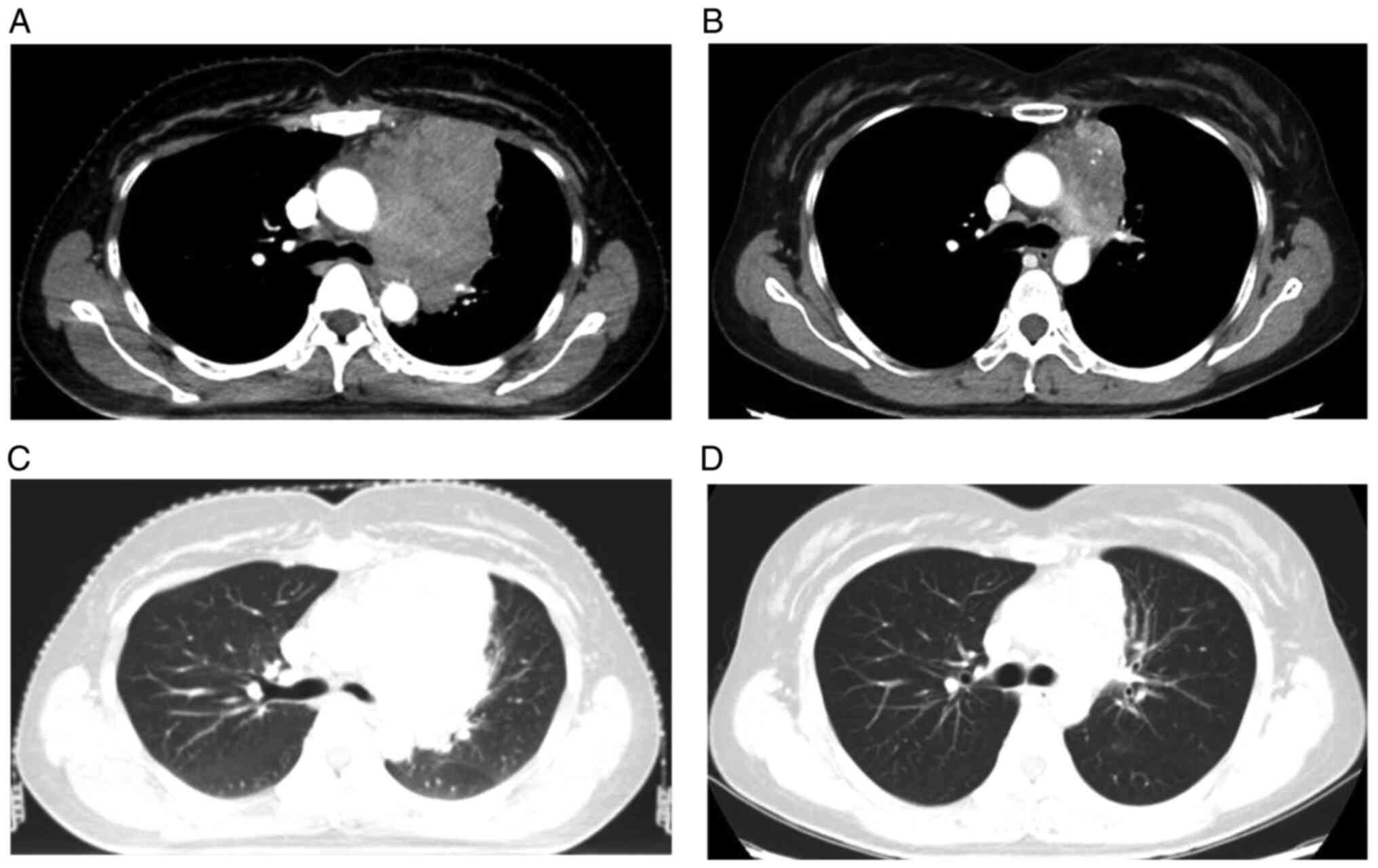
cells/l). After 36 Gy in 12 fractions (Fig. 1), chest CT scan showed that left
anterior mediastinal mass had greatly shrunken with the formation
of calcification (Fig. 2).
One month after the end of radiotherapy, the patient
was admitted to our hospital because of the appearance of bleeding
spots on the skin and gum bleeding. The CBC test showed normocytic
anemia at 69 g/l, WBC count 6.44x109 cells/l,
reticulocyte count 0.0027x1012 cells/l (range,
0.024-0.084x1012 cells/l), percentage of reticulocytes
was 0.12% (range, 0.5-2.5%) and concomitant thrombocytopenia of
2x109 cells/l. There was no folic acid and vitamin B19
deficiency, while anti-internal factor antibodies were reduced. The
coagulation function was basically normal. The erythropoietin level
was high and the direct Coombs test yielded a positive for 2+.
