Introduction
Distal extremity swelling with pitting edema, also
termed remitting seronegative symmetrical synovitis with pitting
edema (RS3PE) syndrome, is a well-recognized extra-articular
feature of rheumatoid arthritis (RA), first described by Kalliomaki
and Vastamaki in 1968(1), although
its association with psoriatic arthritis (PsA) has been
demonstrated to be atypical (2-9).
Unlike RA with pitting edema, in PsA the upper limbs are the most
common sites of involvement, with the right side being
preferentially affected. Approximately half of the patients with
PsA have bilateral edema; to the best of our knowledge, no
correlation has been established between the severity of the
arthritis and the characteristic features of pitting edema in PsA
(3,8,10).
The management of RS3PE syndrome in patients with
PsA presents a challenge. In the majority of cases, the
introduction of, or a change in, conventional synthetic
disease-modifying antirheumatic drug (csDMARD) therapy has been
demonstrated not to result in any improvements in edema due to the
underlying pathogenesis (11). A
total of two main underlying mechanisms have been demonstrated to
be associated with RS3PE in patients with PsA: Defective lymphatic
drainage and inflammation of the tenosynovial structures (5). Magnetic resonance imaging (MRI) scans
and lymphoscintigraphy are used to differentiate between the two
different mechanisms, thus, these techniques may be of therapeutic
value (5). Inflammation of the
tenosynovial structures has been demonstrated to respond well to
csDMARD therapy, whereas defects in the lymphatic vessels do not
appear to be responsive (3).
Biological (b)DMARDs have been established as a therapy for the
treatment of rheumatic disorders and case reports have demonstrated
the use of bDMARDs in the treatment of patients with PsA and
pitting edema (10,12). The aim of the present study was to
systematically analyze the medical data of patients with PsA, with
or without RS3PE syndrome, from The Affiliated Hospital of Qingdao
University (Qingdao, China).
Patients and methods
Patients
Medical charts of consecutive in- and outpatients
with PsA admitted to The Affiliated Hospital of Qingdao University
(Qingdao, China) between September 2008 and September 2018 were
systematically analyzed, including demographic data, clinical
features, laboratory findings (including blood tests) and treatment
strategies, as well as the effectiveness of the treatment. PsA was
diagnosed according to the criteria for the classification of PsA
(13), and disease activity was
evaluated according to the Disease Activity Score using 28 joint
counts (DAS28) guidelines (14).
The diagnosis of RS3PE syndrome was confirmed by diffuse
unsymmetrical or symmetrical swelling of the upper or lower
extremities, or both, usually distributed along a well-defined
tenosynovial structure associated with pitting (15), at the time of admission or recorded
in the chart of the patient. In patients with RS3PE syndrome, a
color Doppler ultrasound was used to exclude venous or arterial
occlusion and to evaluate the joints involved. Furthermore,
quantitative 99mTc-labelled nanocolloid
lymphoscintigraphy and MRI were performed on the affected sites to
reveal the underlying mechanism. Patients with PsA that met both
the inclusion and exclusion criteria were included in the present
study. The inclusion criteria were as follows: i) Complete clinical
data for the patient was available; and ii) the patient was aged
≥14 years, if the patient was aged <18 years, the consent of the
patient's guardian was obtained). The exclusion criteria were as
follows: i) History of alcohol, drugs or chemical abuse; ii) the
patient had been previously diagnosed with cancer, primary or
secondary immunodeficiency or other autoimmune disease; iii) venous
or arterial occlusion was present and iv) incomplete clinical data
was available for the patient.
The patients were prescribed sulfasalazine (SSZ; 2
g/day) and methotrexate (MTX; ≤15 mg/week), both in combination
with non-steroidal anti-inflammatory drugs (NSAIDs; diclofenac
sodium at a dose of 75 mg/day or celecoxib at a dose of 200 mg/day)
to control the PsA. If the edema remained unchanged after 1 month
following the aforementioned prescribed course of treatment,
etanercept (ETN) was administrated subcutaneously at a dose of 50
mg/week. The present study was performed following approval by the
Medical Ethics Committee of The Affiliated Hospital of Qingdao
University (approval no. QYFY WZLL 23519). Written informed consent
was obtained from all participants in the study.
Statistical analysis
Data analysis was performed using SPSS 23.0 for
Windows (IBM Corp.). Continuous data with a normal distribution are
expressed as the mean ± standard deviation. To detect statistical
differences in age, duration of disease, ESR, CRP and DAS28,
two-tailed unpaired t-test was used. To detect statistical
differences in sex and HLA B27, χ2-test was used while
Fisher's exact test was used for other characteristics. P<0.05
was considered to indicate a statistically significant
difference.
