Introduction
Endometriosis is a common disease affecting up to
15% of women of reproductive age (1). It is characterized by the growth of
endometrium-like tissue outside the uterus. Endometriosis of the
appendix is relatively rarely, arising in 2.8% of patients with
endometriosis and in ~0.3% of appendectomy specimens (2). Appendiceal endometriosis is often
asymptomatic or presents with acute or chronic abdominal pain. The
definitive diagnosis is usually established following
histopathological examination of the appendix after appendectomy
and is easy to recognize. However, endometriosis can have unusual
appearances, including metaplasia, absence of the epithelial
component and destruction to the structure, and then cause
diagnostic confusion. Metaplasia involving the epithelial component
occurs most often in endocervical type and infrequently with
intestinal-type (1,3). Intestinal metaplasia is found in 13%
of cases of appendiceal endometriosis and can mimic low-grade
appendiceal mucinous neoplasms (LAMNs) (1,4).
Patients with appendiceal endometriosis require management by
appendectomy and additional imaging evaluation is recommended to
exclude any possibility of multifocal disease only. However, those
with LAMN can develop intraperitoneal recurrence, or be associated
with tumours of gastrointestinal tract, ovary, endometrium and
breast (3), suggesting that
prolonged follow-up (~10 years) or cytoreductive surgery followed
by hyperthermic intraperitoneal chemotherapy is necessary (5). Therefore, despite its rarity, it is
important to distinguish appendiceal endometriosis with intestinal
metaplasia from intestinal mucinous neoplasms. The present study
reports a case of appendiceal endometriosis clinically presenting
with recurrent bouts of abdominal pain diagnosed preoperatively as
chronic appendicitis. The present study was approved by the Clinic
Trial Ethics Committee of Longgang District People's Hospital,
Shenzhen, China (approval no. 2022013).
Case report
A 47-year-old G2P2 Cantonese woman was admitted to
Longgang District People's Hospital, Shenzhen, China on December
25, 2021. She was previously healthy and asymptomatic with no
family history of abdominal disease but presented with 3 months of
recurrent, dull abdominal pain in the right lower quadrant of her
abdomen. Associated symptoms, such as anorexia, nausea, vomiting,
diarrhoea, dysuria or dysmenorrhea, were not present. She had
regular menstrual cycles and was on the 14th day of her menstrual
cycle. A general physical examination did not reveal any abdominal
abnormalities. A gynaecological examination was not conducted due
to the absence of pelvic symptoms. Preoperative laboratory tests
showed elevated white blood cell count (16.5x1012/l) and
normal C-reative protein (CRP) level (0.6 mg/dl), haemoglobin and
albumin concentrations (125 and 45.4 g/l, respectively). The urine
pregnancy test was negative.
Laparoscopic appendectomy was performed following a
preoperative diagnosis of chronic appendicitis. Intraoperatively,
the appendix measured 5x0.9 cm at the widest diameter and exhibited
hyperaemia, oedema and had nearby circumferential serosal
adhesions. Haemorrhaging was noticeable on the serosal surface.
There were no signs of endometriosis throughout the other
intra-abdominal locations in the operative field. The appendix was
excised and fixed, embedded in wax, sectioned and stained with
haematoxylin and eosin (H&E). Serial sections (4 µm) were also
prepared for Masson's trichrome (Masson), Alcian blue-periodic acid
Schiff (AB-PAS) and immunohistochemistry staining. Masson and
immunohistochemistry staining were performed according to the
protocols established in Department of Pathology, Jinan University
School of Medicine previously (6).
The following primary antibodies were used: cluster of
differentiation (CD)10 (cat. no. SP67), CD34 (cat. no. BEQND/10),
estrogen receptor (ER; cat. no. SP1) were purchased from Roche
Diagnostics GmbH. Caudal type homeobox transcription factor 2
(CDX2; cat. no. EP25), cytokeratin 7 (CK7; cat. no. UMAB161), CK20
(cat. no. EP23), mucin 2 (MUC2; cat. no. Ccp58), α-SMA (cat. no.