Bone marrow aspiration suggested that myeloid cells
were normal, while granulocytic hyperplasia with toxic-like
changes, with no red or megakaryocytic lineage detected.
Fluorescence in situ hybridization (FISH) test, leukemia
fusion gene WT1 test and flow-through immunophenotyping were all
negative. Therefore, the combination of all the patient's findings
led to a diagnosis of acquired PRCA and AAMT. We regret that the
lack of hematological images was a limitation of our study because
the patient's bone marrow aspiration was performed at The
Affiliated Hospital of Guizhou Medical University and its
hematological images were not available for loan, so we did not
obtain the hematological images.
Initially, the patient received supportive therapy
such as hemostasis, Recombinant Human Erythropoietin Injection to
relieve anemia, recombinant human platelet thrombopoietin,
Eltrombopag Olamine Tablets and platelet transfusion to raise
platelets, which led to partial alleviation of symptoms. However,
PRCA and AAMT recurred after the patient stopped the above
treatment on her own. The CBC results were shown below: hemoglobin
43 g/l, WBC 7.82x109 cells/l, and platelets
2x109 cells/l. The treatment regimen was then adjusted
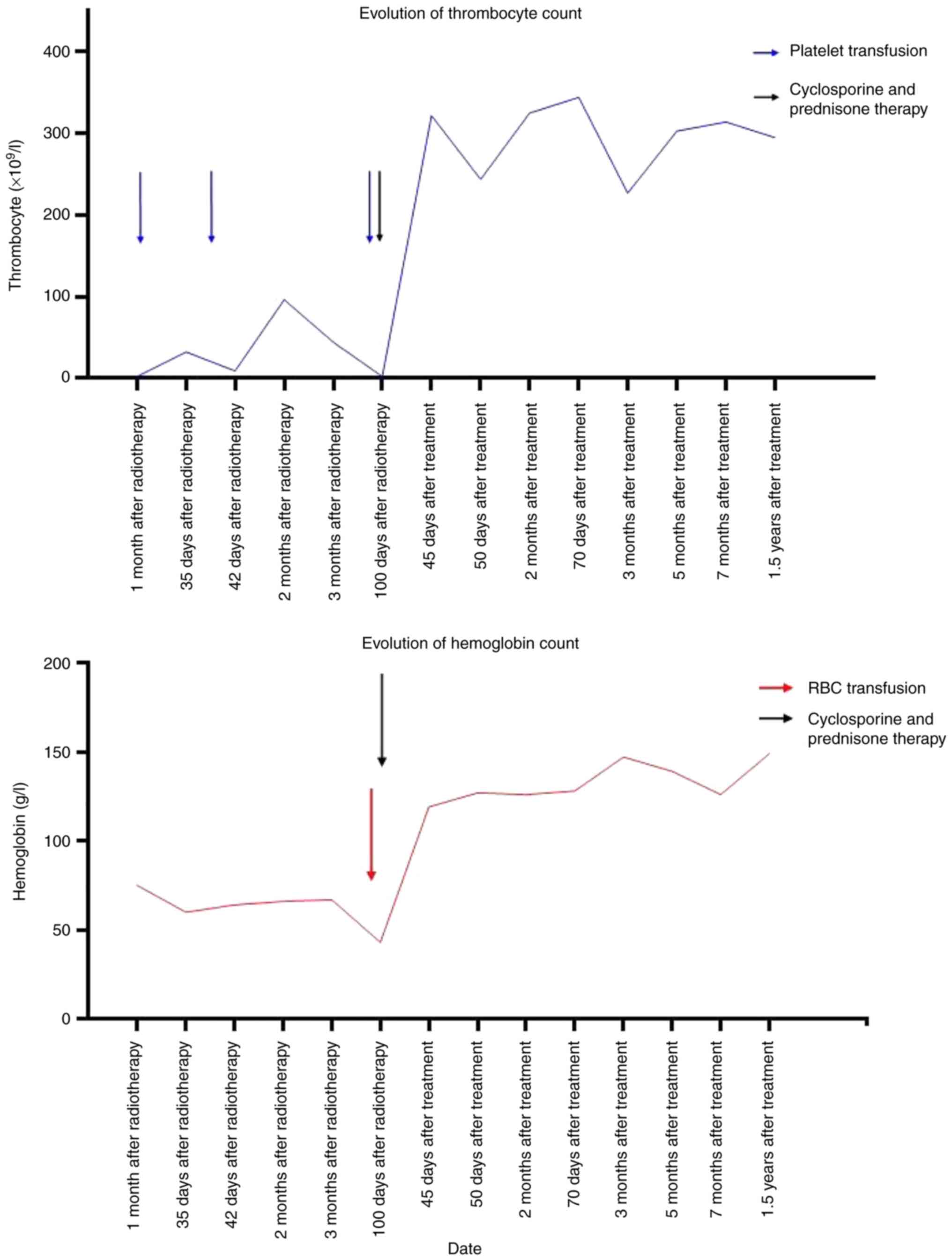
to cyclosporine in combination with prednisone therapy. After more
than one month, the CBC results indicated that the patient's
indicators had returned to the normal range and there was no
recurrence (Fig. 3). Due to low
hemoglobin and platelets, the patient was transfused with 6 units
of platelet and 2 units of red blood cell suspension during the
whole treatment (Fig. 3).