Results
Differences between patients with PsA
with and without pitting edema
A total of 167 patients with PsA were evaluated,
comprising 99 men (59.3%) and 68 women (40.7%) with a mean age of
51.0±13.7 years (range, 14-86 years). Of the 167 patients with PsA,
16 (9.58%) also exhibited RS3PE syndrome during the course of the
illness (Table I). Female patients
were more likely to be affected with PsA and pitting edema compared
PsA without pitting edema (68.8 vs. 36.3%). Blood test results
revealed that patients with PsA and pitting edema presented with a
significantly higher erythrocyte sedimentation rate (ESR) and
concentration of C-reactive protein (CRP), as well as higher DAS28
score (a measure of disease activity in RA), compared with patients
with PsA without pitting edema. The measurements of the three
parameters for ESR, CRP and the DAS28 scores were 47.5±8.4 vs.
24.6±3.3 mm/h, 33.2±9.1 vs. 13.4±3.0 mg/l and 5.3±1.3 vs. 3±0.9 for
patients with PsA with and without pitting edema, respectively.
 | Table IClinical features of patients with PsA
with and without pitting edema. |
Table I
Clinical features of patients with PsA
with and without pitting edema.
| Characteristic | Patients with PsA and
pitting edema (n=16) | Patients with PsA
without pitting edema (n=151) | P-value |
|---|
| Sex, female/male | 11/5 | 57/94 | 0.016a |
| Mean age, years | 53.8±9.1 | 50.7±14.1 | 0.324b |
| Mean duration of
disease, years | 8.8±2.6 | 10.2±1.4 | 0.012b |
| Mean ESR, mm/h | 47.5±8.4 | 24.6±3.3 |
<0.001b |
| Mean CRP, mg/l | 33.2±9.1 | 13.4±3.0 |
<0.001b |
| RF, + | 1 | 11 | 1.000a |
| ACPA, + | 0 | 10 | 0.600a |
| HLA B27, + | 7 | 73 | 0.726a |
| Mean DAS28 | 5.3±1.3 | 3.0±0.9 |
<0.001b |
Characteristics of patients with PsA
and pitting edema
The present study included 16 patients with PsA (11
female and 5 male) and pitting edema (Table II). The mean age of the patients
was 53.81±9.1 years (range, 38-68 years), and the mean duration of
the disease was 8.81±10.2 years (range, 1-36 years). The onset of
pitting edema was associated with the disease activity of PsA. In
patients with PsA and pitting edema, the upper extremities were the
most common sites of involvement (12/16 patients), with the right
side (11/16 patients) being preferentially affected, and the lower
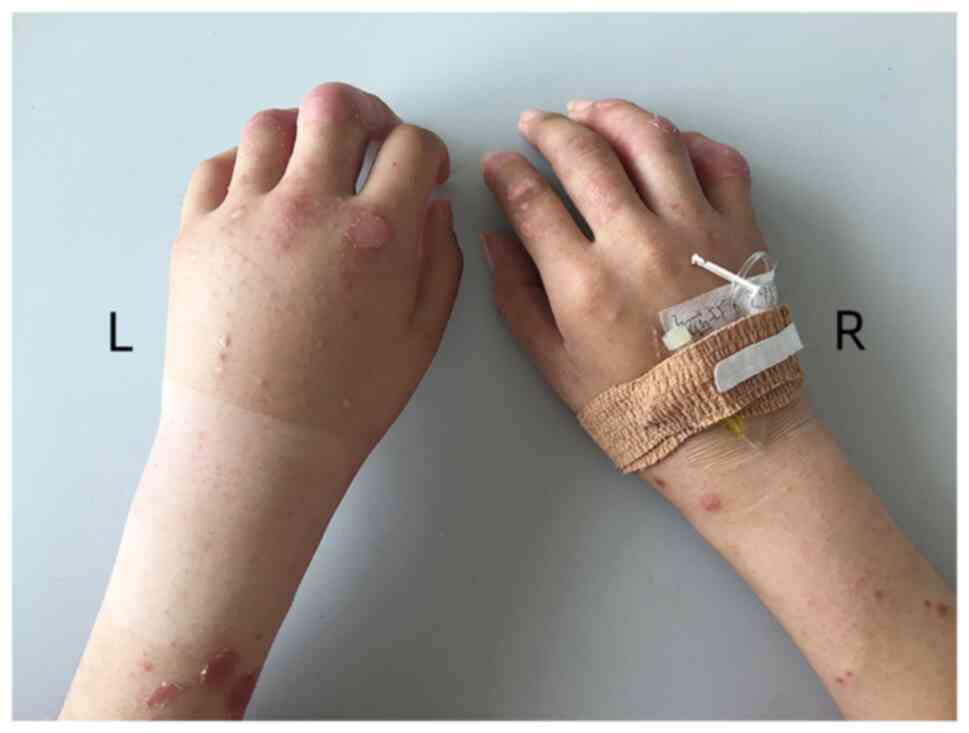
extremities were involved in 5 (27.8%) episodes (Fig. 1). The involvement of extremities
was bilateral in 2 (12.5%) episodes, and unilateral in 14 (87.5%)
episodes. The involvement of upper extremities was
symmetrical-bilateral in 1 (7.7%) episode, and asymmetrical in 12
(92.3%) episodes. Finally, lower extremities were bilaterally
affected in 1 (20%) episode and unilaterally affected in 4 (80%)
episodes.
