Introduction
At present, cardiovascular diseases are among the
leading causes of death worldwide. A growing body of evidence
indicates an association between stem progenitor cells and vascular
disease. Several studies indicated that the origin, differentiation
and abnormal function of stem/progenitor cells may be key factors
in the occurrence and development of cardiovascular disease
(1-3).
Vascular wall remodeling following vascular injury may contribute
to a number of cardiovascular diseases, including atherosclerosis,
hypertension and restenosis following vascular reconstruction
(4,5). Past studies focused on vascular
endothelial cells and vascular smooth muscle cells (VSMCs), but
more recently, the role of the adventitia in vascular remodeling
has attracted increasing attention (6,7).
Adventitial fibroblasts(AFs) are one of the principal cells of the
adventitia. Early atherosclerosis is results in proliferation of
adventitia rather than the intima and AFs are activated and
transformed into myofibroblasts, which express α-smooth muscle
actin (SMA) and migrate to the subintima (8,9).
Atherosclerosis is characterized by abundant vasa vasorum in the
adventitia; neo-proliferative vasa vasorum develops in the
adventitia of the arteries at an early stage (10). Stem/progenitor cells from the
adventitia of the arteries and bone marrow-derived stem cells
migrate into the intima via the vasa vasorum to participate in
vascular remodeling. AFs activated by vascular injury secrete large
amounts of matrix and collagen and abnormally express a number of
adhesion factors and cytokines, such as increased secretion of
TGF-β1 (11,12), some of which may contribute to stem
cell mobilization and homing (13,14).
Norepinephrine (NE) is a neurohumoral regulating
hormone belonging to the catecholamine family. It is mainly
released by sympathetic nerve endings as well as by the adrenal
medulla in small amounts to exert a strong vasoconstriction effect
that is primarily mediated through receptors. There are mainly
sympathetic nerve endings located in the adventitia. As a result of
increased NE release under sympathetic nervous system activation,
oxidative stress, infection injury and changes in blood flow
dynamics, smooth muscle cells and AFs occur, resulting in
vasoconstriction, increased blood pressure, lumen stenosis and
vascular remodeling (15,16).
In the present in vitro study, NE was added
to cell culture medium to activate AFs to differentiate into
myoblasts, simulate the micro-environment of vascular injury,
investigate if differentiation and migration of bone marrow
mesenchymal stem cells (BMSCs) are associated with AFs activation
and examine the mechanism by which BMSCs participate in vascular
remodeling. The present results may provide novel insight into the
mechanisms of restenosis and atherosclerosis development following
vascular injury.
Materials and methods
Experimental animals
The Experimental Animal Center of Binzhou Medical
University (Yantai, China) provided 36 3-week-old male Sprague
Dawley (SD) rats weighing 80-100 g. The animals were housed in
standard cages and maintained under standard conditions at constant
room temperature of 20-25˚C, 40-70% humidity and 12/12-hlight/dark
cycle, with unrestricted access to food and water. For anesthesia,
3% pentobarbital sodium (50 mg/kg body weight) was administered
intraperitoneally, and SD rats were sacrificed using CO2
inhalation, with a fill rate of 60% of the chamber volume/min.
Death was confirmed using lack of pulse, breathing, corneal reflex,
response to a firm toe pinch and graying of the mucous membranes,
which conformed to the Action against Medical Accidents Guidelines
for the Euthanasia of Animals: 2020 Edition (17). After SD rats were sacrificed, 75%
alcohol was used to disinfect them. Under aseptic conditions,
bilateral femurs and the thoracic aorta of rats were removed and
isolated. The present study was approved by the Animal Ethics
Committee of Binzhou Medical University (approval no. 2020105;10
December 2020).
Culture and identification of
BMSCs
Cell-adhesive screening was used to isolate and
culture BMSCs. The bone marrow of the femur of 6 SD rats was rinsed
with L-DMED culture medium (Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd.) using a 5 ml syringe, collected in
a sterile centrifuge tube and centrifuged in 1,000 x g for 5 min at
4˚C. The supernatant was discarded and L-DMED was added to the
centrifuge tube with 15% fetal bovine serum (FBS; cat. no.
11011-8611; Hangzhou Sijiqing Biological Engineering Materials Co.,
Ltd.) and cells were resuspended. The cell concentration was
adjusted to 4x105/ml and transferred into a culture
flask. The culture medium was changed after 2 days incubation at
37˚C with 5% CO2, cells in suspension were discarded and
the culture was continued for further 2 days. In the following 6
days, the medium was changed every 3 days. Each day, an inverted
phase contrast microscope (magnification, x200; Olympus
Corporation) was used to observe the proliferation and morphology
of the cells. The rapid proliferation of cells occurred due to
adhesion to the petri dish bottom. After reaching 70-80%
confluence, cells were sub-cultured. Flow cytometry assay
(NovoCyte, Agilent; software version no. FlowJo 10.8.1, BD company)
was performed to analyze the cells after they were sub-cultured for
2-3 generations. Cells were collected, washed in cold PBS,
centrifuged in 1,500 x g for 5 min at 4˚C and re-suspended in cold
PBS. Flow cytometry was used to analyze cells that were labeled
with CD105, CD44, and CD34 antibodies (all 1:1,000; cat. nos.
A02997-2, A00052 and A00885-1, respectively; all Boster Biological
Technology).
Culture and identification of AFs
Under aseptic conditions, the adventitia of the
thoracic aorta of the rats was isolated from the media and intima.
The adventitia was then cut into 0.5-1 mm thick tissue pieces and
cultured with complete medium supplemented with 15% FBS. The
primary culture of AFs adopted the tissue block adherent culture
method, in which pieces were stored in a petri dish and cultivated
in an incubator for 2-3 days at 37˚C. After being detached from the
tissue block, the cells started dividing and proliferating. For 6-7
days, the cells were added to the bottom of the flask, digested and
sub-cultured using 0.08% trypsinase (Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd.), and the culture medium was
changed every other day. AFs at generation 2-3 and 70-80%
confluence were sub-cultured in 6-well plates filled with culture
medium supplemented with 10% FBS at a density of 1x104
cells/cm2. After washing three times with PBS, fixing
with 4% paraformaldehyde at room temperature for 15 min,
immunocytochemical staining was performed to identify the AFs using
1:100 rabbit anti-Vimentin primary antibody (cat. no. PB9359,
Boster Biological Technology). The cells in the plate were washed
in PBS, and endogenous peroxidase activities were blocked by 3%
H2O2 at room temperature for 15 min. After
washing with PBS three times, the plates were blocked with goat
serum at room temperature for 20 min. Subsequently, the liquid was
discarded and the plate dried. The cells were incubated over night
at 4˚C with the primary antibody followed by incubation with
FITC-conjugated goat anti-rabbit secondary antibody (1:3,000; cat.
no. A0562, Beyotime Institute of Biotechnology) for 30 min at 37˚C.