HUC1-1) and paired-box (PAX)8 (cat. no. OTI6H8) were purchased from
OriGene Technologies, Inc. Sections were incubated in the presence
of the primary antibody overnight at 4˚C after a heat-induced
antigen retrieval step. Biotinylated goat anti-mouse or anti-rabbit
IgG was used as the secondary antibody. Streptavidin-peroxidase
system (cat. no. KIT-9901; Elivision plus; Maxim Biotech Inc.) and
diaminobenzidine tetrahydrochloride substrate (DAB kit, Maxim
Biotech Inc.) kits were used to visualize the staining. For AB-PAS
staining, deparaffinized sections were treated with high iron
diamine for 10 min followed by incubation with Alcian blue solution
at room temperature for 10-20 min. Schiff reagent was poured until
it fully covered the section and kept at 37˚C for 20 min and
counterstained with haematoxylin at room temperature for 1 min.
Images of the slides were captured using an Olympus
IX51epi-fluorescence microscope (Olympus Corporation).
The postoperative course was uneventful. The patient
was subjected to an ultrasonic imaging examination to search for
potential deep pelvic endometriosis, but no abnormalities were
found. The patient was discharged without any adjuvant treatment
and denied the recurrence of abdominal pain during a regular
follow-up for 12 months.
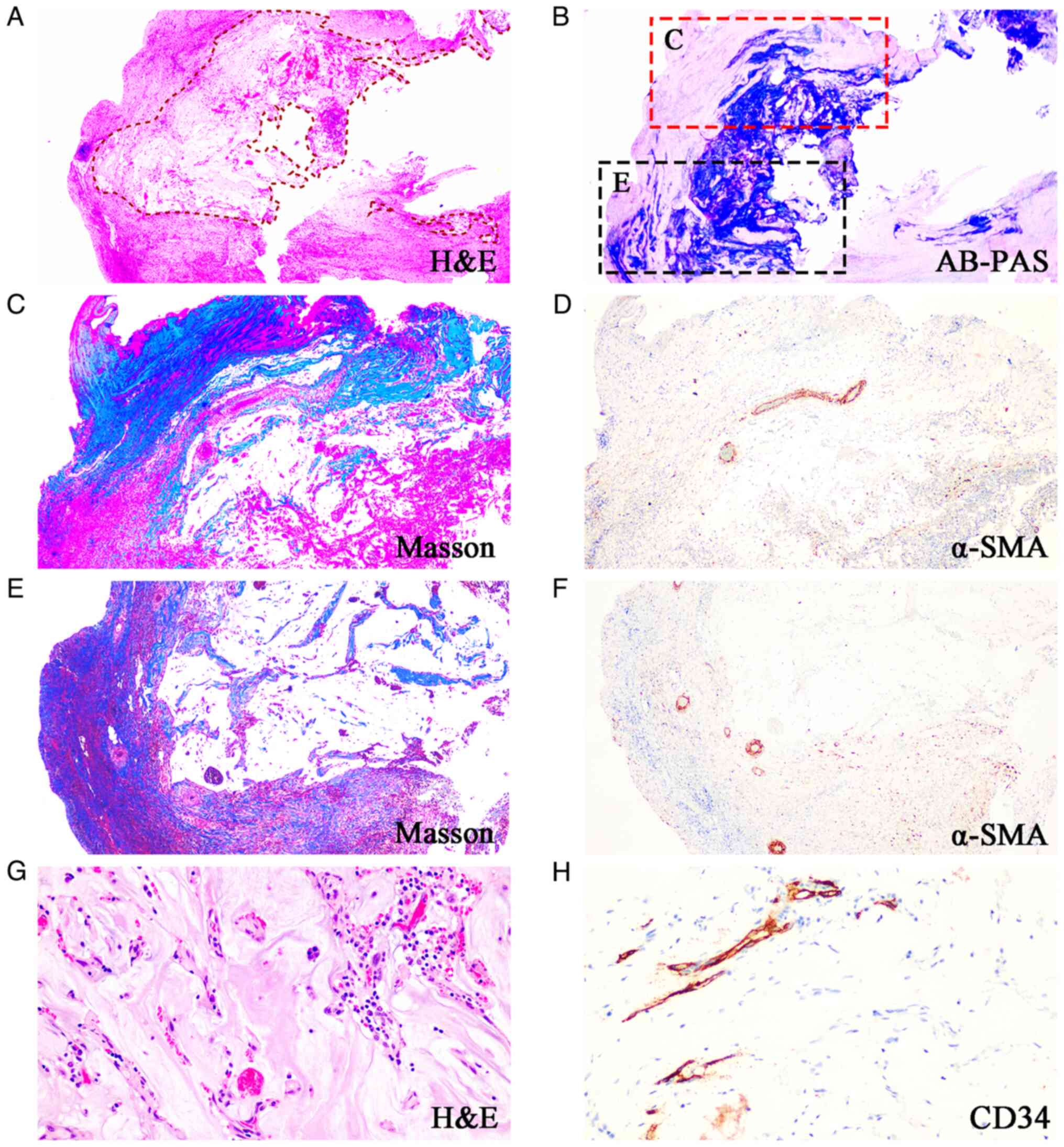
Gross evaluation revealed that the appendix was
torn, with a greyish-black colour. No masses, cysts or luminal
obliteration were present. Microscopic examination revealed no
