Introduction
Patients with a refractive error of <-6 degrees
or an axial length of >26.5 mm and typical pathological changes
in the fundus are diagnosed with pathologic myopia (PM). PM occurs
in 1-3% of adults (1,2). Certain phenotypic features of PM,
including patchy atrophy, the thinning of choroid and
choriocapillaris, lacquer cracks and choroidal neovascularization
(CNV) in the fellow eye may increase the risk of myopic CNV (mCNV)
(3-5).
In addition, CNV has been reported to be a vision-threatening
complication in 5.2-10.2% of highly myopic eyes (6).
In recent years, the anti-vascular endothelial
growth factor (VEGF) agents used in patients with mCNV have
demonstrated considerable success in visual acuity gains and have
led to an improvement in the patients' quality of life (7). Intravitreal and subtenon steroids,
photodynamic therapy, transpupillary thermotherapy and various
other treatments have had varying degrees of success in preventing
visual loss in patients with CNV (8,9). Due
to the good efficacy of anti-VEGF biological agents, including
bevacizumab, ranibizumab and aflibercept, these have been proposed
as a first-line treatment for mCNV (10). The visual prognosis and natural
progression of mCNV are more favourable than those of CNV secondary
to age-related macular degeneration (AMD) and patients with mCNV
have shown a good response to anti-VEGF therapy (11).
Bevacizumab and ranibizumab, which are respectively
a whole anti-VEGF antibody and an antibody fragment, have been
mostly used in mCNV treatment targeting VEGF-A, according to
studies conducted after 2006 (12,13).
Aflibercept is a new recombinant fusion protein consisting of a
homodimeric glycoprotein formed by the fusion of the extracellular
domain of human VEGF receptor (extracellular domain 2 of VEGF
receptor 1 and extracellular domains 3 and 4 of VEGF receptor 2)
and the Fc portion of human immunoglobulin G1(14). It has high affinity for placental
growth factors and VEGF isoforms (15). The MYRROR study indicated that
aflibercept is well-tolerated and effective in the treatment of
mCNV. In this trial, patients in the intravitreal aflibercept group
received two injections between weeks 0 and 8(16). A recent study on the treatment of
mCNV with different anti-VEGF agents pointed out that
afliberceptwas more effective to resolution of CNV activation and
may be preferred to maintain anatomical and visual yields in eyes
with PM for longer follow-ups (17).
Studies on high-dose anti-VEGF therapy used to treat
neovascular AMD have been conducted. Broadhead et al
(18) found that higher dose
anti-VEGF therapy may obtain improved anatomic outcomes and
maintain vision, but frequent injections are required to achieve
this . Another study by You et al (19) suggested that an intravitreal
aflibercept injection at a high dose and frequency is an effective
treatment for patients with wet AMD. Nguyen et al (20) reported that an intravitreal
injection of 4 mg aflibercept is safe and well-tolerated. In the
same study, the 4-mg dose significantly reduced foveal thickening,
improved best corrected visual acuity (BCVA) and reduced repeat
injections in patients with neovascular AMD. Current studies on the
use of aflibercept for the treatment of mCNV usually use a dose of
2 mg (16,21). To date, research on the use of
high-dose aflibercept for mCNV has been limited.
The number of injections varies among different
studies. In the study performed by Bruè et al (22), 68.4% of patients received one or
two injections and 31.6% required three to five injections over the
18-month follow-up. Another study indicated that BCVA improvement
was achieved with a median of two injections and was sustained for
up to 12 months (21). In the
study by Korol et al (23),
patients received a mean of 2.6 intravitreal injections over 12
months. Based on previous studies (18,19)
and our experience, a 4 mg [2+ pro re nata (PRN) scheme]
aflibercept injection was selected in the present study to observe
the outcomes for patients with mCNV for 12 months of follow-up and
to compare the present results with those of other studies.
