1. Introduction
Endometriosis is a common gynecological disease in
women of reproductive age that is characterized by the development
of functional endometrial tissue located outside the uterus
(1). Endometriosis is a
pathologically benign disease that is classified into ovarian
endometriosis, superficial peritoneal disease, or deep infiltrating
endometriosis. Endometriosis most commonly affects the ovaries and
is associated with pelvic pain, infertility, or malignant
transformation (1). Endometriosis
is a possible precursor lesion of endometriosis-associated ovarian
cancer (EAOC), including clear cell carcinoma of the ovary (CCC)
and endometrioid ovarian carcinomas (2). CCC is the most common subtype of EAOC
in Japan (70%) followed by endometrioid carcinoma (3).
The molecular mechanisms underlying the malignant
transformation of endometriosis remain unclear; however, redox
homeostasis imbalance might be involved. Endometriotic cysts
contain high levels of iron, and endometriotic cells survive in
reactive oxygen species (ROS)-rich environments (4). Hemoglobin, heme, and iron derivatives
cause DNA damage and mutations, which promotes endometrial cell
survival and proliferation and causes ectopic implantation
(5-7).
However, high levels of iron lead to cell death due to severe
oxidative stress. It is necessary to elucidate the adaptive
mechanisms that allow endometriotic cells to survive harsh
environments and the key mediators that regulate redox homeostatic
balance (7). Due to the greater
number of patients with CCC in Japan compared with Western
countries (25% vs. 8%), special attention has been paid to ensure
the early diagnosis of malignant transformation of endometriosis
(3).
The aim of this review is to summarize information
on the clinicopathological characteristics, molecular mechanisms
underlying redox homeostasis, and innovative diagnostic methods for
malignant transformation of endometriosis. Finally, we discuss the
current challenges and future directions.
2. Literature search
Search strategy and selection
criteria
A computerized literature search was performed to
identify relevant studies in English. The PubMed and Google Scholar
electronic databases were searched for studies published between
January 2000 and February 2022. The search terms included
endometriosis, endometriosis-associated ovarian
cancer, oxidative stress, antioxidant, energy
metabolism, macrophages, imaging, and
serodiagnosis. The references of each article were searched
to identify potentially relevant studies. Publications of original
studies and review papers were included. Given the heterogeneity in
the research theme, data from studies were synthesized using a
descriptive review design with narrative methods. Fig. 1 shows the first identification
phase that includes records identified through a database search.
Terms in the titles and abstracts were searched during the first
screening. During the second screening, duplicates were removed,
and titles, abstracts, and full-text articles were read to remove
inappropriate papers. The final eligibility phase included
full-text articles for analysis after excluding those wherein
detailed data cannot be extracted.
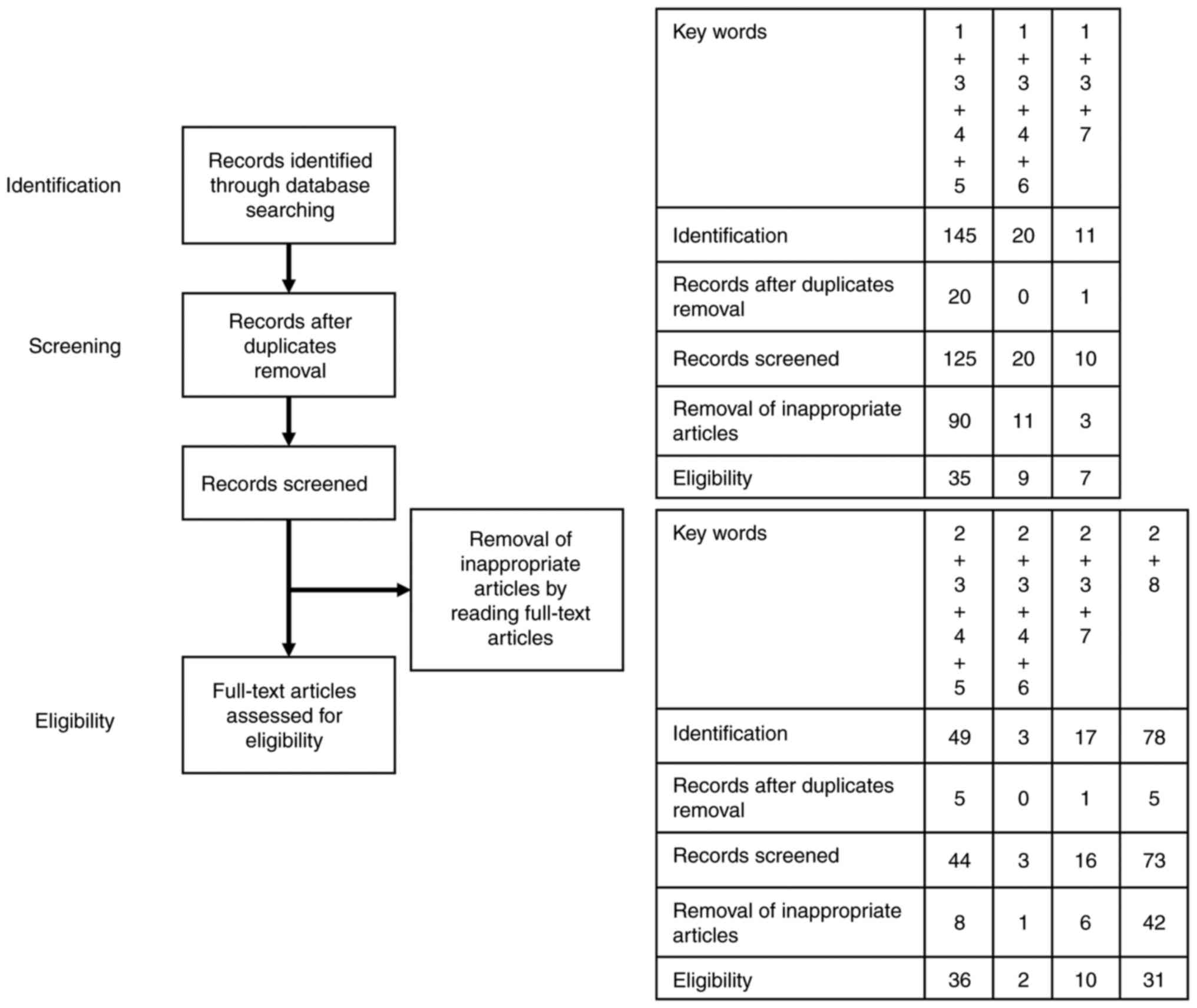 | Figure 1Number of articles identified. This
figure shows the number of articles identified by key word
combinations and the number of records identified through database
searching, records after duplicate removal, records screened,
removal of inappropriate articles by reading full-text articles and
full-text articles assessed for eligibility. Key words: 1,
‘endometriosis’; 2, ‘endometriosis-associated ovarian cancer’; 3,
‘oxidative stress’; 4, ‘antioxidant’; 5, ‘energy metabolism’; 6,
‘macrophages’; 7, ‘imaging’; and 8, ‘serodiagnosis’. For example,
‘1+3+4+5’ in key words means a combination of the key words 1
(‘endometriosis’), 3 (‘oxidative stress’), 4 (‘antioxidant’) and 5
(‘energy metabolism’). As a result of literature search using
‘1+3+4+5’, 145 articles were included in the identification step,
125 articles were selected in the screening step and finally 35
articles remained in the eligibility step. |
3. Current understanding of malignant
transformation of endometriosis
Current challenges in the malignant
transformation of endometriosis in clinical practice
Most cases of endometriosis are benign and regress
spontaneously after menopause. Sampson reported the first case of a
malignant neoplasm arising from pre-existing endometriosis in
1925(8). Malignant transformation
of endometriosis is rare, with an incidence of approximately
<1.0% (2,9). Advances in genomics and molecular
biology have made it possible to elucidate the potential mechanisms
in the malignant transformation of endometriosis. Some researchers
have proposed the clonal evolution model, wherein normal
endometrial cells transform into neoplastic cells via endometriosis
and atypical precursors based on oncogenic driver gene mutations
(i.e., AT-rich interacting domain-containing protein 1A [ARID1A]
gene) (10). Murakami et al
(11) recently proposed a
carcinogenic mechanism that ‘EAOC might not occur as a result of
malignant transformation of endometrial cysts and might be caused
by eutopic endometrial glandular epithelial cells that are refluxed
to engraft in the ovary’. Genomic and epigenomic studies are
required to better understand why and how endometriosis causes
malignant transformation.