chronic appendicitis and few lymphoid follicles in the mucosa.
However, there was an irregularly thickened wall where clusters of
glands were embedded in the mucosa, muscular and serous layers. The
scattered glands were composed of simple cuboidal epithelium. A
densely packed, basophilic stromal component was present. All these
findings suggest endometriosis.
Furthermore, the endometriosis was composed of
regional intestinal-type epithelium, lined by tall mucinous cells
or goblet cells (Fig. 1A and
B). The focal intestinal-type
epithelium bordered the endometrial-type endometrium.
Immunohistochemical staining analyses demonstrated an inverse
staining pattern for the endometrial and intestinal epithelium. The
endometrial stromal cells expressed CD10 (Fig. 1C). The simple low cuboidal
epithelial cells expressed CK7, PAX8 and ER (Fig. 1D-F), consistent with Müllerian
origin. The mucinous epithelium showed the following contrasting
pattern: Lack of expression of CK7, PAX8 and ER and expression of
CK20, CDX2 and MUC2 (Fig. 1D-I),
and negative for MUC5AC and MUC6 (data not shown), consistent with
intestinal origin. The intestinal-type glands were confirmed to be
endometriosis as abundant clusters of CD10-positive stromal cells
were found.
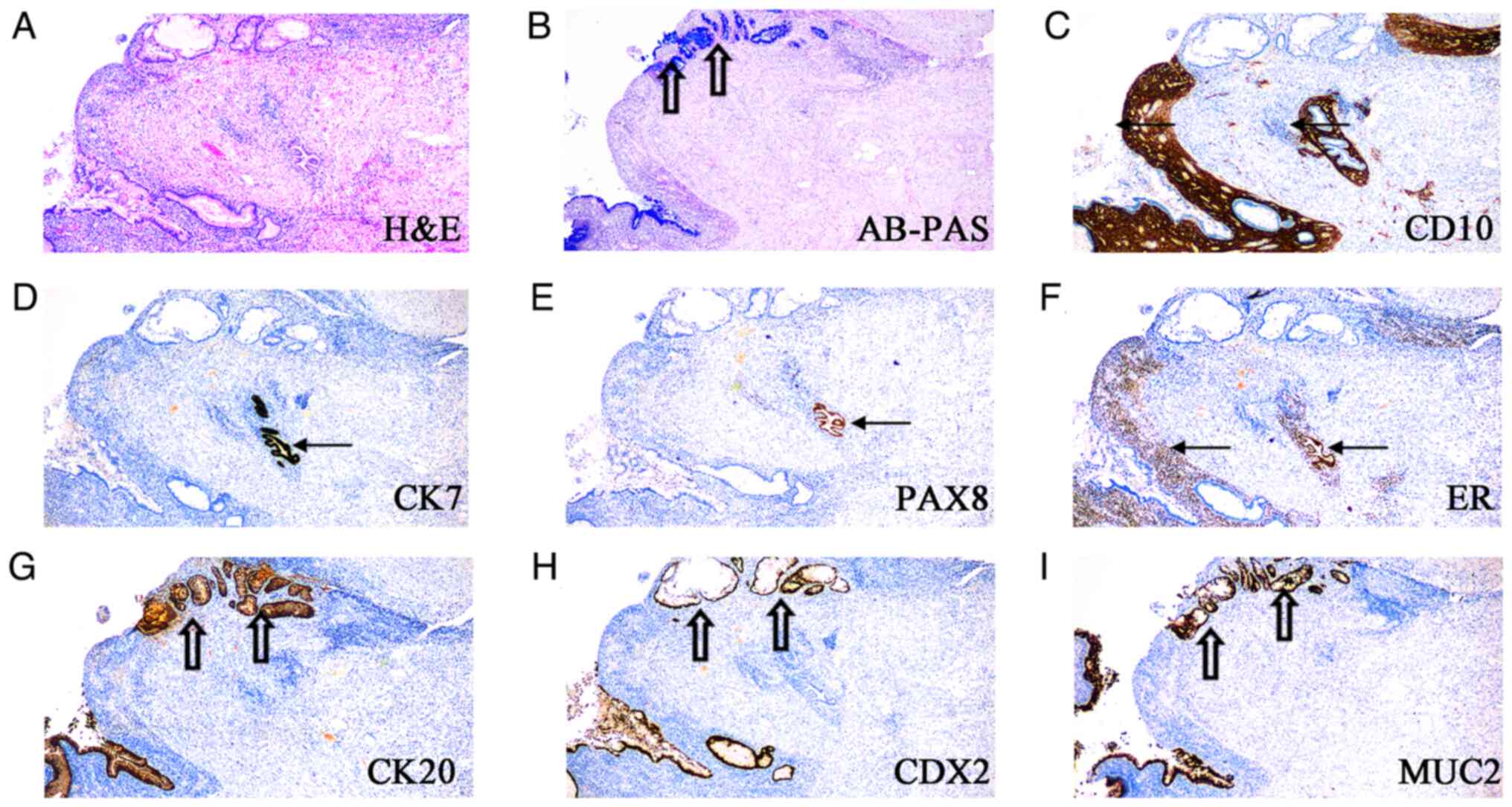 | Figure 1Endometriosis in the appendiceal wall,
partially lined with glands lined by endometrial-type and
intestinal-type epithelium. (A) H&E staining, (B) AB-PAS
staining, immunohistological staining for (C) CD10, (D) CK7, (E)
