Introduction
Lung cancer remains one of the most common and
serious types of cancer worldwide, and it has been estimated that
at present there are 8.2 million mortalities annually (1). Lung cancer is divided into the
classifications of small cell lung cancer (SCLC) and non-SCLC
(NSCLC) (2). According to the
pathophysiology and histological morphology, NSCLC, which comprises
~85% of lung cancer cases, is primarily split into lung
adenocarcinoma and lung squamous cell carcinoma (LSCC) (3). Although significant advances have
been made in the integration of targeted therapies in LSCC
treatment, the overall 5-year survival rate remains low and the
recurrence rate is <20% due to distal metastasis after operation
(4). Therefore, it is of great
importance to identify new molecular mechanisms underlying the
process of LSCC and to discover molecular targets and novel drugs
for improving survival.
MicroRNAs (miRNAs) are a class of short, highly
conserved non-coding RNAs consisting of ~22 nucleotides that
regulate gene expression by targeting the 3'-untranslated region
(3'-UTR) of mRNAs (5,6). Accumulating evidence has shown that
miRNAs play important roles in various biological processes of
tumorigenesis, such as cell proliferation, apoptosis, migration and
invasion (7,8). Functionally, several miRNAs have been
described as tumor suppressors or oncogenes in LSCC (9,10).
For example, Liu et al (11) showed that miR-155-5p, as an
oncogene, negatively regulates fibroblast growth factor 9
expression to promote squamous cell carcinoma (SCC) occurrence and
development in the lungs. Hu et al (12) demonstrated that overexpressed
miR-497-5p markedly inhibits cancer progression by targeting cell
division cycle associated protein 4. Shan et al (13) revealed that miR-448 targeting can
regulate cell proliferation and inhibit apoptosis by targeting
doublecortin-like kinase 1 in LSCC cells. These data suggest that
miRNAs may be a new direction in LSCC diagnosis and treatment.
Therefore, more extensive investigations on the identification of
tumor-suppressive or oncogenic miRNAs are the first step in the
construction of a new treatment strategy for the disease.
miR-494, a miRNA located on chromosome 14q32.31,
participates in various stages of tumorigenesis (14). Extensive studies have shown that
miR-494-3p is an oncogene that has a central role in numerous solid
tumors. For example, Lin et al (15) demonstrated that miR-494 promotes
human hepatocellular carcinoma progression by targeting the
PTEN/PI3K/AKT pathway. Furthermore, Li et al (16) reported that miR-494-3p can promote
glioma cell invasion and proliferation and inhibit its apoptosis
through the suppression of PTEN expression. Another study performed
by Zhang et al (17)
indicated that miR-494 promotes cancer progression and targets
adenomatous polyposis coli in colorectal cancer. However, the roles
and potential mechanisms of miR-494 in NSCLC are still largely
unknown.
In the present study, an miRNA dataset from the GEO
database was analyzed to investigate the expression of miRNAs in
LSCC tissues, and miR-494 was selected for further analysis. The
effects of miR-494 on cell proliferation and apoptosis were then
explored. The precise molecular mechanism of miR-494 in LSCC cells
was further investigated, as well as the correlation between
miR-494 and PUMA-α. The study aimed to determine whether miR-494
may be a potential target for LSCC treatment and may be important
in the development of LSCC.
Materials and methods
Collection of clinical samples
A total of 22 freshly frozen LSCC specimens and 22
adjacent non-tumor tissues (used as the normal control) (at least 5
cm away from the carcinoma) were obtained from patients who
underwent pneumonectomy at the Department of Oncology, The First
Affiliated Hospital of Xinxiang Medical College (Weihui, China)
from January 2019 to July 2020. The inclusion criteria in present
study were as follows: i) LSCC; ii) no radiotherapy or chemotherapy
prior to surgery; iii) a patient age of ≥18 years; iv) weight loss
of ≤10% in the 3 months before diagnosis; and v) Karnofsky
performance status ≥70% (18). The
exclusion criteria were as follows: i) Histology other than SCC;
ii) metastatic lung cancer; iii) patients with serious medical or
psychiatric illness, or a history of serious cardiac disease; iv)
prior radiation therapy to the thorax or total surgical resection;
v) prior systemic chemotherapy; and vi) an age of >80 years. The
clinicopathological information of the patients is shown in
Table I. The present study was
approved by the Ethics Committee of The First Affiliated Hospital
of Xinxiang Medical College (Weihui, China), and written informed
consent was obtained from each patient.
 | Table ICharacteristics of the lung squamous
cell carcinoma cases. |
Table I
Characteristics of the lung squamous
cell carcinoma cases.
| Characteristic | Subcategory | n (%) |
|---|
| Age, years | <60 | 14 (63.6) |
| | ≥60 | 8 (36.4) |
| Sex | Male | 17 (77.3) |
| | Female | 5 (22.7) |
| Smoking
history | <30
pack-years | 4 (18.2) |
| | ≥30 pack-years | 11 (50.0) |
| | Never smoked | 7 (31.8) |
| Stage | I | 9 (40.9) |
| | II-IV | 13 (59.1) |
| Lymphatic
invasion | Absent | 3 (13.6) |
| | Present | 19 (86.4) |
|
Differentiation | Well | 4 (18.2) |
| | Moderately | 16 (72.7) |
| | Poorly | 2 (9.1) |
| Recurrence | Present | 10 (45.5) |
| | Absent | 12 (54.5) |
miRNA microarray
The miRNA dataset (GSE74190) was searched and
downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). The GSE74190
dataset was based on the Agilent-019118 Human miRNA Microarray V1
G4470A platform (19). GEO2R
(www.ncbi.nlm.nih.gov/geo/geo2r/), an interactive web
tool, was applied to compare the samples in two different groups
under the same experimental condition. Fold change ≥2 and P<0.05
served as basic screening parameters. Hierarchical clustering of
differentially expressed miRNA was performed using the Multiple
Experiment Viewer 4.7.1 software program (The Institute for Genomic
Research, USA).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA of the cultured cells and tissues was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) as described previously (20). The TaqMan Reverse Transcription kit
(Takara Biotechnology Co., Ltd.) was used to obtain cDNA for mRNA
detection (42˚C for 1 h), while the TaqMan MicroRNA Reverse
Transcription kit (Takara Biotechnology Co., Ltd.) was used for
miRNA detection (42˚C for 1 h). qPCR was performed using SYBR
Premix Ex Taq (Takara Bio, Inc.) on an ABI PRISM 7500 Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Primers for cDNA amplification were as follows: miR-494
forward, 5'-TGACCTGAAACATACACGGGA-3', and universal reverse primer,
5'-TATCGTTGTACTCCACTCCTTGAC-3'; U6 forward,
5'-GCTTCGGCAGCACATATACTAAAAT-3', and reverse,
5'-CGCTTCACGAATTTGCGTGTCAT-3'; PUMA-α forward,
5'-CGGCGGAGACAAGAGGAGC-3', and reverse,
5'-CAGGGTGTCAGGAGGTGGGAG-3'; and GAPDH forward,
5'-TCAACGACCCCTTCATTGACC-3', and reverse,
5'-CTTCCCGTTGATGACAAGCTTC-3'. The reaction mixtures were denatured
at 95˚C for 3 min, followed by 40 cycles of 95˚C for 10 sec and
60˚C for 30 sec. The relative expression levels of miRNA and mRNA
were normalized to that of U6 and GAPDH, respectively. The relative
expression of each gene was calculated and normalized using the
2-ΔΔCq method (21).