The immunofluorescence was observed using a laser confocal
microscope (magnification, x200; FV3000; Olympus Corporation).
Culture of BMSCs in supernatant of the
AFs
A vascular injury model was simulated in
vitro and BMSCs at generation 2-3 were co-cultured with the
supernatant of the AFs culture medium. L-DMED supplemented with 10%
FBS was used as a complete culture medium and maintained at 37˚C
with 5% CO2. These conditions were used for all
incubations. In Model 1, AFs were cultured for 2 days in complete
culture medium supplemented with NE (cat. no. N7960; Beijing
Solarbio Biological Technology) at a final concentration of 10
ng/ml. Subsequently, the medium was replaced with a fresh complete
culture medium without NE. Cells were cultured for 1 day and
supernatant of the culture medium was collected. BMSCs were
cultured with supernatant for 6 days and the AFs supernatant was
changed every other day. In Model 2, AFs were cultured for 2 days
in complete culture medium without NE, followed by 1 day in fresh
complete culture medium and supernatant collection. BMSCs were
cultured with the AFs supernatant for 6 days and the supernatant
was changed every other day. In the control group, BMSCs were
cultured for 6 days in complete culture medium without the
supernatant of AFs. The complete culture medium was changed every
other day. An inverted optical light microscope (magnification,
x200) was used to observe the morphological changes of BMSCs
cultured in AFs supernatant for 6 days. BMSCs from the three groups
were collected and washed with PBS to be used in subsequent
experiments
Immunofluorescence of BMSCs
BMSCs were fixed with 4% paraformaldehyde for 10 min
at room temperature. BMSC nuclei were stained with DAPI (0.02 g/l;
Santa Cruz Biotechnology, Inc.) overnight at 4˚C and incubated with
rabbit anti-α-SMA, TGF-β1 and SMAD3 (1:200; cat. nos. AF0048,
AF0297 and AF1501, respectively; Beyotime Institute of
Biotechnology) primary antibodies at 37˚C for 60 min, followed by
incubation with FITC- or Cy3-conjugated goat anti-rabbit IgG
(1:300; cat. no. A 0562, A0516; Beyotime Institute of
Biotechnology) secondary antibodies at 37˚C for 60 min. Stained
cells were observed under a laser confocal microscope
(magnification, x200; FV3000; Olympus Corporation).
Western blot analysis
Total protein was extracted from BMSCs using
ice-cold RIPA lysis buffer supplemented with 1 mM phenyl methane
sulfonyl fluoride (Beyotime Institute of Biotechnology). Total
protein was quantified using the Enhanced BCA Protein Assay kit
(cat. no. P0006; Beyotime Institute of Biotechnology) and 40 µg
protein/lane was separated by SDS-PAGE on 8-12% gel. The separated
proteins were transferred onto a polyvinylidene fluoride membrane
(EMD Millipore; Millipore Sigma) and blocked with 5% skimmed milk
for 15 min at room temperature. Membranes were incubated with
rabbit anti-α-SMA, anti-TGF-β1, anti-SMAD3 and GAPDH (1:1,000; cat.
nos. AF0048, AF0297, AF1501 and AF1186, respectively; Beyotime
Institute of Biotechnology) primary antibodies at 4˚C overnight.
Following primary incubation, membranes were incubated with the
horseradish peroxidase-conjugated goat anti-rabbit IgG (1:3,000;
cat. no. SA00001-2, Proteintech Group, Inc.) secondary antibody at
room temperature for 2 h. The bands were visualized using Millipore
Immobilon ECL (Cat. No. WBKLS0100, Millipore Sigma). Protein
expression was quantified using Scion Image (version 1.8.0; Scion
Corporation) with GAPDH as the loading control.
Proliferation of BMSCs
BMSCs in the logarithmic growth phase were seeded
into 96-well plates at a density of 5x103 cells/well
(100 µl/well). The cells were cultured at 37˚C with 5%
CO2 for 12, 24, 36 and 48 h to ensure that they adhered
to the plate wall. Cell Counting Kit-8 (CCK-8) reagent (10 µl; cat.
no. c0037, Beyotime Institute of Biotechnology) was added to each
well and incubated for 4 h. A Bio-Tek microplate reader
(TecanGroup, Ltd.) was used to measure the absorbance at 450 nm for
the determination of BMSC proliferation.
Migration of BMSCs
Transwell plates with 8 µm pore membranes were used
to investigate the BMSC migration. In Model 1, AFs were cultured in
complete culture medium with 10 ng/ml NE for 2 days, whereas in
Model 2 the AFs were cultured in the complete culture medium
without NE for 2 days as afore mentioned The AFs in Model 1 and 2
were then centrifuged in 1,500 x g for 5 min at 4˚C and rinsed with
PBS solution and the AFs were added separately into the lower
chamber of the Transwell plate, while the upper chamber was seeded
with 2x105 cells/cm2. For the control group,
BMSCs (2x105 cells/cm2) were cultured in the
upper chamber without AFs in the lower chamber. BMSCs in the three
groups were cultured in the complete culture medium at 37˚C with 5%
CO2 for 6 days to observe morphological changes,
proliferation and migration under an inverted microscope. The
membrane was collected and the cells on the upper surface of the
membrane were removed with cotton swabs. The membrane was washed
with PBS and cells adhering to the membrane were fixed with 4%
paraformaldehyde at room temperature for 15 min. BMSC nuclei were
stained with DAPI (0.02 mg/ml; cat. no. c1002; Beyotime Institute
of Biotechnology) overnight at 4˚C and rinsed five times with PBS
to wash away the unbound DAPI. Stained cells were observed in five
randomly selected fields of view under laser confocal microscopy
(magnification, x100) to measure the migration of BMSCs.