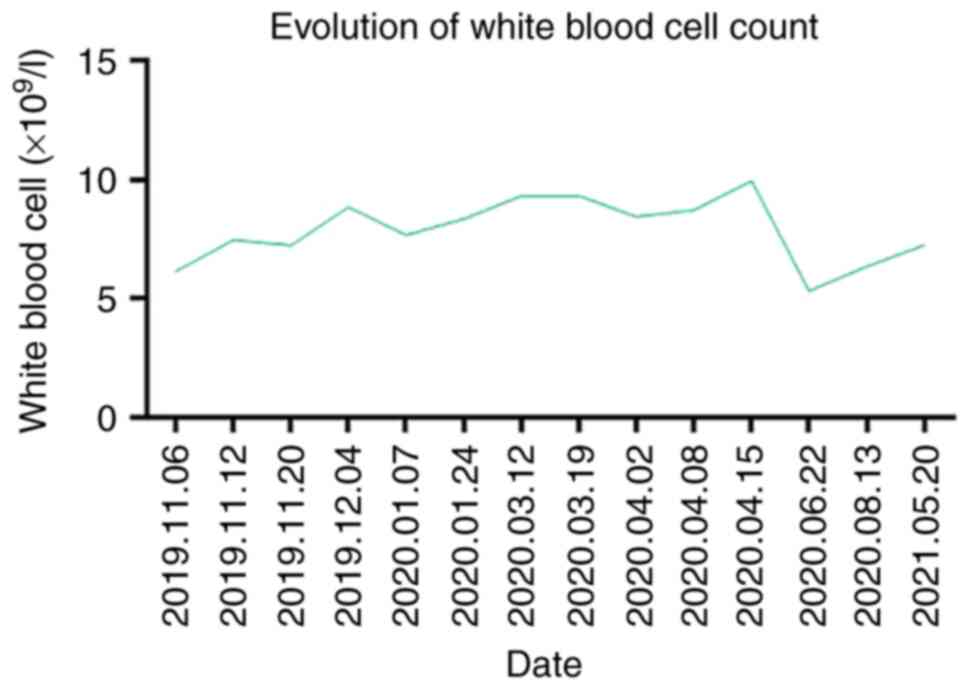
Throughout the course of treatment, the patient's white blood cell
count was normal (Fig. 4).