 | Table IIClinical characteristics of 16 cases
of psoriatic arthritis with pitting edema. |
Table II
Clinical characteristics of 16 cases
of psoriatic arthritis with pitting edema.
| Case no. | Age, years | Sex | Localization of
edema | Distribution of
arthritis | Edema elimination
time, days |
|---|
| 1 | 48 | M | Right upper
limb | Spine and
sacroiliac | 6 |
| 2 | 63 | F | Right lower
limb | Spine, sacroiliac,
hip, knee and ankle | 9 |
| 3 | 57 | F | Left lower
limb | Spine, sacroiliac,
hip and knee | 10 |
| 4 | 65 | M | Right upper
limb | Sacroiliac, DIP,
PIP, MCP, elbow, wrist and knee | 14 |
| 5 | 42 | M | Right upper
limb | Spine, DIP, MCP,
elbow, knee and ankle | 7 |
| 6 | 55 | F | Right upper
limb | Spine, sacroiliac,
knee, ankle, DTP and PTP | 14 |
| 7 | 38 | F | Right upper
limb | Spine, DIP, PIP,
MCP, wrist, ankle and DTP | 7 |
| 8 | 52 | F | Right upper
limb | Sacroiliac, DIP,
elbow, knee, MTP, PTP and DTP | 12 |
| 9 | 55 | M | Bilateral lower
limb | Elbow, knee, ankle
and heel | 14a |
| 10 | 66 | F | Right upper
limb | Spine, knee, MTP
and PTP | 15 |
| 11 | 56 | M | Right upper
limb | Spine, DIP, elbow
and knee | 20 |
| 12 | 43 | F | Bilateral upper
limb | Sacroiliac, PIP,
MCP, MTP and PTP | 14 |
| 13 | 46 | F | Left lower
limb | Spine, DIP, elbow,
knee, ankle and heel | 14 |
| 14 | 59 | F | Right upper
limb | DIP, PIP, MCP,
knee, MTP and PTP | 11 |
| 15 | 68 | F | Left upper
limb | Spine, DIP, PIP,
MCP and knee | 8 |
| 16 | 48 | F | Right hand | Spine, PIP and
MCP | 7 |
All patients with PsA and pitting edema were
examined using ultrasound, MRI scans and lymphoscintigraphy, which
revealed that the associated edema might have been caused by
inflammation of the tenosynovial structures and normal lymphatic
drainage (Fig. 2).