PAX8, (F) ER, (G) CK20, (H) CDX2 and (I) MUC2. Endometrial-type
gland (arrow) and intestinal-type gland (hollow arrow).
Magnification, x40)AB-PAS, Alcian blue-periodic acid-Schiff; CK,
cytokeratin; CDX2, caudal type homeobox transcription factor 2;
H&E, Hematoxylin and eosin; MUC2, mucin 2; PAX8, paired-box 8;
ER, estrogen receptor. (magnification, x40). |
In some segments, mucus filled the appendix. The
mucin was acellular and became deeply invasive, similar to what was
affected by appendiceal mucinous neoplasm. They dissected into the
appendiceal wall and there was loss of the normal architecture of
the appendix, with replacement by fibrosis and with no discernible
residual smooth muscle (Fig.
2A-F). Mucinous deposits are associated with a granulation
tissue-like response and numerous small capillaries containing red
cells were seen coursing through the mucin (Fig. 2G and H). These findings generated doubt
regarding the diagnosis of a mucinous neoplasm of the appendix
(2,3). However, no villous mucinous
proliferation was present. The vast majority of the intestinal-type
mucinous epithelium was rested in the stroma of endometriotic-type.
However, stroma of the endometriotic-type was absent and the
epithelium was in direct contact with intense fibrous tissue or
smooth muscle.
Discussion
Endometriosis is a benign condition characterised by
the presence of ectopic endometrial epithelium and/or stroma. It is
less frequently described in distant extra-abdominal sites.