Statistical analysis
Data are presented as the mean ± standard error of
six independent experimental repeats. The significance of the
differences was examined by one-way ANOVA followed by Duncan's
multiple range tests, Statistical analysis was performed using
SPSS20.0 (IBM Corp.) and GraphPad Prism 6 (GraphPad Software;
Dotmatics000.0). P<0.05 was considered to indicate a
statistically significant difference.
Results
Morphology and immunofluorescence of
BMSCs
By using the cell adherent screening method, BMSCs
were isolated, cultured and observed under an inverted microscope.
After 3 days of culture, round monocytes adhered to the wall,
whereas hematopoietic stem cells did not adhere to the wall,
allowing them to be discarded in the exchange fluid of culture
medium. The primary culture of BMSCs showed a short shape and
mostly round and unextended. A few extended cells started to appear
and proliferate at 3-5 days. Cells were typically spindle-shaped
with 2-3 long protrusions, while a few cells were polygonal and
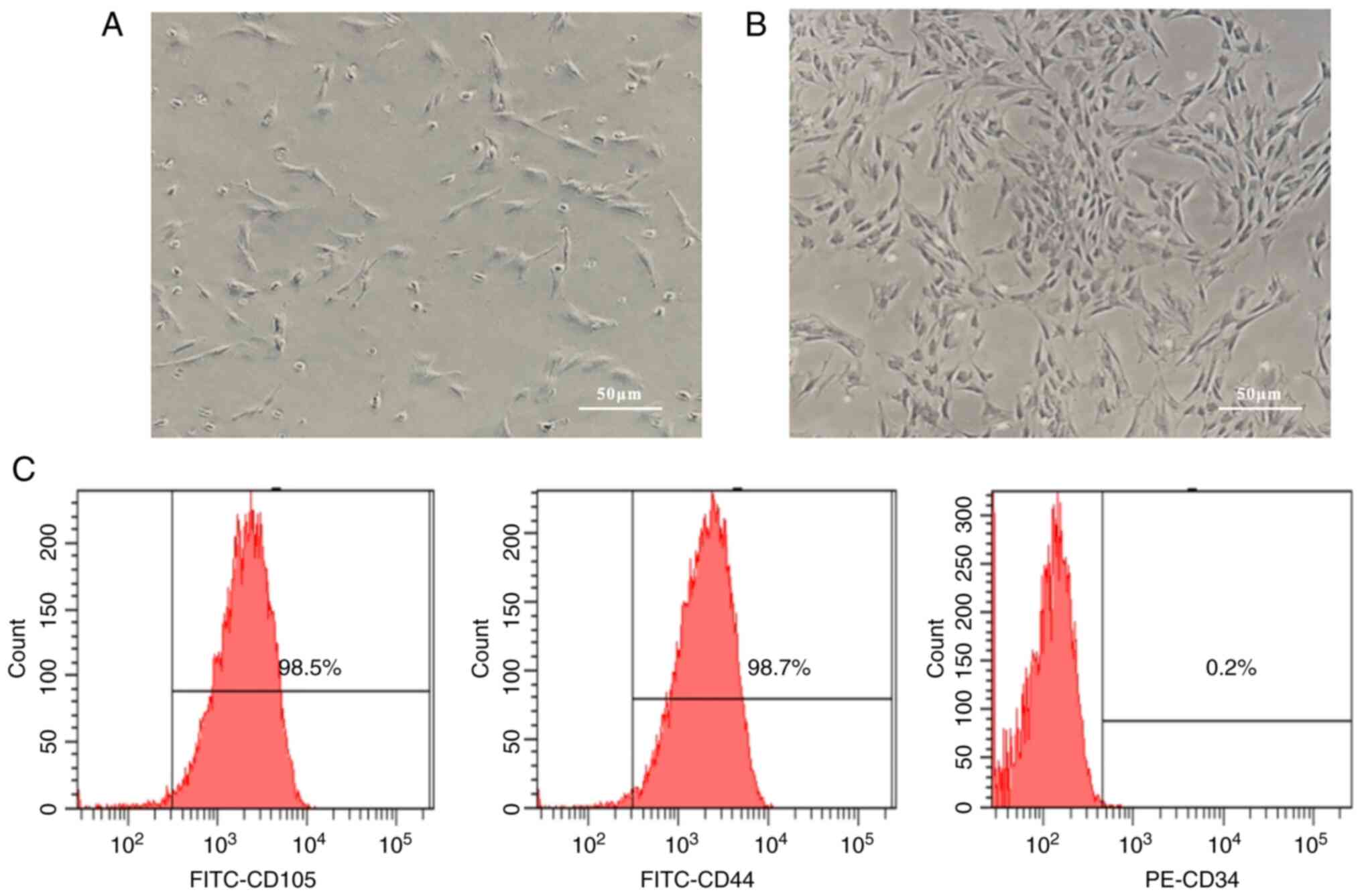
rod-shaped with large oblate nuclei and 1-2 nucleoli (Fig. 1A). Cells were sub-cultured after
5-6 days of cell culture. In cell culture, cell morphology
gradually homogenized into long spindle shape, cells proliferated
rapidly and exhibited swirls (Fig.
1B). As soon as 2-3 generations of BMSCs were obtained, flow
cytometry analysis revealed that the BMSCs were negative for CD34,
while they were positive for CD44 and CD105 (Fig. 1C). According to these findings,
cultured cells had morphological and immunophenotypical
characteristics similar to those of BMSCs.
