Introduction
Suppurative pleural effusion caused by intrathoracic
infection is the main manifestation of acute empyema (1). Severe empyema can be complicated by
acute respiratory distress syndrome (ARDS) or, in severe cases, by
respiratory and circulatory failure which can lead to death.
Conventional treatments for acute empyema and ARDS include thoracic
drainage and mechanical ventilation, extracorporeal membrane
oxygenation (ECMO), and continuous renal replacement therapy (CRRT)
may be necessary to provide effective respiratory and circulatory
support and correct water and electrolyte imbalances (2). Additionally, a surgical procedure
should be considered and may be critical to remove the focus of the
infection completely, especially when conservative treatment has
not been effective despite the extreme risk. Herein, we describe
the case presentation of a 51-year-old man with acute empyema
secondary to an acute lung abscess rupture complicated by a sudden
cardiac arrest caused by rapidly progressing severe hypoxemia who
was successfully treated with ECMO combined with CRRT and
thoracoscopic minimally invasive surgery for persistent alveolar
fistula.
Case report
A 51-year-old man was admitted to emergency
intensive care unit (EICU) of the Second Affiliated Hospital of
Jiaxing University (Jiaxing, China) in July, 2020, due to a cough
accompanied by left chest pain and high fever. On the afternoon of
admission, he experienced sudden shortness of breath and a cough
with yellow purulent foul-smelling sputum accompanied by systemic
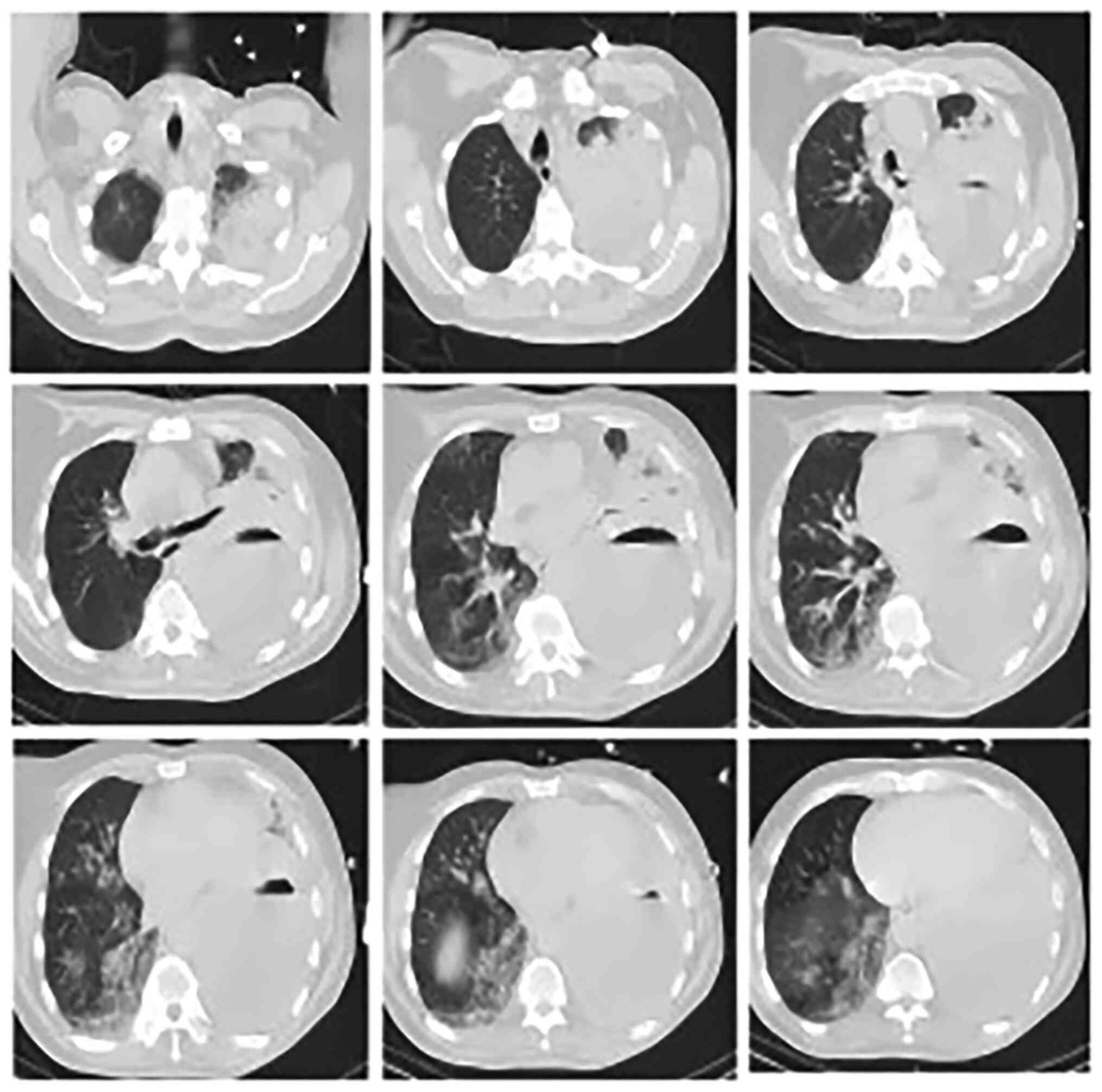
profuse sweating. Chest computed tomography (CT) was performed
immediately (Fig. 1) and showed a
left upper lung abscess and left empyema, considered indicative of
infection. A cardiac color ultrasound showed blunt and paradoxical