All reactions were conducted in triplicate.
Cell culture
A total of four LSCC cell lines (NCI-H520, SW900,
EBC-1 and SK-MES-1) were purchased from the American Type Culture
Collection (ATCC) and cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(Sigma-Aldrich; Merck KGaA), 100 U/ml penicillin and 100 µg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C
in a 5% CO2 atmosphere. A human bronchial epithelial
cell line (16HBE; ATCC) was used as the control. 16HBE cells were
cultured in medium provided by Procell Life Science &
Technology (cat. no. CM-0249) at 37˚C with 5% CO2.
Cell transfection
miR-494 mimics (5'-UGAAACAUACACGGGAAACCUC-3'),
negative control (NC) mimics (5'-CACGAUAAACAAUACGGGUACC-3'),
inhibitor (5'-GAGGUUUCCCGUGUAUGUUUCA-3') and inhibitor NC
(5'-UGUGUCGUUAACUAUGGGCUCU-3') were obtained from Shanghai
GenePharma Co., Ltd. SW900 and EBC-1 cells were transfected with 20
nM miR-494 mimic/inhibitor and mimic NC/inhibitor NC using
Lipofectamine™ RNAiMAX (Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol at 37˚C in a 5% CO2
incubator. Following 48 h of transfection, cells were collected for
cell proliferation, RT-qPCR and western blot analysis. PUMA-α
overexpression plasmid (pcDNA3.1-PUMA-α) and pcDNA vector, siRNAs
against PUMA-α (si-PUMA-α) and the corresponding scrambled NC
(si-NC) were designed and synthesized by Guangzhou RiboBio Co.,
Ltd. The sequences of si-PUMA-α and si-NC are as follows: si-PUMA-α
sense, 5'-GGGUCCUGUACAAUCUCAUCAUGGG-3' and antisense,
5'-CCCAUGAUGAGAUUGUACAGGACCC-3'; and si-NC sense,
5'-GGGUGUCAACACUCUACUAUCUGGG-3' and antisense,
5'-CCCAGAUAGUAGAGUGUUGACACCC-3'. A total of 50 nM si-PUMA-α and
si-NC were co-transfected with 50 nM miR-494 inhibitor or inhibitor
NC into SW900 and EBC-1 cells at 37˚C using Lipofectamine™ RNAiMAX
according to the manufacturer's instructions. SW900 and EBC-1 cells
were cultured at 37˚C for 24 h prior to transfection. After 48 h of
transfection, cells were harvested and used for analysis. All the
transfections were repeated >3 times independently.
Cell viability
Cell viability was measured using the Cell Counting
Kit (CCK)-8 (Dojindo Laboratories, Inc.) assay. Briefly, SW900 and
EBC-1 cells were seeded into a 96-well plate at a density of 3,000
cells/well and cultured at 37˚C for 24 h prior to transfection.
Then, cells were transfected with corresponding oligonucleotides.
After 24, 36 or 48 h, 10 µl CCK-8 solution was added into each well
of the plate. The plates were incubated at 37˚C for 1 h, and the
absorbance at 450 nm was measured. The experiment was repeated
three times.
Apoptosis detection by flow
cytometry
SW900 and EBC-1 cells were harvested 48 h after cell
transfection, and then washed twice with phosphate-buffered saline.
Next, the cells were stained with Annexin V (FITC) and propidium
iodide reagent (Thermo Fisher Scientific, Inc.) at 37˚C for 10 min
in the dark at room temperature according to the manufacturer's
instructions. Cell apoptosis rates were detected via FACSAria flow
cytometry (BD Biosciences) and analyzed using FlowJo 7.6 software
(FlowJo, LLC).
Western blotting
For western blotting, SW900 and EBC-1 cells were
lysed using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Protein concentrations were determined with the
bicinchoninic acid assay (Beyotime Institute of Biotehnology). Cell
lysate (40 µg total protein per lane) were separated on 15% gels
using SDS-PAGE and transferred to polyvinylidene difluoride
membranes (Roche Applied Science). The membranes were first blocked
with 5% skimmed milk at room temperature for 30 min, after which
the membranes were incubated with primary antibodies overnight at
4˚C. Membranes were incubated with primary antibodies against
PUMA-α (1:1,000; cat. no. sc-377015; Santa Cruz Biotechnology,
Inc.), cleaved caspase-3 (1:1,000; cat. no. ab2302; Abcam) and
Bcl-2 (1:1,000; cat. no. ab32124; Abcam) overnight at 4˚C, followed
by incubation with HRP-conjugated anti-rabbit secondary antibody
(1:10,000; cat. no. ab205718; Abcam) for 1 h at room temperature.
β-actin antibody (1:1,000; cat. no. ab8227; Abcam) was used as an
internal control. The protein bands were developed using an ECL kit
(GE Healthcare) and quantified using ImageJ Software (version 1.46;
National Institutes of Health).