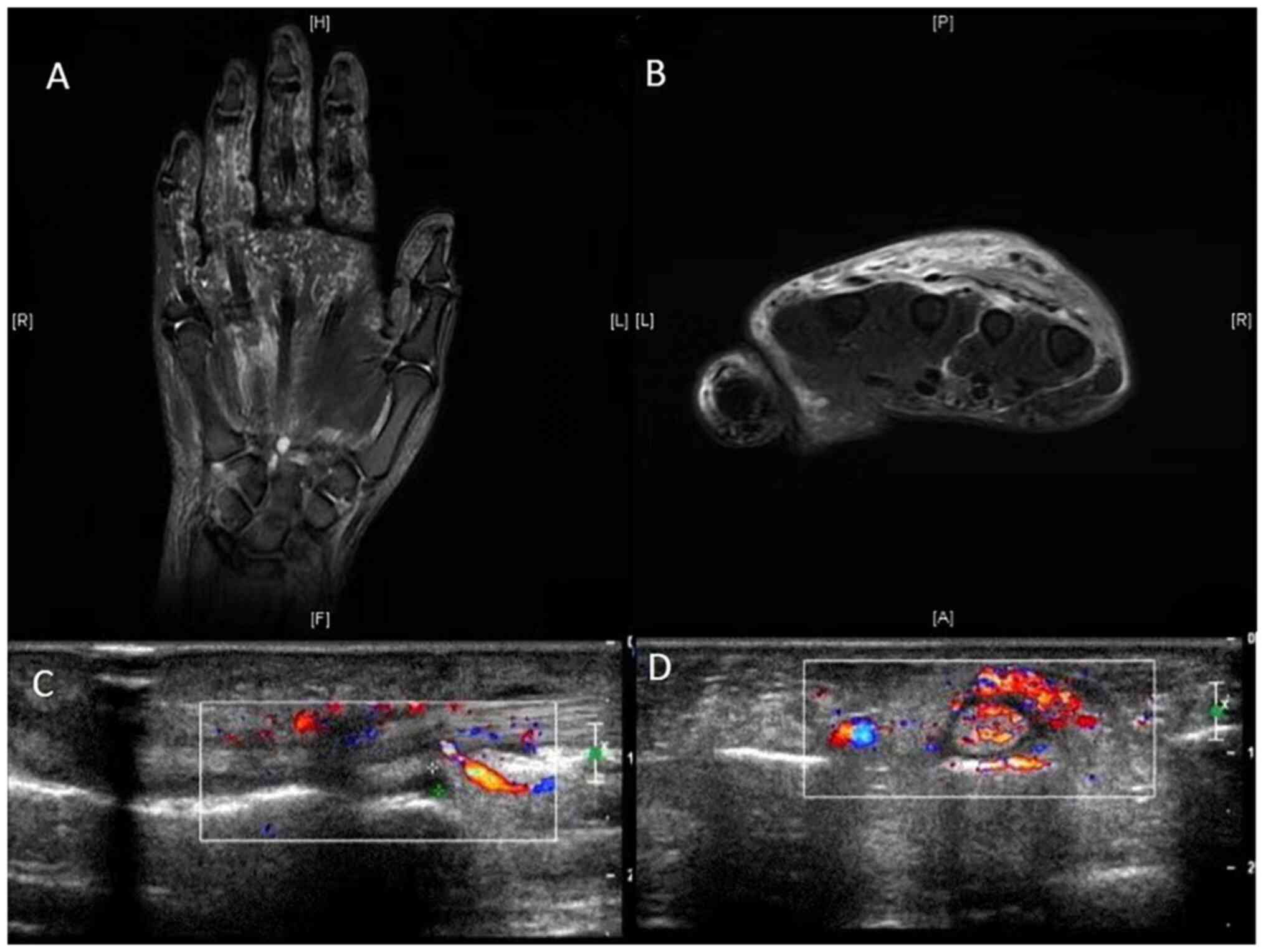 | Figure 2MRI and ultrasound of the hand in
patients with PsA and RS3PE. MRI (A) coronal view revealing
swelling of subcutaneous soft tissue of the back of the hand and
fingers, marked by diffuse strips of fat suppressed T2WI on the
ring finger and (B) transverse view revealing diffuse edema at the
proximal metacarpal. The subcutaneous soft tissue on the back of
the hand was clearly swollen. Ultrasound revealing (C) subcutaneous
soft tissue swelling on the back of the hand with an uneven
internal echo and (D) annular hypoechoic area around the extensor
tendon, indicating subcutaneous soft tissue swelling and an uneven
internal echo. MRI, magnetic resonance imaging; R, right; H, head;
L, left; P, posterior; F, foot; A, anterior; T2WI, T2-weighted
imaging. |
Management of patients with PsA and
pitting edema
During the course of treatment with NSAIDs SSZ and
MTX, 10 patients with PsA experienced RS3PE syndrome. Following
administration of ETN at a dose of 50 mg/week, the edema went into
complete remission within 2 weeks. For three patients where the
features and symptoms of PsA developed concurrently with RS3PE
syndrome, after having been prescribed with NSAIDs for 1 month, the
symptoms of PsA went into partial remission, but this therapy
failed to adequately control edema. Subsequently, ETN was
administered at a dose of 50 mg/week and the symptoms of edema were
eliminated after 6-15 days. In 3/16 (18.8%) of the patients (all
female), RS3PE syndrome presented as a first, isolated
manifestation of PsA. These patients were treated with NSAIDs for a
period of 1 month without any effect on the edema; after this time,
ETN was administered subcutaneously at a dose of 50 mg/week. The
episodes of edema were relieved after 7-21 days; however, the
symptoms of arthritis were only modestly improved after 1 month. In
one patient, the episodes of distal swelling with pitting edema
went into relapse after the therapy had been stopped (Fig. 3).
All patients with PsA and RS3PE syndrome were
available for follow-up and completed a 4-year follow-up; no
malignancy was detected in any of these patients (Fig. 3).