apex movement and mild aortic valve, mitral valve, and tricuspid
regurgitation. His blood tests showed metabolic acidosis and
hypoxemia and white blood cell (WBC) count of
65.5x109/l. The patient had a history of ankylosing
spondylitis, a kind of autoimmune disease, and had been taking
immunosuppressive drugs, including salazosulfadimidine and
adrenocortical hormone. However, his family medical history was
unremarkable.
After admission, bedside left thoracic closed
drainage was performed immediately. After placement of a 24F
thoracic drainage tube, a large amount of yellow pus and
foul-smelling pleural effusion were drained. Simultaneously,
meropenem (1.0 g Q6h) and Vancomycin (0.5 g Q12H) was administered
empirically as anti-infection measures. According to the
bacteriological results of the pleural effusion after thoracic
closed drainage, both gram-positive cocci and gram-negative bacilli
were found in the pleural fluid smear, and the bacterial culture
results indicated Streptococcus constellations. The blood culture
was negative at that time. His chest distress improved, but his
oxygenation levels remained unstable. A dynamic blood gas
reanalysis revealed continuous deterioration of hypoxia and
acidosis. He was ventilated with an endotracheal intubation
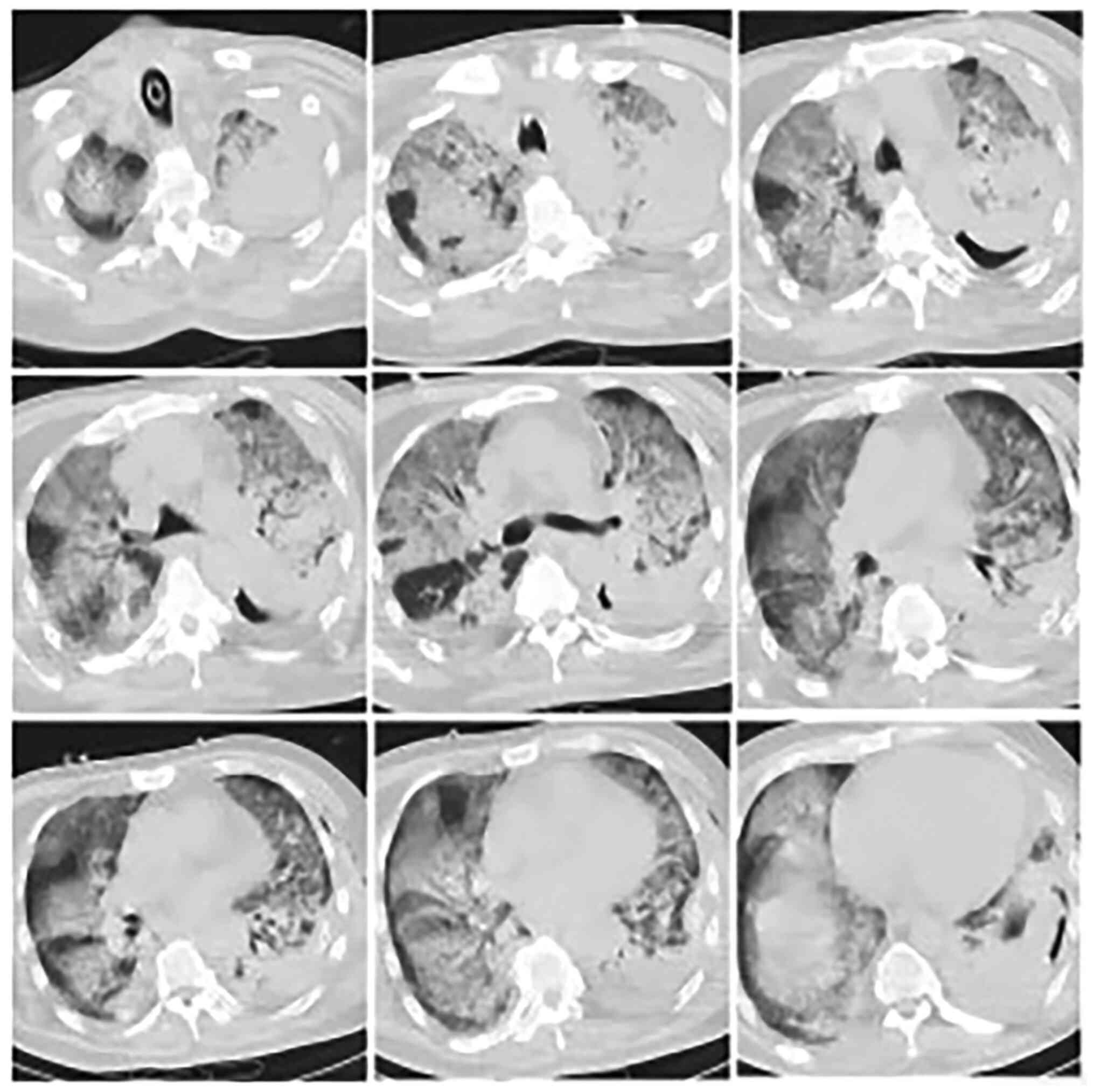
ventilator 32 h after admission, and a second chest CT (Fig. 2) was performed immediately; it
showed multiple infections in both lungs and a large consolidation
in the left lung. A mega dose of methylprednisolone (500 mg) was
administered to reduce inflammation. Compared with previous
examinations, these conditions progressed significantly and
rapidly, and he continued to present with low oxygenation under
pure oxygen ventilation with endotracheal intubation. Thirty-four
