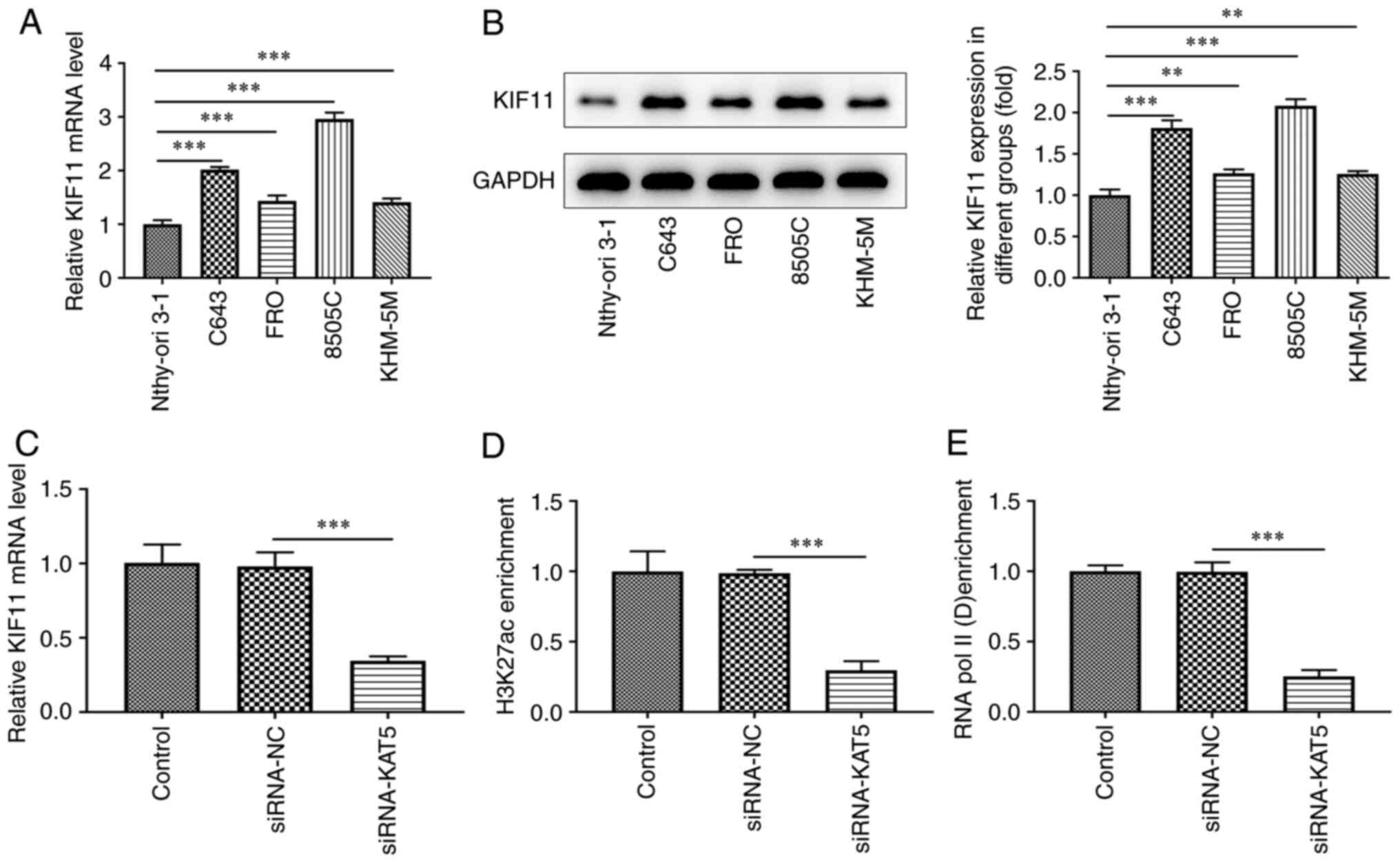
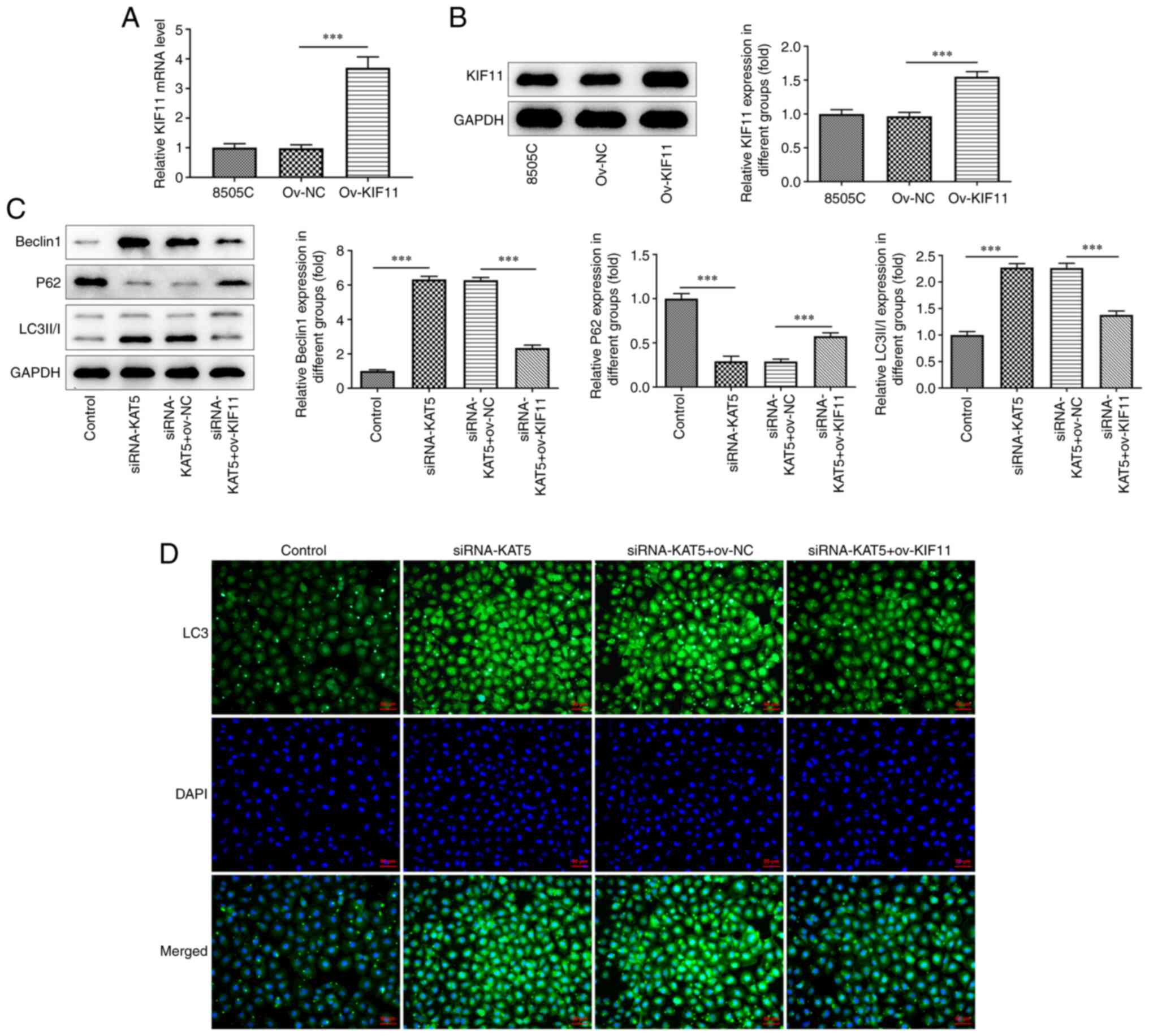