Discussion
PsA is an inflammatory rheumatic disorder with
unknown etiology and a heterogeneous clinical spectrum of symptoms.
The prevalence rates of PsA (after 1987 until December 2006) varied
between 0.001% (Japan) to 0.42% (Italian), and was characterized by
having arthritis in association with psoriasis (16). In certain patients, psoriasis is
associated with arthritis (5-42% of patients) (17). Nearly 15% of patients with PsA
experience onset of arthritis prior to the onset of psoriasis
(18). PsA belongs to a group of
conditions collectively termed spondyloarthritis. A total of five
conditions have been defined: Mono- or oligoarticular,
polyarticular, distal interphalangeal joint predominant disease,
axial disease with or without associated peripheral arthritis and
arthritis mutilans (19).
Regarding the immunopathogenesis of RS3PE syndrome,
TNF-α exerts a role in cartilage degradation via promotion of the
production of matrix metalloproteinases (MMPs), which induces
cartilage erosion and increases the expression of both vascular
endothelial growth factor (VEGF) and transforming growth factor-β
(TGF-β) (20). VEGF and TGF β are
more highly expressed in PsA synovium, thus illustrating the
increased vascularity with a representational winding of the
vascular bed of PsA synovium with respect to RA (21,22).
Within the joint, TNF-α overexpression induces increased production
of MMPs and cartilage destruction, thereby causing abnormal bone
remodeling that is a characteristic feature of PsA.
RS3PE syndrome is a non-specific clinical feature
occurring in a broad spectrum of disorders with an incidence rate
of ~0.09% in the elderly (age ≥60 years), with a higher rate of
onset in males compared with females (23,24).
Retrospective and perspective studies have indicated that the mean
prevalence rate of cancer in RS3PE syndrome is 20% (15,25-27).
In the present study, RS3PE syndrome was observed in 16 patients
with PsA, who were followed up for at least 4 years, and no
malignancy was discovered. Other than neoplasms, various types of
rheumatic disease (e.g. Sjögren's syndrome, ankylosing spondylitis,
reactive arthritis, polymyalgia rheumatica, sarcoidosis etc.)
occurring in RS3PE syndrome have been reported (28-32).
To date, only a limited number of studies have been published on
PsA and RS3PE syndrome (2-9).
One case-control study (33)
demonstrated that 21% of patients with PsA have RS3PE syndrome;
furthermore, in 20% of patients, this feature is the first
presentation of PsA, edema is unilateral, and the lower extremities
are most commonly involved (33).
In the present case series, 9.6% of patients with PsA exhibited
RS3PE syndrome; for 18.8% of the patients with this feature, it
presented as the first, isolated manifestation of PsA. Furthermore,
onset of pitting edema was associated with disease activity and the
upper extremities were predominantly asymmetrically affected in the
present study (Tables I and
II). This different distribution
of edema compared with another case-control study might be
explained by the choice of cases, and the difference of ethnicity
(Asian vs. Caucasian populations with RS3PE with vs. without PsA)
(23). The present study
hypothesized that edema in patients with PsA may be considered as
an atypical RS3PE syndrome, since it is mainly unilateral and
predominantly involves the upper limbs, rather than the lower limbs
(28).
Two different pathogenic mechanisms causing RS3PE
syndrome in patients with PsA have been proposed (5,9). As
aforementioned, VEGF and TNF-α have a role in the
immunopathogenesis of PsA, and these pro-inflammatory molecules may
contribute to development of atypical RS3PE syndrome in patients
with PsA (21). VEGF has a
vasodilatory effect, leading to an increase in vasopermeability
(34), which has an essential role
in development of the RS3PE syndrome (35). It has been postulated that
neoplasia, other rheumatic disease (e.g. polymyalgia rheumatica and
giant cell arteritis) and drugs (e.g. Tirofiban) might result in
the production of VEGF and other molecules (e.g. tumor necrosis
factor-α), which promote polyarthritis/polysynovitis and
subcutaneous pitting edema of the extremities (15,17,36).
MRI, ultrasound and lymphoscintigraphy are used to
reveal the underlying mechanisms of RS3PE syndrome as the
inflammation of tenosynovial structures is responsive to therapy,
whereas the lymphatic vessels are unresponsive to pharmacological
treatment (9). MRI is able to
discern inflammatory changes within peripheral joints, tendons
sheaths and entheses that occur early in inflammatory changes.
Furthermore, MRI is used to evaluate inflammation of the sacroiliac
joints and the spine. Compared with radiographs, MRI is more
sensitive in being able to detect early structural damage.
