Introduction
Lung cancer is the most common type of malignant
tumour and has the highest mortality rate worldwide, accounting for
18% of cancer-related deaths (1).
Small cell lung cancer (SCLC) accounts for 15-20% of all cases of
lung cancer and ~70% of patients have extensive-stage SCLC
(ES-SCLC) at diagnosis (2).
Limited stage SCLC, which has not metastasized is potentially
curable, whereas ES-SCLC is not. Chemotherapy, radiotherapy and
anti-angiogenic therapy are key treatments for ES-SCLC (3). Although this comprehensive treatment
model is effective, the 5-year survival rate is <8% (4). Therefore, there is an urgent need for
a more effective treatment plan.
Immune checkpoint inhibitors (ICIs), including
monoclonal antibodies targeting programmed cell death 1 (PD-1) and
PD ligand 1, have become widely used in lung cancer (2,5).
Moreover, PD-1 inhibitors combined with anti-angiogenic agents are
effective in patients with non-SCLC (NSCLC), significantly
prolonging progression-free survival (PFS) and overall survival
(OS) (6). However, the application
of PD-1 inhibitors in SCLC is limited because SCLC is able to
damage the immune system, downregulate components like PD-1 to
present fewer targets for therapeutic drugs like PD-1 inhibitors,
and improve the ability of tumour cells to evade detection by the
host immune system (7).
The present study reports the case of a patient with
ES-SCLC who experienced disease progression after several courses
of chemotherapy but responded to combination therapy with a PD-1
inhibitor (toripalimab) and anti-angiogenic therapy (anlotinib).
The present study was conducted in accordance with the CARE
reporting checklist (8).
Case presentation
A 66-year-old man with performance status (PS) 3
visited The First People's Hospital of Yongkang City (Yongkang,
China) in February 2020 with a chief complaint of chest tightness
and haemoptysis for 5 months. The patient had a 40 pack-year
smoking history and a history of resection of the right kidney and
ureter due to stones in 2004.
Chest CT (SOMATOM Definition AS; Siemens AG; imaging
parameters: WL 350/50, magnification x0.78, thickness 7, ctawp66653