Patients and methods
Patients
This retrospective study reviewed the charts of
patients with mCNV encountered at the Central Hospital Affiliated
to Shandong First Medical University (Jinan, China) between January
2019 and August 2021. A total of 16 consecutive subjects (7 males
and 9 females; age range, 26-38 years) with mCNV were enrolled in
this retrospective study. The study was approved by the ethics
committee of the Central Hospital Affiliated to Shandong First
Medical University (Jinan, China; approval no. 2022-130-01) and was
performed according to the Declaration of Helsinki. All subjects
provided written informed consent prior to treatment. CNV was
diagnosed by clinical examination, optical coherence tomography
(OCT; Cirrus HD-OCT 4000; Carl Zeiss Meditec, Inc.) and fluorescein
angiography (FA) and/or indocyanine angiography. All patients
underwent computerized optometry using a Topcon KR-800 (Beijing
Dakang Instrument Co., Ltd.), axial length measurement
(IOLMaster®; Carl Zeiss AG) and intraocular pressure
(TOPCON CT-800). The inclusion criteria were as follows: i) High
myopia with a refractive error of <-6 diopters; ii) axial length
of >26.5 mm; iii) myopic retinal pathological changes (posterior
staphyloma, chorioretinal atrophy, papillary crescent and lacquer
cracks); iv) OCT evidence of hyperreflective lesion; v) FA
detection of subfoveal active CNV; and vi) BCVA of ≥0.5 logMAR
prior to treatment. The exclusion criteria were as follows: i)
Patients with different macular diseases, such as ARMD and diabetic
macular oedema; ii) patients with previous subfoveal or juxtafoveal
laser treatment; iii) history of trauma; iv) ophthalmic surgery; v)
presumed ocular histoplasmosis syndrome; vi) hereditary eye
disease; and vii) any other cause of secondary CNV or spheric
equivalent, such as astigmatisms.
Treatments and follow-up
All patients received an intravitreal injection of 4
mg aflibercept (Bayer AG). The lids and conjunctiva were
disinfected with 10% iodophor and 5% povidone iodine, respectively.
The conjunctiva was anesthetized with 1% oxybuprocaine. Aflibercept
(4 mg) was injected using a 30-g needle through the pars plana (4
mm from the limbus of the phakic eye) into the vitreous and an eye
patch was placed over the eye following treatment. Gatifloxacin eye
drops (China Otsuka Pharmaceutical Co., Ltd.) were prescribed to be
instilled four times a day for 7 days, starting on the second day
after surgery. The second injection was administered 35 days later.
Ophthalmic examination and OCT were performed at the baseline and
at 1, 2, 4, 6, 8, 10 and 12 months after the initial injection. At
each follow-up visit, a thorough ophthalmic assessment was
performed, including an evaluation of BCVA and retinal morphology
with OCT. FA was performed if the activity of the lesion could not
be estimated by clinical expression and OCT assessment at each
follow-up visit. BCVA, macular appearance, OCT and FA findings were
used to decide if the subject should have received another
intravitreal injection of aflibercept. A decrease in BCVA,
aggravation of metamorphopsia, presence of macular oedema or
haemorrhage, increased central retinal thickness (CRT) or central
macular thickness (CMT), or increased leakage created the need for
additional treatment with aflibercept.
Statistical analysis
Statistical analysis using SPSS version 26.0 (IBM
Corp.). All values in the text are presented as the mean ± standard
deviation. The outcomes at different time-points following
treatment were compared with baseline values of BCVA and CRT
individually. Pairwise comparisons were performed at different
postoperative time-points. The data were evaluated for normality
using normality tests. Normally distributed data were assessed
using repeated-measures ANOVA and pairwise comparisons were
performed using Friedman's test. Homogeneity of variance was tested
prior to ANOVA. Non-normally distributed data were assessed using
the Kruskal-Wallis H-test. Dunn's test was used for pairwise
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient disposition, baseline
characteristics and exposure
All 16 eyes were administered an aflibercept
intravitreal injection. Basic information of the two groups is
provided in Table I. Follow-up
images were obtained at 1, 2, 4, 6, 8, 10 and 12 months after
treatment. None of the patients developed glaucoma following the
high-dose aflibercept injection and intraocular pressure (IOP) in
all eyes was controlled at <21 mmHg at each visit. During the
follow-up period, no cardiovascular or cerebrovascular embolism and
no death events were recorded.
 | Table IBaseline demographic and clinical
characteristics (patients, n=16; eyes, n=16). |
Table I
Baseline demographic and clinical
characteristics (patients, n=16; eyes, n=16).
