Introduction
Essential hypertension (EH) is a highly
heterogeneous disorder that influenced by genetic and environmental
factors and their interactions (1). It is reported that 27.2% of the adult
population aged 35-74 years suffer from EH (2). Genetic factors are estimated to
account for ~30-50% of variation in blood pressure (BP) levels.
Great efforts have been made to identify the genes and chromosomal
loci associated with blood pressure traits or hypertension over the
past decades. EH is often accompanied by metabolic abnormalities
such as insulin resistance (IR) and ~50% of hypertensive patients
have abnormal glucose tolerance or non-insulin-dependent diabetes
(3). IR and secondary
hyperinsulinemia may increase blood pressure and participate in the
occurrence and development of EH (4). However, the mechanism of EH
concomitant IR remains to be elucidated.
Insulin must be mediated by insulin receptor (INSR)
to perform its function. INSR gene mutation is often
detected in IR patients with moderate to high blood pressure,
suggesting that INSR gene may be one of the candidate genes
for EH (5). Hanis and Bertin
(6) have found that nucleotide at
6244 in human INSR exon 8 was mutated from G to A, resulting
in a NsiI polymorphism. Schrader et al (7) found that the restriction fragment
polymorphism of INSR NsiI was associated with EH in an
Australian population. Similar results were detected in a Korean
population (8). However,
inconsistent results were obtained in Chinese populations (9,10).
In addition, insulin receptor substrate 1 (IRS-1) is
a signaling protein widely distributed in the cytoplasm of
insulin-sensitive tissues and serves a key role in signal
transduction (11). The
IRS-1 gene is a key factor for insulin signaling, which is
transmitted radially in different directions from IRS-1(12). IRS-1 G972R (rs1801278)
polymorphism was shown to be associated with insulin resistance and
may be a candidate gene for EH (13). While Wang et al (14) and Xu et al (15) report no association of IRS-1
G972R polymorphism with EH risk.
Considering the limited sample sizes and
inconclusive results in individual studies, it is necessary to
evaluate more precise results on the genetic association between
the INSR and ISR-1 gene polymorphisms and EH
risk.
Materials and methods
Literature strategy
The present meta-analysis followed the Cochrane
Collaboration definition and PRISMA 2009 guidelines for
meta-analysis and systematic review. A systematically search was
carried out in the databases including PubMed, EMBASE, Web of
Science and China National Knowledge Infrastructure. All the
related publications up to January 20th, 2023 were screened by
using the following search strategy: ‘insulin receptor’ or
‘INSR’ and ‘insulin receptor substrate-1’ or ‘ISR-1’
and ‘polymorphism’ or ‘single nucleotide polymorphism’ or ‘SNP’ or
‘variant’ and ‘hypertension’ or ‘essential hypertension’ or ‘EH’
without restrictions on language. In addition, references from the
relevant literature were screened manually.
Selection criteria
Inclusion criteria: i) Papers were case-control
designed; ii) studies referred to the genetic association between
INSR and ISR-1 polymorphisms and EH risk; and iii)
publications had available genotype frequencies for estimating an
odds ratio (OR) and their 95% confidence interval (CI).
Exclusion criteria: i) Papers were duplicates; ii)
papers were abstracts, letters, short communication, review or case
report; iii) studies had unavailable data in case or control
group.
Data abstraction
The following information was extracted: First
author's name, year of publication, ethnicity, mean ages, sex ratio
(male%), body mass index, systolic pressure, diastolic blood
pressure, methods of genotyping, number of cases and controls,
number of genotypes in cases and controls. Authors Yan Wang and Qin
Xiang conducted the data extraction independently. When there were
discrepancies between the two authors, discussion was applied to
resolve the inconsistencies. The quality assessment was performed
by using Newcastle-Ottawa Scale (NOS). Studies with a score of ≥6
was included in the present meta-analysis study.
Statistical analysis
Combined ORs and 95% CIs were used to evaluate the
relationship of the INSR Nsil, RsaI and ISR-1 G972R
polymorphisms with EH susceptibility under different genetic models
(allele, dominant and recessive models). The significance of the
combined ORs were determined via a Z-test. A
chi-squared-based Cochran's Q test and I2
statistics were applied for evaluating the heterogeneity between
included articles. The random-effects model was used. Subgroup
analyses were conducted stratified by ethnicity in the present
meta-analysis. Sensitivity analysis by systematically removing one
study at a time was performed to estimate the stability of the
results. The possible publication bias was evaluated by the Begg's
funnel plot and the Egger linear regression test. The statistical
tests were performed using the STATA 12.0 software (Stata Corp) and
RevMan 5.2 (Cochrane Collaboration). P<0.05 was considered to
indicate a statistically significant difference.