and thorax CE 7.0 B30f) revealed a mass in the left lung invading
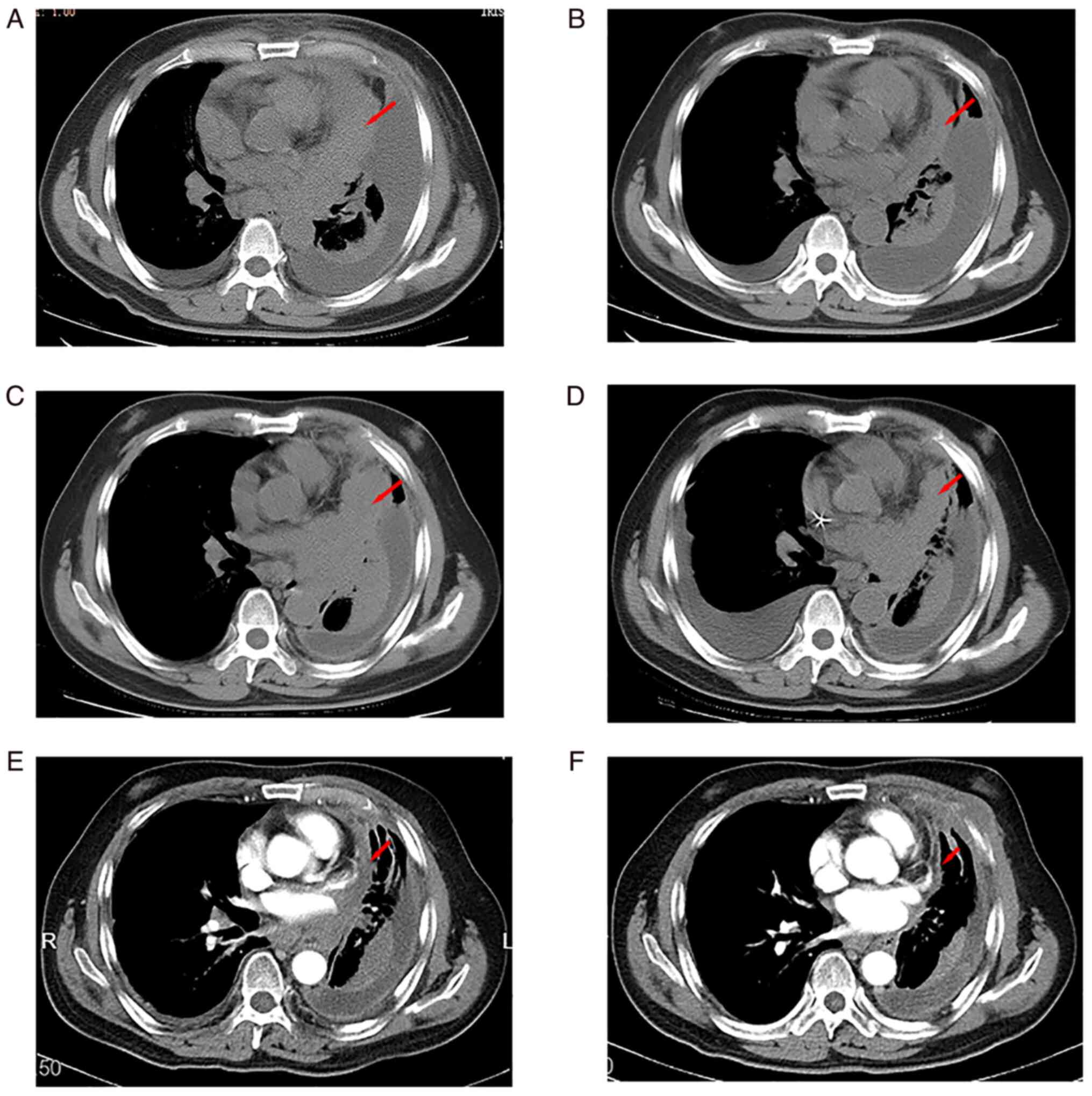
the left hilar region and mediastinum (Fig. 1A). The patient underwent a lung
biopsy. The lung biopsy tissue was immersed in 4% paraformaldehyde
for 4 h at 25˚C and transferred to 70% ethanol. Individual lobes of
lung biopsy material were placed in processing cassettes and
dehydrated using a serial ascending ethanol gradient. After 6 h,
the dehydrated tissue was soaked in xylene, and then soaked in
paraffin wax (Histowax) at 62˚C for 70 min, to embed it in paraffin
wax blocks. Then, 5-µm-thick lung tissue sections were dewaxed in
xylene at room temperature (~25˚C), rehydrated in a descending
ethanol series, washed in phosphate-buffered saline, and stained
with haematoxylin and eosin for 2-5 min at ~25˚C. Following
staining, sections were dehydrated using increasing concentrations
of ethanol and xylene at ~25˚C. Finally, the sections were examined
under an optical microscope (Axio Lab.A1; Zeiss). Under high
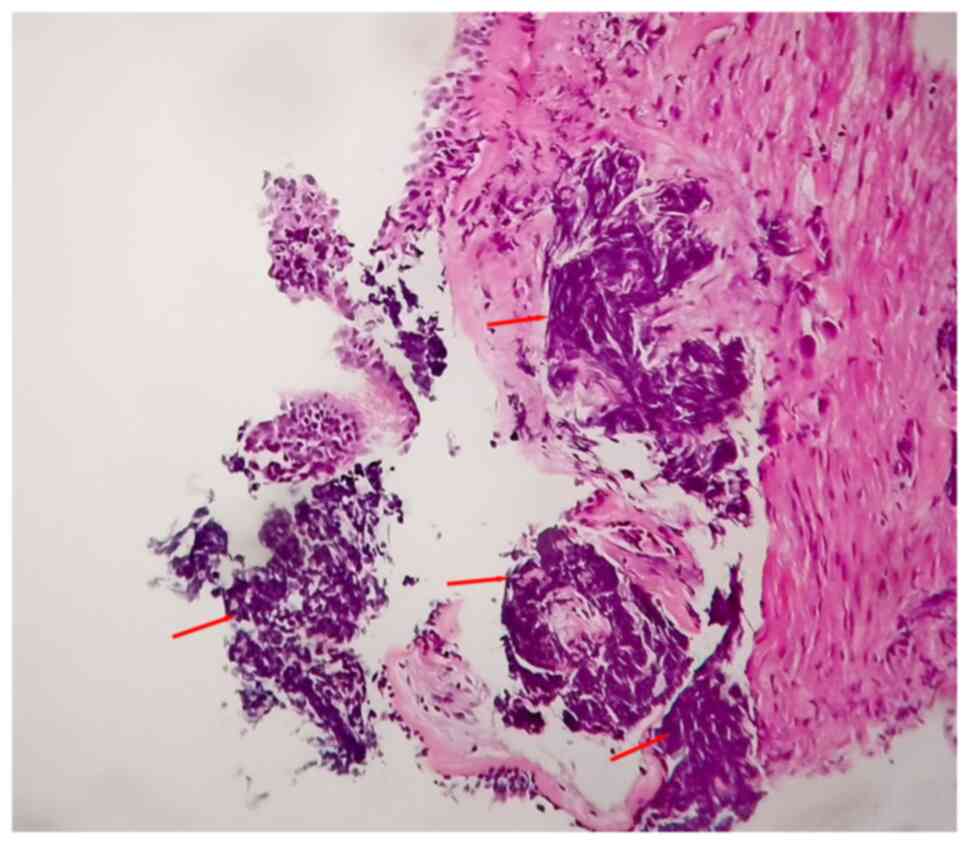
magnification (x100), the cancer cells appeared small, round or
oval, lymphocyte-like but larger in size (Fig. 2). Some cells were spindle- or
oat-shaped, with little cytoplasm and naked, dark nuclei and thick
chromatin. The cancer cells were diffusely separated or arranged in
sheets and strips. Crush injury was evident. The histological
characteristics, including cell crush injury, deeply stained
nuclei, thick chromatin and absence of nucleoli, were typical of
SCLC. Moreover, B-ultrasonography (LOGIQ E9; GE Healthcare; imaging
parameters: FR 35, Frq 15, Gn 44, E/A 1/1, D 4 cm, DR 69 and AO
100%) indicated cervical lymph node metastasis, which was confirmed
by a cervical lymph node biopsy. Histopathology confirmed the
metastasis of poorly differentiated carcinoma.
According to the American Joint Committee on Cancer
8th edition tumour-node-metastasis staging system (9), the tumour was T4N3M1a stage IVA
ES-SCLC. After obtaining consent from the patient, six cycles of
etoposide [100 mg/m2, day 1-3 (d1-3)] and carboplatin