Epidemiological studies revealed that hysterectomy
and unilateral oophorectomy reduces the risk of EAOC by 50-80%
(12,13). Therefore, more than half of cases
of EAOC originate from endometrial tissue fragments that have been
regurgitated during menstruation, while other cases may have been
caused by already existing endometriotic cysts (11). In fact, histopathological
examination revealed that some cases of EAOC showed a continuous
transition from benign endometriosis to atypical endometriosis and
finally to invasive carcinoma (14-16).
The prevalence of atypical endometriosis varies considerably,
ranging from 1.7 to 40% (17-20).
The difference in the results may be due to the lack of a unified
international consensus regarding the definition of atypical
endometriosis. In clinical practice, endometriosis is diagnosed
based on clinical symptoms combined with diagnostic techniques,
including transvaginal ultrasound (TVS) and blood biomarkers (e.g.,
carbohydrate antigen 125 [CA-125]) (21). TVS is the first-line diagnostic
method for discriminating benign from malignant ovarian tumors. As
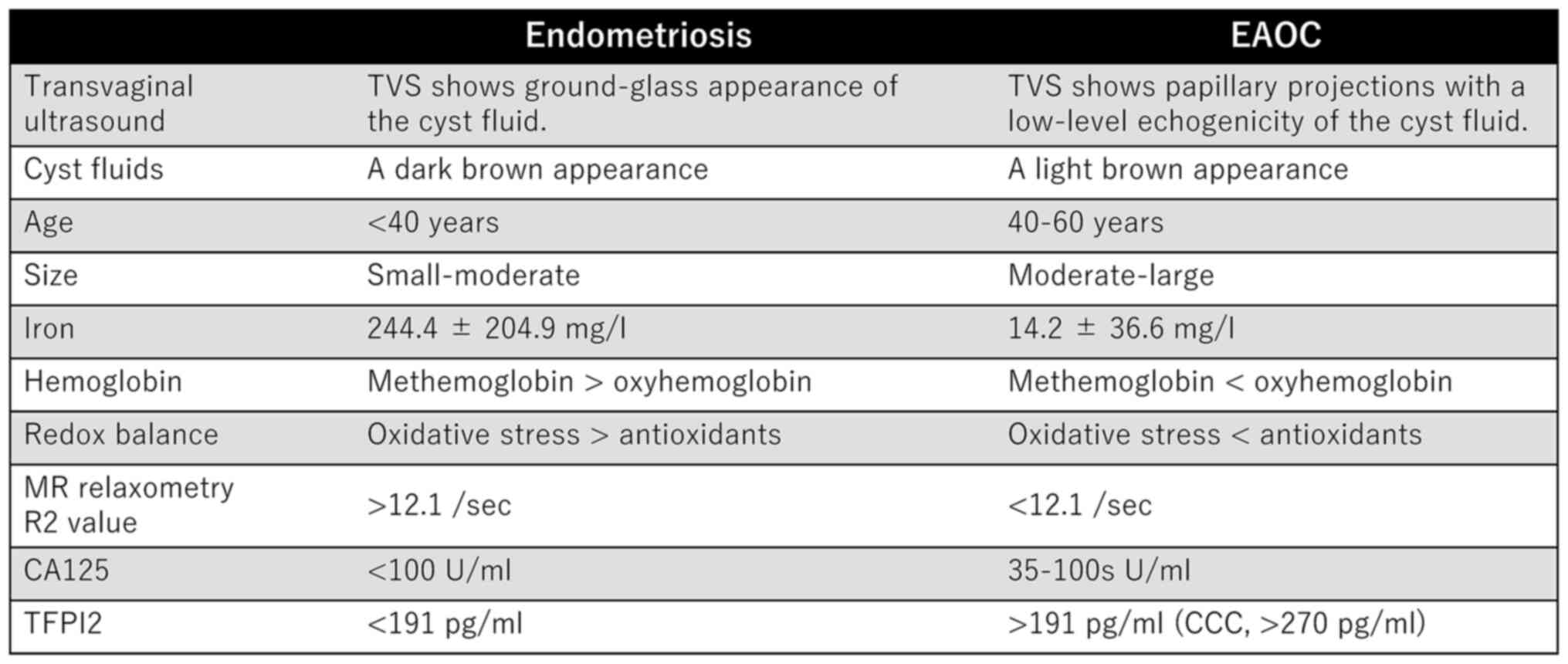
shown in Fig. 2, EAOC is more
common in women aged over 40 years, and the size of the cyst is
larger than that of endometriosis (9). Benign ovarian endometriosis may
pertain to any of the following: 1) pathologically benign
endometriosis; 2) endometriosis with atypical lesions; and 3)
endometriosis with clinically undetectable early-stage ovarian
cancer. Although endometriosis with atypical lesions and with
undetectable early-stage cancer may develop into ovarian cancer,
patients with these conditions cannot be diagnosed as having
cancer. Therefore, current screening options for early detection of
EAOC are warranted.
Ovarian cancer initiation and
progression via persistent oxidative stress mediated by iron
overload
Oxidative stress is caused by an imbalance in the
redox homeostasis between ROS overproduction and the antioxidant
defense system (22). Iron-induced
redox imbalance plays an important role in the pathophysiology of
endometriosis, resulting in either cell death (anti-tumorigenic) or
survival (pro-tumorigenic) (4,6,7).
Repeated hemorrhage occurs in endometriotic cysts and in the
peritoneal cavity during menstruation (2,7).
Hemoglobin, heme, and iron are released following hemolysis from
red blood cells (2,23). Cellular iron is imported through
enhanced divalent metal transporter-1 (DMT1) and exported through
ferroportin (FPN) (24). The
differential expression of transporters (i.e., DMT1 upregulation
and FPN downregulation) is associated with increased levels of
intracellular iron (24). Iron
content is reportedly elevated in the peritoneal cavity of women
with endometriosis, in endometriotic cysts, in macrophages
throughout the stroma of endometriotic cysts, and in the fimbriae
of the fallopian tubes (24,25).
Autoxidation (22) and the Fenton
reaction (26) from the
transformation of ferrous Fe2+ (oxyhemoglobin) to ferric
Fe3+ (methemoglobin) produce large amounts of superoxide
radicals (O2-) and hydroxyl radicals (OH).
Accumulation of oxidative stress biomarkers (e.g., methemoglobin,
8-hydroxy-2'-deoxyguanosine, and lipid ROS) and inactivation of
antioxidant enzymes (e.g., glutathione peroxidase 4) have been
noted in endometriotic cysts (22,27).
High levels of hemoglobin, heme, and iron derivatives in
endometriotic cysts induce redox imbalance (22), leading to cell death through
persistent DNA damage (7).
Moreover, excessive accumulation of lipid ROS triggers ferroptotic
cell death in an iron-dependent manner (28). In contrast, antioxidants protect
cells from DNA damage by neutralizing excess ROS (29). A sublethal dose of iron overload
participates in mutagenesis and aberrant pro-tumorigenic signaling
to activate cell survival and promote carcinogenesis (29,30).
Thus, low to moderate oxidative stress may contribute to the
development of EAOC (26,27,29-31).
This suggests that not only endometriosis but also conditions of
iron overload, such as hemochromatosis, viral hepatitis B and C,
and asbestos exposure, are risk factors for cancer development
(32).
Metallobiology revealed diverse features involved in
redox imbalance and the pathogenesis of malignant transformation of
endometriosis (27). Compared to
endometriosis, the contents of EAOC cysts have high antioxidant
capacity due to lower levels of iron (14.2±36.6 mg/l vs.
244.4±204.9 mg/l) and higher levels of oxyhemoglobin (33) (Fig.