Therefore, it is a useful technique for detecting disease activity,
differential diagnosis and supporting the therapeutic
decision-making processes (37).
Doppler ultrasound has been validated less for PsA compared with
for RA (37). Ultrasound may be
used to identify and investigate enthesitis, joint effusions,
synovial proliferation and erosions. It may also be used to exclude
venous or arterial occlusion-induced edema. Through detecting
hyperemia, ultrasound indirectly reveals inflammation and
differentiates acute synovial proliferation from effusion (38). Ultrasound and MRI studies have been
useful in demonstrating subcutaneous edema and tenosynovitis, as
well as in detecting joint involvement at an early stage (37,38).
However, unlike these techniques, lymphoscintigraphy offers an
objective and reliable approach to diagnosing and characterizing
the severity of lymphedema, which is difficult to diagnose,
especially in its early stages. On the basis of the
lymphoscintigraphic image pattern, it is possible to determine
whether the limb swelling is due to lymphedema (39). In the present study, through the
use of ultrasound, MRI scans and lymphoscintigraphy, it was
possible to demonstrate that the patients with PsA and edema had
edema that may have been caused by inflammation of the tenosynovial
structures, rather than defective lymphatic drainage.
The management of RS3PE syndrome in patients with
PSA has not yet been standardized. The use of systemic
corticosteroids elicits a rapid response for patients with RS3PE
syndrome, although these drugs should be used with caution in
patients with PsA, as withdrawal may trigger a relapse of psoriasis
(12,40). csDMARDs are effective in treating
peripheral PsA, but were do not effectively treat RS3PE syndrome
(6); this is consistent with
results of the present study. Previous studies have provided
evidence for the efficacy of bDMARDs to control the symptoms of
PsA, and to either impede or arrest radiological disease
progression (12,41). In addition, case reports have
previously been published regarding the use of bDMARDs as a therapy
for patients with PsA and pitting edema (10,12).
The present study suggested that ETN might be used to treat the
atypical RS3PE syndrome that was resistant to csDMARD management in
patients with PsA.
However, there were a number of limitations
associated with the present study. Firstly, the present study was a
single-center study, so it was difficult to avoid the problem of
small sample size. In the future, multi-center studies with large
sample sizes should be conducted. Inclusion of other ethnic groups
and regions should be considered in future studies to validate the
results of the present study. At the same time, prospective studies
should be performed. Given that the precise mechanism of
pathogenesis of PsA has yet to be fully elucidated, future research
should investigate other kinds of biological agent for treatment of
distal extremity swelling with pitting edema in patients with PSA
that responds inadequately to conventional therapy. Future advances
in genetic analysis and targeted therapies may facilitate genetic
and immune profile modification therapies.
In conclusion, distal extremity swelling with
pitting edema in patients with PsA is an atypical RS3PE syndrome
that may be initially apparent as a symptom of PsA. TNFi may be an
effective treatment.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant nos. 81241094 and 81671600) and
The Natural Science Foundation of Shandong Province (grant no.
ZR2016HM13).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CZ conceived and designed the study and wrote the
manuscript. BingL, YY and KY analyzed and interpreted data for the
work. BZ performed the investigation. BinL contributed to study
design and interpretation of data and edited the manuscript. BingL
and BinL confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted following approval
by the Medical Ethics Committee of The Affiliated Hospital of
Qingdao University (approval no. QYFY WZLL 23519).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kalliomaki JL and Vastamaki M: Chronic
diffuse oedema of the rheumatoid hand: A sign of local lymphatic
involvement. Ann Rheum Dis. 27:167–169. 1968.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Salvarani C, Macchioni PL, Veneziani M,
Rossi F, Lodi L, Baricchi R, Boiardi L and Portioli I: Upper limb
lymphedema in psoriatic arthritis. J Rheumatol. 17:273–274.
1990.PubMed/NCBI
|
|
3
|
Mulherin DM, FitzGerald O and Bresnihan B:
Lymphedema of the upper limb in patients with psoriatic arthritis.
Semin Arthritis Rheum. 22:350–356. 1993.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kiely PD, Bland JM, Joseph AE, Mortimer PS
and Bourke BE: Upper limb lymphatic function in inflammatory
arthritis. J Rheumatol. 22:214–217. 1995.PubMed/NCBI
|
|
5
|
Salvarani C, Cantini F, Olivieri I,
Niccoli L, Senesi C, Macchioni L, Boiardi L and Padula A: Distal
extremity swelling with pitting edema in psoriatic arthritis:
Evidence of 2 pathological mechanisms. J Rheumatol. 26:1831–1834.