| Characteristic | Value |
|---|
| Gender | |
|
Male | 7 |
|
Female | 9 |
| Age, years | 30.5±3.35 |
| Spherical equivalent,
D | -7.31±0.90 |
| Axial length, mm | 27.17±0.89 |
| Duration of symptoms,
months | 0.96±0.67 |
| Number of
injections | 2.13±0.5 |
| Follow-up duration,
months | 13.41±1.17 |
Key outcomes
The Snellen BCVA was changed into a logarithm of the
minimum angle of resolution (logMAR). The changes in BCVA and CRT
reached statistical significance at the 1-, 2-, 4-, 6-, 8-, 10- and
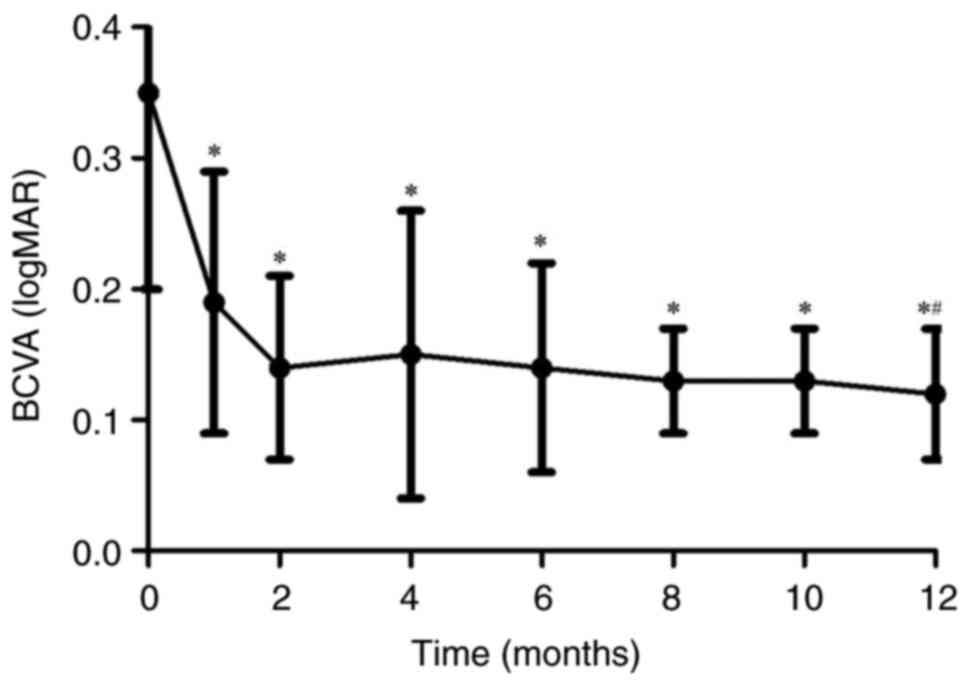
12-month follow-ups compared with the baseline. The mean BCVA
improved from 0.35±0.15 logMAR at the baseline to 0.12±0.05 logMAR
at the final follow-up (P<0.05; Fig. 1). The reduction in metamorphopsia
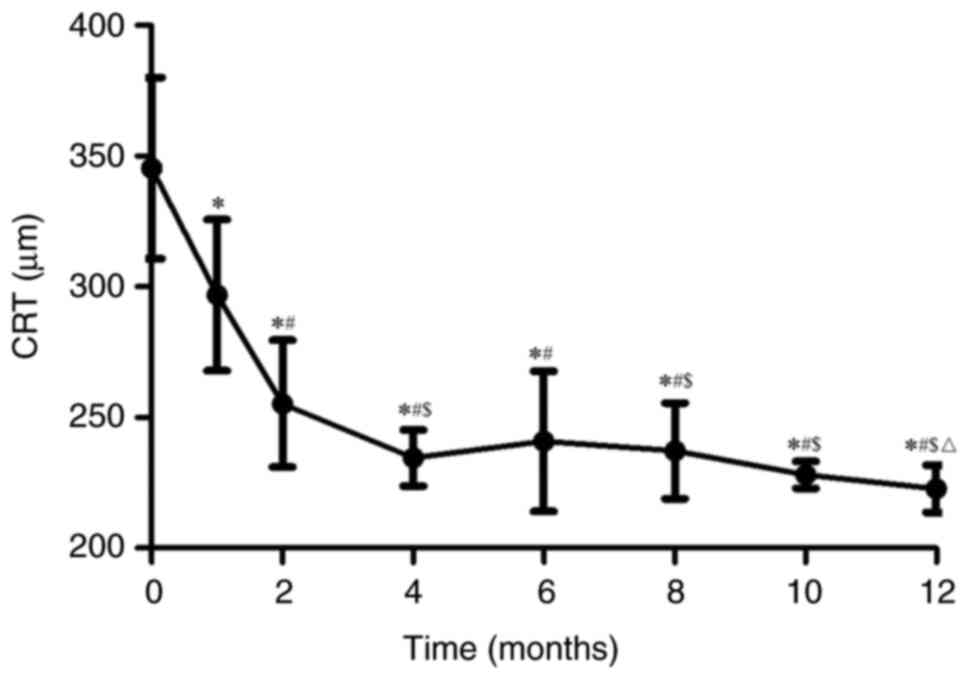
was obvious. The mean CRT at the last follow-up was reduced from
345.38±34.69 µm (pre-treatment levels) to 222.75±8.98 µm
(P<0.05; Fig. 2). There was a
significant difference in BCVA at 12 months after surgery compared
with the 1-month follow-up (P<0.05). There were significant
differences in CRT at the 2-, 4-, 6-, 8-, 10- and 12-month
follow-ups, as compared with the 1-month follow-up after the
initial injection (P<0.05). In addition, significantly different
changes were observed at 4-months of follow-up compared with the
2-month follow-up. Most of the retinal fluid was absorbed and the
choroidal neovascularization was diminished within the subretinal
space. The favourable results of the present study strengthened the
confidence in the efficacy of high-dose aflibercept for mCNV
treatment.
Case of a patient
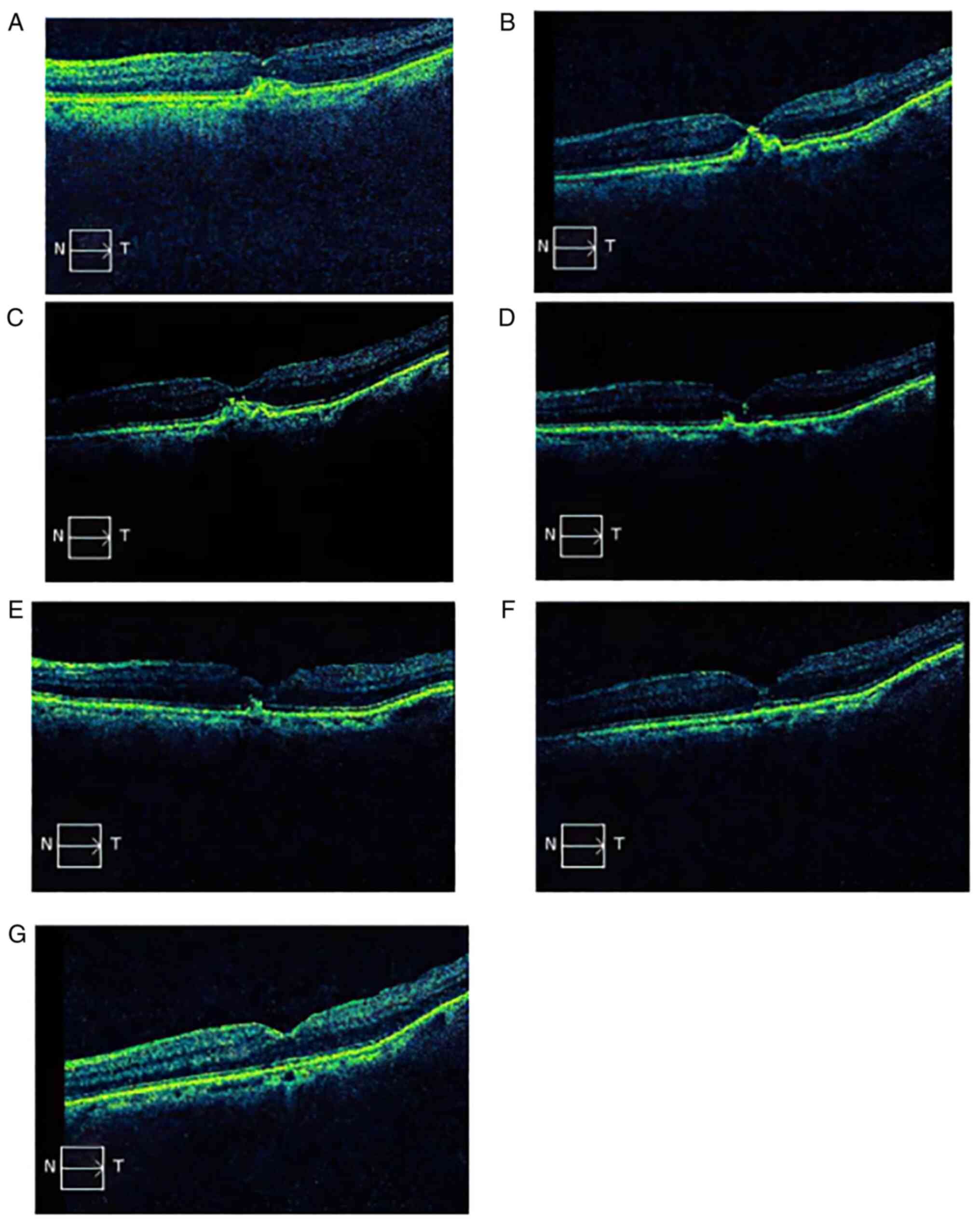
Fig. 3A-G presents
the changes in the OCT scan of a representative patient (female, 29
years old) diagnosed with mCNV during the follow-up. At diagnosis,