Luciferase reporter assays
The potential binding site between PUMA-α and
miR-494 was searched in TargetScan (http://www.targetscan.org) and PicTar (https://pictar.mdc-berlin.de/). A whole fragment of
3'UTR PUMA-α mRNA and a mutant form were cloned into pGL-3-Luc
(Promega Coporation). The 293T cells were seeded in 24-well plates
at 5x104 cells per well and co-transfected with the
pGL-3-PUMA-α wild-type or mutant portion and TK100 Renilla
combined with the aforementioned miR-494 mimic, miR-494 inhibitor,
mimic NC or inhibitor NC using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). Cells were harvested 48 h after
transfection, and luciferase activity was measured using a
Dual-Luciferase® Reporter System (Promega Corporation)
according to the manufacturer's protocol. Relative firefly
luciferase activity was normalized to Renilla luciferase
activity. All the dual-luciferase reporter assays were performed in
triplicate within each experiment.
Statistical analysis
Data are reported as the mean ± SD (unless otherwise
presented). Statistical significance among different groups was
determined using one-way ANOVA followed by Tukey's post-hoc test
and the significance of the difference in means between two groups
was evaluated using unpaired Student's t-test. The paired Student's
t-test was used to compare data from cancerous vs. non-cancerous
tissues. The correlation between PUMA-α and miR-494 expression was
assessed using a two-tailed Pearson's correlation analysis.
Statistical significance was analyzed using GraphPad Prism software
(version 5.0; GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
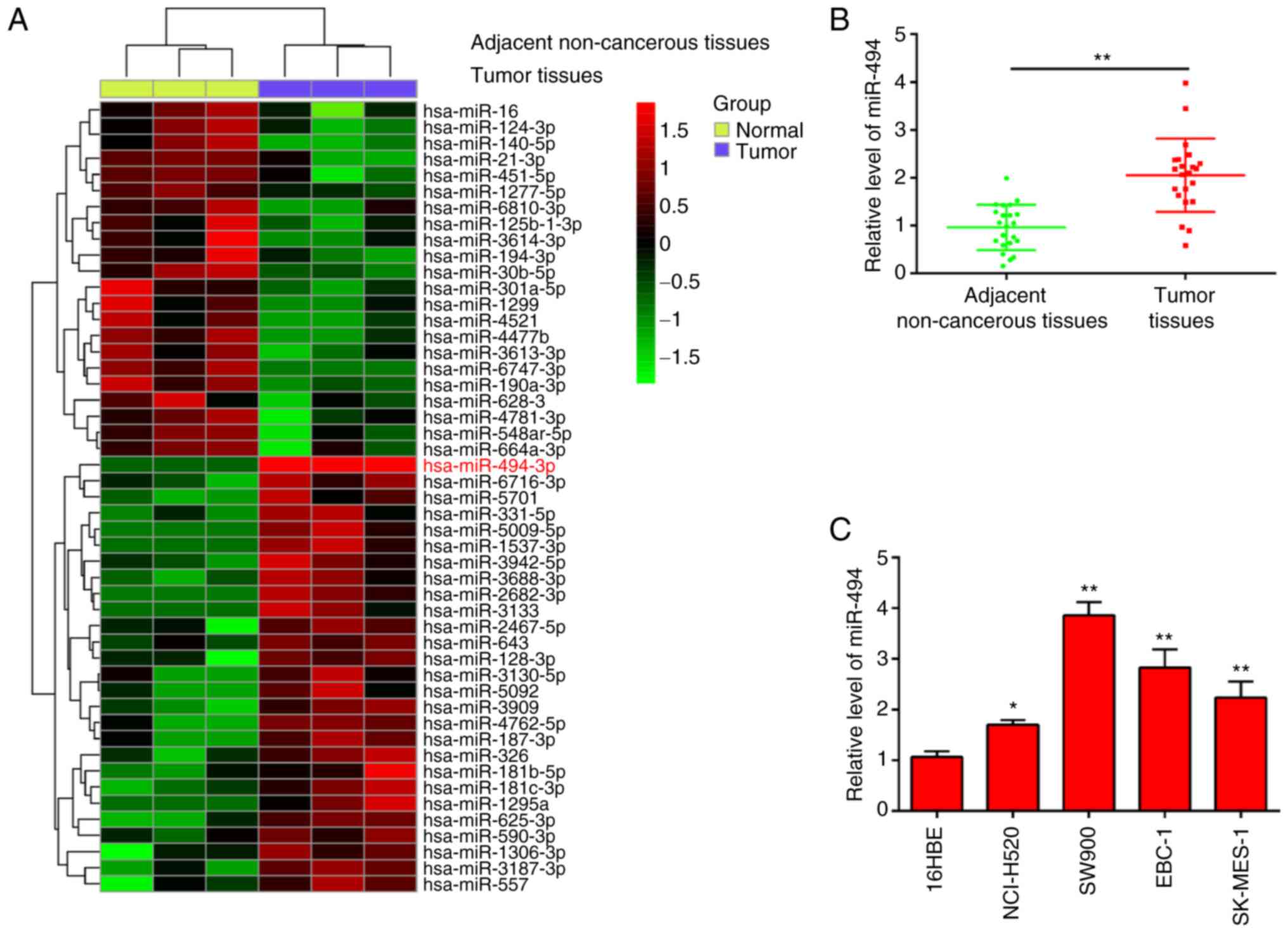
miR-494 is upregulated in LSCC tissues
and cell lines
To explore the role of miRNAs in LSCC, miRNA
expression patterns were initially profiled using GSE74190
microarray data. Cluster analysis based on the miRNA expression
pattern indicated a significant difference between LSCC tissues and
adjacent non-cancerous tissues (Fig.
1A). Among the aberrantly expressed miRNAs, miR-494 was chosen
for further study, which has been reported as an oncogenic miRNA in
several types of human cancer (16,22-24).
Moreover, several studies have revealed that miR-494 acts as an
oncomir and is involved in tumor development, progression and
metastasis, and confers resistance to chemotherapeutic drugs by
targeting a number of molecules in several types of human cancer,
such as endometrial cancer and hepatocellular carcinoma (25,26).