hours after admission, he experienced ventricular fibrillation and
sudden cardiac arrest three times in the following two hours.
Aggressive treatment, including cardiac compression, electrical
defibrillation, and drug treatment, was immediately initiated. ECMO
using veno-arterial catheterization (VA-ECMO) (flow rate, 2.8
l/min) was performed immediately after cardioversion. One hour
after ECMO placement, arterial blood gas analysis showed a partial
pressure of oxygen of 131 mmHg, suggesting a substantial
improvement in his hypoxia and acidosis.
Four hours after ECMO implantation, the patient had
continuous anuria and a urea nitrogen level of 8.9 mmol/l, and his
creatinine levels rose to 150.7 µmol/l. Bedside CRRT was performed
immediately. The treatment mode was set to continuous venovenous
hemofiltration (flow rate, 2,000 ml/h). The patient's condition
gradually improved after this. On the 8th day after admission, both
ECMO and CRRT were withdrawn after six days of treatment. On the
11th day, chest CT showed that the pulmonary infection had improved
significantly. Tracheal extubation was then performed. After the
patient was transferred to the thoracic surgery ward for subsequent
treatment, he continued to have air bubble overflow in the left
chest tube (1-2 degrees of air leakage) while coughing. On the 20th
day after admission, he developed a low-grade fever and leukopenia.
Routine blood tests showed a WBC count of 1.8x109/l,
neutrophil count of 0.2x109/l (11.3%), and hemoglobin
level of 67 g/l. Drug-induced myelosuppression was considered after
discussion with a multidisciplinary team (MDT). All drugs,
including antibiotics, were discontinued, and a subcutaneous
injection of granulocytes was subsequently administered. Three days
later, his body temperature was back to normal.