[area under the curve5, 400 mg, d1; every 21 days (Q21d)] were
administered between 13 February 2020 and 7 July 2020. Chest CT
revealed partial response (PR) after two cycles (Fig. 1B) and stable disease (SD) over the
next four cycles. The patient was PS 2. The lesion area was too
large to receive radiotherapy; therefore, the patient was followed
up with observation after chemotherapy. Periodic reviews indicated
SD until November 2020. At that point, chest CT revealed
enlargement of the primary lesion and mediastinal lymph nodes
(Fig. 1C). The PFS with first-line
chemotherapy (PFS1) was 9 months.
The patient received two cycles of irinotecan (65
mg/m2, d1 and d8) and cisplatin (30 mg/m2, d1
and d8; Q21d), after which chest CT showed SD. While using this
regimen for the third cycle, the patient developed IV
thrombocytopenia, which posed a difficult recovery, and d8
chemotherapy was interrupted. After 40 days, the patient recovered
from myelosuppression but refused to continue chemotherapy. Chest
CT revealed SD (Fig. 1D); PFS2 was
3 months.
At that time, the patient was repeatedly advised to
undergo biopsy and genetic testing for guiding immediate
management. The financial burden of long-term anti-tumour treatment
was substantial. The patient refused all invasive operations and
expensive genetic testing.
Due to shortness of breath, the patient could walk
only ~100 m and PS was 2. Dall'Olio et al (10) found that PS 2 patients treated with
chemotherapy had worse outcomes compared with those of PS 0-1
patients. However, PD-1 inhibitors are well tolerated in PS 2
patients (10). Moreover, Liu
et al (2) reported a case
in which a combination of carrelizumab and anlotinib was used to
treat a patient diagnosed with SCLC and staged as ES-SCLC. After 26
cycles, the curative effect was evaluated as complete remission.
Considering the present patient's poor PS and large economic
burden, toripalimab, the least expensive domestic PD-1 inhibitor,
was chosen and combined with anlotinib. Starting on 8 March 2021,
the patient received a PD-1 inhibitor (toripalimab 240 mg, d1,
Q21d) and anti-angiogenic therapy (anlotinib 10 mg, d1-14, Q21d).