OCT indicated fibrovascular pigment epithelial detachment and
increased retinal thickness in the fovea. The BCVA was 0.9 logMAR.
At one month after the initial injection, the fibrovascular pigment
epithelial detachment had partially retreated and the retinal
thickness decreased from 341 µm (pre-treatment levels) to 221 µm. A
strong dot reflection may be seen at the top. The BCVA was 0.4
logMAR. After the second aflibercept 4 mg injection, the OCT scan
showed that the neovascularization further retreated. It was
observed that the neovascularization gradually subsided, the
retinal thickness returned to normal, the morphology of the macular
fovea was almost normal in late follow-ups and the BCVA was
improved and stable. The BCVA was 0.3, 0.3, 0.2, 0.1 and 0.1 logMAR
at 2, 4, 6, 8 and 12 months, respectively, following the initial
aflibercept injection (data not shown).
Discussion
mCNV is one of the sight-threatening complications
of PM. The application of anti-VEGF drugs in choroidal
neovascularization of high myopia has markedly improved the visual
quality of patients, particularly the working population. Among the
anti-VEGF drugs, bevacizumab and ranibizumab exhibited a similar
efficacy in restoring functional and anatomical parameters.
However, ranibizumab was designed and approved for ocular
administration. It appears to be the preferred treatment for mCNV,
as it achieves efficacy with a short treatment duration and
frequency and few adverse effects (10,23).
Aflibercept as a new generation of anti-VEGF agent was originally
approved for the treatment of AMD. Fauser and Muether compared the
VEGF suppression times in their study on AMD treatment and reported
that aflibercept has significantly longer VEGF suppression times