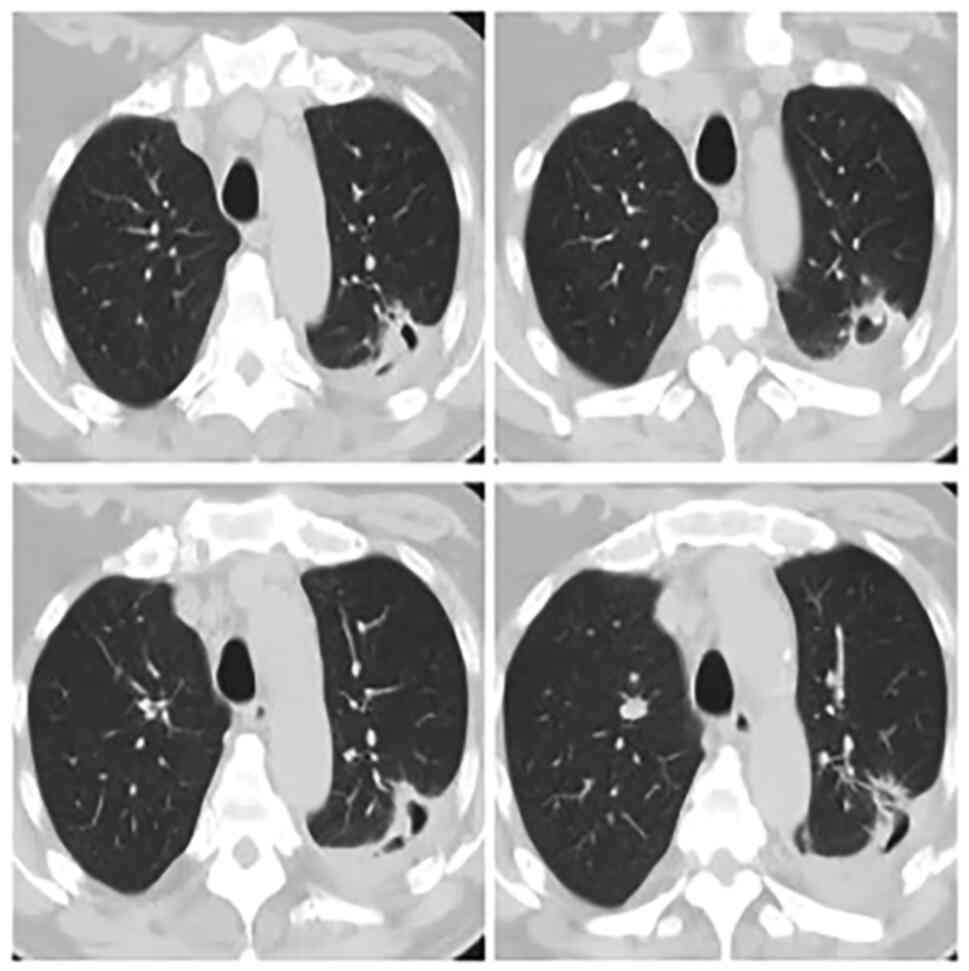
A chest CT on the 35th day after admission (Fig. 3) demonstrated that the exudation in
both lungs was absorbed, but a left upper lung abscess with
bronchopleural fistula was considered as a possible diagnosis.