Although appendiceal endometriosis is rare, several studies have
reported a high incidence of histopathologically proven appendiceal
endometriosis in women with chronic pelvic pain (1-4).
When forming the differential diagnoses, it is essential to rule
out appendiceal pathologies in women with endometriosis and chronic
pelvic pain. Some researchers argue that peri-appendiceal
inflammation plus adhesions may suggest appendiceal endometriosis
grossly (7). It is considered that
fibrotic changes result from a continuous inflammatory process,
which may represent chronic appendicitis or endometriosis (2). Thus, endometriosis of the appendix
can mimic appendicitis and gross abnormalities are essential.
However, the patient had a history of lower abdominal pain
unrelated to her menstruation and showed normal findings on gross
inspection, no diagnostic clues were present to cause suspicion of
endometriosis before microscopic examination, thereby no other
solutions contribute to predict such patients thus far.
Appendiceal endometriosis is rare and an appendiceal
mucinous neoplasm (AMN) is caused secondarily to the overt growth
of endometriosis (8). AMN is
classified as LAMN or high-grade appendiceal mucocele neoplasm and
forms following abnormal intraluminal accumulation of mucin; the
amount of mucin increasing as the tumour grows. Additionally,
dysplastic intestinal-type metaplasia may involve the epithelial
component of endometriosis (9),
which can resemble LAMN (8).
Previous reports suggest that mucinous neoplasms produce abundant
extracellular mucin and have clinically aggressive behaviour,
infiltrating through the appendiceal wall (2,3,7).
Acellular mucin has been considered as a risk for tumour recurrence
and AMN staging (10). A breach in
the appendix wall with acellular mucinous secretions may prompt
consideration for appendiceal neoplasms, especially invasive types
(5). However, in the present case,
the acellular mucin lakes infiltrating the appendix wall with
granulation tissue-like response and neovascularization and the
presence of appendiceal perforation, are uncommon hispathological
findings in endometriosis, making distinction from a LAMN difficult
in the setting of abundant extracellular mucin. Immunohistochemical
staining of CK7, PAX8, CD10 and ER may help diagnose an endometrial
origin. Even if there is no remarkable cuff of endometrial stroma
surrounding mucinous glands, lack of a recognisable intestinal
primary lesion and the presence of conventional endometriosis will
be helpful in diagnosis (1).
Acellular mucin with neovascularization has rarely described in
endometriosis, but together these data may suggest a possible role
for chronic inflammation in mucin production in endometriosis.
Hapke and Bigelow (11) noted that the appendiceal pathology
originates from appendicitis or luminal obstruction caused by the
adhesion formed around the appendix in patients with endometriosis,
as occurs with other diseases. The mucin products in in the present
case appeared obstructive and dissected through the wall of the
appendix, which may be related to intestinal metaplasia. Although
no study, to the best of the authors' knowledge, has established
the mechanism by which it occurs, endometriosis with intestinal
metaplasia carries a significant risk of malignancy and is becoming
increasingly recognised (7,3).
Alterations in several genes are predisposing factors in the
progression of endometriotic lesions to endometrial or ovarian
cancer (9,12), such as KRAS mutations,
Microsatellite instability and DNA mismatch repair protein
deficiency (12-14).
In the appendix, a more advanced investigation is required to
elucidate the molecular genetic profiling involved in the
intestinal metaplasia of endometriosis.
In conclusion, the present study presented a rare
case of endometriosis with intestinal metaplasia. Lesions are
usually superficial and small in previously reported cases but are
deeply invasive, similar to that which was affected by appendiceal
mucinous neoplasm in the present case. Diagnosable imaging
findings, histological and immunohistochemical features facilitate
a precise diagnosis for this rare case. An awareness that
endometriosis with intestinal metaplasia may involve the appendix
and mimic an appendiceal mucinous neoplasm is vital for preventing
misdiagnosis and guiding patient management. More studies are
warranted to evaluate the molecular profiles of intestinal
metaplasia in appendiceal endometriosis for early detection.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by National Natural
Science Foundation of China (grant no. NSFC 81971395) and Guangdong
Natural Science Foundation (grant no. 2021A1515011221).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MW recruited the patient and obtained specimens. PL
conceived the idea, provided financial support and wrote
manuscript. BH and JL analysed the data and prepared the figures.