2). The contents of endometriotic and EAOC cysts are often dark
and light brown, respectively, which may reflect iron
concentrations and hemoglobin species. A study examining the
expression of iron transport proteins showed that iron excretion is
greater in CCC cells than in endometriotic cells (24), indicating that the intracellular
levels of iron in CCC cells are maintained at low levels. Iwabuchi
et al (27) provided a
detailed characterization of hemoglobin species, such as
methemoglobin and oxyhemoglobin, in endometriotic and EAOC cysts.
The major components of hemoglobin species in endometriotic and
EAOC cysts are methemoglobin and oxyhemoglobin, respectively
(27) (Fig. 2). High levels of antioxidants allow
EAOC cells to escape the lethal effects of excessive oxidative
stress-induced cell death. Thus, iron levels should be maintained
within a narrow range to prevent endometriotic cell death, which
may in turn increase the risk of cancer progression when the
cellular antioxidant capacity exceeds ROS production (2,6,27,29).
Dual role of antioxidants in the
different stages of malignant transformation of endometriosis
Nagayasu et al (34) presented a comprehensive review on
the endogenous antioxidant defense systems in endometriosis. The
generated ROS can be scavenged by enzymatic (e.g., superoxide
dismutase [SOD], catalase, reduced glutathione [GSH], glutathione
peroxidase [GPX], and thioredoxin) and non-enzymatic (e.g.,
estradiol, melatonin, vitamin E, and vitamin C) antioxidants
(35,36). Increased levels of oxidants (e.g.,
iron, ROS, nitric oxide [NO], lipid peroxidation, or advanced
oxidation protein products) and decreased levels of antioxidants
(e.g., SOD, catalase, GSH, GPX, glutathione reductase, total
antioxidant capacity, vitamin C, or vitamin E) were observed in the
serum, peritoneal fluid, follicular fluid, and tissue samples
collected from patients with endometriosis compared to those in
patients without endometriosis (37-39).
Estradiol is a potent antioxidant that overcomes excessive
oxidative stress by directly scavenging ROS or increasing the
expression of SOD (34), and it is
also involved in the development, maintenance, and progression of
endometriosis. This may be the reason why endometriosis
spontaneously regresses after menopause.
Overproduction of ROS and upregulation of endogenous
antioxidant genes and signaling pathways are the main biological
features of human cancers (29).
Antioxidants have a dual role in tumor development and progression
as both a tumor suppressor and promoter (29). Since nuclear factor erythroid
2-related factor 2 (NRF2) and CD44 variant isoform 9 (CD44v9) are
well-studied regulators of antioxidants, we explain the role of
these genes as an example. In genetically engineered mouse model,
antioxidant (e.g., NRF2) depletion promotes carcinogenesis,
demonstrating that antioxidants are involved in inhibiting cancer
initiation and development (40).
For example, vitamin supplementation may decrease the risk of
developing human cancers (41). In
contrast, treatment with antioxidant inhibitors reduced the growth
of pre-existing cancer in xenograft animal models (42). Antioxidant inhibitors increase
intracellular ROS and accelerate oxidative damage that induces cell
death. For example, redox homeostasis in ovarian cancer is
regulated by endogenous antioxidants, including NRF2 and
CD44v9(29). Concurrent inhibition
of CD44v9 and NRF2 triggers apoptotic cell death via the
ROS-dependent p38 and p21 pathways (29). Animal experiments revealed that
sublethal oxidative stress (low to moderate toxicity) is involved
in the early phase of cancer initiation, while sufficient
antioxidant capacity is necessary in the late phase of cancer
progression. Similar results have been observed in changes in redox
homeostasis in the process of malignant transformation of
endometriosis (22,43). In the EAOC group, 8-OHdG levels in
cystic fluids were significantly decreased, while levels of
antioxidants (e.g., heme oxygenase-1 [HO-1], cytochrome P450
family, and glutathione transferase family) were increased compared
with those in the endometriosis group (22,43).
EAOC cells might be protected against excessive oxidative stress by
an effective antioxidant defense (22). A fine-tuned pattern in redox
homeostasis supports the idea that pro- and antioxidants may be
involved in the initiation and progression of EAOC, respectively
(7,23). EAOC is a disease that involves
redox homeostasis and has predominant antioxidant capacity. Taken
together, iron homeostatic pathways contribute to cancer initiation
and progression via two major processes (Fig. 3). In the first step, hemoglobin,
heme, and iron cause DNA damage and mutations by producing ROS,
leading to cancer initiation. In the next step, antioxidants
influence cancer progression.
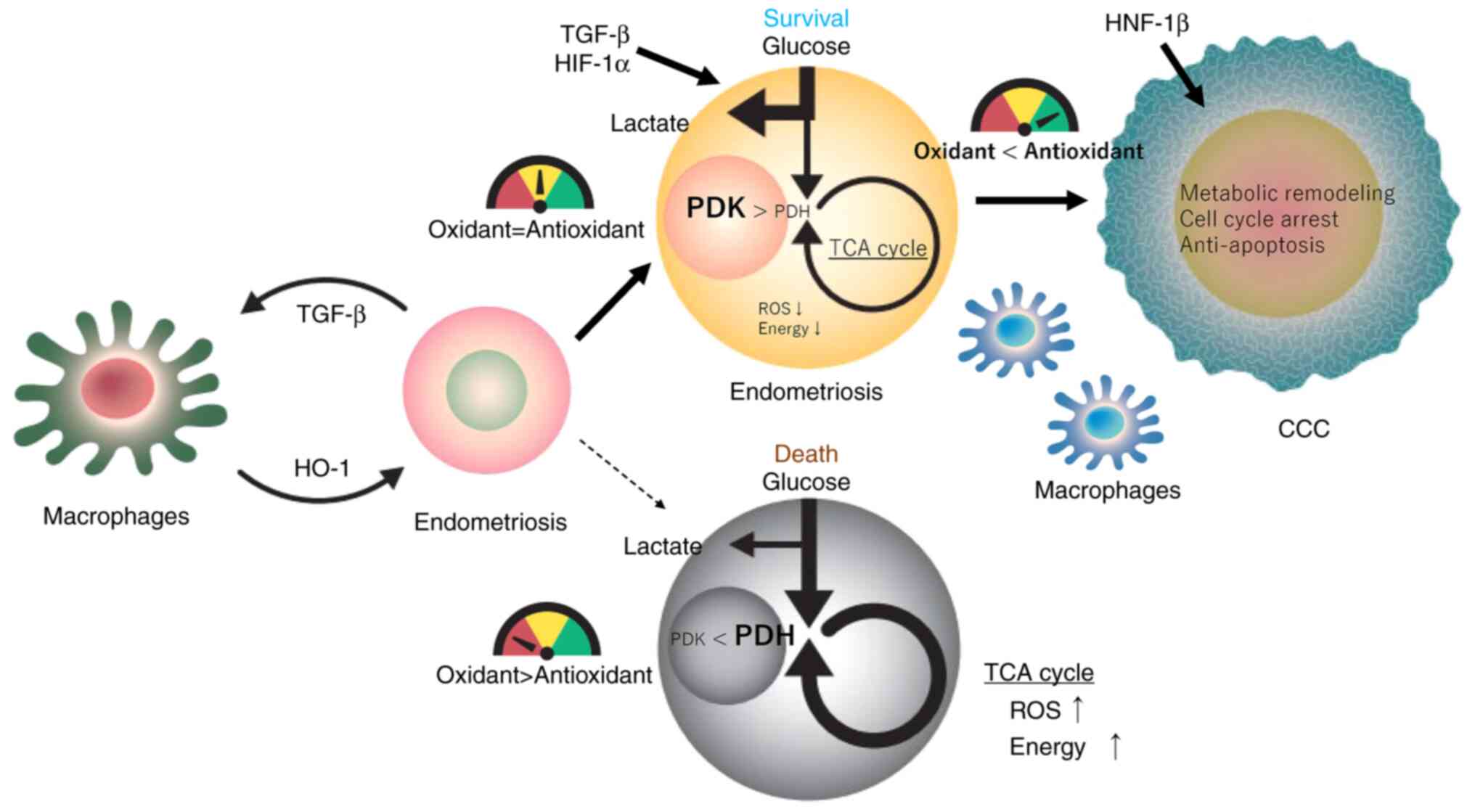 | Figure 3Redox balance and metabolic switch
regulating endometriotic cell fate (survival or death) during
malignant transformation. Redox and metabolic changes caused by
exposure of endometriotic cells to hemoglobin, heme and
iron-induced oxidative stress are described. Excessive oxidative
stress and a metabolic shift to mitochondrial oxidative
phosphorylation interfere with biological processes associated with
endometriotic cell growth and survival (oxidant > antioxidant).