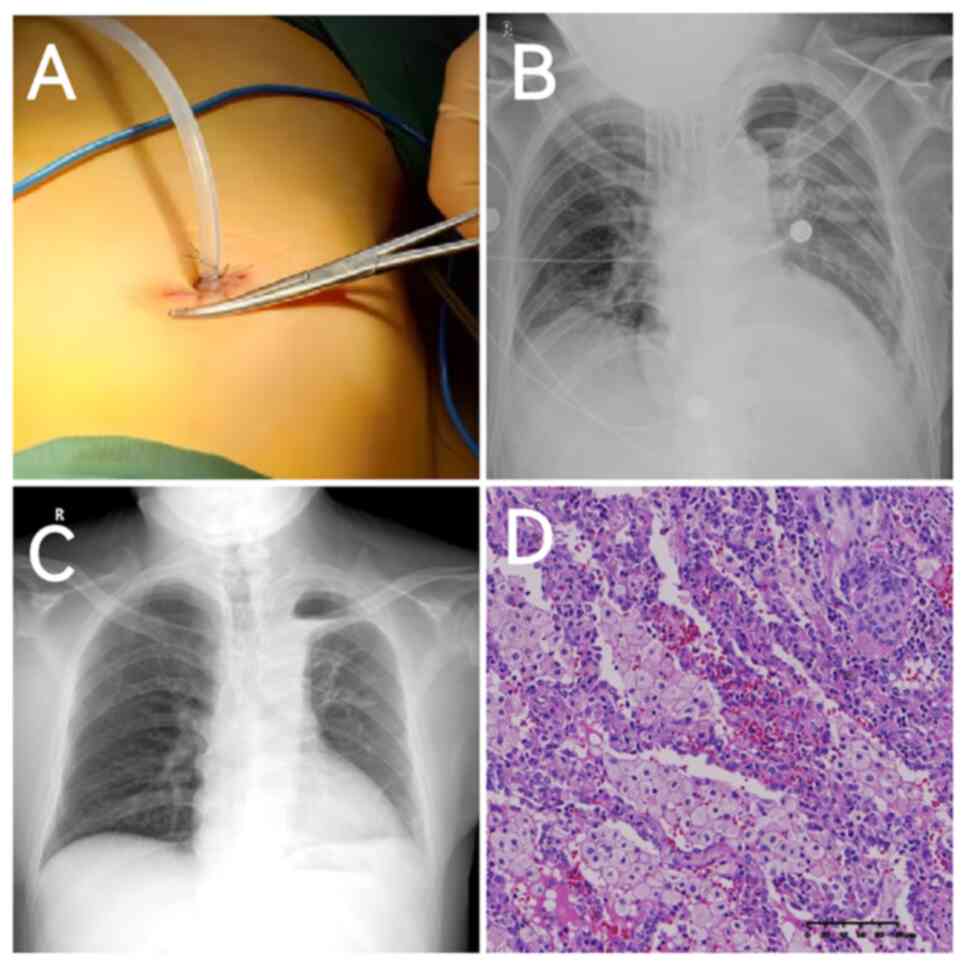
After MDT discussions, minimally invasive surgery-having only a
single-hole (Fig. 4A)-was
performed the next day, including wedge resection of the left upper
lung lesion and empyema removal. Intraoperative exploration
revealed a peripheral abscess with an alveolar fistula in the left
upper lung lesion. The total operation time was ~70 min. During
intraoperative exploration, lesions were observed in the visceral
pleura. The surrounding lung tissues showed inflammatory changes
and demonstrated a brittle texture. After the lesion was removed
using Endo-GIA staplers (Ethicon, Somerville, NJ, USA), 4-0 prolene
thread (Ethicon) was used for continuous suture reinforcement of
the wound surfaces to reduce postoperative air leakage.
Chest radiography performed the day after surgery
revealed no significant hydropneumothorax in the left thoracic
cavity (Fig. 4B). There was no air
leakage in the left thoracic duct one week postoperatively. The
chest tube was removed two weeks postoperatively and chest
radiography at three weeks (Fig.