Morphology and immunofluorescence of
AFs
As observed under an inverted microscope, AFs grew
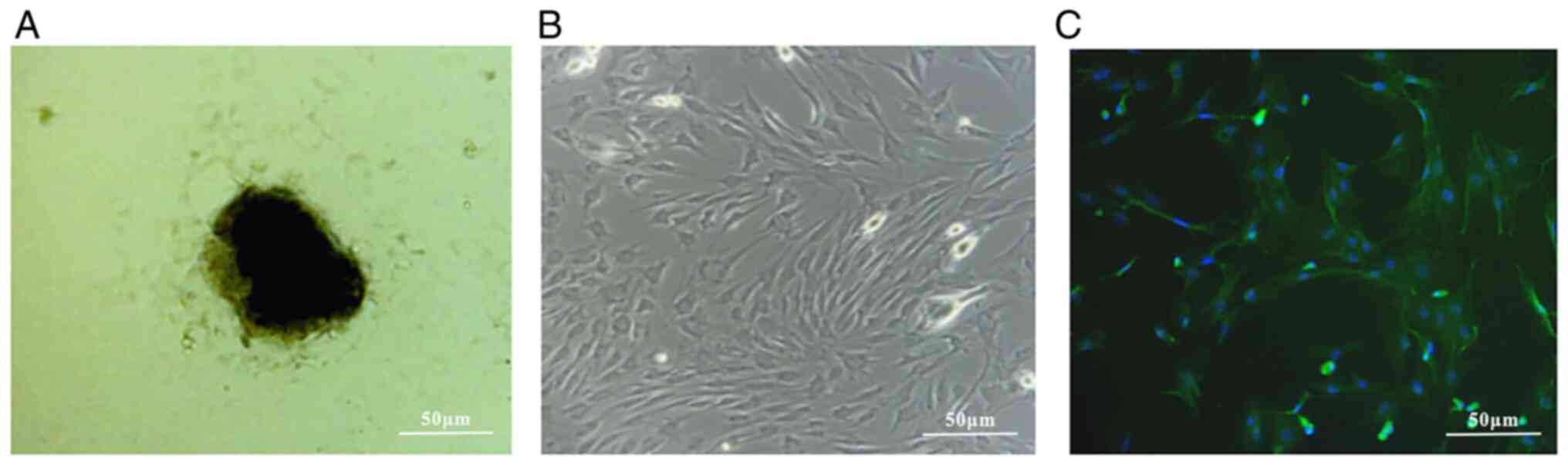
from the edge of the vascular adventitial tissue block and divided
2-3 days later. The primary cultured AFs also demonstrated adherent
growth with large and oblate nuclei (Fig. 2A). Under the microscope, the
adherent cells were irregular polygons or fusiform, growing
vigorously and overlapping in crista-like layers (Fig. 2B). Fluorescence staining indicated
that AFs cells were positive for vimentin expression (Fig. 2C).
Morphology and immunofluorescence
identification of BMSCs cultured in AFs supernatant
Induction and differentiation of BMSCs were
performed using cells at generations 2-3. An inverted microscope
was used to observe the morphology of the cultured cells. After 6
days of BMSCs culture with supernatant of AFs, the BMSCs had
exhibited 70-80% confluence in Models 1 and 2. BMSCs morphology had
lost the characteristic swirls state, while some cells changed
their long spindle shape and exhibited an elliptical appearance.
There was no notable change in cell morphology in the control
group. A total of three groups of cell slides were prepared for
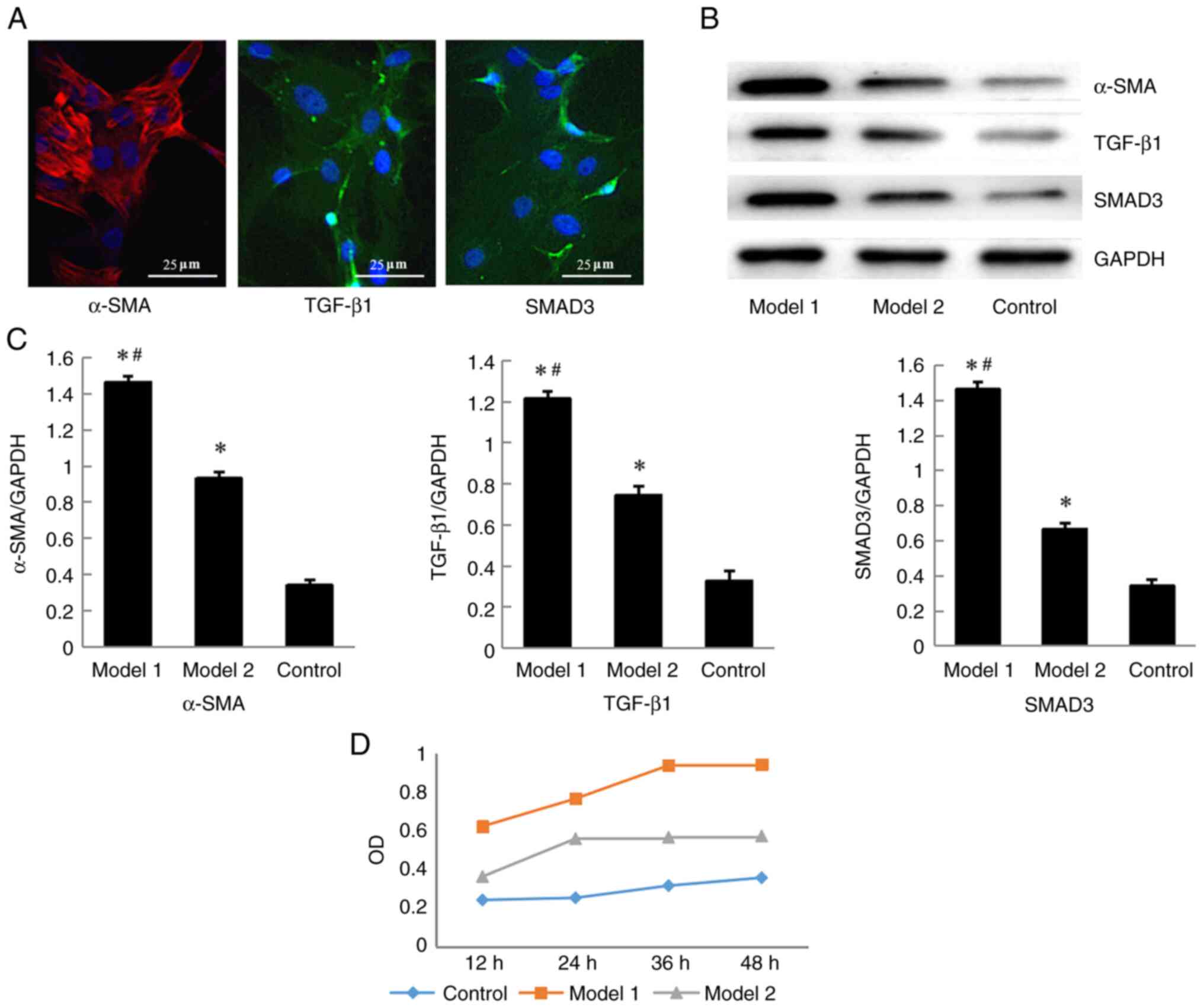
immunofluorescence staining. The cytoplasm expressing α-SMA showed
red fluorescence due to the Cy3-conjugated second antibody goat
anti-rabbit IgG, while positive expression of TGF-β1 and SMAD3 in
the cytoplasm was indicated by a green fluorescence due to the
FITC-conjugated secondary goat anti-rabbit IgG. Immunofluorescence
showed that after cell culture for 6 days, Model 1 exhibited the
strongest staining compared with that in model 2 and the Control
group (Fig. 3A).