Results
Studies selection and
characteristics
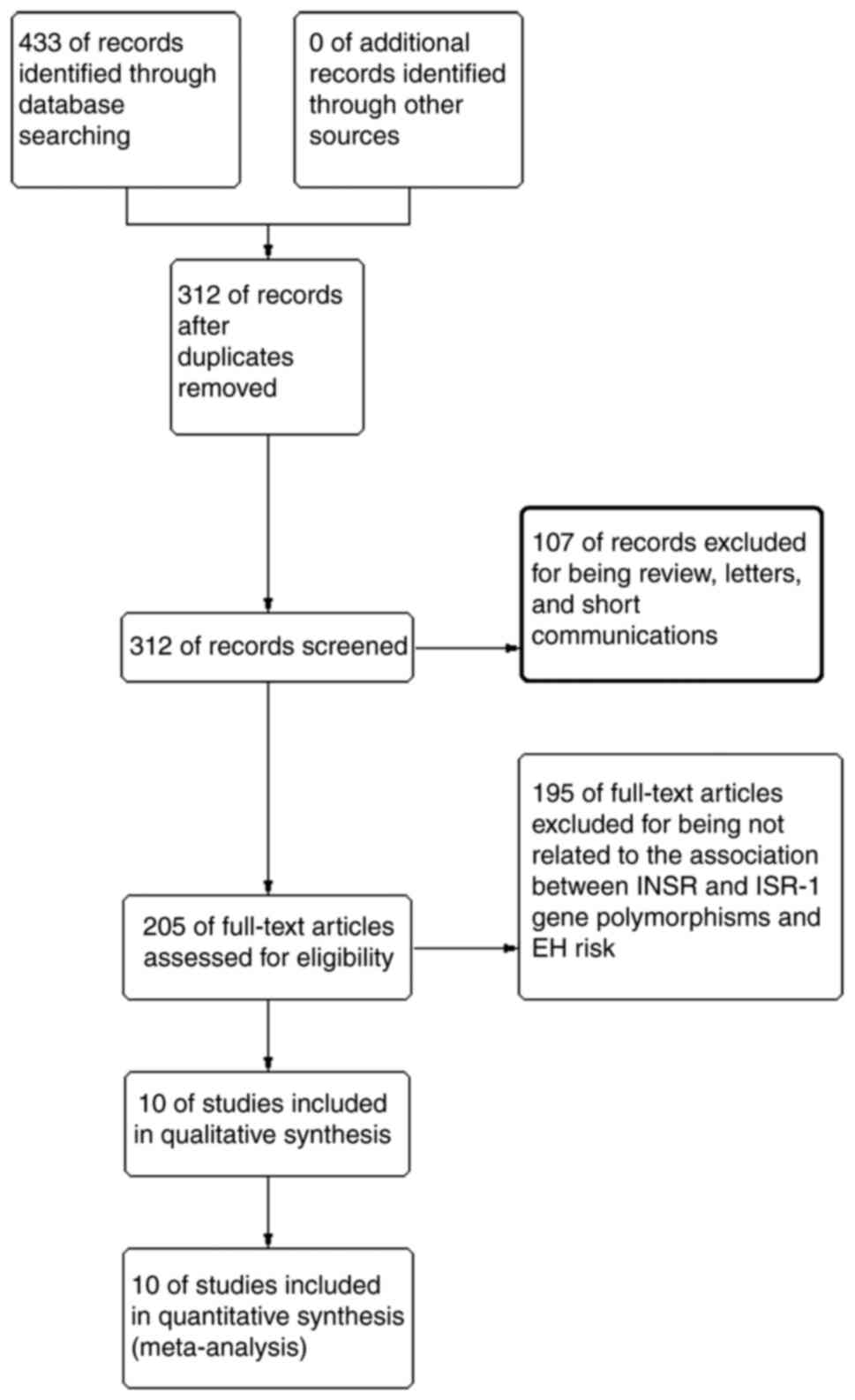
A total of 433 studies were originally obtained.
Among which, 121 studies were excluded for being duplicates, 185
studies were excluded for being unrelated to the association of
INSR and ISR-1 gene polymorphisms and hypertension
risk, 107 studies were excluded for not being original articles.
Among the excluded studies, Zee et al (16) found that INSR intron 9 polymorphism
was related to EH. Qiu et al (17), found that the polymorphism of INSR
exon 17 was related to EH in a Chinese population. Finally, 10
eligible publications were chosen for data extraction and quality
assessment (7-10,14,15,18-21;
Fig. 1). All the original
published articles had detected the polymorphisms for human blood.
Among the eligible studies, six articles were related to the
relationship of INSR Nsil and EH risk and two were related
to the association between INSR Rsal and EH risk. A total of
two studies were conducted on the association between ISR-1
G972R and EH risk. In addition, seven studies were conducted in
Asian populations and three in Caucasian populations. The number of
the included studies reporting the genetic associations between the
INSR (Nsil and Rsal) and ISR-1 (G972R) polymorphisms and EH risk
was enough to perform a meta-analysis. Thus, the present study
chose the current polymorphisms for the analysis. The relevant
information for eligible studies is listed in Table I.
 | Table ICharacters of eligible studies in the
present study. |
Table I
Characters of eligible studies in the
present study.
| First author,
year | Ethnicity | Age | Sex (M%)
(case/control) | BMI
(case/control) | SBP
(case/control) | DBP
(case/control) | Genotyping
methods | Case | Control | NOS | (Refs.) |
|---|
| Ao, 2006 | Chinese | 52.40±12.34/
43.54±9.79 | 38.1/39.7 | 26.65±4.37/
25.40±3.61 | NA | NA | PCR-RFLP | 84 | 199 | 6 | (10) |
| Kang, 2000 | Korean | NA/NA | NA | NA | NA | NA | PCR-RFLP | 86 | 134 | 6 | (8) |
| Lin, 2000 | Chinese | 53. 36±10.12/ 49.
47±9.59 | 55.0/62.8 | 24.91±3.22/
25.08±2.74 | 169.15±28.36/
170.48±10.36 | 7.98±16.15/
8.76±8.44 | PCR-RFLP | 120 | 86 | 8 | (9) |
| Schrader, 1996 | Australian | 52±12/46±10 | 46.3/62.7 | 26.2±4.5/
25.4±4.3 | 176±24/ 116±9 | 111±18/ 73±8 | PCR-RFLP | 134 | 126 | 8 | (7) |
| Yu, 2007 | Chinese | 53.36±10.12/
49.47±9.59 | 36.7/44.6 | 23.00±3.54/
24.88±4.25 | 177.01±17.25/
114.00±11.53 | 98.31±13.54/
75.23±9.14 | PCR-RFLP | 221 | 204 | 8 | (21) |
| Zhu, 2009 | Chinese | 63.52±4.60/
63.94±4.84 | 55.8/60.6 | 25.37±2.69/
24.34±2.60 | 162±12/ 123±11 | 95±9/77±7 | PCR-RFLP | 190 | 94 | 8 | (18) |
| Morris, 1993 | Australia | 50±14/46±19 | 54.0/61.0 | 25±5/NA | 176±22/ 113±7 | 114±22/ 71±5 | PCR-RFLP | 85 | 100 | 8 | (19) |
| Ying, 1991 | Australia | 51±14/40±11 | NA | NA | NA | NA | PCR-RFLP | 67 | 75 | 6 | (20) |
| Wang, 2007 | Chinese | 51.22±10.86/
53±11.85 | 55.8/58.0 | 26.48±4.09/ 24.