Macrophages protect endometriotic cells from oxidative injury
through the upregulation of HO-1. TGF-β and HIF-1α induce
glycolysis in endometriotic cells and are associated with a
metabolic shift to anaerobic glycolysis, reduction of ROS levels,
and increased survival (oxidant=antioxidant). HNF-1β prevents
oxidative damage via metabolic remodeling, cell cycle arrest and
anti-apoptosis (oxidant < antioxidant). Redox imbalance may be
directly or indirectly involved in the malignant transformation of
endometriosis. The redox meter shows red when oxidative stress is
predominant, green when antioxidant is predominant and yellow when
redox homeostasis is maintained. CCC, clear cell carcinoma of the
ovary; HIF-1α, hypoxia-inducible factor-1α; HNF-1β, hepatocyte
nuclear factor-1β; HO-1, heme oxygenase-1; PDK, pyruvate
dehydrogenase kinase; PDH, pyruvate dehydrogenase; ROS, reactive
oxygen species; TCA, tricarboxylic acid. |
Altered survival signals in
endometriosis and EAOC
Energy production is required for cell adaptation
and survival in stressful environmental conditions. Glucose is
metabolized through glycolysis and oxidative phosphorylation
(OXPHOS) to produce adenosine triphosphate (ATP), an important
energy source. Endometriosis and EAOC, especially CCC, are
characterized by common metabolic and molecular alterations
(5,44,45).
The key metabolic features include increased glucose uptake,
anaerobic glycolysis, lactate production, and metabolic conversion
from mitochondrial OXPHOS to aerobic glycolysis (44-47).
Kirsten rat sarcoma (KRAS) and
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit
alpha (PIK3CA) mutations found in endometriosis and EAOC are
associated with glucose metabolism (10,48,49).
However, much of our understanding about energy metabolism by
mutated genes has come primarily from in vitro studies using
other types of cancer cells rather than those in endometriosis or
EAOC.
Transforming growth factor-beta (TGF-β) and
hypoxia-inducible factor-1alpha (HIF-1α) have attracted attention
as potential target genes related to energy metabolism in
endometriosis (44,45) (Fig.
3). TGF-β is expressed in response to hypoxia, mitochondrial
stress, oxidative stress, or tissue damage (44,45,50).
TGF-β signaling enhances aerobic glycolysis by promoting glucose
transporter 1 (GLUT1) expression, which converts glucose to lactate
(44,45,50).
HIF-1α induced by TGF-β1 modifies the expression of
metabolism-related enzymes (44).
Overexpression of HIF-1 inhibits pyruvate dehydrogenase (PDH)
activation via upregulation of pyruvate dehydrogenase kinase (PDK)
expression (44,45). PDH converts pyruvate to acetyl-CoA
and stimulates the transfer of acetyl-CoA to the tricarboxylic acid
cycle, enhancing the formation of ATP. Therefore, TGF-β and HIF-1α
are major players in the metabolic switch from OXPHOS to glycolysis
through the PDK-PDH pathway (44,45).
Mitochondria not only produce ATP but also induce oxidative stress
via the overproduction of ROS. Therefore, cells that survive in
unfavorable environments (e.g., endometriotic and cancer cells)
suppress ROS production in favor of glycolysis over mitochondrial
OXPHOS even if they reduce energy production (44,45).
Indeed, endometriotic lesions had higher expression of TGF-β and
HIF-1α genes compared with eutopic endometrium (44). TGF-β plays multiple roles in
tumorigenesis, possibly through the activation of the
HIF-1α-PDK-PDH pathway (44,45).
A recent study demonstrated that overexpression of PDK2 was linked
to poor prognosis in patients with CCC due to decreased production
of mitochondrial ROS (51).
Emerging evidence indicates that TGF-β, HIF-1α, and
glycolysis-related genes play critical roles in the pathogenesis
and progression of endometriosis and CCC.
Hepatocyte nuclear factor-1 beta (HNF-1β) is a
transcription factor that is overexpressed in almost all CCCs
(52). HNF-1β is expressed only in
CCC and not in other types of ovarian cancers (53). HNF-1β is also expressed in
approximately 40% of endometriotic lesions (52). HNF-1β is a promising new marker for
molecular diagnosis of both benign and malignant lesions (54). Single-nucleotide polymorphisms
(rs11651755) in HNF-1β also correlated with the risk for ovarian
cancer and endometriosis (55,56).
Several papers suggested that HNF-1β affects a wide range of
biological events, including tumorigenesis. For example, HNF-1β
promotes glycogen accumulation in the cytoplasm of normal and
gestational endometrial and CCC cells (28,57).
HNF-1β is a key regulator of gene expression regulating metabolic
remodeling, cell cycle arrest, and anti-apoptosis. First, HNF-1β
induces glucose uptake into CCC cells via the overexpression of
glucose transporter GLUT1 and facilitates glycolytic activity,
lactate production, and glutathione synthesis, all of which are
involved in the maintenance of redox homeostasis through enhanced
anaerobic metabolism, decreased mitochondrial OXPHOS, and reduced
ROS accumulation (46,53). Second, HNF-1β inhibits cell
proliferation by blocking G1/S cell cycle progression through
direct suppression of SMAD6-induced cyclin D1 expression (46,58).
Furthermore, HNF-1β promotes G2/M cell cycle arrest in CCC cells
through activation of the deubiquitinase ubiquitin-specific
protease 28 (USP28)-Claspin-checkpoint kinase 1 (Chk1) signal
transduction cascade (59).
Finally, HNF-1β promotes cell proliferation and survival of
endometriotic and CCC cells by upregulating the transcriptional
regulator nuclear factor kappa B-induced antiapoptotic BCL2 gene
expression (60,61). Thus, HNF-1β can facilitate DNA
damage repair by inducing transient cell cycle arrest. HNF-1β
protects endometriotic and CCC cells from oxidative damage, thereby
contributing to the promotion of cell growth and survival.
Taken together, the potential target genes (i.e.,
KRAS, PIK3CA, TGF-β, HIF-1α and HNF-1β) confer a survival advantage
against oxidative stress. Genome-scale reconstructions of
metabolic, redox homeostasis, cell cycle, and survival signals are
critical factors in disease progression (47,53).
Accumulating evidence demonstrates that common metabolic
reprogramming for cell survival, namely the Warburg effect, has
been identified not only in cancer cells but also in endometriotic
cells (20,44-46,53).
Functional roles of macrophages in the
endometriotic/EAOC microenvironment
In response to inflammation, hypoxia, and oxidative
stress, endometriosis creates a unique microenvironment composed of
epithelium, stroma, mesenchyme, and infiltrative immune cells
(62). Endometriotic cells adapt
to and survive in hypoxic and oxidative stress conditions due to
the high levels of hemoglobin, heme, iron, angiogenic factors
(e.g., vascular endothelial growth factor), inflammatory cytokines
and chemokines (e.g., TGF-β1, tumor necrosis factor alpha,
interleukin-6 and -8, regulated on activation normal T cell
expressed and secreted, and monocyte chemotactic protein-1
(62,63). These key mediators act as
chemoattractants and enhance macrophage recruitment into
endometriotic lesions. Iron-laden macrophages, especially M2
macrophages, are detected throughout the stroma of endometriotic
cysts and CCC lesions (16,24,64).
M2 macrophages scavenge iron and secrete antioxidants that relieve
and eliminate excess oxidative stress in the surrounding
environment (7). Heme oxygenase 1
(HO-1) is a key factor that contributes to antioxidant defenses
(22). Co-culture experiments with
macrophages and endometriotic cells have demonstrated that
macrophage-derived HO-1 expression was induced by TGF-β1 produced
by endometriotic cells (65).