compared with ranibizumab (24).
There is now substantial evidence in favour of the
use of aflibercept for mCNV. Toto et al (7) reported the most recent data from the
articles of anti-VEGF therapy for mCNV. In their study on
intravitreal aflibercept injection for mCNV, Chen et al
(25) found that a single
aflibercept 2.0 mg injection resolved mCNV in ~50% of the patients.
The median number of injections was two within 12 months. Another
prospective 12-month cohort study reported promising results; the
efficacy of the intravitreal aflibercept (2 mg) 2+PRN regimen in 31
eyes of 30 patients with mCNV was evaluated and the mean decimal
BCVA improved from 0.2±0.1 at the baseline to 0.35±0.16 at the
12-month follow-up (23). In
addition, studies showing the efficacy of the initial dosing
regimens, such as the 1+PRN and 3+PRN regimens, indicated no
statistically significant difference in BCVA. In a retrospective
study, Kung et al (26)
reported that there was no significant difference in visual
improvement between the eyes with a single intravitreal injection
and the eyes with a loading dose of 3 monthly injections . The mean
number of injections was 2.32 and 3.57, respectively. Korol et
al (27) reported a series of
47 eyes with 24-month follow-up in which there was an increase in
BCVA and decrease in central foveal thickness. The total mean
number of injections in their study was 2.8±1.1. In another study
by our group on mCNV treatment using conbercept, it was found that
the effect of one injection was not ideal; the CRT was 261.50±21.66
µm at 1 month after the first injection and 247.06±23.85 µm at 1
month after the second injection (28). Furthermore, the mean CNV area was
0.22 mm2 at 1 month after the first injection and was
0.07 mm2 at 1 month after the second injection.