25±3.56 | NA | NA | PCR-RFLP | 120 | 100 | 7 | (14) |
| Xu, 2006 | Chinese | 52.71±12.16/
44.03±10.55 | 37.6/47.3 | 26.88±4.31/
25.55±3.97 | NA | NA | PCR-RFLP | 182 | 375 | 7 | (15) |
Meta-analyses for INSR and ISR-1
polymorphisms and EH risk
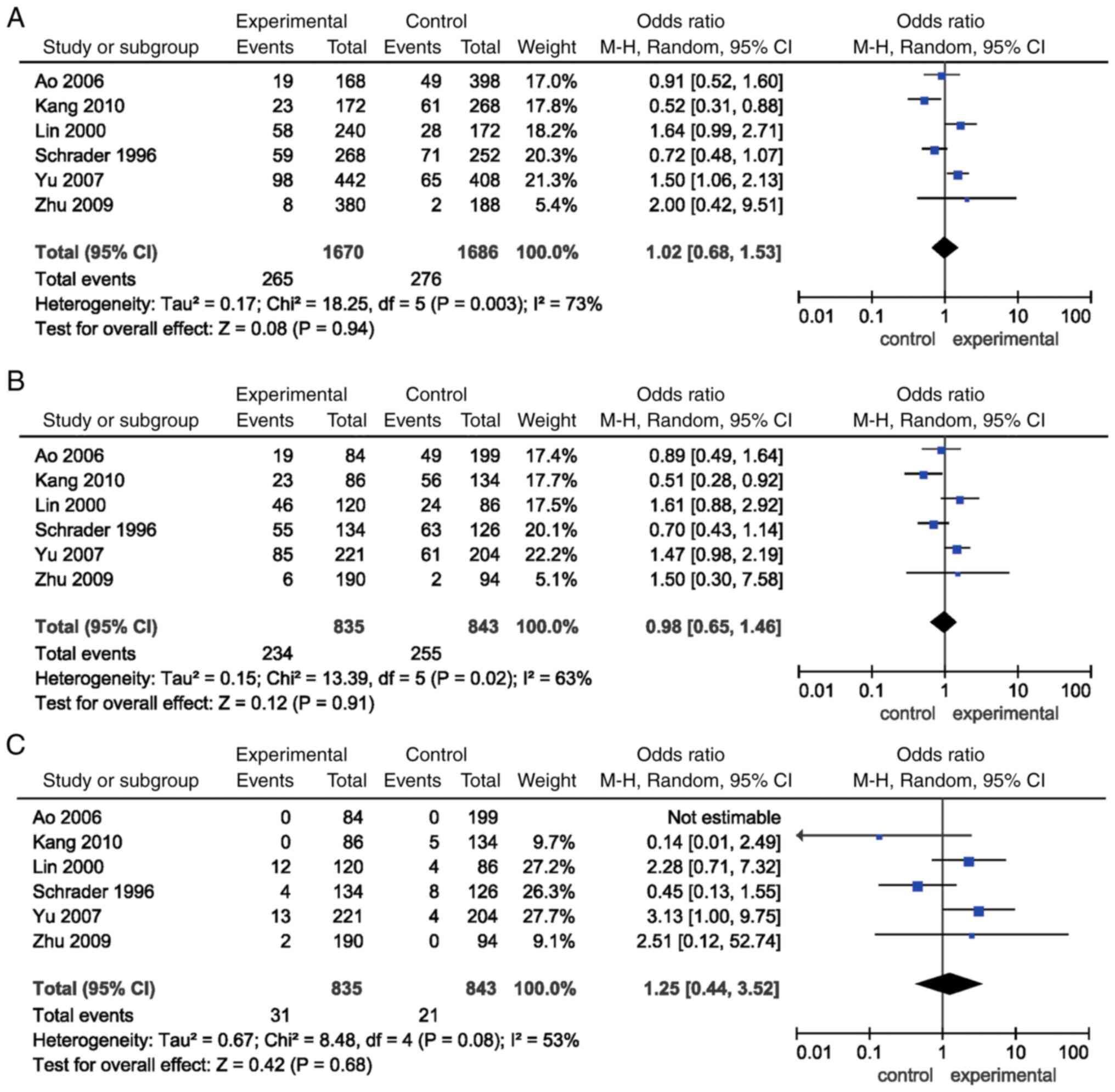
The combined ORs for the allele [P=0.94, OR=1.02,
(95% CI)=(0.68, 1.53)], dominant [P=0.91, OR=0.98, (95% CI)=(0.65,
1.46)] and recessive models [P=0.68, OR=1.25, (95% CI)=(0.44,
3.52)] of INSR Nsil polymorphism failed to detect an
association. Subgroup analysis according to ethnicity showed no
association between the allele, dominant and recessive models of
INSR Nsil polymorphism and EH risk either in Asian or in
Caucasian populations (P>0.05; Table II; Fig. 2).
 | Table IIGenetic association between the
INSR (Nsil and Rsal) and ISR-1 (G972R) genes and
hypertension risk. |
Table II
Genetic association between the
INSR (Nsil and Rsal) and ISR-1 (G972R) genes and
hypertension risk.
| | Test of
association | | Test of
heterogeneity |
|---|
|
Gene/polymorphism | Minor allele
frequency | Genetic models | Ethnicity | Number of
studies | OR | 95% CI | P-value | Model | P-value | I2
(%) |
|---|
| INSR/Nsil | Unavailable | Allele | Total | 6 | 1.02 | (0.68, 1.53) | 0.94 | R | 0.003 | 73 |
| | | | Asian | 5 | 1.11 | (0.69, 1.78) | 0.66 | R | 0.007 | 71 |
| | | | Caucasian | 1 | 0.72 | (0.48, 1.07) | 0.11 | - | - | - |
| | | Dominant | Total | 6 | 0.98 | (0.65, 1.46) | 0.91 | R | 0.02 | 63 |
| | | | Asian | 5 | 1.06 | (0.67, 1.69) | 0.80 | R | 0.03 | 63 |
| | | | Caucasian | 1 | 0.70 | (0.43, 1.14) | 0.15 | - | - | - |
| | | Recessive | Total | 6 | 1.25 | (0.44, 3.52) | 0.68 | R | 0.08 | 53 |
| | | | Asian | 5 | 1.97 | (0.75, 5.22) | 0.17 | R | 0.25 | 27 |