However, the function and underlying molecular mechanism of miR-494
in LSCC has not been fully elucidated. To validate the expression
trend of miR-494 in the LSCC tissues, RT-qPCR was performed to
detect miR-494 expression in 22 pairs of LSCC tissues and adjacent
non-cancerous tissues. As presented in Fig. 1B, the expression level of miR-494
in LSCC tissues was significantly higher compared with that in
adjacent non-cancerous tissues. In addition, miR-494 levels were
assessed in four LSCC cell lines (NCI-H520, SW900, EBC-1 and
SK-MES-1) and in 16HBE, which acted as a normal control. miR-494
expression in all LSCC cells was upregulated compared with 16HBE,
especially in SW900 and EBC-1 cells (Fig. 1C). These findings suggest that
upregulation of miR-494 could be involved in the progression of
LSCC.
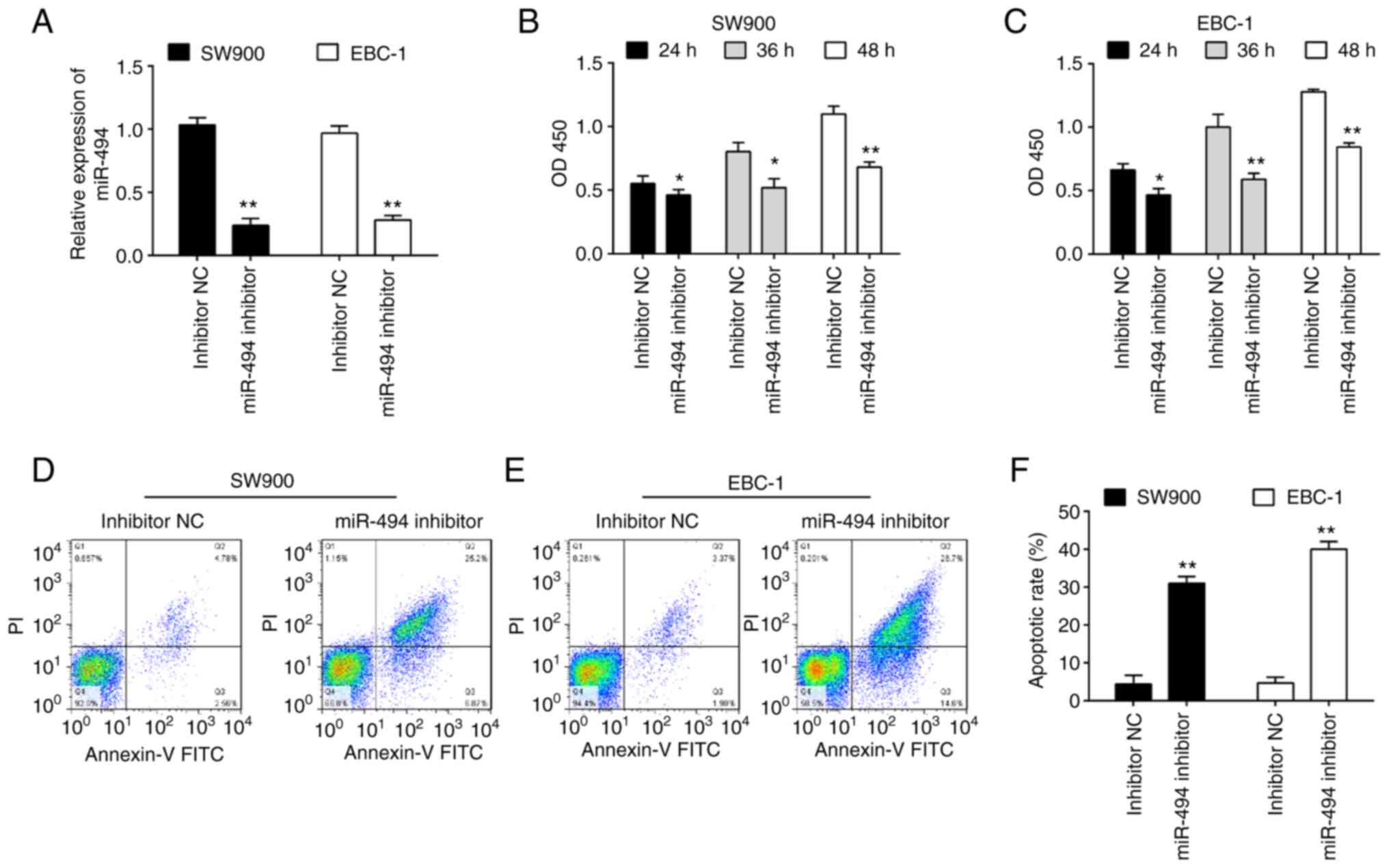
miR-494 knockdown suppresses cell
viability and promotes apoptosis
Given the upregulation of miR-494 in LSCC tissues,
it was predicted that miR-494 may function as an oncogene. To
verify this hypothesis, miR-494 inhibitor was transfected into two
LSCC cell lines, namely SW900 and EBC-1. The miR-494 level was
significantly downregulated in both SW900 and EBC-1 cells after
miR-494 inhibitor transfection (Fig.
2A). To evaluate whether miR-494 could affect cell
proliferation, a CCK-8 assay was performed, and it was revealed
that knockdown of miR-494 significantly suppressed the viability of
SW900 and EBC-1 cells compared with inhibitor NC transfected cells
(Fig. 2B and C). The effects of miR-494 knockdown on
cell apoptosis were further examined. As shown in Fig. 2D-F, knockdown of miR-494 promoted
apoptosis in both SW900 and EBC-1 cells compared with the inhibitor
NC. Taken together, these data suggest that miR-494 may function as
an oncogene in LSCC.
PUMA-α is a direct target of
miR-494
To uncover the potential molecular mechanisms
involved in the suppressive role of miR-494 inhibition in LSCC
cells, the potential downstream targets of miR-494 were searched
for using publicly available databases, including TargetScan and
PicTar. According to bioinformatics analysis, PUMA-α was selected
for further study due to its promotive role in apoptosis (27). The binding sites between miR-494
and PUMA-α are illustrated in Fig.