SW and PX performed the histological experiment. MW and PL confirm
the authenticity of all the raw data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Clinic Trial
Ethics Committee of Longgang District People's Hospital, Shenzhen,
China (approval no. 2022013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chitul M, Chivu M, Chitul A, Popa I,
Becheanu G, Cristian D and Grama F, Popa I, Becheanu G, Cristian D
and Grama F: Appendiceal endometriosis with intestinal metaplasia
mimicking appendiceal mucinous neoplasm-A case report and a concise
review for the practicing pathologist. Int J Surg Pathol: Jul 14,
2022 (Epub ahead of print).
|
|
2
|
Klingbeil KD, Azab B and Moller MG:
Low-grade appendiceal mucinous neoplasm and endometriosis of the
appendix. World J Surg Oncol. 15(226)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alboni C, Spanò Bascio L, Camacho Mattos
L, Gallo G, Botticelli L, Cabry F, Facchinetti F and Gelmini R:
Ileocecal deep infiltrating endometriosis with intestinal mucinous
metaplasia and high-grade dysplasia. J Obstet Gynaecol.
42:1593–1596. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vyas M, Wong S and Zhang X: Intestinal
metaplasia of appendiceal endometriosis is not uncommon and may
mimic appendiceal mucinous neoplasm. Pathol Res Pract. 213:39–44.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Valasek MA and Pai RK: An update on the
diagnosis, grading, and staging of appendiceal mucinous neoplasms.
Adv Anat Pathol. 25:38–60. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gao L, Chen H, Liu J, Wang M, Lin F, Yang
G, Lash GE and Li P: Extravillous trophoblast invasion and
decidualization in cesarean scar pregnancies. Acta Obstet Gynecol
Scand. 101:1120–1128. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mai KT and Burns BF: Development of
dysplastic mucinous epithelium from endometriosis of the appendix.
Histopathology. 35:368–372. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kang DW, Kim BH, Kim JM, Kim J, Chang HJ,
Chang MS, Sohn JH, Cho MY, Jin SY, Chang HK, et al:
Gastrointestinal Pathology Study Group of the Korean Society of
Pathologists. Standardization of the pathologic diagnosis of
appendiceal mucinous neoplasms. J Pathol Transl Med. 55:247–264.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tomohiro M, Matsumoto T, Miura R, Oguri Y,
Yokoi A, Tochimoto M and Saegusa M: Alterations in β-catenin,
microsatellite instability, and HNF-1β levels are independently
associated with ovarian endometriosis-associated tumorigenesis. Hum
Pathol. 89:10–23. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Umetsu SE and Kakar S: Staging of
appendiceal mucinous neoplasms: Challenges and recent updates. Hum
Pathol. 132:65–76. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hapke MR and Bigelow B: Mucocele of the
appendix secondary to obstruction by endometriosis. Hum Pathol.
8:585–589. 1977.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Anglesio MS, Papadopoulos N, Ayhan A,
Nazeran TM, Noë M, Horlings HM, Lum A, Jones S, Senz J, Seckin T,
et al: Cancer-Associated Mutations in Endometriosis without Cancer.
N Engl J Med. 376:1835–1848. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yoo JY, Kim TH, Fazleabas AT, Palomino WA,
Ahn SH, Tayade C, Schammel DP, Young SL, Jeong JW and Lessey BA:
KRAS Activation and over-expression of SIRT1/BCL6 contributes to
the pathogenesis of endometriosis and progesterone resistance. Sci
Rep. 7(6765)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Misdraji J, Burgart LJ and Lauwers GY:
Defective mismatch repair in the pathogenesis of low-grade
appendiceal mucinous neoplasms and adenocarcinomas. Mod Pathol.
17:1447–1454. 2004.PubMed/NCBI View Article : Google Scholar
|