The outcomes of 16 consecutive eyes with mCNV
treated with 4 mg aflibercept intravitreal injection were
retrospectively reviewed and followed up for 12 months. All eyes
initially underwent two injections 35 days apart, followed by an
additional injection based on monthly visits. The present study
revealed several clinical effects of the intravitreal injection of
aflibercept in the treatment of mCNV. The BCVA improved from
0.35±0.15 logMAR at the baseline to 0.12±0.05 logMAR at the final
follow-up visit. The BCVA improved significantly 1 month after the
initial injection and further after the second injection. There was
a significant difference in BCVA at 1 and 12 months after surgery.
An ideal visual acuity was achieved and remained stable after two
injections. The CRT was decreased significantly from 345.38±34.69
to 296.88±28.93 µm at 1 month after the initial injection. At 2
months after treatment, the CRT reached 255.31±24.25 µm. At 4
months from surgery, the CRT was further reduced and reached
234.56±10.74 µm. The CRT remained stable and was 222.75±8.98 µm at
the 12-month follow-up visit. In the present study, the CRT reached
stable levels at 2 and 4 months after the initial injection. This
outcome is different from what was previously reported in the
MYRROR trial (16). The MYRROR
trial, which adopted an as-needed aflibercept treatment for mCNV,
indicated that the CMT rapidly decreased and reached stable levels
between weeks 4 and 8. Such an early improvement of CMT was
consistent with another retrospective clinical study, which
compared bevacizumab to aflibercept in the treatment of mCNV
(29). At week 48, in the MYRROR
trial, the decrease in CRT was 86.2 µm, whereas in the present
study, it was 122.63 µm (16).
Furthermore, in the present study, aflibercept had significant
advantages in terms of the VEGF suppression times in the treatment
of mCNV. Only 3/16 mCNV cases were injected for the third time and
the mean number of injections during the 12-month follow-up was
2.13±0.5, which was lower than that in other studies (23,26,27,30).
In the present study, it was found that the 4 mg aflibercept 2+PRN
scheme was able to markedly eliminate neovascularization. In
addition, none of the cases had any recurrence or significant
increase in CRT, while the visual acuity remained stable during the
12-month follow-up. The present results showed that aflibercept may
represent an ideal choice for mCNV treatment. Following the
diagnosis of mCNV, a timely intravitreal injection of aflibercept
may provide an improved curative effect.
As with any common intraocular surgery, intravitreal
injections of anti-VEGF drugs are accompanied by risks of bleeding,
infection, cataracts and glaucoma (31). Elevated IOP and ocular inflammation
following intravitreal injection are the most frequently reported
serious ocular adverse events (32). Therefore, a comprehensive
ophthalmic examination should be performed in subjects with mCNV
prior to intravitreal injections. In the present study, no systemic
or ocular safety issues were noted in any of the patients treated
with high-dose aflibercept. No cases of increased IOP or
intraocular inflammation were noted in patients treated with 4 mg
aflibercept. The safety and efficacy of high-dose therapy for mCNV
needs to be confirmed by large-scale and rigorous clinical
trials.
There were several limitations to the present study
that may impact or influence the interpretation and
generalizability of the findings. First, due to the retrospective
design, the lack of randomization somewhat reduced the power of the
results. Furthermore, the outcomes should be interpreted with
caution due to the small sample size. As another limitation, the
study lacked a control group. In addition, the follow-up time was
short (12 months) and continuous follow-up is necessary. The
present study also has its advantages. To the best of our
knowledge, this is the first clinical observation obtained using
high-dose aflibercept (4 mg) in the treatment of mCNV. According to
these results, the 4 mg aflibercept 2+PRN scheme proved effective
for mCNV, but future studies, require to be conducted in order to
further evaluate the effect of this scheme.