3A. To examine whether PUMA-α is a direct target of miR-494, a
luciferase activity assay was conducted. As shown in Fig. 3B, miR-494 overexpression reduced
the luciferase reporter activity of the PUMA-α 3'UTR, while
inhibition of miR-494 had the opposite effect. Additionally, PUMA-α
3'UTR luciferase reporter activity was unaffected by point
mutations in the miR-494-binding seed region. Moreover, it was
observed that miR-494 overexpression inhibited the mRNA and protein
expression levels of PUMA-α, whereas miR-494 knockdown enhanced the
expression levels of PUMA-α (Fig.
3C and D). These results
suggested that miR-494 suppressed the expression of PUMA-α at the
transcriptional level.
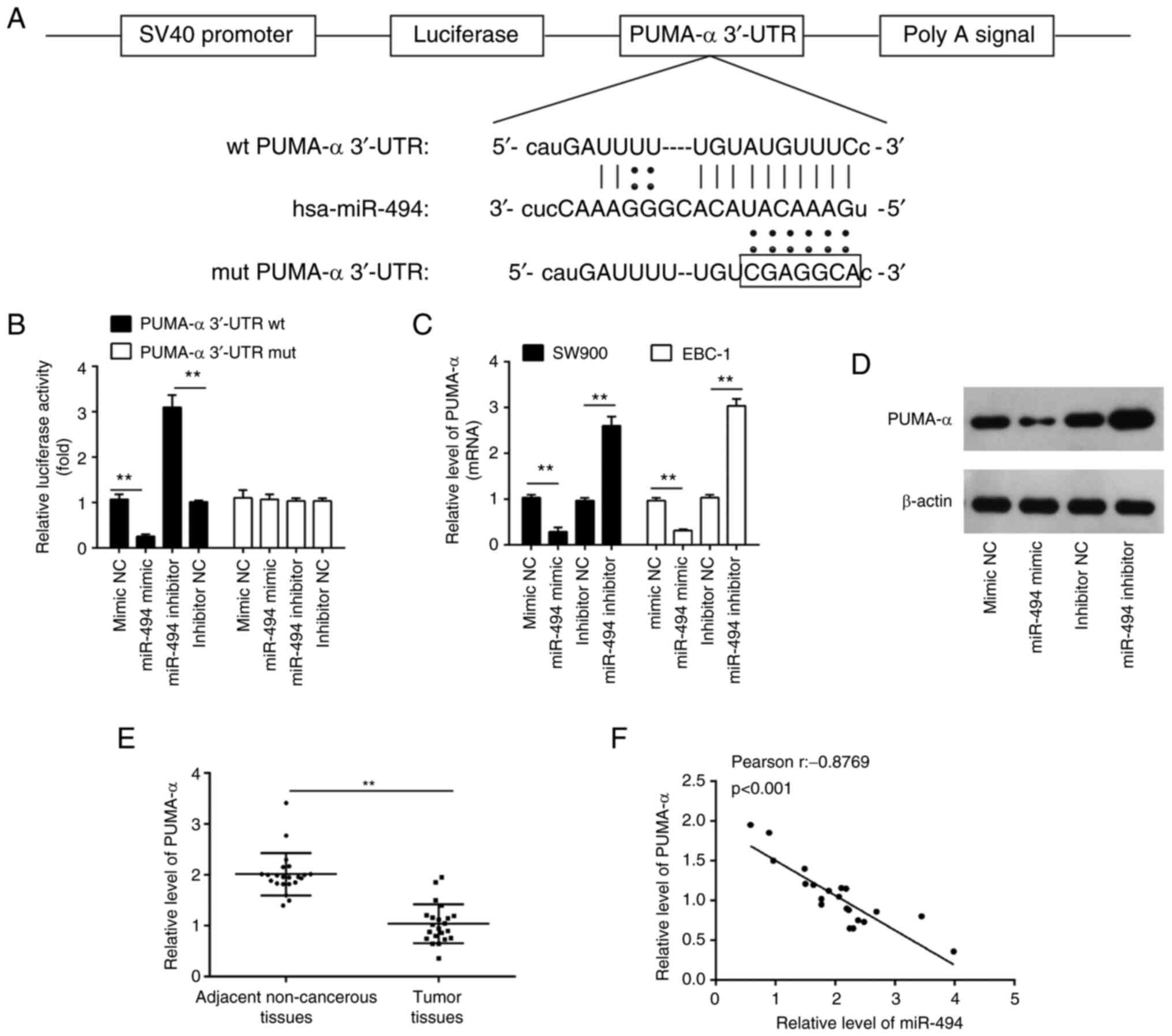 | Figure 3miR-494 directly targets PUMA-α. (A)
miR-494 binds to the predicted site of the 3'-UTR of PUMA-α. (B)
Luciferase activities; 293T cells were co-transfected with firefly
luciferase constructs containing PUMA-α wt or mut 3'-UTRs and
miR-494 mimic, mimic NC, miR-494 inhibitor or inhibitor NC. PUMA-α
(C) mRNA and (D) protein levels in the indicated cells transfected
with 20 nM miR-494 mimic, mimic NC, miR-494 inhibitor or inhibitor
NC were measured using RT-qPCR and western blotting, respectively.
(E) Expression levels of PUMA-α in 22 pairs of LSCC tissues and
adjacent non-cancerous tissues were determined using RT-qPCR. (F)
Analysis of the correlation between levels of PUMA-α and miR-494
expression in LSCC tissues (r=-0.8769; P<0.001). Data are shown
as the mean ± SD of three separate experiments.
**P<0.01. PUMA-α, p53 upregulated modulator of
apoptosis-α; UTR, untranslated region; NC, negative control;
RT-qPCR, reverse transcription-quantitative PCR; LSCC, lung
squamous cell carcinoma; wt, wild-type; mut, mutated; miR,
microRNA. |
In addition, RT-qPCR was used to determine the
PUMA-α expression levels in 22 pairs of LSCC tissues and adjacent
non-tumor tissues. It was demonstrated that the PUMA-α expression
levels were significantly decreased in LSCC tissues compared with
the adjacent non-tumor tissues (Fig.