| | | | Caucasian | 1 | 0.45 | (0.13, 1.55) | 0.21 | - | - | - |
| INSR/Rsal | Unavailable | Allele | Total | 2 | 0.58 | (0.42, 0.80) | 0.0008 | R | 0.96 | 0 |
| | | | Asian | 0 | - | - | - | - | - | - |
| | | | Caucasian | 2 | 0.58 | (0.42, 0.80) | 0.0008 | R | 0.96 | 0 |
| | | Dominant | Total | 2 | 0.59 | (0.38, 0.92) | 0.02 | R | 0.87 | 0 |
| | | | Asian | 0 | - | - | - | - | - | - |
| | | | Caucasian | 2 | 0.59 | (0.38, 0.92) | 0.02 | R | 0.87 | 0 |
| | | Recessive | Total | 2 | 0.38 | (0.20, 0.72) | 0.003 | R | 0.93 | 0 |
| | | | Asian | 0 | - | - | - | - | - | - |
| | | | Caucasian | 2 | 0.38 | (0.20, 0.72) | 0.003 | R | 0.93 | 0 |
| ISR-1/G972R | T=0.062104/ | Allele | Total | 2 | 1.66 | (0.52, 5.28) | 0.39 | F | 0.99 | 0 |
| | 11306 (ALFA) | | Asian | 2 | 1.66 | (0.52, 5.28) | 0.39 | F | 0.99 | 0 |
| | | | Caucasian | 0 | - | - | - | - | - | - |
| | | Dominant | total | 2 | 1.67 | (0.52, 5.33) | 0.39 | F | 0.99 | 0 |
| | | | Asian | 2 | 1.67 | (0.52, 5.33) | 0.39 | F | 0.99 | 0 |
| | | | Caucasian | 0 | - | - | - | - | - | - |
| | | Recessive | Total | 2 | 1.66 | (0.52, 5.28) | 0.39 | F | 0.99 | 0 |
| | | | Asian | 2 | 1.66 | (0.52, 5.28) | 0.39 | F | 0.99 | 0 |
| | | | Caucasian | 0 | - | - | - | - | - | - |
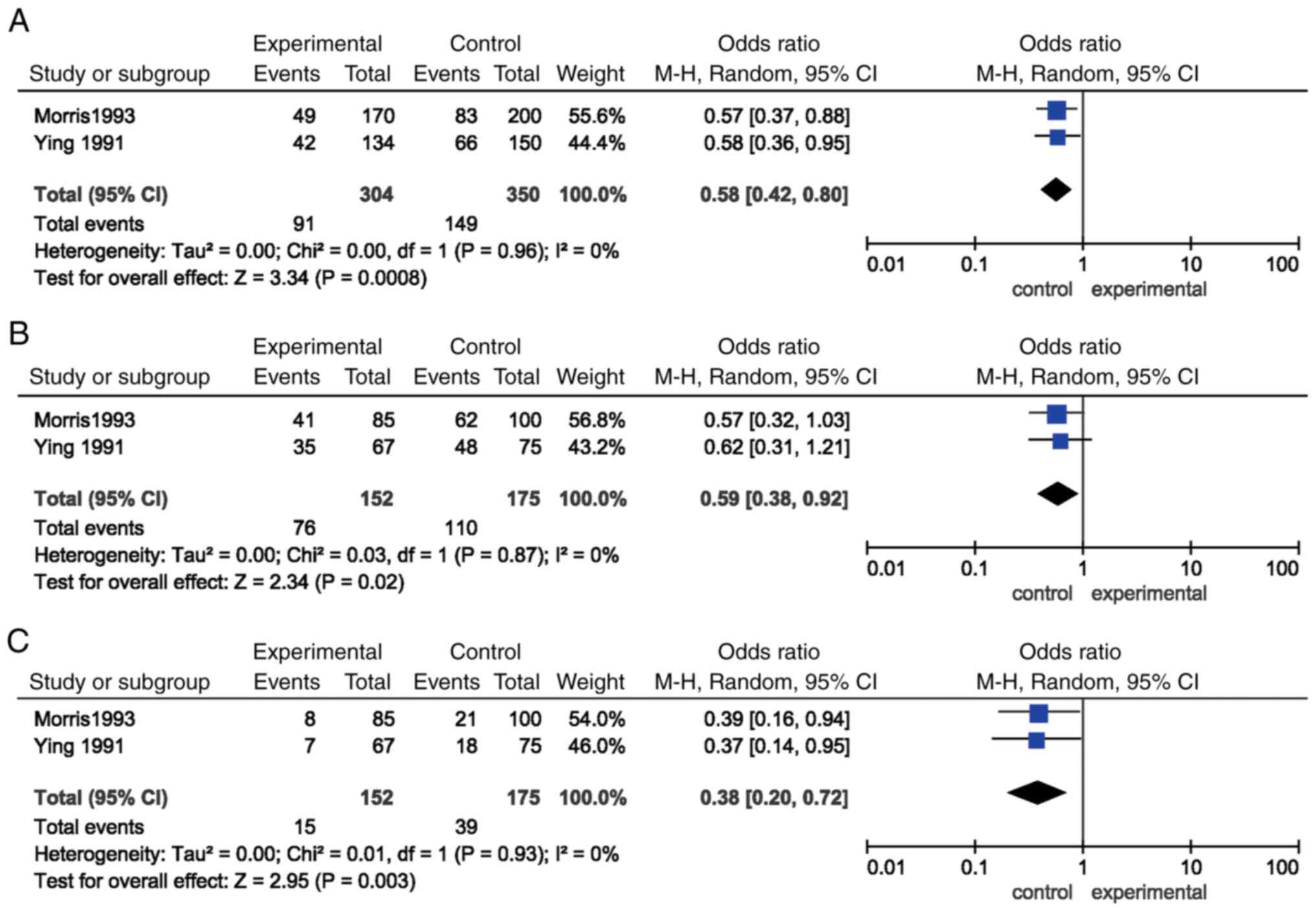
In addition, significant associations were found for
the allele [P=0.0008, OR=0.58, (95% CI)=(0.42, 0.80)], dominant
[P=0.02, OR=0.59, (95% CI)=(0.38, 0.92)] and recessive models
[P=0.003, OR=0.38, (95% CI)=(0.20, 0.72)] of INSR Rsal
polymorphism and EH susceptibility. Subgroup analysis according to
ethnicity showed that the significant associations between the
allele, dominant and recessive models of INSR Rsal
polymorphism and EH risk were observed in Caucasian, but not in
Asian populations (P>0.05; Table
II; Fig. 3).