After two cycles, an evaluation by chest CT showed PR (Fig. 1E). The patient's condition
gradually improved to PS 1. Tumour re-examinations were performed
every 3 months, including CT of the brain and chest, and
B-ultrasonography of cervical lymph nodes and abdomen. The most
recent review was on 13 November 2022 (Fig. 1F). Compared with the previous
review on 20 April 2021, the measurable width of the tumour was
reduced by ~45% and the response was evaluated as PR. The patient's
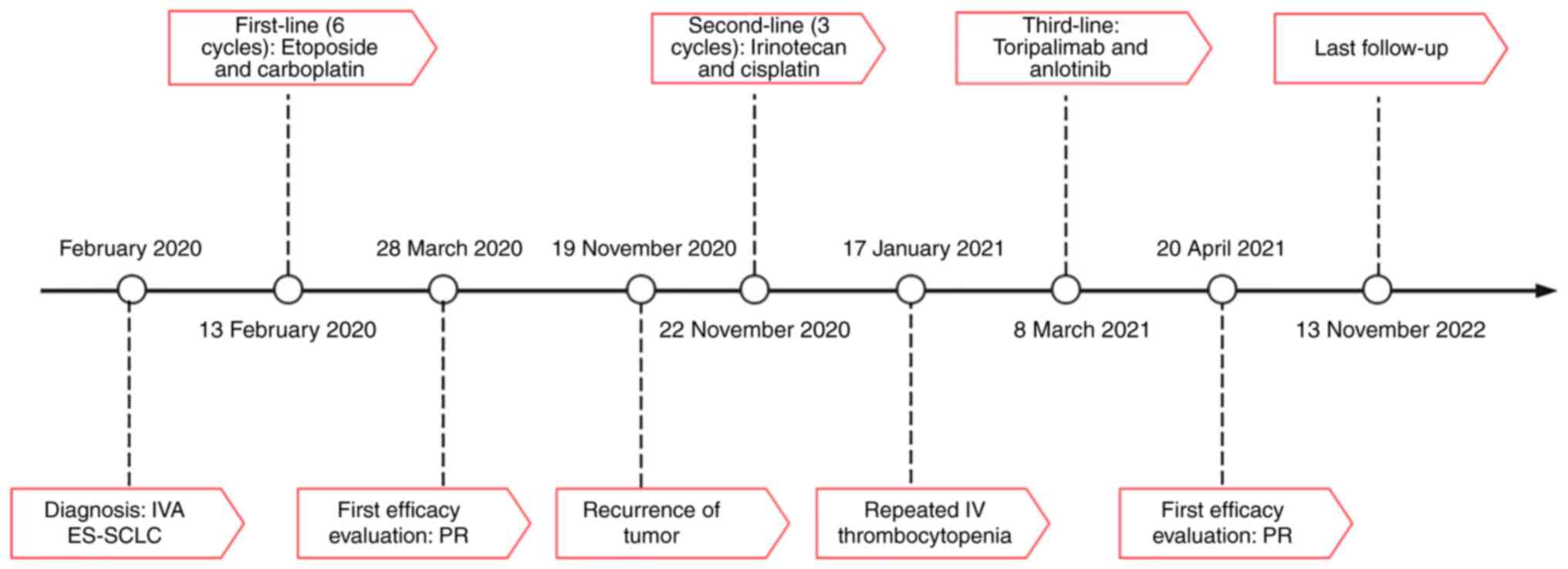
diagnosis and treatment timeline are shown in Fig. 3. During the administration of
anlotinib, the primary adverse reaction was onset of the hand-foot
syndrome, grade 3, according to the National Cancer Institute
Common Toxicity Criteria for Adverse Events version 4.0(11). Consequently. the dose of anlotinib
was decreased from 10 to 8 mg, which downgraded the hand-foot
syndrome reaction to grade 1.
The study was approved by the Ethics Committee of
the First People's Hospital of Yongkang City. Written informed
consent was obtained from the patient.
Discussion
Platinum-based doublet chemotherapy is considered
the standard of treatment for SCLC, with a response rate as high as
75-95%. However, patients who respond to platinum therapy are prone
to relapse and the median PFS is <6 months (12). In the present case, PFS1 was 9
months and PFS2 was only 3 months.