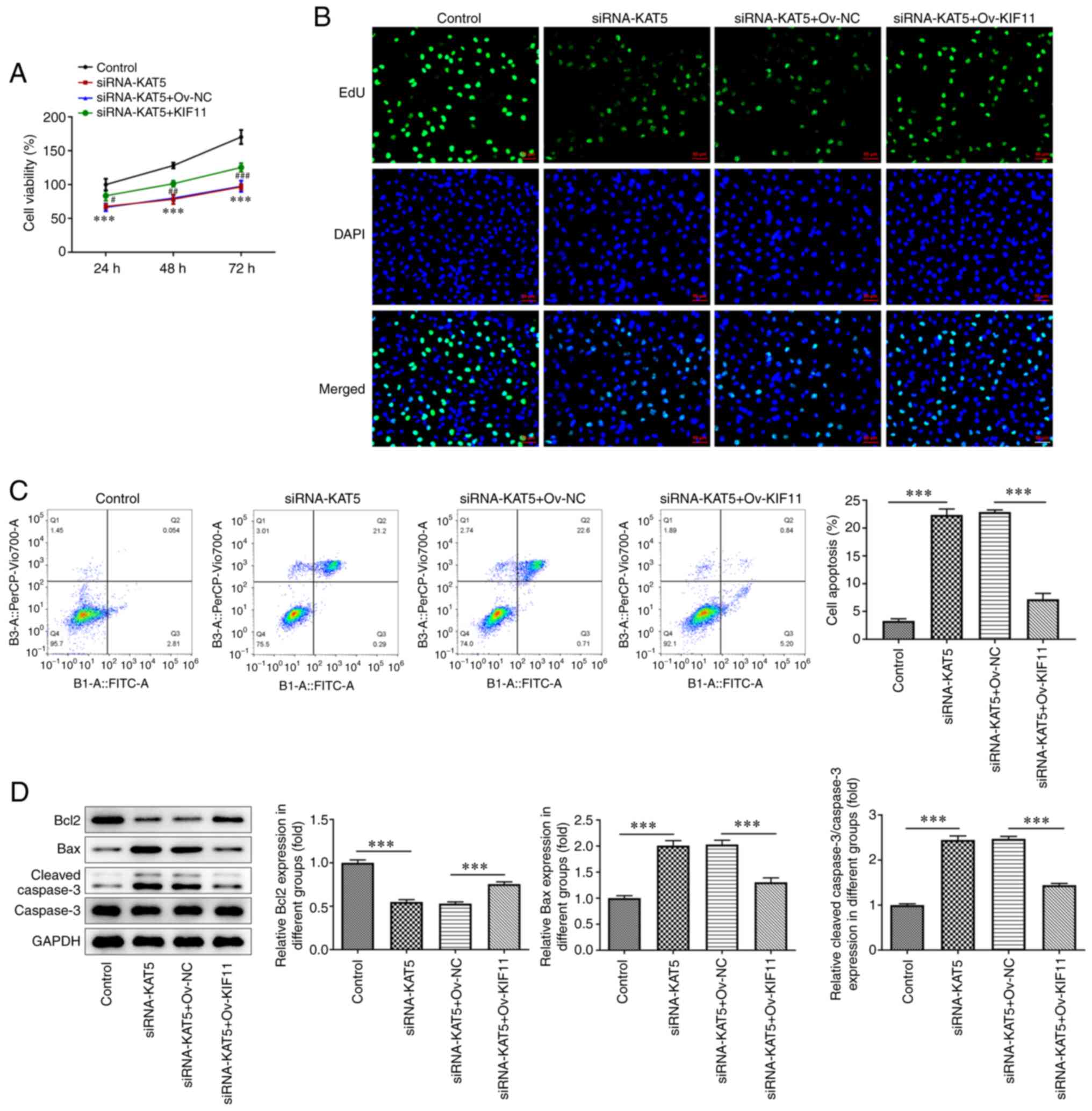
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Laha D, Nilubol N and Boufraqech M: New
therapies for advanced thyroid cancer. Front Endocrinol (Lausanne).