Western blot analysis
Western blotting was used to analyze expression of
α-SMA and TGF-β1/SMAD3 signaling pathway proteins in the BMSCs
(Fig. 3B). Models 1 and 2 differed
significantly from the control (P<0.05). Compared with those in
the control group, the expression levels of α-SMA, TGF-β1 and SMAD3
in BMSCs in models1 and 2 were increased (P<0.05; Fig. 3C), with model 1 showing the highest
increase.
Proliferation of BMSCs
The proliferation of BMSCs was evaluated using the
CCK-8 method. BMSCs from Model 1 and 2 were cultured with AFs
supernatant for 12, 24, 36 and 48 h, while BMSCs cultured in normal
medium were used as the control group (Fig. 3D). There were all have significant
differences among the three groups of the proliferation of BMSCs
(P<0.05), and the Model 1showing the highest proliferation
rate.
Migration of BMSCs
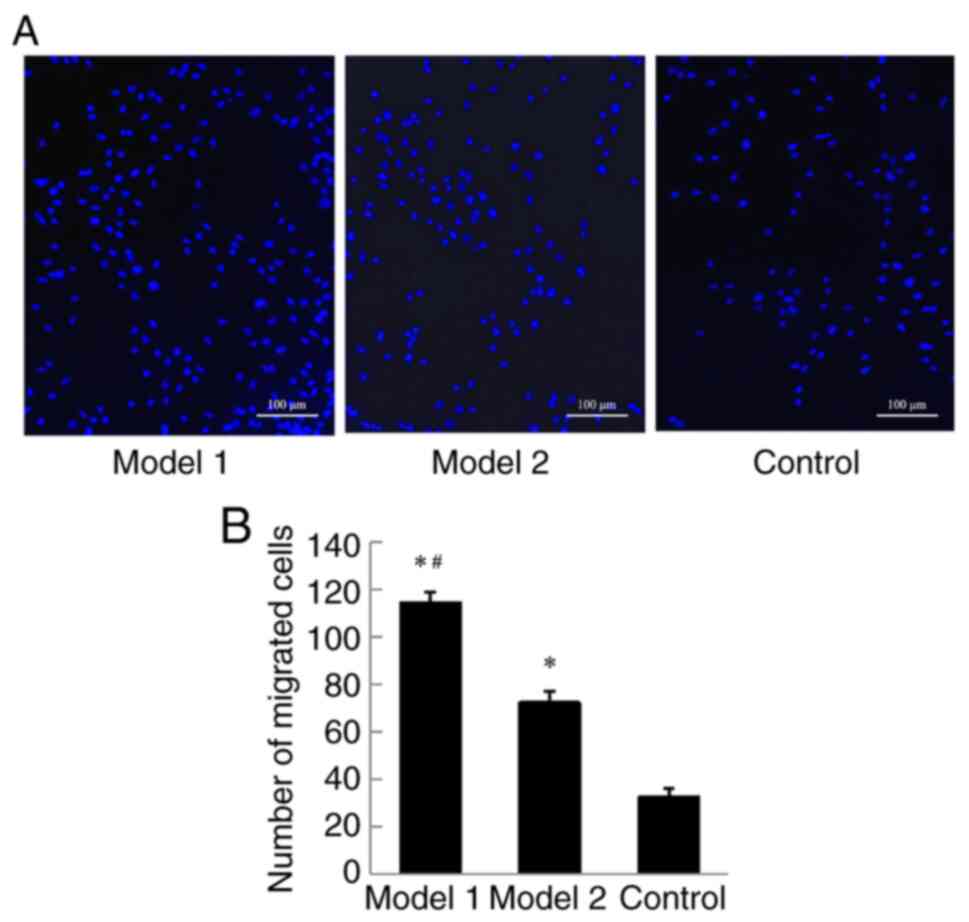
According to Transwell migration assay, BMSCs
migrated from the upper to the lower chamber through the membrane
in all three groups. BMSCs migration in Model 1 was significantly
higher than that in Model 2 and Control after 6 days of culture
(P<0.05; Fig. 4A and B).
Discussion
At present, the incidence of cardiovascular diseases
is increasing globally, which accounts for a large proportion of
the global disease burden, nearly doubling from 271 million in 1990
to 523 million in 2019, and the mortality rate is also on the rise
(18). Atherosclerosis is the most
common cause of cardiovascular disease, including intima injury,
vascular wall pathological remodeling and lumen stenosis (19,20).
It is hypothesized that cell proliferation in the intima and media
of arteries contributes to vascular remodeling, whereas the
adventitia of the arteries is not involved (21). It is hypothesized that the
adventitia provides only physical and nutritional support to the
vascular wall. In addition to being composed of loose connective
tissue, the adventitia is rich in vasa vasorum, lymphatic vessels
and adrenergic nerves and is surrounded by perivascular adipose
tissue (8). A previous study
demonstrated that various cells in the adventitia of the blood
vessels, particularly fibroblasts, have high metabolic activity
(22). Secretion of numerous
cytokines, enzymes and chemokines by AFs following injury is
increased compared with smooth muscle cells. These cytokines,
enzymes and chemokines, including TGF-β1, monocyte chemotactic
protein-1, interleukins, TNF-α, endothelin-1 and matrix metallo
proteinases, induce an inflammatory response and vascular
remodeling (23), which may
contribute to the mobilization of stem cells. Previous studies have
demonstrated that the source of vascular remodeling of smooth
muscle cells following vascular injury is not only the cells of the
vascular wall itself but some BMSCs are also involved through
adhesion differentiation (24,25).