4C) showed that the left lung had recovered well. Postoperative
pathology (Fig. 4D) revealed
fibrous hyperplasia with massive neutrophil and plasma cell
infiltration, and massive foam cell accumulation in the alveolar
space in the left upper lung. His vital signs were stable, and he
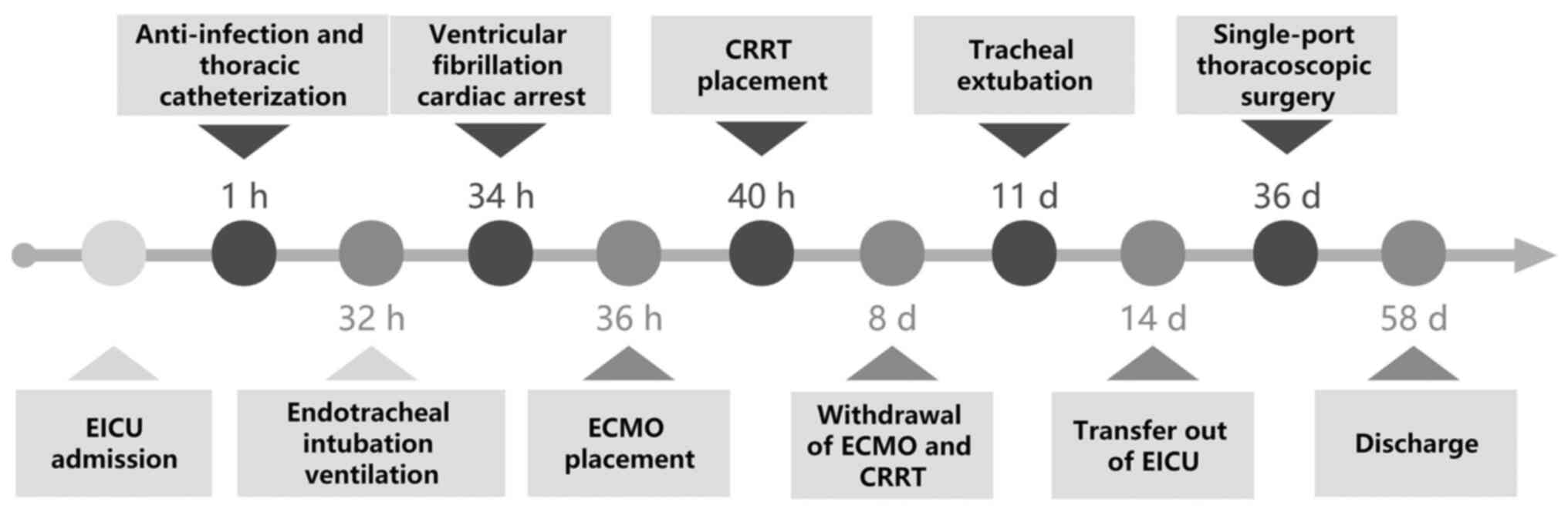
was discharged uneventfully. The timeline of the treatment process
is shown in Fig. 5. The critical
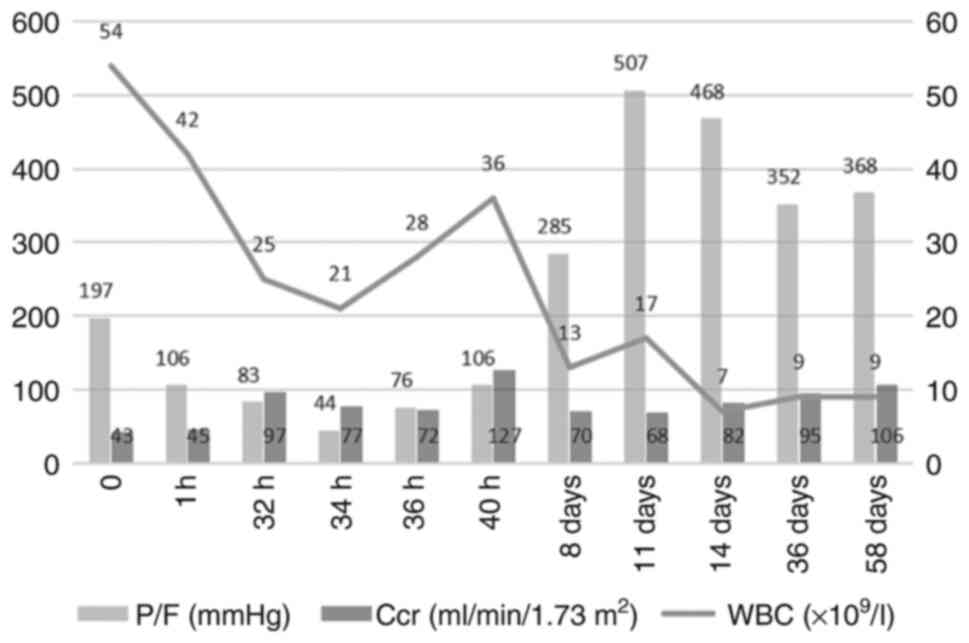
information that reflects the severity of the disease included
partial pressure of oxygen (PaO2)/fraction of inspired
oxygen (FiO2) ratio (P/F ratio) and endogenous
creatinine clearance rate (Ccr) are shown in Fig. 6. After one year of postoperative
follow-up, his cardiopulmonary function continued to be stable,
with no recurrence of infection.