One month later, she underwent a complete resection
of mediastinal tumor on April 14, 2020 (Fig. 5). On the first postoperative day,
the patient's CBC results were as follows: hemoglobin 141 g/l, WBC
10.99x109 cells/l and platelets 205x109
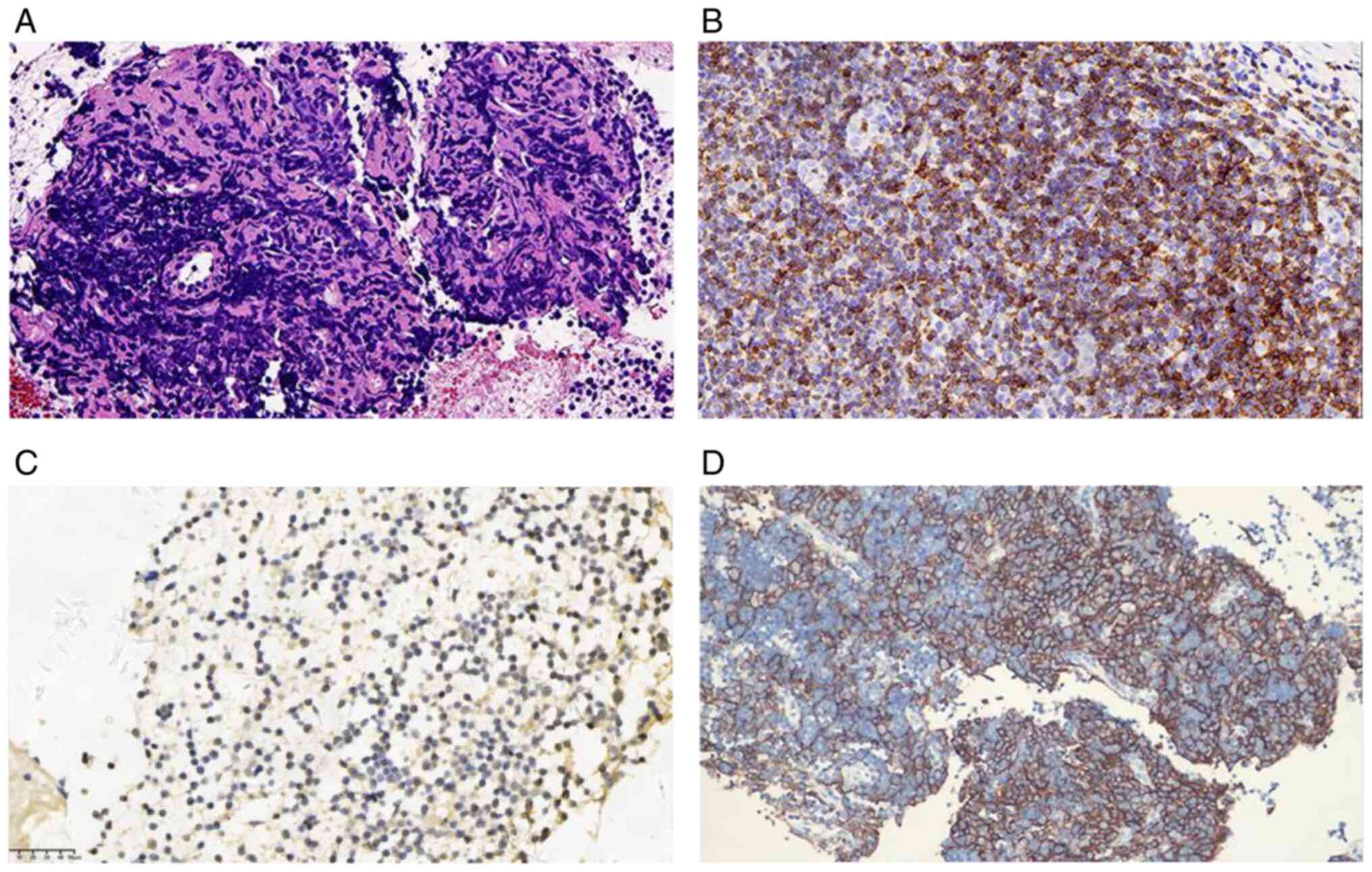
cells/l. The tissue pathology indicated type AB thymoma, with a
predominance of B2 type, about 70-80% (Fig. 6A). Immunohistochemical analysis
showed the epithelial cells to be positive for PCK, CK19, P63, EMA,
but negative for CD117, CgA, Syn, while background lymphocytes were
positive for CD5 and TdT, and the MIB-1 positivity rate was
approximately 80% (Fig. 6B and
C). Next-generation sequencing
(NGS) was performed. The NGS was performed by a company (Shanghai
Siludi Medical Laboratory) separate to the study, and the results
obtained by the company are reported in the present study.
Expression of PD-L1 was high with TPS=40% and CPS=40 (Fig. 6D), and immune status was in favor
of immunotherapy. Notably, NGS indicated that MSH3 variants in DNA
damage repair pathway-related genes was mutated with p.A57P in
abundance of 9.21%. Other than that, the patient's PRCA and AAMT
did not recur after the surgery. The CBC was performed on
2022-01-19 with the following results: hemoglobin 113 g/l, WBC
9.10x109 cells/l and platelets 268x109
cells/l. By telephone follow-up on October 20, 2022, we were
informed that the patient had no tumor recurrence, no recurrence of
PRCA and AAMT, and the CBC results in the normal range.
Discussion
Thymoma has a strong correlation with paraneoplastic
immune disorders, and PRCA is the most common hematologic
paraneoplastic manifestation of thymoma (12). Thymoma is rarely associated with
AAMT, with only 8 cases reported in the literature (4-11).