11(82)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chmielik E, Rusinek D, Oczko-Wojciechowska
M, Jarzab M, Krajewska J, Czarniecka A and Jarzab B: Heterogeneity
of thyroid cancer. Pathobiology. 85:117–129. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Prete A, Borges de Souza P, Censi S, Muzza
M, Nucci N and Sponziello M: Update on fundamental mechanisms of
thyroid cancer. Front Endocrinol (Lausanne). 11(102)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bible KC, Kebebew E, Brierley J, Brito JP,
Cabanillas ME, Clark TJ Jr, Di Cristofano A, Foote R, Giordano T,
Kasperbauer J, et al: 2021 American thyroid association guidelines
for management of patients with anaplastic thyroid cancer. Thyroid.
31:337–386. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Molinaro E, Romei C, Biagini A, Sabini E,
Agate L, Mazzeo S, Materazzi G, Sellari-Franceschini S, Ribechini
A, Torregrossa L, et al: Anaplastic thyroid carcinoma: From
clinicopathology to genetics and advanced therapies. Nat Rev
Endocrinol. 13:644–660. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gervasi R, Orlando G, Lerose MA, Amato B,
Docimo G, Zeppa P and Puzziello A: Thyroid surgery in geriatric
patients: A literature review. BMC Surg. 12 (Suppl
1)(S16)2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang J and Barletta JA: Anaplastic thyroid
carcinoma. Semin Diagn Pathol. 37:248–256. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fiorentino F, Mai A and Rotili D: Lysine
acetyltransferase inhibitors: Structure-activity relationships and
potential therapeutic implications. Future Med Chem. 10:1067–1091.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Numata A, Kwok HS, Zhou QL, Li J,
Tirado-Magallanes R, Angarica VE, Hannah R, Park J, Wang CQ,
Krishnan V, et al: Lysine acetyltransferase Tip60 is required for
hematopoietic stem cell maintenance. Blood. 136:1735–1747.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li P, Ge J and Li H: Lysine
acetyltransferases and lysine deacetylases as targets for
cardiovascular disease. Nat Rev Cardiol. 17:96–115. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Proietti G, Wang Y, Punzo C and Mecinović
J: Substrate scope for human histone lysine acetyltransferase KAT8.
Int J Mol Sci. 22(846)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li Y, Huang H, Zhu M, Bai H and Huang X:
Roles of the MYST family in the pathogenesis of Alzheimer's disease
via histone or non-histone acetylation. Aging Dis. 12:132–142.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ghobashi AH and Kamel MA: Tip60: Updates.
J Appl Genet. 59:161–168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu Z, Chen T, Mo H, Guo C and Liu Q: BRD8,
which is negatively regulated by miR-876-3p, promotes the
proliferation and apoptosis resistance of hepatocellular carcinoma
cells via KAT5. Arch Biochem Biophys. 693(108550)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ren X, Yu J, Guo L and Ma H: Circular RNA
circRHOT1 contributes to pathogenesis of non-small cell lung cancer
by epigenetically enhancing C-MYC expression through recruiting
KAT5. Aging (Albany NY). 13:20372–20382. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Du J, Fu L, Ji F, Wang C, Liu S and Qiu X:
FosB recruits KAT5 to potentiate the growth and metastasis of
papillary thyroid cancer in a DPP4-dependent manner. Life Sci.
259(118374)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wei X, Cai S, Boohaker RJ, Fried J, Li Y,
Hu L, Pan Y, Cheng R, Zhang S, Tian Y, et al: KAT5 promotes
invasion and metastasis through C-MYC stabilization in ATC. Endocr
Relat Cancer. 26:141–151. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Abe I and Lam AK: Anaplastic thyroid
carcinoma: Current issues in genomics and therapeutics. Curr Oncol
Rep. 23(31)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Perrier ND, Brierley JD and Tuttle RM:
Differentiated and anaplastic thyroid carcinoma: Major changes in
the American Joint Committee on Cancer eighth edition cancer
staging manual. CA Cancer J Clin. 68:55–63. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lu X, Bao L, Pan Z and Ge M: Immunotherapy
for anaplastic thyroid carcinoma: The present and future. Zhejiang
Da Xue Xue Bao Yi Xue Ban. 50:675–684. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Haroon Al Rasheed MR and Xu B: Molecular
alterations in thyroid carcinoma. Surg Pathol Clin. 12:921–930.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bozorg-Ghalati F and Hedayati M: Molecular
biomarkers of anaplastic thyroid carcinoma. Curr Mol Med.