Addition of ROS to the co-culture system enhanced HO-1 production
from macrophages (65). This
crosstalk significantly enhances the antioxidant defense mechanism
and protects endometriotic cells against oxidative injury,
contributing to the progression of endometriosis (65). Moreover, a mouse model of
endometriosis demonstrated that targeted depletion of macrophages
blocks the growth of endometriotic lesions (66). Preclinical research suggests that
endometriosis-macrophage crosstalk can provide a promising survival
advantage to endometriotic cells (65,66).
Tumor-associated M2 macrophages contribute to tumor
growth, invasion, metastasis, and treatment resistance in ovarian
cancer (67); however, the effects
of macrophages on the malignant transformation of endometriosis
yielded inconsistent results. There were no statistically
significant differences in the number of M2 macrophages in
endometriotic cysts and CCC cells (24). In contrast, it was reported that
the number of HO-1-positive M2 macrophages was significantly lower
in the CCC group compared to the endometriotic cyst group (64). Additionally, CDC42-positive
macrophages may prevent malignant transformation of endometriosis
(68). CDC42, a small GTPase of
the Rho-subfamily, regulates cell cycle progression. The number,
polarization, and characteristics of infiltrating macrophages as
well as their spatial distribution (e.g., closer proximity of M2
macrophages to target cells) play an important role in the
carcinogenic process.
4. A promising tool for the early diagnosis
of malignant transformation of endometriosis
Non-invasive diagnostic imaging
TVS is an effective imaging technique for the
evaluation of ovarian masses, while magnetic resonance imaging may
be used to identify TVS-indeterminate lesions. Increasing tumor
size, rapidly growing mural nodules, papillary excrescences, or
irregular and thick septations are features highly suspicious of
malignancy (69). The conventional
diagnostic modalities cannot distinguish benign from malignant
lesions before the appearance of various morphological and
anatomical changes in the cyst. Therefore, early-stage disease with
little or no morphological changes may be missed during imaging
surveillance. Histological examination is the gold standard for
diagnosis, but it is an invasive procedure. Non-invasive diagnostic
approaches to distinguish between endometriosis and EAOC are
warranted. In 2008, Yamaguchi et al (4) showed for the first time that iron
levels in endometriotic cysts are higher than in other benign
ovarian cysts. Total iron, heme iron, and free iron levels in
cystic fluids are useful markers in the differential diagnosis of
endometriosis and EAOC (33).
Total iron levels in patients with endometriosis were markedly
higher than those with EAOC (median ± SD, 244.4±204.9 mg/l vs.
14.2±36.6 mg/l, P<0.001) (33).
The total iron level that distinguishes endometriosis from EAOC
provided 90.9% sensitivity and 100% specificity, with an optimal
cut-off value of 64.8 mg/l (33).
Recent advances in metallobiology have enabled the non-invasive
quantification of iron levels. MR transverse relaxometry quantifies
iron concentrations in endometriotic cysts using non-invasive
techniques (70). MR relaxometry
calculates the R2 value as a predicted value of iron concentration
using a single-voxel multi-echo MR sequence (HISTO) by a 3T-MR
system (70). R2 values highly
correlated with iron concentrations, allowing for the rapid and
non-invasive differentiation of endometriotic cysts from EAOC
preoperatively, with a sensitivity of 86% and a specificity of 94%
(70). MR relaxometry is a
promising alternative for the early detection of malignant
transformation of endometriosis (71-73).
Additionally, real-time in vivo imaging methods used for the
diagnosis of malignant transformation of endometriosis include
electronic absorption spectroscopy and near infrared approach in
addition to MR transverse relaxometry (74). However, diagnostic imaging
techniques other than MR relaxometry are not available in clinical
practice. Endometriosis causes changes in biochemical markers,
including iron, during malignant transformation; hence, novel
diagnostic modalities may allow physicians to detect biochemical
changes early in the disease before anatomical changes occur. The
non-invasive quantification of iron levels will aid in the early
diagnosis and provide information that improves disease management
strategies.
Potential serodiagnostic
biomarkers
The final part highlights new serum biomarkers that
may distinguish between benign and malignant ovarian tumors and
improve the diagnostic accuracy for ovarian cancer. CA-125 is
widely used in the diagnosis and monitoring of patients with
ovarian cancer (75). However, the
use of CA-125 is limited by its low sensitivity for CCC and a high
false positivity rate in endometriosis (76). As CCC is the prevalent type of EAOC
in Japan, novel non-invasive biomarkers that can accurately
distinguish CCC from endometriosis are urgently needed. Human
epididymis protein 4 (HE4) levels are not elevated in benign
diseases, such as endometriosis, and are not affected by the
menstrual cycle or hormone therapy. HE4 also has higher specificity
than CA-125 (0.93 vs. 0.75, respectively) (77,78).
Hence, HE4 could be useful in distinguishing suspected malignant
ovarian tumors from endometriosis. However, HE4 is affected by
several factors, including menopausal status, age, smoking, and
renal dysfunction (79). Serum HE4
levels were elevated in 90% of patients with high-grade serous
ovarian cancer and 69% of patients with CCC tumors, indicating that
HE4 is reliable in diagnosing serous ovarian cancer, while it seems
to be a less useful marker for CCC (80). Moreover, scoring systems and
prediction algorithms may better assess the risk for ovarian cancer
(81,82). The Risk of Ovarian Malignancy
Algorithm and Copenhagen Index, including CA-125, HE4, and
menopausal status or age, have high diagnostic performance for
differentiating benign from malignant lesions (81,82).
Additionally, recent advances in secretome analysis and
bioinformatics revealed a serine protease inhibitor, namely tissue
factor pathway inhibitor 2 (TFPI2), as a potential biomarker for
the detection of ovarian cancer, especially CCC (76,83).
TFPI2 is elevated in the serum and tumor tissues of patients with
CCC, suggesting that serum TFPI2 may be a clinically useful
biomarker for diagnosing CCC (76,83).
A prospective validation study revealed that TFPI2 provided
sufficient specificity for predicting CCC (79.5%) (84). Additionally, CA-125 had a high
false-positive rate (71.4% [15/21]) in endometriosis, whereas none
of the patients with elevated CA-125 levels had elevated TFPI2
levels (84). Miyagi et al
(84) evaluated the diagnostic
value of CA-125 and TFPI2 for distinguishing endometriosis and CCC,
demonstrating that TFPI2 is superior to CA-125 as a marker of CCC
(AUC 0.855 vs. 0.520). Based on these results, TFPI2 testing became
available for ovarian cancers under the national health insurance
in Japan since April 2021. We believe that TFPI2 can be used as a
reliable diagnostic and treatment marker for malignant
transformation of endometriosis into CCC.
5. Discussion and future challenges
We discuss the current challenges and future
directions for malignant transformation of endometriosis. Fig. 3 shows the redox balance and
metabolic switch regulating the endometriotic cell fate (death or
survival) during malignant transformation. Redox homeostasis is
regulated in response to environmental changes. Hemoglobin, heme,
and iron are abundant in endometriotic cysts and cause oxidative
stress (5-7,22).
In the oxidative microenvironment, endometriotic cells stimulate
the production of HO-1 from the surrounding macrophages via TGF-β
production (64,65). HO-1 is a key regulator of redox
homeostasis that eliminates excessive ROS in endometriotic cells
and prevents cell death (64,65).
This crosstalk can help macrophages promote endometriotic cell
survival. On the other hand, the response of endometriotic cells to
harsh environmental conditions requires energy generated through a
metabolic shift from aerobic glycolysis to mitochondrial oxidation.
In endometriotic cells under excessive oxidative stress (a redox
meter showing the red area in Fig.
3), a metabolic shift to oxidative phosphorylation ultimately
results in the death of endometriotic cells via extra ROS
production from mitochondria. Proper energy homeostasis is
regulated by specific control systems (43,44).
The PDK-PDH axis plays a key role in mitochondrial dynamics during
energy switching (44,45). When redox homeostasis is balanced
in an oxidative microenvironment (a meter showing the yellow area
in Fig. 3), HIF-1α stimulates PDK
activation via TGF-β1 expression. As a result, ROS accumulation is
reduced by fermentative glycolysis through a metabolic shift from
mitochondrial OXPHOS to aerobic glycolysis. Accumulation of ROS at
sublethal doses may increase tumorigenic potential via DNA
mutagenesis (29,30).