3E). Correlation analysis revealed a strong negative
correlation between the expression levels of miR-494 and PUMA-α
mRNA levels in LSCC tissues (Fig.
3F). These data indicated that PUMA-α is a functional target of
miR-494.
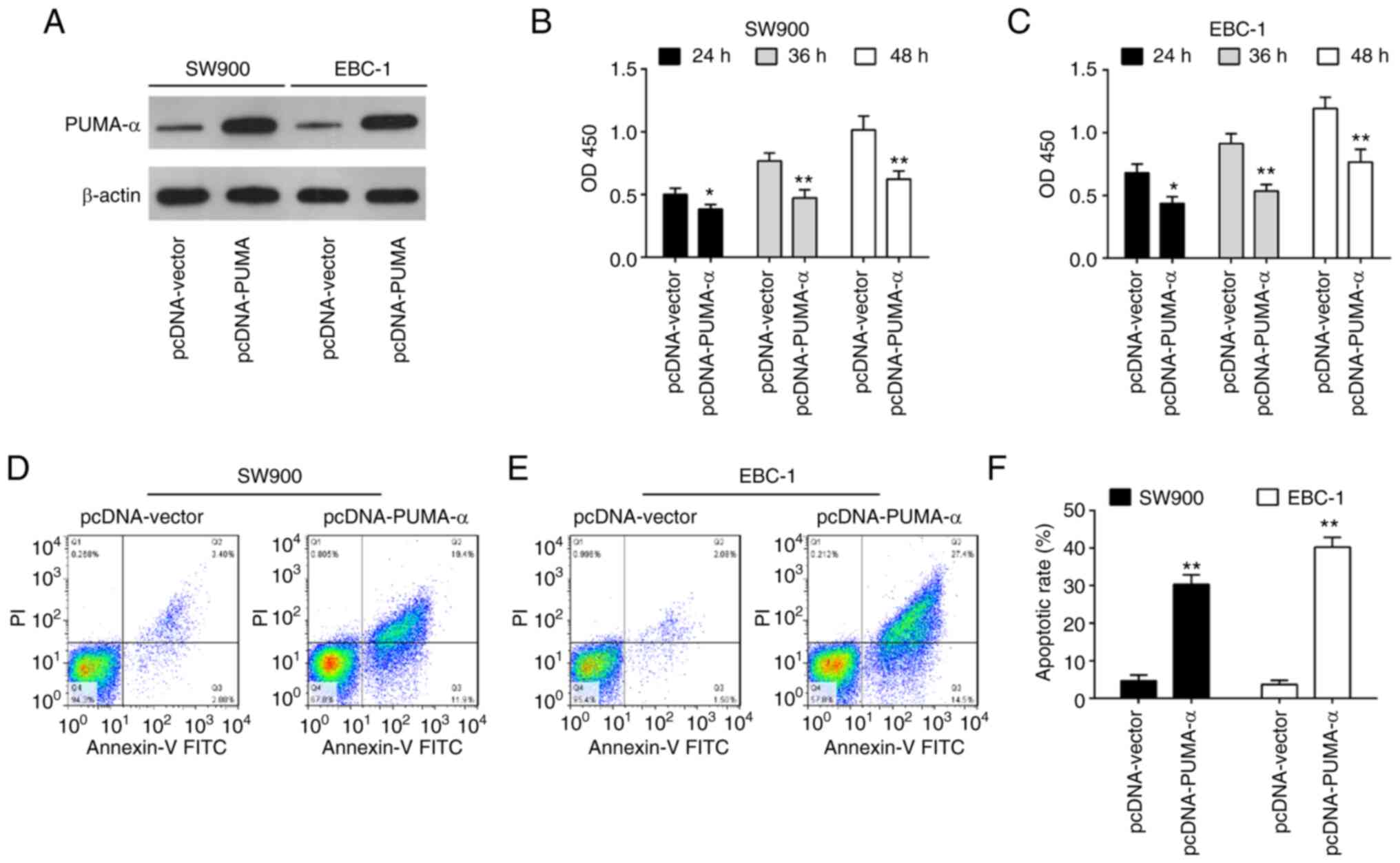
Overexpression of PUMA-α inhibits cell
viability and promotes apoptosis
To investigate the function of PUMA-α in SW900 and
EBC-1 cells, SW900 and EBC-1 cells were transiently transfected
with pcDNA-PUMA-α for 24 h. As shown in Fig. 4A, PUMA-α expression levels were
increased in SW900 and EBC-1 cells after pcDNA-PUMA-α transfection
compared with the pcDNA-vector group. CCK-8 assay demonstrated that
overexpression of PUMA-α significantly inhibited the proliferation
of SW900 and EBC-1 cells compared with the pcDNA-vector group
(Fig. 4B and C). Additionally, a significant increase
in the percentage of apoptotic cells was also observed in the
pcDNA-PUMA-α-transfected cells compared with the pcDNA-vector
transfected cells (Fig. 4D-F).
These findings suggest that overexpression of PUMA-α has a similar
role to miR-494 inhibition in SW900 and EBC-1 cells.
Knockdown of PUMA-α attenuates the
inhibitory effects of miR-494 inhibition on the SW900 and EBC-1
cells
Since PUMA-α was identified as the target gene of
miR-494 in LSCC cells, whether PUMA-α is involved in
miR-494-mediated roles in LSCC cells was determined. As shown in
Fig. 5A, PUMA-α knockdown could
rescue the suppression effect of miR-494 inhibitor on SW900 and
EBC-1 cell viability. Furthermore, the data showed that miR-494
inhibition significantly increased the expression levels of
pro-apoptotic protein PUMA-α and cleaved caspase-3, and also
decreased the expression levels of anti-apoptotic protein Bcl-2 in
the SW900 and EBC-1 cells compared with the Blank group. However,
the effects of the miR-494 inhibitor were partially attenuated by
the knockdown of PUMA-α (Fig.
5B-E). Subsequently, it was confirmed that the promotion of
apoptosis mediated by miR-494 inhibition was also attenuated by the
knockdown of PUMA-α (Fig. 5F).