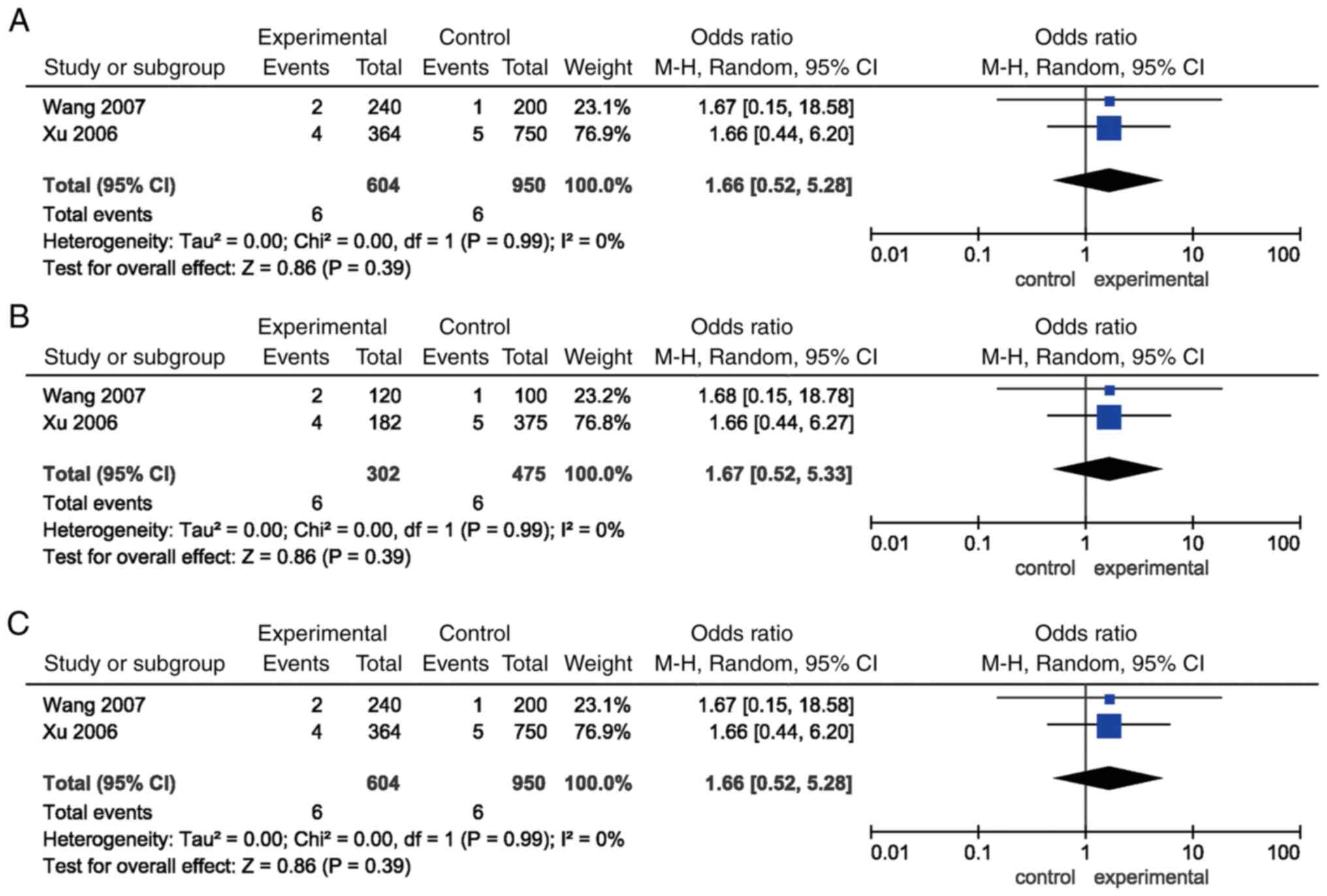
Furthermore, the pooled ORs for all the genetic
models of ISR-1 G972R polymorphism failed to show an
association with EH risk (P>0.05). Similar results were detected
in the subgroup analysis stratified by ethnicity (Table II; Fig. 4).
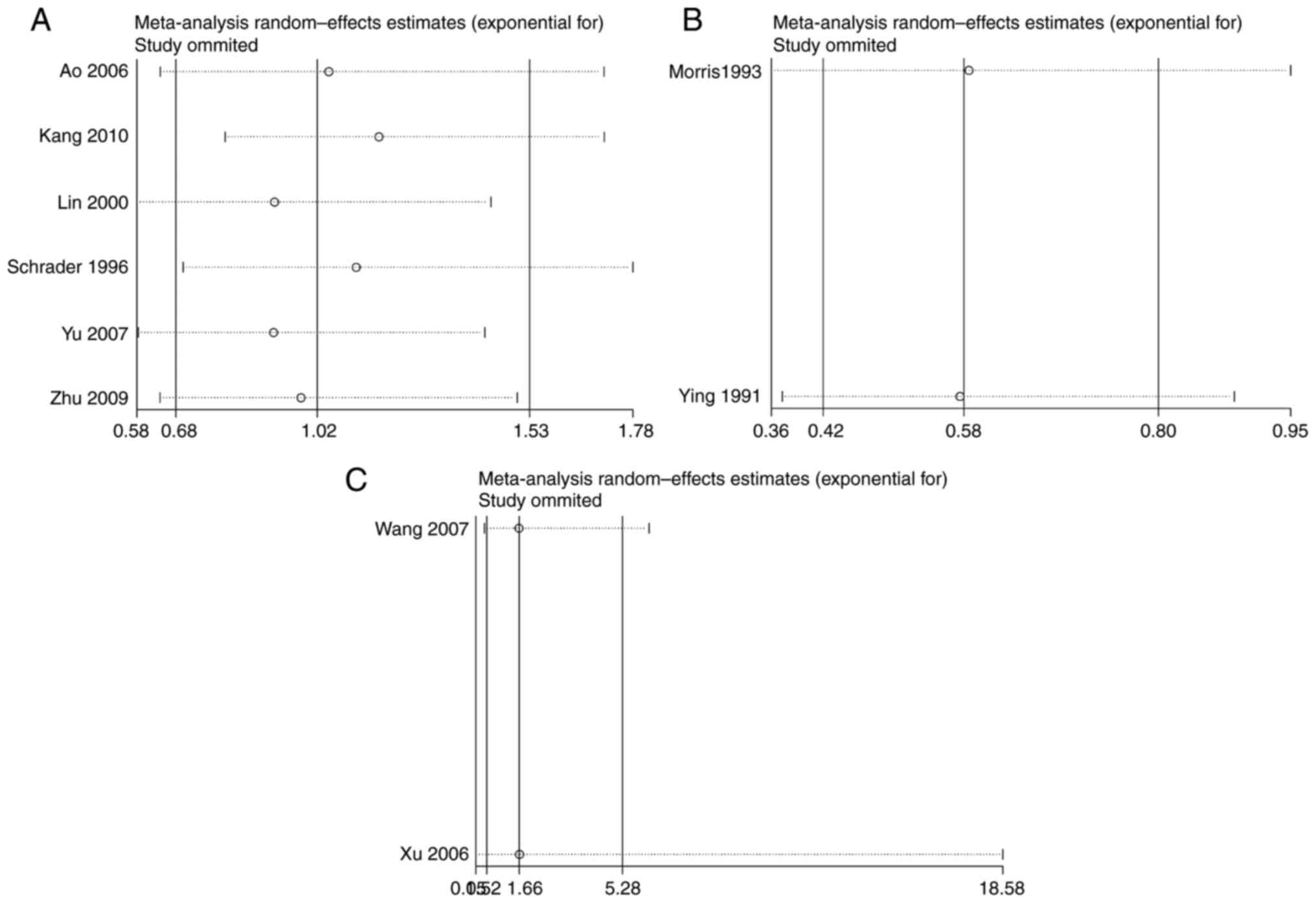
Source of heterogeneity
Significant heterogeneities were found in the
allele, dominant, and recessive models of INSR Nsil
polymorphism. The studies conducted by Schrader et al
(7) and Kang et al
(8) contributed mainly to the
significant heterogeneity. A 0% (P>0.05) heterogeneity was
obtained after removing these two studies (Fig. 5).