Ni et al (26) reported
that TGF-β1 expression was increased in neointimal lesion of
transplanted angiogenesis, and TGF-β1 stimulated c-kit + cells to
differentiate into smooth muscle cells. It is hypothesized that
proliferating smooth muscle cells in damaged blood vessels are
derived from stem/progenitor cells that exist in situ within
the vessel wall (3). A previous
study demonstrated MMP8 secreted by macrophages promotes the
differentiation of vasculo membrane stem cells into smooth muscle
cells by TGF-β and expression of α-SMA gene by binding the binding
site of the smooth muscle cell gene promoter through NOTCH1;
moreover, vascular injury disease is associated with neointimal
hyperplasia caused by differentiation of adventitial membrane stem
cells into smooth muscle cells (27). Another study showed that Dickkopf-3
induced the differentiation of Spinocerebellar ataxia type
1-positive vascular progenitor cells into smooth muscle cells via
the activation of TGF-β/activating transcription factor 6 and Wnt
signaling pathways, leading to maintenance of atherosclerotic
plaque stability (28).
BMSCs are among the most studied mesenchymal stem
cells due to their relatively simple culture, fast proliferation,
high genetic stability, ability to multi-differentiate and low
immunogenicity (29). The primary
pathological target cells for vascular remodeling are VSMCs,
although, some studies have suggested that vascular stem/progenitor
cells may contribute to smooth muscle cell development in vascular
remodeling lesions (30). Due to
their complexity, the origins of these stem/progenitor cells and
their inducer remains unclear; therefore; discovering the key
inducer of stem/progenitor cells is important. According to a
previous study, miR-503 promoted the mesenchymal stem cell
differentiation induced by TGF-β1 in a VSMC model by targeting
SMAD7(31). In addition, miR-128
inhibited the differentiation of human hair follicle mesenchymal
dry cells into smooth muscle induced by TGF-β1 by targeting
SMAD2(32).
Our previous study examined the interaction between
BMSCs and AFs to discover the mechanism of cell differentiation and
vascular remodeling (33). In
response to vascular injury, certain cytokines and chemokines
mobilize stem/progenitor cells into the local adhesion of the
injury (34). Among these, TGF-β1
was shown to play a fundamental role as it promoted cell
proliferation, migration and stroma secretion and was involved in
vascular remodeling (35,36). In the present study, NE was added
to medium to activate AFs, express α-SMA, differentiate into
myoblasts and secrete increased TGF-β1 to simulate in vitro
the micro-environment of vascular injury. BMSCs were cultured with
supernatant of AFs to observe the effect on cell differentiation
and migration. After 2 days of culture, BMSCs showed increased
expression of α-SMA, TGF-β1 and SMAD3. According to these findings,
NE-induced activation of AFs during vascular injury induced
differentiation and migration of BMSCs and facilitated
differentiation into smooth muscle-like cells, likely through the
TGF-β1/Smad3 signaling pathway involved in vascular remodeling.
An example of a classic neurotransmitter is NE.
Sympathetic and parasympathetic nerves are primarily distributed in
the vascular adventitia. Catecholamines, such as NE, are released
from sympathetic nerve extremities and diffuse or enter the
bloodstream to act on VSMCs and endothelial cells. As an α-and
β-receptor agonist, NE plays an important role in the regulation of
the cardiovascular system (37).
Sympathetic activity is directly associated with the plasma NE
levels and the severity of hypertension (38). The implementation of device-based
therapeutic interventions to decrease renal and systemic
sympathetic activity is used to attenuate hypertension in patients
with resistant hypertension. This technique aims to decrease renal
sympathetic activation by the destruction of renal sympathetic
nerves located in the adventitia of the renal artery (39). A previous study demonstrated that
NE induces epithelial-mesenchymal transformation of lung cancer
cells and promotes invasion and metastasis of lung cancer via the
TGF-β1 signaling pathway (40).
CD147 expression upregulated by stress hormone NE via the
β-adrenergic/β-arrestin1/ERK1/2-selective promoter factor 1 pathway
promotes secretion of MMP-2 and levels of extracellular lactic
acid, which accelerates invasion and metastasis of glioma cells
in vitro (41). NE is
released from the sympathetic ends of the heart and exerts its
biological role by stimulating cardiac adrenergic receptors
(42). A recent study showed that
NE-induced activation of AFs of rats promoted AFs phenotypical
transformation and proliferation, which was mediated by α-receptor
(16,43). NE promotes AFs-derived small
extracellular vesicle release of angiotensin converting enzyme.
miR-155-5p and miR-135a-5p, which were detected in extracellular
vesicles using reverse transcription-quantitative PCR, were shown
to be involved in adjusting VSMC proliferation in hypertension
(16).
Sympathetic nerve endings, which are primarily
located in the arterial vascular adventitia, release NE. The
sympathetic nerves primarily innervate the adventitia of the artery
but rarely innervate the media of the artery (44). However, as a key regulator in
neurohumoral regulation, the roles of NE in vascular adventitia
remain unclear. To the best of our knowledge, the present study is
the first to use NE to promote the activation of AFs, which induced
differentiation and migration of BMSCs via the vascular remodeling
process. This finding suggested that inhibition of NE-induced
activation may be a potential therapeutic target for excessive
sympathetic activation-associated vascular remodeling.
According to a previous study (45), early implementation of certain
interventions in the adventitia may delay or suspend the process of
atherosclerotic vascular remodeling. Adventitial Sca1+cells
transduced with ETV2 are committed to the endothelial fate and
improve vascular remodeling following injury. However, stem cell
migration and differentiation are influenced by several factors and
are complex processes, such as smooth muscle derived chemokines
CCL2 and CXCL1 induced vascular stem/progenitor cell contributes to
neointima formation (23). It has
been reported that DKK3 (Dikkopf-3) transdifferentiates fibroblasts
into functional endothelial cells (46). DKK3 alters atherosclerotic plaque
phenotype involving vascular progenitor and fibroblast
differentiation into smoot muscle (28). So on the one hand, activated stem
cells contribute to the repair of damaged tissue; on the other
hand, these cells may contribute to vascular remodeling and
aggravate atherosclerosis. Therefore, it is essential to have a
thorough understanding of the mechanism of stem cell migration and
differentiation in pathological conditions to improve the
understanding of the pathogenesis of cardiovascular disease,
discover new targets and provide novel preventive strategies
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Shandong Province (grant nos. ZR2017MH046 and
ZR2020MH080) and the Science Foundation of Binzhou Medical
University (grant nos. BY2020KJ46, BY2019XRX06 and
BY2019XRX07).
Availability of data and materials
The dataset used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WY, DY and JG were responsible for study design,
data collection, statistical analysis and manuscript preparation.