1999.PubMed/NCBI
|
|
6
|
Bohm M, Riemann B, Luger TA and Bonsmann
G: Bilateral upper limb lymphoedema associated with psoriatic
arthritis: A case report and review of the literature. Br J
Dermatol. 143:1297–1301. 2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Diez-Porres L, Munoz-Fernandez S, Aguado
P, Alonso M and Martin-Mola E: Remitting seronegative symmetrical
synovitis with pitting oedema as the first manifestation of
psoriatic arthropathy. Rheumatology (Oxford). 41:1333–1335.
2002.PubMed/NCBI
|
|
8
|
Yamamoto T and Nishioka K: Psoriasis
arthropathy and lymphedema. J Dermatol. 29:812–814. 2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Quarta L, Corrado A, d'Onofrio F, Maruotti
N and Cantatore FP: Two cases of distal extremity swelling with
pitting oedema in psoriatic arthritis: The different pathological
mechanisms. Rheumatol Int. 30:1367–1370. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Almodovar R, Zarco P, Quiros FJ and
Mazzucchelli R: Infliximab treatment efficacy in lymphoedema
associated with ankylosing spondylitis. Rheumatology (Oxford).
43(1456)2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lubrano E, Perrotta FM, Scriffignano S,
Coates LC and Helliwell P: Sustained very low disease activity and
remission in psoriatic arthritis patients. Rheumatol Ther.
6:521–528. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lekpa FK, Economu-Dubosc A, Fevre C,
Claudepierre P and Chevalier X: Efficacy of etanercept in
lymphedema associated with psoriatic arthritis. J Rheumatol.
36:207–208. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Taylor W, Gladman D, Helliwell P,
Marchesoni A, Mease P, Mielants H and Group CS: Classification
criteria for psoriatic arthritis: Development of new criteria from
a large international study. Arthritis Rheum. 54:2665–2673.
2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Helliwell PS, FitzGerald O, Fransen J,
Gladman DD, Kreuger GG, Callis-Duffin K, McHugh N, Mease PJ, Strand
V, Waxman R, et al: The development of candidate composite disease
activity and responder indices for psoriatic arthritis (GRACE
project). Ann Rheum Dis. 72:986–991. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gan Y, Sun Y, Jin J, Wang Y, Chen J, Chung
Y, Li X and Ye H: bFGF could be a biomarker of malignancy in
RS3PE syndrome: An ambispective single-center cohort
analysis of 51 patients. Arthritis Res Ther. 23(261)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kerschbaumer A, Fenzl KH, Erlacher L and
Aletaha D: An overview of psoriatic arthritis-epidemiology,
clinical features, pathophysiology and novel treatment targets.
Wien Klin Wochenschr. 128:791–795. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gladman DD and Brockbank J: Psoriatic
arthritis. Expert Opin Investig Drugs. 9:1511–1522. 2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gottlieb AB, Bakewell C and Merola JF:
Musculoskeletal imaging for dermatologists: Techniques in the
diagnosis and management of psoriatic arthritis. Dermatol Ther
(Heidelb). 11:1199–1216. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Szczerkowska-Dobosz A, Krasowska D,
Bartosinska J, StawczykMacieja M, Walczak A, Owczarczyk-Saczonek A,
Reich A, Batycka-Baran A, Czajkowski R, Dobrucki IT, et al:
Pathogenesis of psoriasis in the ‘omic’ era. Part IV. Epidemiology,
genetics, immunopathogenesis, clinical manifestation and treatment
of psoriatic arthritis. Postepy Dermatol Alergol. 37:625–634.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kenzaka T: The relationship between
remitting seronegative symmetrical synovitis with pitting edema and
vascular endothelial growth factor and matrix metalloproteinase 3.
Intern Med. 59:1021–1022. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fearon U, Griosios K, Fraser A, Reece R,
Emery P, Jones PF and Veale DJ: Angiopoietins, growth factors, and
vascular morphology in early arthritis. J Rheumatol. 30:260–268.
2003.PubMed/NCBI
|
|
22
|
Veale DJ and Fearon U: The pathogenesis of
psoriatic arthritis. Lancet. 391:2273–2284. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bucaloiu ID, Olenginski TP and Harrington
TM: Remitting seronegative symmetrical synovitis with pitting edema
syndrome in a rural tertiary care practice: A retrospective
analysis. Mayo Clin Proc. 82:1510–1515. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kimura M, Tokuda Y, Oshiawa H, Yoshida K,
Utsunomiya M, Kobayashi T, Deshpande GA, Matsui K and Kishimoto M:
Clinical characteristics of patients with remitting seronegative
symmetrical synovitis with pitting edema compared to patients with
pure polymyalgia rheumatica. J Rheumatol. 39:148–153.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gisserot O, Cremades S, Landais C, Leyral
G, Bernard P and de Jaureguiberry JP: RS3PE revealing recurrent
non-Hodgkin's lymphoma. Joint Bone Spine. 71:424–426.