From the available reported literature, thymoma in combination with
AAMT was always accompanied by PRCA, and in three of these cases
the patients eventually developed aplastic anemia (AA) despite
immunotherapy (6,9,10).
Therefore, we support the theory that PRCA and AAMT may be early
manifestations of aplastic anemia (10), and early intervention is needed for
patients with combined PRCA and AAMT to avoid further progression
to AA.
AAMT and PRCA secondary to thymoma frequently occur
before or after chemotherapy and thymectomy (9). However, in our case, the simultaneous
appearance of AAMT and PRCA after a favorable response to
radiotherapy for thymoma is the first to be reported. Single
nucleotide polymorphisms (SNPs) in genes associated with biological
responses to radiation damage may affect the radiosensitivity of
clinically normal tissues (13).
MSH3, a member of DNA mismatch repair-related genes, while its
genetic variant was found to be associated with the development of
radiosensitivity in breast cancer patient (14), increasing the risk of acute skin
toxicity after radiotherapy in breast cancer patients (14,15).
In addition, MSH3 variant genotype has been reported to alter the
toxicity of cisplatin-based chemotherapy and response to
radiotherapy in patients with squamous cell carcinoma of the head
and neck (16). Thus mismatch
repair mechanisms may be involved in the cellular response to
radiation therapy, and genetic polymorphisms may be valid
candidates for predicting radiosensitivity (14). In this case, NGS detection revealed
MSH3 gene variants, so we speculate that the pathogenesis may be
related to the enhanced radiosensitivity caused by MSH3 variants,
which may involve increased apoptosis of tumor cells and normal
cells through an endogenous pathway, leading to a prolonged
response and causing severe side effects (17).
Patients with AAMT in combination with PRCA may
present with anemia, such as weakness, fatigue, pallor or dyspnea,
or with bleeding due to thrombocytopenia, such as mucocutaneous or
skin bleeding (9). The CBC test
showed markedly reduced hemoglobin and platelets, as well as
markedly reduced reticulocytes; bone marrow biopsy shows severe
depletion of megakaryocytes and red lineage precursor cells, while
myeloid cells are normal (4-11,18).
In this case, the patient was presented with weakness, bleeding
gums and bleeding spots on the skin, while her CBC tests and bone
marrow aspiration were consistent with previous reports.
Unfortunately, we did not obtain hematological images because the
patients' bone marrow aspiration was not performed at our hospital,
which is a limitation of the present study.
The treatment options for patients with thymoma
combined with AAMT and PRCA remain unclear, but most patients
achieve remission on cyclosporine-based therapy, combined or not
with corticosteroid (4,7,8).
After failure of first-line cyclosporine combined with
corticosteroids, the addition of antithymocyte globulin may be
effective (4). Allogenic stem cell
transplantation may be considered if the patient fails to respond
to these treatments or eventually progresses to Aplastic anemia
(6). Successful immunotherapy with
azathioprine or rituximab in patients with AAMT has also been
reported (19,20). Corticosteroids alone may be
ineffective, and one patient with unresolved thrombocytopenia after
prednisolone treatment eventually died of intracranial hemorrhage
(11). Iterative transfusions and
platelet transfusions seems may not be effective (5,6,9,10).
However, this patient achieved partial remission with symptomatic
supportive therapy, AAMT and PRCA recurred after cessation of
therapy and complete remission after treatment with cyclosporine
combined with prednisone.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Youth Project of
Sichuan Natural Science Foundation (reference no.
2023NSFSC1892).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL designed the study, edited and approved the
manuscript. YX drafted the manuscript. YX and QW were involved in
the process of diagnosis, treatment, follow-up of the patient, and
revised the article. QW, FX and DP collected and analyzed the data.
All authors were involved in writing the manuscript. YX, QW and YL
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
subject for the publication of any potentially identifiable images
or data included in this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Riedel RF and Burfeind WR Jr: Thymoma:
Benign appearance, malignant potential. Oncologist. 11:887–894.
2006.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bernard C, Frih H, Pasquet F, Kerever S,
Jamilloux Y, Tronc F, Guibert B, Isaac S, Devouassoux M,
Chalabreysse L, et al: Thymoma associated with autoimmune diseases:
85 cases and literature review. Autoimmun Rev. 15:82–92.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rosenow EC III and Hurley BT: Disorders of
the thymus. A review. Arch Intern Med. 144:763–770. 1984.PubMed/NCBI
|
|
4
|
Gay CM, William WN Jr, Wang SA and Oo TH:
Thymoma complicated by acquired amegakaryocytic thrombocytopenia
and pure red cell aplasia. J Natl Compr Canc Netw. 12:1505–1509.