LL, DZ and LC were responsible for data collection, statistical
analysis and literature search. XS, XW, PD and WY supervised the
project, designed the study, analyzed data and wrote the
manuscript. All authors have read and approved the final
manuscript. WY and PD confirm the authenticity of all raw data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Binzhou Medical University
(approval no. 2015-008).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yu B, Chen Q, Le Bras A, Zhang L and Xu Q:
Vascular stem/progenitor cell migration and differentiation in
atherosclerosis. Antioxid Redox Signal. 29:219–235. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jiang L, Sun X, Deng J, Hu Y and Xu Q:
Different roles of stem/progenitor cells in vascular remodeling.
Antioxid Redox Signal. 35:192–203. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang L, Issa Bhaloo S, Chen T, Zhou B and
Xu Q: Role of resident stem cells in vessel formation and
arteriosclerosis. Circ Res. 122:1608–1624. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang D, Wang Z, Zhang L and Wang Y: Roles
of cells from the arterial vessel wall in atherosclerosis.
Mediators Inflamm. 2017(8135934)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Durham AL, Speer MY, Scatena M, Giachelli
CM and Shanahan CM: Role of smooth muscle cells in vascular
calcification: Implications in atherosclerosis and arterial
stiffness. Cardiovasc Res. 114:590–600. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Patzelt M, Kachlik D, Stingl J, Sach J,
Stibor R, Benada O, Kofronova O and Musil V: Morphology of the vasa
vasorum in coronary arteries of the porcine heart: A new insight.
Ann Anat. 223:119–126. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen Y, Chen Y, Jiang X, Shi M, Yang Z,
Chen Z, Hua X, Chen J and Wang Y: Vascular adventitial
fibroblasts-derived FGF10 promotes vascular smooth muscle cells
proliferation and migration in vitro and the neointima formation in
vivo. J Inflamm Res. 25:2207–2223. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nava E and Llorens S: The local regulation
of vascular function: From an inside-outside to an outside-inside
model. Front Physiol. 10(729)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zou F, Li Y, Zhang S and Zhang J: DP1
(prostaglandin D2 receptor 1) activation protects against vascular
remodeling and vascular smooth muscle cell transition to
myofibroblasts in angiotensin II-induced hypertension in mice.
Hypertension. 79:1203–1215. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sedding DG, Boyle EC, Demandt JAF, Sluimer
JC, Dutzmann J, Haverich A and Bauersachs J: Vasa vasorum
angiogenesis: Key player in the initiation and progression of
atherosclerosis and potential target for the treatment of
cardiovascular disease. Front Immunol. 9(706)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li XD, Hong MN, Chen J, Lu YY, Ye MQ, Ma
Y, Zhu DL and Gao PJ: Adventitial fibroblast-derived vascular
endothelial growth factor promotes vasa vasorum-associated
neointima formation and macrophage recruitment. Cardiovasc Res.
116:708–720. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G,
Vaziri ND and Zhao YY: New insights into TGF-β/Smad signaling in
tissue fibrosis. Chem Biol Interact. 292:76–83. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yang JX, Pan YY, Wang XX, Qiu YG and Mao
W: Endothelial progenitor cells in age-related vascular remodeling.
Cell Transplant. 27:786–795. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lv BK, Li F, Fang J, Xu L, Sun C, Han J,
Hua T, Zhang Z, Feng Z and Jiang X: Hypoxia inducible factor 1α
promotes survival of mesenchymal stem cells under hypoxia. Am J
Transl Res. 9:1521–1529. 2017.PubMed/NCBI
|
|
15
|
DeLalio LJ, Sved AF and Stocker SD:
Sympathetic Nervous system contributions to hypertension: Updates
and therapeutic relevance. Can J Cardiol. 36:712–720.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ye C, Zheng F, Xu T, Wu N, Tong Y, Xiong
XQ, Zhou YB, Wang JJ, Chen Q, Li YH, et al: Norepinephrine acting
on adventitial fibroblasts stimulates vascular smooth muscle cell
proliferation via promoting small extracellular vesicle release.
Theranostics. 12:4718–4733. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Leary S, Underwood W, Anthony R, et al:
AVMA Guidelines for the euthanasia of animals: 2020 Edition.
Schaumburg: American Veterinary Medical Association, 2020.
|
|
18
|
Roth GA, Mensah GA, Johnson CO, Addolorato
G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ,
Benziger CP, et al: Global Burden of cardiovascular diseases and
risk fisk factors,1990-2019: Update From the GBD 2019 study. J Am
Coll Cardiol. 76:2982–3021. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Soehnlein O and Libby P: Targeting
inflammation in atherosclerosis-from experimental insights to the
clinic. Nat Rev Drug Discov. 20:589–610. 2021.PubMed/NCBI View Article : Google Scholar : Lee J and Choi JH:
Deciphering macrophage phenotypes upon lipid uptake and
atherosclerosis. Immune Netw 20: e22, 2020.
|
|
20
|
Ma Z, Mao C, Jia Y, Fu Y and Kong W:
Extracellular matrix dynamics in vascular remodeling. Am J Physiol
Cell Physiol. 319:C481–C499. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tinajero MG and Gotlieb AI: Recent
developments in vascular adventitial pathobiology: The dynamic
adventitia as a complex regulator of vascular disease. Am J Pathol.
190:520–534. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Han X, Wu A, Wang J, Chang H, Zhao Y,
Zhang Y, Mao Y, Lou L, Gao Y, Zhang D, et al: Activation and
migration of adventitial fibroblasts contributes to vascular
remodeling. Anat Rec (Hoboken). 301:1216–1223. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Yu B, Wong MM, Potter CM, Simpson RM,
Karamariti E, Zhang Z, Zeng L, Warren D, Hu Y, Wang W and Xu Q:
Vascular stem/progenitor cell migration induced by smooth muscle
cell-derived chemokine (C-C Motif) ligand 2 and chemokine (C-X-C
motif) ligand 1 contributes to neointima formation. Stem Cells.
34:2368–2380. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gu W, Nowak WN, Xie Y, Le Bras A, Hu Y,
Deng J, Issa Bhaloo S, Lu Y, Yuan H, Fidanis E, et al: Single-Cell
RNA-Sequencing and metabolomics analyses reveal the contribution of
perivascular adipose tissue stem cells to vascular remodeling.