In conclusion, the present study indicated that an
intravitreal injection of high-dose aflibercept (4 mg 2+PRN scheme)
was effective in promoting vision improvement and stabilization. It
also significantly alleviated metamorphopsia and reduced the CRT in
patients with mCNV. During the 12-month follow-up, the BCVA and CRT
were stable. The present results suggested that the aflibercept 4
mg 2+PRN scheme may be an ideal choice for mCNV treatment,
prompting further studies to evaluate the effect of this
regimen.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ and CH designed the study and drafted and revised
the manuscript. YH and ZY performed data acquisition, analysis and
interpretation. All authors have read and approved the final
manuscript. CH and WZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
This study followed the tenets of the Declaration of
Helsinki and was approved by the ethics committee of the Central
Hospital Affiliated to Shandong First Medical University (Jinan,
China; approval no. 2022-130-01). Written informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Neelam K, Cheung CM, Ohno-Matsui K, Lai TY
and Wong TY: Choroidal neovascularization in pathological myopia.
Prog Retin Eye Res. 31:495–525. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wong TY, Ferreira A, Hughes R, Carter G
and Mitchell P: Epidemiology and disease burden of pathologic
myopia and myopic choroidal neovascularization: An evidence-based
systematic review. Am J Ophthalmol. 157:9–25.e12. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ohno-Matsui K, Yoshida T, Futagami S,
Yasuzumi K, Shimada N, Kojima A, Tokoro T and Mochizuki M: Patchy
atrophy and lacquer cracks predispose to the development of
choroidal neovascularization in pathological myopia. Br J
Ophthalmol. 87:570–573. 2003.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wakabayashi T and Ikuno Y: Choroidal
filling delay in choroidal neovascularization due to pathological
myopia. Br J Ophthalmol. 94:611–15. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ikuno Y, Jo Y, Hamasaki T and Tano Y:
Ocular risk factors for choroidal neovascularization in pathologic
myopia. Invest Ophthalmol Vis Sci. 51:3721–3725. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Grossniklaus HE and Green WR: Pathologic
findings in pathologic myopia. Retina. 12:127–133. 1992.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Toto L, Di Antonio L, Costantino O and
Mastropasqua R: Anti-VEGF therapy in myopic CNV. Curr Drug Targets.
22:1054–1063. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ehrlich R, Kramer M, Rosenblatt I,
Weinberger D, Mimouni K, Priel E and Axer-Siegel R: Photodynamic
therapy for choroidal neovascularization in young adult patients.
Int Ophthalmol. 30:345–351. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sickenberg M, Schmidt-Erfurth U, Miller
JW, Pournaras CJ, Zografos L, Piguet B, Donati G, Laqua H,
Barbazetto I, Gragoudas ES, et al: A preliminary study of
photodynamic therapy using verteporfin for choroidal
neovascularization in pathologic myopia, ocular histoplasmosis
syndrome, angioid streaks and idiopathic causes. Arch Ophthalmol.
118:327–336. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cohen SY: Anti-VEGF drugs as the 2009
first-line therapy for choroidal neovascularization in pathologic
myopia. Retina. 29:1062–1066. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Teo KY, Ng WY, Lee SY and Cheung CM:
Management of myopic choroidal neovascularization: Focus on
anti-VEGF therapy. Drugs. 76:1119–1133. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ferrara N, Damico L, Shams N, Lowman H and
Kim R: Development of ranibizumab, an anti-vascular endothelial
growth factor antigen binding fragment, as therapy for neovascular
age-related macular degeneration. Retina. 26:859–870.
2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Laud K, Spaide RF, Freund KB, Slakter J
and Klancnik JM Jr: Treatment of choroidal neovascularization in
pathologic myopia with intravitreal bevacizumab. Retina.
26:960–963. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Howaidy A and Eldaly ZH: Comparison of
structural and functional outcome of aflibercept versus ranibizumab
in patients with myopic choroidal neovascularization. Eur J
Ophthalmol. 31:211–217. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu Z, Zhou P, Li X, Wang H, Luo D, Qiao H,
Ke X and Huang J: Structural characterization of a recombinant
fusion protein by instrumental analysis and molecular modeling.