2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dahal S, Sharma E, Dahal S, Shrestha B and
Bhattarai B: Acquired amegakaryocytic thrombocytopenia and pure red
cell aplasia in thymoma. Case Rep Hematol.
2018(5034741)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Simkins A, Maiti A, Short NJ, Jain N,
Popat U, Patel KP and Oo TH: Acquired amegakaryocytic
thrombocytopenia and red cell aplasia in a patient with thymoma
progressing to aplastic anemia successfully treated with allogenic
stem cell transplantation. Hematol Oncol Stem Cell Ther.
12:115–118. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Onuki T, Kiyoki Y, Ueda S, Yamaoka M,
Shimizu S and Inagaki M: Invasive thymoma with pure red cell
aplasia and amegakaryocytic thrombocytopenia. Hematol Rep.
8(6680)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fujiwara A, Inoue M, Kusumoto H, Shintani
Y, Maeda T and Okumura M: Myasthenic crisis caused by preoperative
chemotherapy with steroid for advanced thymoma. Ann Thorac Surg.
99:e11–e13. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Dragani M, Andreani G, Familiari U, Marci
V and Rege-Cambrin G: Pure red cell aplasia and amegakaryocytic
thrombocytopenia in thymoma: The uncharted territory. Clin Case
Rep. 8:598–601. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Maslovsky I, Gefel D, Uriev L, Ben Dor D
and Lugassy G: Malignant thymoma complicated by amegakaryocytic
thrombocytopenic purpura. Eur J Intern Med. 16:523–524.
2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cho AR, Cha YJ, Kim HR, Park EK and Cha
EJ: Acquired amegakaryocytic thrombocytopenia after thymectomy in a
case of pure red cell aplasia associated with thymoma. Korean J Lab
Med. 30:244–248. 2010.PubMed/NCBI View Article : Google Scholar : (In Korean).
|
|
12
|
Thompson CA and Steensma DP: Pure red cell
aplasia associated with thymoma: Clinical insights from a 50-year
single-institution experience. Br J Haematol. 135:405–407.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Andreassen CN, Alsner J, Overgaard M and
Overgaard J: Prediction of normal tissue radiosensitivity from
polymorphisms in candidate genes. Radiother Oncol. 69:127–135.
2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mangoni M, Bisanzi S, Carozzi F, Sani C,
Biti G, Livi L, Barletta E, Costantini AS and Gorini G: Association
between genetic polymorphisms in the XRCC1, XRCC3, XPD, GSTM1,
GSTT1, MSH2, MLH1, MSH3, and MGMT genes and radiosensitivity in
breast cancer patients. Int J Radiat Oncol Biol Phys. 81:52–58.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Terrazzino S, La Mattina P, Masini L,
Caltavuturo T, Gambaro G, Canonico PL, Genazzani AA and Krengli M:
Common variants of eNOS and XRCC1 genes may predict acute skin
toxicity in breast cancer patients receiving radiotherapy after
breast conserving surgery. Radiother Oncol. 103:199–205.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nogueira GAS, Costa EFD, Lopes-Aguiar L,
Lima TRP, Visacri MB, Pincinato EC, Lourenço GJ, Calonga L, Mariano
FV, Altemani AMAM, et al: Polymorphisms in DNA mismatch repair
pathway genes predict toxicity and response to cisplatin
chemoradiation in head and neck squamous cell carcinoma patients.
Oncotarget. 9:29538–29547. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lopes-Aguiar L, Visacri MB, Nourani CML,
Costa EFD, Nogueira GAS, Lima TRP, Pincinato EC, Moriel P, Altemani
JMC and Lima CSP: Do genetic polymorphisms modulate response rate
and toxicity of Cisplatin associated with radiotherapy in laryngeal
squamous cell carcinoma?: A case report. Medicine (Baltimore).
94(e578)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Means RT Jr: Pure red cell aplasia. Blood.
128:2504–2509. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Deeren D and Dorpe JV: Effective use of
rituximab for acquired amegakaryocytic thrombocytopenia. Am J
Hematol. 85:977–978. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chang H and Tang TC: Successful treatment
of amegakaryocytic thrombocytopenia with azathioprine. Acta
Haematol. 126:135–137. 2011.PubMed/NCBI View Article : Google Scholar
|