Recent genomic studies revealed that somatic
mutations accumulated in cancer-related genes (e.g., ARID1A,
PIK3CA, and KRAS proto-oncogenes) not only in epithelial cells of
endometriosis but also in the normal endometrium, and that
mutagenesis in a certain gene (i.e., apolipoprotein B mRNA Editing
Enzyme Catalytic Subunit) is involved in the genomic heterogeneity
of endometriosis (85). Candidate
gene mutations may affect cell survival via changes in
inflammation, redox homeostasis, and metabolism. Genetic diversity
occurs over time (85).
Endometriosis clones that have adapted to harsh environments have
also fueled adaptation to a suitable environment for
carcinogenesis. Since the ARID1A gene is mutated in 46-70% of
patients with CCC (10),
epigenetic inactivation of this gene also contributes to the
development of CCC. Methemoglobin and oxyhemoglobin represent the
major hemoglobin species of endometriosis and EAOC cystic fluid,
respectively (27). Endometriotic
cells surviving in oxyhemoglobin-rich antioxidant environments (a
meter showing the green area in Fig.
3) have a greater ability for carcinogenesis possibly through
(epi)genetic modulations of CCC susceptibility genes, leading to
the initiation and progression of malignant transformation.
Furthermore, some antioxidants (e.g., HO-1) produced by macrophages
may play a role in tumor promotion and progression. Therefore, the
main hallmarks of malignant transformation are the changes in redox
signaling, energy metabolism, and tumor immune microenvironment.
Understanding the molecular mechanisms underlying these biological
features will open new possibilities for the diagnosis, treatment,
and management of the disease.
In clinical practice, conventional biomarkers and
diagnostic imaging techniques are not sufficient for the early
detection of malignant transformation of endometriosis. Novel
imaging modalities (e.g., MR relaxometry) and biomarkers (e.g.,
TFPI2) are promising technologies. MR relaxometry offers the
possibility of early diagnosis as changes in iron levels can be
quantified quickly and non-invasively (70-73).
Compared with conventional diagnostic imaging, MR relaxometry more
accurately detects malignant transformation; however, there is
limited data on its use. TFPI2 may become a useful biomarker for
discriminating between endometriosis and CCC (76,83,84).
In April 2021, TFPI2 testing was covered by the Japanese national
health insurance system, enabling multicenter collaborative
research to evaluate its diagnostic accuracy. Understanding the
pathophysiology of malignant transformation may lead to the
development of novel diagnostic imaging techniques and biomarkers.
This review described the mechanisms of redox homeostasis, energy
metabolism, and crosstalk with macrophages to better understand the
malignant transformation of endometriosis and summarized the
potential diagnostic and monitoring techniques for early diagnosis.
Further studies are needed to determine how endometriotic cell
clones that have acquired survival benefits undergo malignant
transformation.
Acknowledgements
Figure 3 was
created by Mrs. Toyomi Kobayashi (Ms.Clinic MayOne, Kashihara,
Nara, Japan).
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HK was in charge of conceptualization, data
curation, formal analysis, funding acquisition, investigation,
methodology, project administration, resources, supervision,
validation, visualization, original draft writing, review and
editing. Data authentication is not applicable. The author read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that they have no competing
interests.
References
|
1
|
Giudice LC: Clinical practice.
Endometriosis. N Engl J Med. 362:2389–2398. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wei JJ, William J and Bulun S:
Endometriosis and ovarian cancer: A review of clinical, pathologic,
and molecular aspects. Int J Gynecol Pathol. 30:553–568.
2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Machida H, Matsuo K, Yamagami W, Ebina Y,
Kobayashi Y, Tabata T, Kanauchi M, Nagase S, Enomoto T and Mikami
M: Trends and characteristics of epithelial ovarian cancer in Japan
between 2002 and 2015: A JSGO-JSOG joint study. Gynecol Oncol.
153:589–596. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yamaguchi K, Mandai M, Toyokuni S,
Hamanishi J, Higuchi T, Takakura K and Fujii S: Contents of
endometriotic cysts, especially the high concentration of free
iron, are a possible cause of carcinogenesis in the cysts through
the iron-induced persistent oxidative stress. Clin Cancer Res.
14:32–40. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Van Langendonckt A, Casanas-Roux F and
Donnez J: Iron overload in the peritoneal cavity of women with
pelvic endometriosis. Fertil Steril. 78:712–718. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mandai M, Matsumura N, Baba T, Yamaguchi
K, Hamanishi J and Konishi I: Ovarian clear cell carcinoma as a
stress-responsive cancer: Influence of the microenvironment on the
carcinogenesis and cancer phenotype. Cancer Lett. 310:129–133.
2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kobayashi H: Potential scenarios leading
to ovarian cancer arising from endometriosis. Redox Rep.
21:119–126. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sampson JA: Endometrial carcinoma of the
ovary, arising in endometrial tissue in that organ. Arch Surg.
10:1–72. 1925.
|
|
9
|
Kobayashi H, Sumimoto K, Moniwa N, Imai M,
Takakura K, Kuromaki T, Morioka E, Arisawa K and Terao T: Risk of
developing ovarian cancer among women with ovarian endometrioma: A
cohort study in Shizuoka, Japan. Int J Gynecol Cancer. 17:37–43.
2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yachida N, Yoshihara K, Yamaguchi M, Suda
K, Tamura R and Enomoto T: How does endometriosis lead to ovarian
cancer? The molecular mechanism of endometriosis-associated ovarian
cancer development. Cancers (Basel). 13(1439)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Murakami K, Kotani Y, Nakai H and
Matsumura N: Endometriosis-Associated ovarian cancer: The origin
and targeted therapy. Cancers (Basel). 12(1676)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Melin A, Sparén P, Persson I and Bergqvist
A: Endometriosis and the risk of cancer with special emphasis on
ovarian cancer. Hum Reprod. 21:1237–1242. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dixon-Suen SC, Webb PM, Wilson LF, Tuesley
K, Stewart LM and Jordan SJ: The association between hysterectomy
and ovarian cancer risk: A population-based record-linkage study. J
Natl Cancer Inst. 111:1097–1103. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
LaGrenade A and Silverberg SG: Ovarian
tumors associated with atypical endometriosis. Hum Pathol.
19:1080–1084. 1988.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stern RC, Dash R, Bentley RC, Snyder MJ,
Haney AF and Robboy SJ: Malignancy in endometriosis: Frequency and
comparison of ovarian and extraovarian types. Int J Gynecol Pathol.
20:133–139. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kondi-Pafiti A, Papakonstantinou E,
Iavazzo C, Grigoriadis C, Salakos N and Gregoriou O:
Clinicopathological characteristics of ovarian carcinomas
associated with endometriosis. Arch Gynecol Obstet. 285:479–483.
2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fukunaga M, Nomura K, Ishikawa E and
Ushigome S: Ovarian atypical endometriosis: Its close association
with malignant epithelial tumours. Histopathology. 30:249–255.
1997.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Van Gorp T, Amant F, Neven P, Vergote I
and Moerman P: Endometriosis and the development of malignant
tumours of the pelvis. A review of literature. Best Pract Res Clin
Obstet Gynaecol. 18:349–371. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Vercellini P, Cribiù FM, Del Gobbo A,
Carcangiu ML, Somigliana E and Bòsari S: The oncofetal protein
IMP3: A novel biomarker and triage tool for premalignant atypical
endometriotic lesions. Fertil Steril. 99:1974–1979. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Varga J, Reviczká A, Háková H, Švajdler P,
Rabajdová M and Ostró A: Predictive factors of endometriosis
progression into ovarian cancer. J Ovarian Res.