Sensitive analysis and Publication
bias
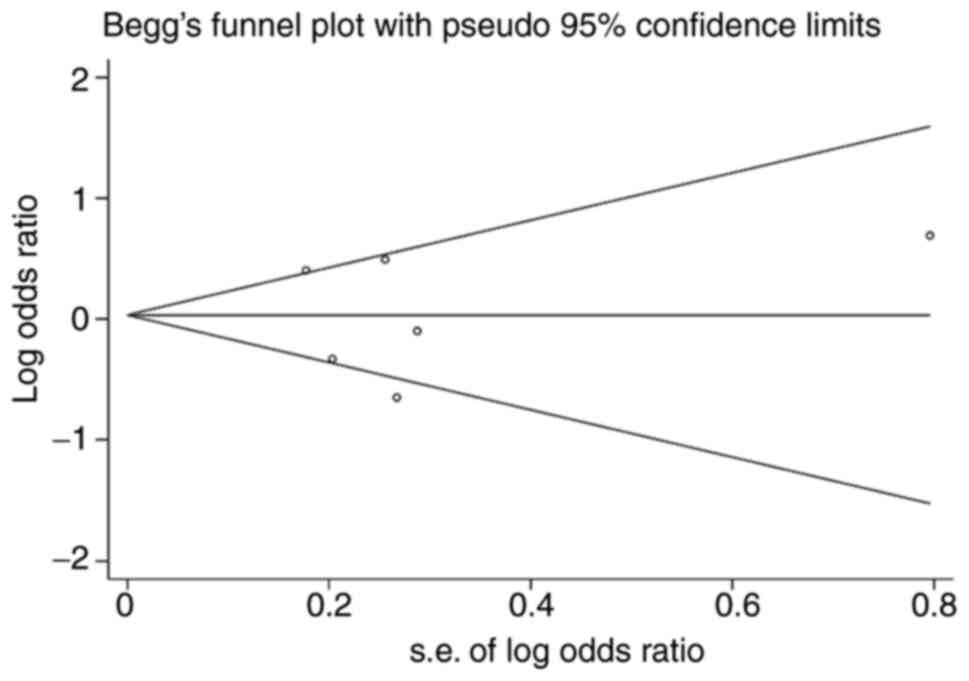
Publication bias in the included studies was
assessed using the Begg's funnel plot and Egger's linear regression
test. The shapes of the Begg's funnel plot did not reveal any
evidence of obvious asymmetry for INSR Nsil polymorphism as
shown in Fig. 6. The Egger's
linear regression test also did not display a strong statistical
evidence of publication bias (t=-0.04, P=0.971). As there were only
two studies in analyzing the relationship of INSR Rsal and
ISR-1 G972R and EH risk, the Egger's linear regression test
were not performed.
Discussion
INSR gene mutation can affect receptor function in
several ways: i) Reduced receptor synthesis rate; ii) abnormal
receptor embedding process; iii) decreased affinity between the
receptor and insulin; iv) decreased tyrosine kinase activity; and
v) accelerated receptor degradation (22). The mutation inhibits INSR recycling
and the receptor degradation is relatively dominant, leading to a
decrease in the number of INSRs on the cell membrane (23). Insulin receptor gene, as a
candidate gene for many diseases, is involved in the occurrence of
hypertension, obesity, atherosclerosis and other diseases (24). The INSR gene is located on
chromosome 19, which contains 22 exons and 21 introns (25). Some studies have confirmed that
INSR gene polymorphism is related to EH. For example, Zee
et al (16) found that
INSR 9 intron polymorphism is related to EH. Ying et
al (20) found that
hypertension is related to INSR gene RsaI polymorphism, but
not to insulin gene polymorphism. Qiu et al (17), found that the polymorphism of
INSR exon 17 is related to EH in Chinese populations.
The nucleotide at INSR exon 8 6244 loci is mutated
from G to A, resulting in NsiI polymorphism (6). Schrader et al (7) found that INSR NsiI
polymorphism is associated with EH in the study of 134 cases in a
Caucasian population. The frequency of N1 allele in EH group is
higher compared with that in the normal control group. Lin et
al (9) report that INSR
NsiI polymorphism is associated with EH risk in the Chinese
population and the frequency of N2 allele is higher compared with
that in the normal group. In addition, the frequency of the N2
allele was higher in male EH patients compared with that in male
controls, but not in the female group. Thus, the N2 allele may be
the susceptible factor for EH in the Chinese male group. Moreover,
no association was found between the INSR NsiI polymorphism
and EH risk in Xinjiang Mongol and Jiangsu Chinese Han populations
(10,18). These inconsistencies may be due to
the regional differences between north and south China. The present
meta-analysis enrolled six studies consisting of 835 cases and 843
controls and found that the INSR NsiI polymorphism was not a
susceptible factor for EH. Subgroup analysis stratified by
ethnicity showed that the INSR NsiI polymorphism was not a
susceptible factor for EH in the Asian or Caucasian populations. It
may be concluded that the INSR NsiI polymorphism is not
associated with EH risk. Notably, INSR NsiI polymorphism
does not cause alteration in amino acid sequences. Therefore, this
nucleotide conversion was not directly involved in the pathogenesis
of EH, but may be due to the linkage disequilibrium between this
site and the pathogenic gene locus of hypertension. The genes that
play a role in the pathogenesis of EH may be in other exons,
introns, or regulatory sequences of INSR, and may also be in other
genes. Finding the key gene of primary hypertension is still the
focus of current EH molecular epidemiology research, which will not
only improve and clarify the molecular mechanism of EH
pathogenesis, but also provide the basis for EH gene therapy
research.
INSR RsaI R1 has been mapped to the 5.3 kb
region of INSR cDNA, located between 1928 and 2478, that is,
near the hinge region separating the receptor α and β strands,
where rare mutations lead to severe insulin resistance. Defects in
EH that lead to insulin resistance are likely to involve defects in
the glucose transport pathway coupled with the insulin receptor and
glucose transport system (26).