Arterioscler Thromb Vasc Biol. 39:2049–2066. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Iso Y, Usui S, Toyoda M, Spees JL, Umezawa
A and Suzuki H: Bone marrow-derived mesenchymal stem cells inhibit
vascular smooth muscle cell proliferation and neointimal
hyperplasia after arterial injury in rats. Biochem Biophys Rep.
16:79–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ni Z, Deng J, Potter CMF, Nowak WN, Gu W,
Zhang Z, Chen T, Chen Q, Hu Y, Zhou B, et al: Recipient c-Kit
lineage cells repopulate smooth muscle cells of transplant
arteriosclerosis in mouse models. Circ Res. 125:223–241.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang F, Chen Q, Yang M, Maguire EM, Yu X,
He S, Xiao R, Wang CS, An W, Wu W, et al: Macrophage-derived MMP-8
determines smooth muscle cell differentiation from adventitia
stem/progenitor cells and promotes neointima hyperplasia.
Cardiovasc Res. 116:211–225. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Karamariti E, Zhai C, Yu B, Qiao L, Wang
Z, Potter CMF, Wong MM, Simpson RML, Zhang Z, Wang X, et al: DKK3
(Dickkopf 3) Alters atherosclerotic plaque phenotype involving
vascular progenitor and fibroblast differentiation into smooth
muscle cells. Arterioscler Thromb Vasc Biol. 38:425–437.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Gong P, Zhang W, He Y, Wang J, Li S, Chen
S, Ye Q and Li M: Classification and characteristics of mesenchymal
stem cells and its potential therapeutic mechanisms and
applications against ischemic stroke. Stem Cells Int.
2021(2602871)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang H, Zhao H, Zhu H, Li Y, Tang J, Li Y
and Zhou B: Sca1 + cells minimally contribute to smooth muscle
cells in atherosclerosis. Circ Res. 128:133–135. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gu X, Hong X, Bras AL, Le Bras A, Nowak
WN, Issa Bhaloo S, Deng J, Xie Y, Hu Y, Ruan XZ and Xu Q: Smooth
muscle cells differentiated from mesenchymal stem cells are
regulated by microRNAs and suitable for vascular tissue grafts. J
Biol Chem. 293:8089–8102. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang Z, Pang L, Zhao H, Song L, Wang Y,
Sun Q, Guo C, Wang B, Qin X and Pan A: miR-128 regulates
differentiation of hair follicle mesenchymal stem cells into smooth
muscle cells by targeting SMAD2. Acta Histochem. 118:393–400.
2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wendan Y, Changzhu J, Xuhong S, Hongjing
C, Hong S, Dongxia Y and Fang X: BMSCs interactions with
adventitial fibroblasts display smooth muscle cell lineage
potential in differentiation and migration that contributes to
neointimal formation. Stem Cells Int. 2016(3196071)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lu W and Li X: Vascular stem/progenitor
cells: Functions and signaling pathways. Cell Mol Life Sci.
75:859–869. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Low EL, Baker AH and Bradshaw AC: TGFβ,
smooth muscle cells and coronary artery disease:A review. Cell
Signal. 53:90–101. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kudryashova TV, Shen Y, Pena A, Cronin E,
Okorie E, Goncharov DA and Goncharova EA: Inhibitory antibodies
against activin A and TGF-β reduce self-supported, but not soluble
factors-induced growth of human pulmonary arterial vascular smooth
muscle cells in pulmonary arterial hypertension. Int J Mol Sci.
19(2957)2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Grassi G, Pisano A, Bolignano D, Seravalle
G, D'Arrigo G, Quarti-Trevano F, Mallamaci F, Zoccali C and Mancia
G: Sympathetic nerve traffic activation in essential hypertension
and its correlates: Systematic reviews and meta-analyses.
Hypertension. 72:483–491. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Oparil S, Acelajado MC, Bakris GL,
Berlowitz DR, Cífková R, Dominiczak AF, Grassi G, Jordan J, Poulter
NR, Rodgers A and Whelton PK: Hypertension. Nat Rev Dis Primers.
4(18014)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Akinseye OA, Ralston WF, Johnson KC,
Ketron LL, Womack CR and Ibebuogu UN: Renal sympathetic
denervation: A comprehensive review. Curr Probl Cardiol.
46(100598)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang J, Deng YT, Liu J, Wang YQ, Yi TW,
Huang BY, He SS, Zheng B and Jiang Y: Norepinephrine induced
epithelial-mesenchymal transition in HT-29 and A549 cells in vitro.
J Cancer Res Clin Oncol. 142:423–435. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang P, Wang Z, Yan Y, Xiao L, Tian W, Qu
M, Meng A, Sun F, Li G and Dong J: Psychological stress
up-regulates CD147 expression through Beta-Arrestin1/ERK to promote
proliferation and invasiveness of glioma cells. Front Oncol.
10(571181)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lymperopoulos A, Cora N, Maning J, Brill
AR and Sizova A: Signalling and function of cardiac autonomic
nervous system receptors: Insights from the GPCR signalling
universe. FEBS J. 288:2645–2659. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ren XS, Tong Y, Qiu Y, Ye C, Wu N, Xiong
XQ, Wang JJ, Han Y, Zhou YB, Zhang F, et al: MiR155-5p in
adventitial fibroblasts-derived extracellular vesicles inhibits
vascular smooth muscle cell proliferation via suppressing
angiotensin-converting enzyme expression. J Extracell Vesicles.
9(1698795)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mackay CDA, Jadli AS, Fedak PWM and Patel
VB: Adventitial fibroblasts in aortic aneurysm: Unraveling
pathogenic contributions to vascular disease. Diagnostics (Basel).
12(871)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Le Bras A, Yu B, Issa Bhaloo S, Hong X,
Zhang Z, Hu Y and Xu Q: Adventitial Sca1 +cells transduced with
ETV2 are committed to the endothelial fate and improve vascular
remodeling after injury. Arterioscler Thromb Vasc Biol. 38:232–244.
2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen T, Karamariti E, Hong X, Deng J, Wu
Y, Gu W, Simpson R, Wong MM, Yu B, Hu Y, et al: DKK3
(Dikkopf-3)transdifferentiates fibroblasts into functional
endothelial cells-brief report. Arterioscler Thromb Vasc Biol.
39:765–773. 2019.PubMed/NCBI View Article : Google Scholar
|