15(5)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dunselman GA, Vermeulen N, Becker C,
Calhaz-Jorge C, D'Hooghe T, De Bie B, Heikinheimo O, Horne AW,
Kiesel L, Nap A, et al: ESHRE guideline: Management of women with
endometriosis. Hum Reprod. 29:400–412. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Iwabuchi T, Yoshimoto C, Shigetomi H and
Kobayashi H: Oxidative stress and antioxidant defense in
endometriosis and its malignant transformation. Oxid Med Cell
Longev. 2015(848595)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kobayashi H, Yamada Y, Kawahara N, Ogawa K
and Yoshimoto C: Integrating modern approaches to pathogenetic
concepts of malignant transformation of endometriosis. Oncol Rep.
41:1729–1738. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Akashi K, Nagashima Y, Tabata T and Oda H:
Immunochemical analysis of iron transporters and M2 macrophages in
ovarian endometrioma and clear cell adenocarcinoma. Mol Clin Oncol.
15(159)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Seidman JD: The presence of mucosal iron
in the fallopian tube supports the ‘incessant menstruation
hypothesis’ for ovarian carcinoma. Int J Gynecol Pathol.
32:454–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yamada Y, Shigetomi H, Onogi A, Haruta S,
Kawaguchi R, Yoshida S, Furukawa N, Nagai A, Tanase Y, Tsunemi T,
et al: Redox-active iron-induced oxidative stress in the
pathogenesis of clear cell carcinoma of the ovary. Int J Gynecol
Cancer. 21:1200–1207. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Iwabuchi T, Yoshimoto C, Shigetomi H and
Kobayashi H: Cyst fluid hemoglobin species in endometriosis and its
malignant transformation: The role of metallobiology. Oncol Lett.
11:3384–3388. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yamaguchi K, Kitamura S, Furutake Y,
Murakami R, Yamanoi K, Taki M, Ukita M, Hamanishi J and Mandai M:
Acquired evolution of mitochondrial metabolism regulated by HNF1B
in ovarian clear cell carcinoma. Cancers (Basel).
13(2413)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kobayashi H, Imanaka S and Shigetomi H:
Revisiting therapeutic strategies for ovarian cancer by focusing on
redox homeostasis. Oncol Lett. 23(80)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vercellini P, Crosignani P, Somigliana E,
Viganò P, Buggio L, Bolis G and Fedele L: The ‘incessant
menstruation’ hypothesis: A mechanistic ovarian cancer model with
implications for prevention. Hum Reprod. 26:2262–2273.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Defrère S, Van Langendonckt A, Vaesen S,
Jouret M, González Ramos R, Gonzalez D and Donnez J: Iron overload
enhances epithelial cell proliferation in endometriotic lesions
induced in a murine model. Hum Reprod. 21:2810–2816.
2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Toyokuni S: Iron as a target of
chemoprevention for longevity in humans. Free Radic Res.
45:906–917. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yoshimoto C, Iwabuchi T, Shigetomi H and
Kobayashi H: Cyst fluid iron-related compounds as useful markers to
distinguish malignant transformation from benign endometriotic
cysts. Cancer Biomark. 15:493–499. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Nagayasu M, Imanaka S, Kimura M, Maruyama
S and Kobayashi H: Nonhormonal treatment for endometriosis focusing
on redox imbalance. Gynecol Obstet Invest. 86:1–12. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Turrens JF: Mitochondrial formation of
reactive oxygen species. J Physiol. 552(Pt 2):335–344.
2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
He L, He T, Farrar S, Ji L, Liu T and Ma
X: Antioxidants maintain cellular redox homeostasis by elimination
of reactive oxygen species. Cell Physiol Biochem. 44:532–553.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ota H, Igarashi S, Hatazawa J and Tanaka
T: Endometriosis and free radicals. Gynecol Obstet Invest. 48
(Suppl 1):S29–S35. 1999.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jana SK, Dutta M, Joshi M, Srivastava S,
Chakravarty B and Chaudhury K: 1H NMR based targeted metabolite
profiling for understanding the complex relationship connecting
oxidative stress with endometriosis. Biomed Res Int.
2013(329058)2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Singh AK, Chattopadhyay R, Chakravarty B
and Chaudhury K: Markers of oxidative stress in follicular fluid of
women with endometriosis and tubal infertility undergoing IVF.
Reprod Toxicol. 42:116–124. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Moon EJ and Giaccia A: Dual roles of NRF2
in tumor prevention and progression: Possible implications in
cancer treatment. Free Radic Biol Med. 79:292–299. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Vahid F and Davoodi SH: Nutritional
factors involved in the etiology of gastric cancer: A systematic
review. Nutr Cancer. 73:376–390. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Müller MF, Florian S, Pommer S, Osterhoff
M, Esworthy RS, Chu FF, Brigelius-Flohé R and Kipp AP: Deletion of
glutathione peroxidase-2 inhibits azoxymethane-induced colon cancer
development. PLoS One. 8(e72055)2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fujimoto Y, Imanaka S, Yamada Y, Ogawa K,
Ito F, Kawahara N, Yoshimoto C and Kobayashi H: Comparison of redox
parameters in ovarian endometrioma and its malignant
transformation. Oncol Lett. 16:5257–5264. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Young VJ, Brown JK, Maybin J, Saunders PT,
Duncan WC and Horne AW: Transforming growth factor-β induced
Warburg-like metabolic reprogramming may underpin the development
of peritoneal endometriosis. J Clin Endocrinol Metab. 99:3450–3459.
2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Young VJ, Ahmad SF, Brown JK, Duncan WC
and Horne AW: ID2 mediates the transforming growth
factor-β1-induced Warburg-like effect seen in the peritoneum of
women with endometriosis. Mol Hum Reprod. 22:648–654.
2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Okamoto T, Mandai M, Matsumura N,
Yamaguchi K, Kondoh H, Amano Y, Baba T, Hamanishi J, Abiko K,
Kosaka K, et al: Hepatocyte nuclear factor-1β (HNF-1β) promotes
glucose uptake and glycolytic activity in ovarian clear cell
carcinoma. Mol Carcinog. 54:35–49. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kobayashi H, Shigetomi H and Imanaka S:
Nonhormonal therapy for endometriosis based on energy metabolism
regulation. Reprod Fertil. 2:C42–C57. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ying H, Kimmelman AC, Lyssiotis CA, Hua S,
Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff
JL, et al: Oncogenic Kras maintains pancreatic tumors through
regulation of anabolic glucose metabolism. Cell. 149:656–670.
2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Bhattacharya B, Low SH, Soh C, Kamal
Mustapa N, Beloueche-Babari M, Koh KX, Loh J and Soong R: Increased
drug resistance is associated with reduced glucose levels and an
enhanced glycolysis phenotype. Br J Pharmacol. 171:3255–3267.
2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhou MY, Cheng ML, Huang T, Hu RH, Zou GL,
Li H, Zhang BF, Zhu JJ, Liu YM, Liu Y and Zhao XK: Transforming
growth factor beta-1 upregulates glucose transporter 1 and
glycolysis through canonical and noncanonical pathways in hepatic
stellate cells. World J Gastroenterol. 27:6908–6926.
2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kitamura S, Yamaguchi K, Murakami R,
Furutake Y, Higasa K, Abiko K, Hamanishi J, Baba T, Matsumura N and
Mandai M: PDK2 leads to cisplatin resistance through suppression of
mitochondrial function in ovarian clear cell carcinoma. Cancer Sci.
112:4627–4640. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kato N, Sasou S and Motoyama T: Expression
of hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors
and endometriosis of the ovary. Mod Pathol. 19:83–89.
2006.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Amano Y, Mandai M, Yamaguchi K, Matsumura
N, Kharma B, Baba T, Abiko K, Hamanishi J, Yoshioka Y and Konishi
I: Metabolic alterations caused by HNF1β expression in ovarian
clear cell carcinoma contribute to cell survival. Oncotarget.