However, neither of these substances is likely to cause EH. Insulin
receptor subunits have complete tyrosine kinase activity and may
participate in the second messenger generation (27). Genetic variation in this receptor
region may lead to reduced glucose transport and insulin
resistance. However, the mechanism by which EH occurs is unclear.
The INSR RsaI polymorphism may itself alter the activity of
the encoded chain tyrosine kinase or may affect other sites
associated with changes in enzyme activity. In addition,
INSR RsaI polymorphism may result in decreased affinity of
insulin receptors, decreased number of receptors, or altered gene
regulation (16). Therefore, the
function of INSR RsaI polymorphism affecting the EH need to
be studied further. The present meta-analysis found an association
between the INSR RsaI polymorphism and EH, which agreed with
previous result of Zee et al (19) and Ying et al (20). However, subgroup analysis in Asian
population failed to find an association between INSR RsaI
polymorphism and EH risk due to lack of data.
The IRS-1 gene is located at 2q36-37 and
serves an important role in insulin signaling (28). Studies have shown significant
increases in blood pressure and triglyceride levels in IRS-1
deficient mice, accompanied by impaired endothelium-dependent
vasodilation (29). Perticone
et al (30) found in a
study of 100 hypertensive patients that IRS-1 gene
polymorphisms may contribute to hypertension by causing endothelial
dysfunction. Federici et al (31) found that IRS-1 G972R
mutation damages the release of NO in endothelial cells, which may
lead to endothelial dysfunction and cardiovascular disease. There
are ethnic differences in the mutation rate of the IRS-1
G972R polymorphism. The mutation rate of IRS-1 G972R
polymorphism in Asian population is lower compared with that in
European and American populations (32-33).
However, the present study found no link between G972R and EH risk.
In the present study, no association was found between all the
genetic models of IRS-1 G972R polymorphism, which was in
contrast with previous work conducted by Wang et al
(14) and Xu et al
(15). Thus, we may conclude that
the IRS-1 G972R polymorphism might not be a susceptible
factor for EH. Due to the small number of samples in the present
combined study, large sample studies are still needed for
confirmation.
Limitations of this study should also be considered.
First, the number of included studies and subjects in the present
study were relatively small, which might partly reduce the
calculation power for the association between the INSR RsaI
and ISR-1 G972R polymorphisms and EH susceptibility. Second,
a subgroup analysis based on ethnicity could not be performed due
to a lack of data. It is necessary to conduct additional studies
among multiple ethnicities in the future. Third, multiple gene
changes are involved in the occurrence and development of EH and
there are strong interactions among multiple mutant genotypes and
environmental risk factors, which greatly increases the risk of EH
susceptibility. However, the present study could not assess the
effect of interaction of these two types of factors in the
development of EH in this study. Fourth, only case-control studies
were included in present study which may partly influence the
accuracy of the genetic polymorphisms and EH. To confirm this
results, other kinds of studies, such as cohort studies, would be
necessary. Fifth, multiple factors were shown to serve a role in
the pathology of EH, However, the present study could not assess
the association between the INSR (Nsil and Rsal) and
ISR-1 (G972R) polymorphisms and EH adjusting for traditional
risk factors, such as gender, diet, stress, smoking, weight and
drugs, for lack of sufficient data. Association studies with
detailed definition of traditional risk factors would be necessary.
Last, the NOS scores were not high in several studies, which may be
due to the study design, Thus, more studies with higher NOS scores
should be included.
The data of the present study suggested that the
INSR Rsal polymorphism is highly probable to be a protective
factor for EH and provided evidence the INSR Rsal
polymorphism is associated with EH risk.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Changsha
Outstanding Innovative Young People Training Scheme (grant nos.
kq2206058 and kq2206056), The Foundation of Project of Hunan Health
and Family Planning Commission (grant no. 202202082739), The
Foundation of the Education Department of Hunan Province (grant no.
21A0586), The Foundation of the Education Department of Guangxi
Province (grant no. 2021KY1959); and The Hunan Key Laboratory
Cultivation Base of the Research and Development of Novel
Pharmaceutical Preparations (grant no. 2016TP1029).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LT and JML participated in study design and data
collection, carried out the initial analysis and drafted the
article. YW and QX aided in data acquisition, data analysis and
statistical analysis. LT and YW carried out literature search, data
acquisition and manuscript editing. QX and JML confirm the
authenticity of all the raw data. TL and JML performed manuscript
review. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lifton RP, Gharavi AG and Geller DS:
Molecular mechanisms of human hypertension. Cell. 104:545–556.
2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gu D, Reynolds K, Wu X, Chen J, Duan X,
Muntner P, Huang G, Reynolds RF, Su S, Whelton PK, et al:
Prevalence, awareness, treatment, and control of hypertension in
China. Hypertension. 40:920–927. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gu S, Wang A, Ning G, Zhang L and Mu Y:
Insulin resistance is associated with urinary albumin-creatinine
ratio in normal weight individuals with hypertension and diabetes:
The REACTION study. J Diabetes. 12:406–416. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bamaiyi AJ, Woodiwiss AJ, Peterson V,
Gomes M, Libhaber CD, Sareli P and Norton GR: Insulin resistance
influences the impact of hypertension on left ventricular diastolic
dysfunction in a community sample. Clin Cardiol. 42:305–311.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Morris BJ: Insulin receptor gene in
hypertension. Clin Exp Hypertens. 19:551–565. 1997.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hanis CL and Bertin TK: Identification of
an insulin receptor exon 8 NsiI polymorphism using the polymerase
chain reaction. Nucleic Acids Res. 18(5923)1990.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schrader AP, Zee RY and Morris BJ:
Association analyses of NsiI RFLP of human insulin receptor gene in
hypertensives. Clin Genet. 49:74–78. 1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kang BY, Kim KT, Eo HS, Lee KH, Hong SS,
Shin JH and Lee CC: Association between genetic variation of the
insulin receptor gene and essential hypertension in the Korean
population. Korean J Biol Sci. 4:87–90. 2000.
|
|
9
|
Lin CR, Wu KG, Xie LD, Ye Q and Chen S:
The association between Insulin receptor gene exon 8 Nsi I
polymorphism and hypertension in Chinese population. Chin J Med
Genet. 17:364–365. 2000.(In Chinese).