17:181–188. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cabanillas ME, Zafereo M, Gunn GB and
Ferrarotto R: Anaplastic thyroid carcinoma: Treatment in the age of
molecular targeted therapy. J Oncol Pract. 12:511–518.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shvedunova M and Akhtar A: Modulation of
cellular processes by histone and non-histone protein acetylation.
Nat Rev Mol Cell Biol. 23:329–349. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sapountzi V and Côté J: MYST-family
histone acetyltransferases: Beyond chromatin. Cell Mol Life Sci.
68:1147–56. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Doyon Y and Côté J: The highly conserved
and multifunctional NuA4 HAT complex. Curr Opin Genet Dev.
14:147–154. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bird AW, Yu DY, Pray-Grant MG, Qiu Q,
Harmon KE, Megee PC, Grant PA, Smith MM and Christman MF:
Acetylation of histone H4 by Esa1 is required for DNA double-strand
break repair. Nature. 419:411–415. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Van Den Broeck A, Nissou D, Brambilla E,
Eymin B and Gazzeri S: Activation of a Tip60/E2F1/ERCC1 network in
human lung adenocarcinoma cells exposed to cisplatin.
Carcinogenesis. 33:320–325. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Feng FL, Yu Y, Liu C, Zhang BH, Cheng QB,
Li B, Tan WF, Luo XJ and Jiang XQ: KAT5 silencing induces apoptosis
of GBC-SD cells through p38MAPK-mediated upregulation of cleaved
Casp9. Int J Clin Exp Pathol. 7:80–91. 2013.PubMed/NCBI
|
|
32
|
Kim CH and Lee DH: KAT5 Negatively
regulates the proliferation of prostate cancer LNCaP cells via the
caspase 3-dependent apoptosis pathway. Anim Cells Syst (Seoul).
23:253–259. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Song ZM, Lin H, Yi XM, Guo W, Hu MM and
Shu HB: KAT5 acetylates cGAS to promote innate immune response to
DNA virus. Proc Natl Acad Sci USA. 117:21568–21575. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Martin BJE, Brind'Amour J, Kuzmin A,
Jensen KN, Liu ZC, Lorincz M and Howe LJ: Transcription shapes
genome-wide histone acetylation patterns. Nat Commun.
12(210)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xiao X, Zeng S, Li Y, Li L and Zhang J:
Aspirin Suppressed PD-L1 expression through suppressing KAT5 and
subsequently inhibited PD-1 and PD-L1 signaling to attenuate OC
development. J Oncol. 2022(4664651)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li H, Liu W, Zhang X, Wu F, Sun D and Wang
Z: Ketamine suppresses proliferation and induces ferroptosis and
apoptosis of breast cancer cells by targeting KAT5/GPX4 axis.
Biochem Biophys Res Commun. 585:111–116. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li L, Zhu M, Huang H, Wu J and Meng D:
Identification of hub genes in anaplastic thyroid carcinoma:
Evidence from bioinformatics analysis. Technol Cancer Res Treat.
19(1533033820962135)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wei D, Rui B, Qingquan F, Chen C, Ping HY,
Xiaoling S, Hao W and Jun G: KIF11 promotes cell proliferation via
ERBB2/PI3K/AKT signaling pathway in gallbladder cancer. Int J Biol
Sci. 17:514–526. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhou J, Jiang YY, Chen H, Wu YC and Zhang
L: Tanshinone I Attenuates the malignant biological properties of
ovarian cancer by inducing apoptosis and autophagy via the
inactivation of PI3K/AKT/mTOR pathway. Cell Prolif.
53(e12739)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Long HL, Zhang FF, Wang HL, Yang WS, Hou
HT, Yu JK and Liu B: Mulberry anthocyanins improves thyroid cancer
progression mainly by inducing apoptosis and autophagy cell death.
Kaohsiung J Med Sci. 34:255–262. 2018.PubMed/NCBI View Article : Google Scholar
|