2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chiappetta N and Gruber B: Remitting
seronegative symmetrical synovitis with pitting edema associated
with acute myeloid leukemia. J Rheumatol. 32:1613–1614.
2005.PubMed/NCBI
|
|
27
|
Tunc SE, Arslan C, Ayvacioglu NB, Sahin M,
Akkus S and Yorgancigil H: Paraneoplastic remitting seronegative
symmetrical synovitis with pitting edema (RS3PE syndrome): A report
of two cases and review of the literature. Rheumatol Int.
24:234–237. 2004.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Schaeverbeke T, Fatout E, Marce S, Vernhes
JP, Halle O, Antoine JF, Lequen L, Bannwarth B and Dehais J:
Remitting seronegative symmetrical synovitis with pitting oedema:
Disease or syndrome? Ann Rheum Dis. 54:681–684. 1995.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Olivieri I, Padula A, Favaro L, Pierro A,
Oranges GS and Ferri S: Dactylitis with pitting oedema of the hand
in longstanding ankylosing spondylitis. Clin Rheumatol. 14:701–704.
1995.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cantini F, Niccoli L, Olivieri I, Barozzi
L, Pavlica P, Bozza A, Macchioni PL, Padula AA and Salvarani C:
Remitting distal lower extremity swelling with pitting oedema in
acute sarcoidosis. Ann Rheum Dis. 56:565–566. 1997.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Okumura T, Tanno S, Ohhira M and Nozu T:
The rate of polymyalgia rheumatica (PMR) and remitting seronegative
symmetrical synovitis with pitting edema (RS3PE) syndrome in a
clinic where primary care physicians are working in Japan.
Rheumatol Int. 32:1695–1699. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Joseph AD, Kumanan T, Aravinthan N and
Suganthan N: An unusual case of remitting seronegative symmetrical
synovitis with pitting edema: Case report and literature review.
SAGE Open Med Case Rep. 8(2050313X20910920)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cantini F, Salvarani C, Olivieri I,
Macchioni L, Niccoli L, Padula A, Falcone C, Boiardi L, Bozza A,
Barozzi L and Pavlica P: Distal extremity swelling with pitting
edema in psoriatic arthritis: A case-control study. Clin Exp
Rheumatol. 19:291–296. 2001.PubMed/NCBI
|
|
34
|
Ku DD, Zaleski JK, Liu S and Brock TA:
Vascular endothelial growth factor induces EDRF-dependent
relaxation in coronary arteries. Am J Physiol. 265:H586–H592.
1993.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Arima K, Origuchi T, Tamai M, Iwanaga N,
Izumi Y, Huang M, Tanaka F, Kamachi M, Aratake K, Nakamura H, et
al: RS3PE syndrome presenting as vascular endothelial growth factor
associated disorder. Ann Rheum Dis. 64:1653–1655. 2005.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Baumgartner I, Rauh G, Pieczek A, Wuensch
D, Magner M, Kearney M, Schainfeld R and Isner JM: Lower-extremity
edema associated with gene transfer of naked DNA encoding vascular
endothelial growth factor. Ann Intern Med. 132:880–884.
2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Eder L, Aydin SZ, Kaeley GS, Maksymowych
WP and Ostergaard M: Options for Assessing Joints and Entheses in
Psoriatic Arthritis by Ultrasonography and Magnetic Resonance
Imaging: How to Move Forward. J Rheumatol Suppl. 94:44–47.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Crespo-Rodriguez AM, Sanz Sanz J, Freites
D, Rosales Z, Abasolo L and Arrazola J: Role of diagnostic imaging
in psoriatic arthritis: How, when, and why. Insights Imaging.
12(121)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Szuba A, Shin WS, Strauss HW and Rockson
S: The third circulation: Radionuclide lymphoscintigraphy in the
evaluation of lymphedema. J Nucl Med. 44:43–57. 2003.PubMed/NCBI
|
|
40
|
Kleinert S, Feuchtenberger M, Kneitz C and
Tony HP: Psoriatic arthritis: Clinical spectrum and diagnostic
procedures. Clin Dermatol. 25:519–523. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kohm M, Burkhardt H and Behrens F:
Anti-TNFalpha-therapy as an evidence-based treatment option for
different clinical manifestations of psoriatic arthritis. Clin Exp
Rheumatol. 33 (Suppl 5):S109–S114. 2015.PubMed/NCBI
|