Discussion
ARDS caused by acute intrathoracic infection usually
progress rapidly because of systemic inflammatory storm, especially
in patients with a history of autoimmune disease. With accumulating
evidence (3,4), ECMO has demonstrated strong potential
for a good prognosis and good application prospects in the
treatment of adult ARDS. For example, venovenous ECMO (VV-ECMO)
plays a definitive role in improving hypoxia, correcting carbon
dioxide retention, and protecting lung tissue (2). However, controversies still remain
regarding the selection of VV-ECMO or VA-ECMO (5). After an MDT discussion, the patient
finally underwent VA-ECMO. The reasons were: first,
echocardiography on admission revealed paradoxical movement of the
ventricular wall, significantly elevated creatine kinase myocardial
band (142 ng/ml, ref 0-5), myoglobin (>3,000 ng/ml, ref 20-80),
and high-sensitivity troponin levels (1,592 ng/l, ref 0-0.04),
suggesting pre-existing infectious myocarditis and myocardial
damage. Second, he had experienced three episodes of ventricular
fibrillation and sudden cardiac arrest due to hypoxia, so the
myocardial damage was substantially aggravated. Third, he
experienced hemodynamic instability after cardiopulmonary
resuscitation. Although VA-ECMO can increase the risk of stroke,
bleeding, renal failure, and cobra syndrome (i.e., selective upper
body hypoxia) compared to that with VV-ECMO (6-8),
this patient experienced no associated adverse events.
Protection of renal function and hemofiltration are
especially important in the presence of multiple organ failure. The
main purpose of CRRT is to remove excessive water and metabolic
waste, correct water and electrolyte imbalances. CRRT is mostly
used for treating acute and chronic kidney diseases. Since CRRT can
also remove various cytokines and inflammatory mediators, it has
likewise been successfully applied to the treatment of severe
liver, lung, heart, pancreas, and other organ damage as well as
sepsis (9). For this patient, we
conducted bedside CRRT to protect the patient's renal function in
the early stages of renal impairment, and thus avoided additional
deterioration of renal function, adjusted the stability of his
internal environment, and provided the proper preconditions for
drug efficacy. It should be also noted that CRRT can remove small
molecules, promoting the recovery of cardiac function (his left
ventricular ejection fraction increased from 39 to 58%). Moreover,
CRRT can also help patients with severe ARDS with hemodynamic
instability by accurately implementing fluid management strategies
(10,11). A previous retrospective study
showed better fluid management with than without CRRT among
surviving patients treated with ECMO, and that fluid overload
during ECMO was an important factor associated with a poor
prognosis (12).
After the patient was transferred to the general
ward at our hospital, chest CT showed a left upper lung abscess
(~1.5 cm in diameter) with an alveolar fistula accompanied by two
clinical problems: persistent air leakage in the left chest tube
and intermittent low-grade fever. A prior study confirmed that
surgical treatment should be selected for patients with small lung
abscesses, localized empyema, symptoms lasting >12 weeks, and
little hope for the efficacy of conservative treatment (13). Additional surgical indications
include massive hemoptysis, persistent septic fever, bronchopleural
fistula, and empyema caused by abscess rupture (14). Recommended surgical methods include
lobectomy, segmentectomy, and pulmonary wedge resection, which are
selected according to the lesion size and location (14). The prognosis for surgery depends on
the patient's systemic status and immune function. Although
postoperative mortality as high as 11-28% was reported, as of 2005
the mortality rate has decreased with the recent popularization of
effective and minimally invasive techniques (15).
In summary, acute empyema may rapidly develop into
ARDS and lead to fatal consequences especially in immunocompromised
patients, thus this critical situation warrants vigilance from
clinicians. Also, timely and effective use of ECMO can increase the
success rate of rescue, and the choice of ECMO mode depends on
comprehensively evaluating the patient's condition. Moreover,
minimally invasive surgery, even for high-risk patients, should
still be considered when conservative treatment fails.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and HW conceived and designed the work. DG
collected and collated the data and wrote the manuscript. CW, JW
and QR implemented the placement and withdrawal of ECMO. HW guided
the use of CRRT. JG, ZL, XW, LX and XH participated in the
operation and the collation of patient information. YB guided the
anti-infection drug treatment and collected data. JZ critically
revised the manuscript. All authors read and approved the final
manuscript. DG and JZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
This report was approved and presented according to
the guidelines of the Ethics Committee of the Second Hospital of
Jiaxing (approval no. JXEY-LWSC080).