These results indicated that the knockdown of miR-494 induced
apoptosis in LSCC cells by targeting PUMA-α.
Discussion
The present study revealed that miR-494 was
upregulated in LSCC tissues and cell lines, and that knockdown of
miR-494 suppressed cell proliferation and induced apoptosis. PUMA-α
was verified as the target of miR-494 in LSCC cells. It was shown
that knockdown of PUMA-α reversed the inhibitory effects of the
miR-494 inhibitor on LSCC cells. These results provide new insights
into LSCC research and therapeutic strategies.
Mounting evidence has indicated that miRNAs play an
important role in the initiation and progression of human cancer
(28,29). Thus, identification of
tumor-associated miRNAs and their target genes is critical for
understanding the roles of miRNAs in tumorigenesis (29). Up to now, several miRNAs have been
identified in LSCC and demonstrated to regulate cell migration,
invasion, proliferation and apoptosis (30-32).
For example, miR-218 is frequently downregulated in LSCC clinical
specimens and appears to function in anti-migration and
anti-invasion roles through targeting tumor protein D52(33). In the present study, using GSE74190
microarray data, it was found that large numbers of miRNAs were
significantly upregulated; in particular, miR-494 was identified as
the most upregulated miRNA in human LSCC tissues, which is
consistent with a previous study that showed that miR-494
expression is increased in LSCC tissues (22), thus indicating that increased
miR-494 may be involved in LSCC carcinogenesis.
In previous studies, miR-494 has been implicated in
several types of human cancer, such as endometrial cancer and
prostate cancer (25,34). It has been reported that miR-494
inhibition can suppress cell proliferation, migration and invasion,
as well as induce apoptosis by targeting downstream genes,
including p190B and mutated in colorectal cancer (35,36).
A previous report demonstrated that miR-494 promotes cell
proliferation in hepatocellular carcinoma by targeting PTEN
(22). However, its biological
roles in LSCC remain largely unknown. In the present study, it was
found that miR-494 knockdown inhibited LSCC cell proliferation and
induced apoptosis, suggesting that miR-494 functioned as an
oncogene in LSCC. However, the underlying molecular mechanisms
involved in miR-494 inhibition-mediated proliferation and apoptosis
suppression have not been completely clarified.
The Bcl-2 family of proteins, which includes the
protein known as PUMA-α, regulates the mitochondrial apoptotic
pathway to maintain the integrity of the outer membrane of the
mitochondria (37). According to
reports, PUMA-α is important for the apoptosis of certain cancer
cells (27,38). For example, Yang et al
(39) showed that, in colon cancer
cells, PUMA-α mediates the pro-apoptotic impact of idelalisib
through the mitochondrial route. In colorectal and lung cancer
cells, overexpression of PUMA-α has been linked to miR-203-induced
cell death, according to a previous study (40). However, whether PUMA-α participates
in the antitumor roles of miR-494 inhibition in LSCC remains
unknown. In the present study, PUMA-α was shown to be a target of
miR-494. Moreover, it was demonstrated that overexpression of
PUMA-α has similar effects to miR-494 inhibition on SW900 and EBC-1
cells, whereas knockdown of PUMA-α reversed the inhibitory effects
of miR-494 inhibition on LSCC cells. Collectively, these results
suggest that miR-494 inhibition upregulates the expression of
PUMA-α, resulting in the promotion of the apoptosis of LSCC
cells.
In conclusion, the present study confirmed that
miR-494 knockdown suppressed LSCC cell proliferation and promoted
apoptosis by targeting PUMA-α. These findings provide a theoretical
basis for the prevention and treatment of LSCC, and implicate
miR-494 as a potential prognostic biomarker and therapeutic target
of LSCC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Overseas
Research and Training Project of Henan Province Health Science and
Technology Talents (grant no. HWYX2019088).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG, XYa and FH performed all the experiments and
collected the data. XYu conceived and designed the study. XYa, XL
and DL wrote the main manuscript and analyzed the data. XG and XYu
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Xinxiang Medical
College (Weihui, China; approval no. XXMC2019-0112) and written
informed consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Erratum: Global cancer statistics 2018:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 70(313)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mengoli MC, Longo FR, Fraggetta F, Cavazza
A, Dubini A, Alì G, Guddo F, Gilioli E, Bogina G, Nannini N, et al:
The 2015 world health organization classification of lung tumors:
New entities since the 2004 Classification. Pathologica. 110:39–67.
2018.PubMed/NCBI
|
|
3
|
Ruiz-Cordero R and Devine WP: Targeted
therapy and checkpoint immunotherapy in lung cancer. Surg Pathol
Clin. 13:17–33. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gao M, Kong W, Huang Z and Xie Z:
Identification of key genes related to lung squamous cell carcinoma
using bioinformatics analysis. Int J Mol Sci.
21(2994)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhong X, Coukos G and Zhang L: miRNAs in
human cancer. Methods Mol Biol. 822:295–306. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jafri MA, Al-Qahtani MH and Shay JW: Role
of miRNAs in human cancer metastasis: Implications for therapeutic
intervention. Semin Cancer Biol. 44:117–131. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang K, Xu K, Leng X, Han Y and Fang Q:
miRNA-9 inhibits proliferation and migration of lung squamous cell
carcinoma cells by regulating NRSF/EGFR. Technol Cancer Res Treat.
19(1533033820945807)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang J, Rao S, Cao R, Xiao S, Cui X and Ye
L: miR-30a-5p suppresses lung squamous cell carcinoma via ATG5 -
mediated autophagy. Aging (Albany NY). 13:17462–17472.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu F, Mao Q, Zhu S and Qiu J:
MicroRNA-155-5p promotes cell proliferation and invasion in lung
squamous cell carcinoma through negative regulation of fibroblast
growth factor 9 expression. J Thorac Dis. 13:3669–3679.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hu J, Xiang X, Guan W, Lou W, He J, Chen
J, Fu Y and Lou G: MiR-497-5p down-regulates CDCA4 to restrains
lung squamous cell carcinoma progression. J Cardiothorac Surg.