|
|
10
|
Ao Y, Xu C and Geer L: Association of the
INSR gene with essential hypertension in Xinjiang Mongol group. J
Xinjiang Med Univ. 29:804–807. 2006.(In Chinese).
|
|
11
|
Annalisa G, Pappalardo MA, Russo GT, Romeo
EL, Alibrandi A, Di Bari F, Vita R, Cucinotta D and Benvenga S:
Influence of peroxisome proliferator-activated receptor-γ exon 2
and exon 6 and insulin receptor substrate (IRS)-1 Gly972Arg
polymorphisms on insulin resistance and beta-cell function in
southern mediterranean women with polycystic ovary syndrome. J Clin
Transl Endocrinol. 13:1–8. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Albegali AA, Shahzad M, Mahmood S and
Ullah MI: Genetic association of insulin receptor substrate-1
(IRS-1, rs1801278) gene with insulin resistant of type 2 diabetes
mellitus in a Pakistani population. Mol Biol Rep. 46:6065–6070.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dziwura J, Bińczak-Kuleta A, Miazgowski T,
Ziemak J and Widecka K: The associations between G972R polymorphism
of the IRS-1 gene, insulin resistance, salt sensitivity and
non-dipper hypertension. Hypertens Res. 34:1082–1086.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang FJ, Xie XQ, Li XD and Li X-Q: The
correlation analysis of IRS-1 polymorphism with CRP in essential
hypertension. J Yunyang Med Coll. 26:141–143. 2007.(In
Chinese).
|
|
15
|
Xu L, Ao YT, Wu M, Song T and Ge E:
Association of the G972R polymorphism of IRS-1 with essential
hypertension in Xinjiang Mongol group. J Xinjiang Med Univ.
29:926–928. 2006.(In Chinese).
|
|
16
|
Zee RY, Lou YK and Morris BJ: Insertion
variant in intron 9, but not microsatellite in intron 2, of the
insulin receptor gene is associated with essential hypertension. J
Hypertens Suppl. 12:S13–S22. 1994.PubMed/NCBI
|
|
17
|
Qiu CC, Zhu XL, Ji TR, Gao Z, Sun M, Guan
B, Guo D and Liu L: Analysis of insulin receptor gene in essential
hypertension. Acta Acad Med Sin. 17:81–85. 1995.(In Chinese).
|
|
18
|
Zhu M, Chen XM, Men S, et al: Relationship
between insulin receptor G6244A gene polymorphism and essential
hypertension in Dongtai rural area, Jiangsu Province. Chin Gen
Pract. 12:2130–2132. 2009.(In Chinese).
|
|
19
|
Morris BJ, Zee RY, Ying LH and Griffiths
LR: Independent, marked associations of alleles of the insulin
receptor and dipeptidyl carboxypeptidase-I genes with essential
hypertension. Clin Sci (Lond). 85:189–195. 1993.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ying LH, Zee RYL, Griffiths LR and Morris
BJ: Association of a RFLP for the insulin receptor gene, but not
insulin, with essential hypertension. Biochem Biophys Res Commun.
181:486–492. 1991.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yu GF, Ma JX, Fu ZT, Liu C, Guo X, Li W,
Su J, Liu H, Chen X, et al: Correlation research in NSII
polymorphism of insulin receptor gene and essential hypertension.
Mod Prev Med. 34:457–459. 2007.(In Chinese).
|
|
22
|
Saiya-Cork K, Collins R, Parkin B,
Ouillette P, Kuizon E, Kujawski L, Erba H, Campagnaro E, Shedden K,
Kaminski M and Malek SN: A pathobiological role of the insulin
receptor in chronic lymphocytic leukemia. Clin Cancer Res.
17:2679–2692. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tuthill A, Semple RK, Day R, Soos MA,
Sweeney E, Seymour PJ, Didi M and O'rahilly S: Functional
characterization of a novel insulin receptor mutation contributing
to Rabson-Mendenhall syndrome. Clin Endocrinol (Oxf). 66:21–26.
2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yip CC: Insulin receptor: Aspects of its
structure and function. Adv Exp Med Biol. 334:79–88.
1993.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Curtain R, Tajouri L, Lea R, MacMillan J
and Griffiths L: No mutations detected in the INSR gene in a
chromosome 19p13 linked migraine pedigree. Eur J Med Genet.
49:57–62. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hariawalai MD, Deshmukh VV and Sellke FW:
Insulin resistance: A common factor in the triad of dyslipidemia,
hypertension, and coronary artery disease? Am J Med Sci.
313:104–106. 1997.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Duggan BM, Cavallari JF, Foley KP, Barra
NG and Schertzer JD: RIPK2 dictates insulin responses to tyrosine
kinase inhibitors in obese male mice. Endocrinology.
161(bqaa086)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Tamemoto H, Kadowaki T, Tobe K, Yagi T,
Sakura H, Hayakawa T, Terauchi Y, Ueki K, Kaburagi Y, Satoh S, et
al: Insulin resistance and growth retardation in mice lacking
insulin receptor substrate-1. Nature. 372:182–186. 1994.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Abe H, Yamada N, Kamata K, Kuwaki T,
Shimada M, Osuga J, Shionoiri F, Yahagi N, Kadowaki T, Tamemoto H,
et al: Hypertension, hypertriglyceridemia, and impaired
endothelium-dependent vascular relaxation in mice lacking insulin
receptor substrate-1. J Clin Invest. 101:1784–1788. 1998.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Perticone F, Sciacqua A, Scozzafava A,
Ventura G, Laratta E, Pujia A, Federici M, Lauro R and Sesti G:
Impaired endothelial function in never-treated hypertensive
subjects carrying the Arg972 polymorphism in the insulin receptor
substrate-1 gene. J Clin Endocrinol Metab. 89:3606–3609.
2004.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Federici M, Pandolfi A, DeFilippis EA,
Pellegrini G, Menghini R, Lauro D, Cardellini M, Romano M, Sesti G,
Lauro R and Consoli A: G972R IRS-1 variant impairs insulin
regulation of endothelial nitric oxide synthase in cultured human
endothelial cells. Circulation. 109:399–405. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Abate N, Carulli L, Cabo-Chan A Jr,
Chandalia M, Snell PG and Grundy SM: Genetic polymorphism PC-1
K121Q and ethnic susceptibility to insulin resistance. J Clin
Endocrinol Metab. 88:5927–5934. 2003.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang DF, Qiu ZX and Zhang J: Study on the
polymorphism of insulin receptor base 1 gene in type 2 diabetic
patients of Han nationality in Northern China. Chin J Pathophysiol.
21:793–796. 2005.(In Chinese).
|