Patient consent for publication
Written informed consent was obtained for the
publication of the patient's data and images in this case
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Man MY, Shum HP, Wu A, Lee RA and Yan WW:
A case of severe empyema with acute respiratory distress syndrome
caused by slackia exigua requiring veno-venous extracorporeal
membrane oxygenation. Anaerobe. 48:7–11. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tandukar S and Palevsky PM: Continuous
renal replacement therapy: Who, when, why, and how. Chest.
155:626–638. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Australia and New Zealand Extracorporeal
Membrane Oxygenation (ANZ ECMO) Influenza Investigators. Davies A,
Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P,
Gattas D, Granger E, et al: Extracorporeal membrane oxygenation for
2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA.
302:1888–1895. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Peek GJ, Mugford M, Tiruvoipati R, Wilson
A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F,
Cooper N, et al: Efficacy and economic assessment of conventional
ventilatory support versus extracorporeal membrane oxygenation for
severe adult respiratory failure (CESAR): A multicentre randomised
controlled trial. Lancet. 374:1351–1363. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kon ZN, Bittle GJ, Pasrija C, Pham SM,
Mazzeffi MA, Herr DL, Sanchez PG and Griffith BP: Venovenous versus
venoarterial extracorporeal membrane oxygenation for adult patients
with acute respiratory distress syndrome requiring precannulation
hemodynamic support: A review of the ELSO registry. Ann Thorac
Surg. 104:645–649. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Delius R, Anderson H III, Schumacher R,
Shapiro M, Otsu T, Toft K, Hirsch J and Bartlett R: Venovenous
compares favorably with venoarterial access for extracorporeal
membrane oxygenation in neonatal respiratory failure. J Thorac
Cardiovasc Surg. 106:329–338. 1993.PubMed/NCBI
|
|
7
|
Oshima K, Kunimoto F, Hinohara H, Ohkawa
M, Mita N, Tajima Y and Saito S: Extracorporeal membrane
oxygenation for respiratory failure: A comparison of venovenous
versus venoarterial bypass. Surg Today. 40:216–222. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Papademetriou MD, Tachtsidis I, Banaji M,
Elliott MJ, Hoskote A and Elwell CE: Optical topography to measure
variations in regional cerebral oxygenation in an infant supported
on veno-arterial extra-corporeal membrane oxygenation. Adv Exp Med
Biol. 737:71–76. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhu CY, Pan AJ, Mei Q and Chen T:
Successful cure of a patient with urosepsis using a combination of
extracorporeal membrane oxygenation and continuous renal
replacement therapy: A case report and literature review. Chin J
Traumatol. 23:372–375. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Han F, Sun R, Ni Y, Hu X, Chen X, Jiang L,
Wu A, Ma L, Chen M, Xv Y and Tu Y: Early initiation of continuous
renal replacement therapy improves clinical outcomes in patients
with acute respiratory distress syndrome. Am J Med Sci.
349:199–205. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Laggner AN, Druml W, Lenz K, Schneeweiss B
and Grimm G: Influence of ultrafiltration/hemofiltration on
extravascular lung water. Contrib Nephrol. 93:65–70.
1991.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cavagnaro F, Kattan J, Godoy L, Gonzáles
A, Vogel A, Rodríguez JI, Faunes M, Fajardo C and Becker P:
Continuous renal replacement therapy in neonates and young infants
during extracorporeal membrane oxygenation. Int J Artif Organs.
30:220–226. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kuhajda I, Zarogoulidis K, Tsirgogianni K,
Tsavlis D, Kioumis I, Kosmidis C, Tsakiridis K, Mpakas A,
Zarogoulidis P, Zissimopoulos A, et al: Lung abscess-etiology,
diagnostic and treatment options. Ann Transl Med.
3(183)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pagès PB and Bernard A: Lung abscess and
necrotizing pneumonia: Chest tube insertion or surgery? Rev Pneumol
Clin. 68:84–90. 2012.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
15
|
Herth F, Ernst A and Becker HD: Endoscopic
drainage of lung abscesses: Technique and outcome. Chest.
127:1378–1381. 2005.PubMed/NCBI View Article : Google Scholar
|