6:26002–26017. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Chandra S, Srinivasan S and Batra J:
Hepatocyte nuclear factor 1 beta: A perspective in cancer. Cancer
Med. 10:1791–1804. 2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Shen H, Fridley BL, Song H, Lawrenson K,
Cunningham JM, Ramus SJ, Cicek MS, Tyrer J, Stram D, Larson MC, et
al: Epigenetic analysis leads to identification of HNF1B as a
subtype-specific susceptibility gene for ovarian cancer. Nat
Commun. 4(1628)2013.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Burghaus S, Fasching PA, Häberle L, Rübner
M, Büchner K, Blum S, Engel A, Ekici AB, Hartmann A, Hein A, et al:
Genetic risk factors for ovarian cancer and their role for
endometriosis risk. Gynecol Oncol. 145:142–147. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yamamoto S, Tsuda H, Aida S, Shimazaki H,
Tamai S and Matsubara O: Immunohistochemical detection of
hepatocyte nuclear factor 1beta in ovarian and endometrial
clear-cell adenocarcinomas and nonneoplastic endometrium. Hum
Pathol. 38:1074–1080. 2007.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lu W, Sun J, Zhou H, Wang F, Zhao C, Li K,
Fan C, Ding G and Wang J: HNF1B inhibits cell proliferation via
repression of SMAD6 expression in prostate cancer. J Cell Mol Med.
24:14539–14548. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ito F, Yoshimoto C, Yamada Y, Sudo T and
Kobayashi H: The HNF-1β-USP28-Claspin pathway upregulates DNA
damage-induced Chk1 activation in ovarian clear cell carcinoma.
Oncotarget. 9:17512–17522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Suzuki E, Kajita S, Takahashi H, Matsumoto
T, Tsuruta T and Saegusa M: Transcriptional upregulation of HNF-1β
by NF-κB in ovarian clear cell carcinoma modulates susceptibility
to apoptosis through alteration in bcl-2 expression. Lab Invest.
95:962–972. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Preya UH, Woo JH, Choi YS and Choi JH:
Hepatocyte nuclear factor-1 beta protects endometriotic cells
against apoptotic cell death by up-regulating the expression of
antiapoptotic genes. Biol Reprod. 101:686–694. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Wendel JRH, Wang X and Hawkins SM: The
endometriotic tumor microenvironment in ovarian cancer. Cancers
(Basel). 10(261)2018.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Capobianco A and Rovere-Querini P:
Endometriosis, a disease of the macrophage. Front Immunol.
4(9)2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Yamada Y, Uchiyama T, Ito F, Kawahara N,
Ogawa K, Obayashi C and Kobayashi H: Clinical significance of M2
macrophages expressing heme oxygenase-1 in malignant transformation
of ovarian endometrioma. Pathol Res Pract. 215:639–643.
2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ogawa K, Liu T, Kawahara N and Kobayashi
H: Macrophages protect endometriotic cells against oxidative damage
through a cross-talk mechanism. Reprod Sci. 29:2165–2178.
2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Bacci M, Capobianco A, Monno A, Cottone L,
Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S,
et al: Macrophages are alternatively activated in patients with
endometriosis and required for growth and vascularization of
lesions in a mouse model of disease. Am J Pathol. 175:547–556.
2009.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Colvin EK: Tumor-associated macrophages
contribute to tumor progression in ovarian cancer. Front Oncol.
4(137)2014.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Canet B, Pons C, Espinosa I and Prat J:
CDC42-positive macrophages may prevent malignant transformation of
ovarian endometriosis. Hum Pathol. 43:720–725. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Guerriero S, Alcazar JL, Coccia ME, Ajossa
S, Scarselli G, Boi M, Gerada M and Melis GB: Complex pelvic mass
as a target of evaluation of vessel distribution by color Doppler
sonography for the diagnosis of adnexal malignancies: Results of a
multicenter European study. J Ultrasound Med. 21:1105–1111.
2002.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Yoshimoto C, Takahama J, Iwabuchi T,
Uchikoshi M, Shigetomi H and Kobayashi H: Transverse relaxation
rate of cyst fluid can predict malignant transformation of ovarian
endometriosis. Magn Reson Med Sci. 16:137–145. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Matsubara S, Kawahara N, Horie A, Murakami
R, Horikawa N, Sumida D, Wada T, Maehana T, Yamawaki A, Ichikawa M,
et al: Magnetic resonance relaxometry improves the accuracy of
conventional MRI in the diagnosis of endometriosis-associated
ovarian cancer: A case report. Mol Clin Oncol. 11:296–300.
2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Liu T, Sumida D, Wada T, Maehana T,
Yamawaki A, Sugimoto S, Kawahara N, Yoshimoto C and Kobayashi H: A
diagnostic challenge of seromucinous borderline tumor: A case
report. Medicine (Baltimore). 98(e15707)2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Kawahara N, Miyake R, Yamanaka S and
Kobayashi H: A novel predictive tool for discriminating
endometriosis associated ovarian cancer from ovarian endometrioma:
The R2 predictive index. Cancers (Basel). 13(3829)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Kobayashi H, Yamada Y, Kawahara N, Ogawa K
and Yoshimoto C: Modern approaches to noninvasive diagnosis of
malignant transformation of endometriosis. Oncol Lett.
17:1196–1202. 2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Molina R, Ojeda B, Filella X, Borras G, Jo
J, Mas E, Lopez JJ and Ballesta A: A prospective study of tumor
markers CA 125 and CA 19.9 in patients with epithelial ovarian
carcinomas. Tumour Biol. 13:278–286. 1992.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Arakawa N, Miyagi E, Nomura A, Morita E,
Ino Y, Ohtake N, Miyagi Y, Hirahara F and Hirano H: Secretome-based
identification of TFPI2, a novel serum biomarker for detection of
ovarian clear cell adenocarcinoma. J Proteome Res. 12:4340–4350.
2013.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Urban N, Thorpe J, Karlan BY, McIntosh MW,
Palomares MR, Daly MB, Paley P and Drescher CW: Interpretation of
single and serial measures of HE4 and CA125 in asymptomatic women
at high risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev.
21:2087–2094. 2012.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Zapardiel I, Gorostidi M, Ravaggi A,
Allende MT, Silveira M and Macuks R: Utility of human epididymis
protein 4 serum marker for the detection of adnexal malignancy: A
multicentric prospective study. Eur J Cancer Prev. 26:346–350.
2017.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Lycke M, Ulfenborg B, Malchau Lauesgaard
J, Kristjansdottir B and Sundfeldt K: Consideration should be given
to smoking, endometriosis, renal function (eGFR) and age when
interpreting CA125 and HE4 in ovarian tumor diagnostics. Clin Chem
Lab Med. 59:1954–1962. 2021.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Blackman A, Mitchell J, Rowswell-Turner R,
Singh R, Kim KK, Eklund E, Skates S, Bast RC, Messerlian G, Miller
MC and Moore RG: Analysis of serum HE4 levels in various histologic
subtypes of epithelial ovarian cancer and other malignant tumors.
Tumour Biol. 43:355–365. 2021.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Dikmen ZG, Colak A, Dogan P, Tuncer S and
Akbiyik F: Diagnostic performances of CA125, HE4, and ROMA index in
ovarian cancer. Eur J Gynaecol Oncol. 36:457–462. 2015.PubMed/NCBI
|
|
82
|
Wang Z, Tao X and Ying C: CPH-I and HE4
Are more favorable than CA125 in differentiating borderline ovarian
tumors from epithelial ovarian cancer at early stages. Dis Markers.
2019(6241743)2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Arakawa N, Kobayashi H, Yonemoto N,
Masuishi Y, Ino Y, Shigetomi H, Furukawa N, Ohtake N, Miyagi Y,
Hirahara F, et al: Clinical significance of tissue factor pathway
inhibitor 2, a serum biomarker candidate for ovarian clear cell
carcinoma. PLoS One. 11(e0165609)2016.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Miyagi E, Arakawa N, Sakamaki K, Yokota
NR, Yamanaka T, Yamada Y, Yamaguchi S, Nagao S, Hirashima Y,
Kasamatsu Y, et al: Validation of tissue factor pathway inhibitor 2
as a specific biomarker for preoperative prediction of clear cell
carcinoma of the ovary. Int J Clin Oncol. 26:1336–1344.
2021.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Revathidevi S, Nakaoka H, Suda K, Fujito
N, Munirajan AK, Yoshihara K, Enomoto T and Inoue I: APOBEC
mediated mutagenesis drives genomic heterogeneity in endometriosis.
J Hum Genet. 67:323–329. 2022.PubMed/NCBI View Article : Google Scholar
|