PLoS One. 8(e57642)2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ikuno Y, Ohno-Matsui K, Wong TY,
Korobelnik JF, Vitti R, Li T, Stemper B, Asmus F, Zeitz O,
Ishibashi T and MYRROR Investigators: Intravitreal aflibercept
injection in patients with myopic choroidal neovascularization: The
MYRROR study. Ophthalmology. 122:1220–1227. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Karasu B and Celebi ARC: The efficacy of
different anti-vascular endothelial growth factor agents and
prognostic biomarkers in monitoring of the treatment for myopic
choroidal neovascularization. Int Ophthalmol. 42:2729–2740.
2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Broadhead GK, Keenan TDL, Chew EY, Wiley
HE and Cukras CA: Comparison of agents using higher dose anti-VEGF
therapy for treatment-resistant neovascular age-related macular
degeneration. Graefes Arch Clin Exp Ophthalmol. 260:2239–2247.
2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
You QS, Gaber R, Meshi A, Ramkumar HL,
Alam M, Muftuoglu IK and Freeman WR: High-dose high-frequency
aflibercept for recalcitrant neovascular age-related macular
degeneration. Retina. 38:1156–1165. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nguyen QD, Campochiaro PA, Shah SM,
Browning DJ, Hudson HL, Sonkin PL, Hariprasad SM, Kaiser PK,
Slakter J, Haller JA, et al: Evaluation of very high-and very
low-dose intravitreal aflibercept in patients with neovascular
age-related macular degeneration. J Ocul Pharmacol Ther.
28:581–588. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pece A and Milani P: Intravitreal
aflibercept for myopic choroidal neovascularization. Graefes Arch
Clin Exp Ophthalmol. 254:2327–2332. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bruè C, Pazzaglia A, Mariotti C, Reibaldi
M and Giovannini A: Aflibercept as primary treatment for myopic
choroidal neovascularisation: A retrospective study. Eye (Lond).
30:139–145. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Korol AR, Zadorozhnyy OS, Naumenko VO,
Kustryn TB and Pasyechnikova NV: Intravitreal aflibercept for the
treatment of choroidal neovascularization associated with
pathologic myopia: A pilot study. Clin Ophthalmol. 10:2223–2229.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fauser S and Muether PS: Clinical
correlation to differences in ranibizumab and aflibercept vascular
endothelial growth factor suppression times. Br J Ophthalmol.
100:1494–1498. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen SL, Tang PL and Wu TT: Result of
intravitreal aflibercept injection for myopic choroidal
neovascularization. BMC Ophthalmol. 21(342)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kung YH, Wu TT and Huang YH: One-year
outcome of two different initial dosing regimens of intravitreal
ranibizumab for myopic choroidal neovascularization. Acta
Ophthalmol. 92:e615–e620. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Korol A, Kustryn T, Zadorozhnyy O,
Pasyechnikova N and Kozak I: Comparison of efficacy of intravitreal
ranibizumab and aflibercept in eyes with myopic choroidal
neovascularization: 24-month follow-up. J Ocul Pharmacol Ther.
36:122–125. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhu W, Hao Y, Yuan Z, Huang C, Liu J and
Ma Y: Long-term outcomes of high-dose conbercept treatment for
myopic cho-roidal neovascularisation and idiopathic choroidal
neovascularisation. Ophthalmic Res, Feb 6, 2023 (Epub ahead of
print).
|
|
29
|
Wang JK, Huang TL, Chang PY, Chen YT,
Chang CW, Chen FT, Hsu YR and Chen YJ: Intravitreal aflibercept
versus bevacizumab for treatment of myopic choroidal
neovascularization. Sci Rep. 8(14389)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Corazza P, Kabbani J, Soomro T, Alam MMR,
D'Alterio FM and Younis S: Three-year real-world outcomes of
intravitreal anti-VEGF therapies in patients affected by myopic
choroidal neovascularization. Eur J Ophthalmol. 31:2481–2487.
2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fung AE, Rosenfeld PJ and Reichel E: The
international intravitreal bevacizumab safety survey: Using the
internet to assess drug safety worldwide. Br J Ophthalmol.
90:1344–1349. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Solomon SD, Lindsley KB, Krzystolik MG,
Vedula SS and Hawkins BS: Intravitreal bevacizumab versus
ranibizumab for treatment of neovascular age-related macular
degeneration: Findings from a cochrane systematic review.
Ophthalmology. 123:70–77. 2016.PubMed/NCBI View Article : Google Scholar
|