16(330)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shan C, Fei F, Li F, Zhuang B, Zheng Y,
Wan Y and Chen J: miR-448 is a novel prognostic factor of lung
squamous cell carcinoma and regulates cells growth and metastasis
by targeting DCLK1. Biomed Pharmacother. 89:1227–1234.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shen PF, Chen XQ, Liao YC, Chen N, Zhou Q,
Wei Q, Li X, Wang J and Zeng H: MicroRNA-494-3p targets CXCR4 to
suppress the proliferation, invasion, and migration of prostate
cancer. Prostate. 74:756–767. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lin H, Huang ZP, Liu J, Qiu Y, Tao YP,
Wang MC, Yao H, Hou KZ, Gu FM and Xu XF: MiR-494-3p promotes
PI3K/AKT pathway hyperactivation and human hepatocellular carcinoma
progression by targeting PTEN. Sci Rep. 8(10461)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li XT, Wang HZ, Wu ZW, Yang TQ, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Huang YL, et al: miR-494-3p
regulates cellular proliferation, invasion, migration, and
apoptosis by PTEN/AKT signaling in human glioblastoma cells. Cell
Mol Neurobiol. 35:679–687. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhang Y, Guo L, Li Y, Feng GH, Teng F, Li
W and Zhou Q: MicroRNA-494 promotes cancer progression and targets
adenomatous polyposis coli in colorectal cancer. Mol Cancer.
17(1)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Buccheri G, Ferrigno D and Tamburini M:
Karnofsky and ECOG performance status scoring in lung cancer: A
prospective, longitudinal study of 536 patients from a single
institution. Eur J Cancer. 32A:1135–1141. 1996.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang J, Wang N, Li ZJ, Yang LJ, Jing YG,
Cheng JM and Li J: Identification of key genes and construction of
microRNA-mRNA regulatory networks in non-small cell lung cancer.
Cancer Genet. 228:47–54. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hayashita Y, Osada H, Tatematsu Y, Yamada
H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y and
Takahashi T: A polycistronic microRNA cluster, miR-17-92, is
overexpressed in human lung cancers and enhances cell
proliferation. Cancer Res. 65:9628–9632. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu K, Liu S, Zhang W, Jia B, Tan L, Jin Z
and Liu Y: miR-494 promotes cell proliferation, migration and
invasion, and increased sorafenib resistance in hepatocellular
carcinoma by targeting PTEN. Oncol Rep. 34:1003–1010.
2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liu L, Jiang Y, Zhang H, Greenlee AR and
Han Z: Overexpressed miR-494 down-regulates PTEN gene expression in
cells transformed by
anti-benzo(a)pyrene-trans-7,8-dihydrodiol-9,10-epoxide. Life Sci.
86:192–198. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang J, Chen H, Liao Y, Chen N, Liu T and
Zhang H and Zhang H: Expression and clinical evidence of miR-494
and PTEN in non-small cell lung cancer. Tumour Biol. 36:6965–6972.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhu L, Wang X, Wang T, Zhu W and Zhou X:
miR4943p promotes the progression of endometrial cancer by
regulating the PTEN/PI3K/AKT pathway. Mol Med Rep. 19:581–588.
2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang J, Zhu Y, Hu L, Yan F and Chen J:
miR-494 induces EndMT and promotes the development of HCC
(hepatocellular carcinoma) by targeting SIRT3/TGF-β/SMAD signaling
pathway. Sci Rep. 9(7213)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yu J, Wang Z, Kinzler KW, Vogelstein B and
Zhang L: PUMA mediates the apoptotic response to p53 in colorectal
cancer cells. Proc Natl Acad Sci USA. 100:1931–1936.
2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ambros V: MicroRNA pathways in flies and
worms: Growth, death, fat, stress, and timing. Cell. 113:673–676.
2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Raponi M, Dossey L, Jatkoe T, Wu X, Chen
G, Fan H and Beer DG: MicroRNA classifiers for predicting prognosis
of squamous cell lung cancer. Cancer Res. 69:5776–5783.
2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Moriya Y, Nohata N, Kinoshita T, Mutallip
M, Okamoto T, Yoshida S, Suzuki M, Yoshino I and Seki N: Tumor
suppressive microRNA-133a regulates novel molecular networks in
lung squamous cell carcinoma. J Hum Genet. 57:38–45.
2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kumamoto T, Seki N, Mataki H, Mizuno K,
Kamikawaji K, Samukawa T, Koshizuka K, Goto Y and Inoue H:
Regulation of TPD52 by antitumor microRNA-218 suppresses cancer
cell migration and invasion in lung squamous cell carcinoma. Int J
Oncol. 49:1870–1880. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cai B and Peng JH: Increased Expression of
miR-494 in serum of patients with prostate cancer and its potential
diagnostic value. Clin Lab. 65:2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kwak SY, Yang JS, Kim BY, Bae IH and Han
YH: Ionizing radiation-inducible miR-494 promotes glioma cell
invasion through EGFR stabilization by targeting p190B rhoGAP.
Biochim Biophys Acta. 1843:508–516. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lim L, Balakrishnan A, Huskey N, Jones KD,
Jodari M, Ng R, Song G, Riordan J, Anderton B, Cheung ST, et al:
MicroRNA-494 within an oncogenic microRNA megacluster regulates
G1/S transition in liver tumorigenesis through suppression of
mutated in colorectal cancer. Hepatology. 59:202–215.
2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ambroise G, Portier A, Roders N, Arnoult D
and Vazquez A: Subcellular localization of PUMA regulates its
pro-apoptotic activity in Burkitt's lymphoma B cells. Oncotarget.
6:38181–38194. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yu J and Zhang L: PUMA, a potent killer
with or without p53. Oncogene. 27 (Suppl 1):S71–S83.
2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang S, Zhu Z, Zhang X, Zhang N and Yao Z:
Idelalisib induces PUMA-dependent apoptosis in colon cancer cells.
Oncotarget. 8:6102–6113. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Funamizu N, Lacy CR, Kamada M, Yanaga K
and Manome Y: MicroRNA-203 induces apoptosis by upregulating Puma
expression in colon and lung cancer cells. Int J Oncol.
47:1981–1988. 2015.PubMed/NCBI View Article : Google Scholar
|