The introduction of ICIs is the first notable
development in therapeutic strategies for SCLC for two decades
without improvements in patient outcomes. Based on the outcomes of
the CheckMate 032 and KeyNote 028/158 trials, the United States
Food and Drug Administration approved nivolumab and pembrolizumab
monotherapy as a third-line treatment for ES-SCLC (13-15).
However, two other studies on nivolumab, CheckMate 331 and
CheckMate 451, failed and Bristol-Myers Squibb withdrew the
indication for nivolumab in SCLC on 30 December 2020 (16,17).
The indication for pembrolizumab in SCLC was also withdrawn because
it did not provide an OS advantage in KeyNote 604(18). Therefore, more attention is needed
to improve the efficacy of PD-1 inhibitors in SCLC.
Several factors are associated with efficacy of
ICIs. One such factor is an abnormal vascular state in the tumour
microenvironment (19). Abnormal
tumour blood vessels interact with immune cells, which hinders
their anti-tumour effect and leads to tumour progression (20). This can be mitigated by combination
therapy with vascular endothelial growth factor (VEGF) inhibitors
such as anlotinib. VEGF inhibitors not only have anti-angiogenic
effects but can also be used as immunomodulators to enhance the
efficacy of immunotherapy (21).
Anlotinib is a domestic multi-target tyrosine kinase
inhibitor that targets VEGF and fibroblast growth factor receptors
(21-24).
Several recent clinical trials have demonstrated the efficacy of
anlotinib in SCLC, such as the ALTER1202 trial, in which anlotinib
significantly prolonged PFS and OS compared with those in the
placebo group (22,23). Anlotinib has been approved for
third- and later-line treatment of SCLC in China. Hand-foot
syndrome is a common anlotinib-associated adverse event. Nan et
al (24) found that hand-foot
syndrome may be a potential clinical marker for the response to
anlotinib in patients with NSCLC: Significant PFS and OS benefits
for patients with the hand-foot syndrome were observed in all
cases.
Toripalimab is a humanised immunoglobulin G4
monoclonal antibody targeting PD-1(25). Currently, toripalimab is used to
treat unresectable or metastatic melanoma following failure of
previous systemic therapy, recurrent or metastatic nasopharyngeal
carcinoma without previous second- or later-line systematic
treatment and locally advanced or metastatic urothelial carcinoma
following platinum-containing chemotherapy failure. In addition,
clinical trials for various malignancies are still in progress and
good results in NSCLC were achieved in a multicentre retrospective
study (25,26).
However, to the best of our knowledge, studies on
the use of toripalimab in SCLC are limited. Several PD-1 drugs,
including pembrolizumab, carrelizumab, and tislelizumab, were
introduced in 2021, but none have been approved for treating SCLC.
Liu et al (2) reported a
case in which a combination of carrelizumab and anlotinib was used
to treat a patient diagnosed with SCLC and staged as ES-SCLC. In
the present case, after 26 cycles, the curative effect was
evaluated as complete remission and the PFS was 24 months. In
consideration of the cost, the present patient chose toripalimab
rather than carrelizumab. The patient's symptoms improved notably.
After two cycles, the patient showed a PR; the curative benefit was
long-lasting, extending over 20 months, with a PS of 1. Before
immunotherapy, the patient had obvious chest tightness and
shortness of breath. The PS score was 2. The patient could not
tolerate chemotherapy, but could tolerate immunotherapy, which was
consistent with the report of Dall'Olio et al (10). For patients with poor PS who cannot
tolerate chemotherapy, ICIs provide a reasonable choice that can
bring some survival benefits, and the adverse reactions are few
(10). In the present case and
that reported by Liu et al (2), biopsy and genetic testing were not
performed as the disease progressed. Therefore, the association
between the expression of PD-1 and the efficacy of immunotherapy in
SCLC could not be determined. However, the good curative effect in
this patient may indirectly indicate that their PD-1 was highly
expressed. To the best of our knowledge, only case reports are
currently available and prospective studies would be needed to
prove the efficacy of PD-1 inhibitors in SCLC. In the present case,
toripalimab (PD-1 inhibitor) combined with anlotinib (an
anti-angiogenic drug) notably improved the outcome of a patient
with recurrent ES-SCLC and the curative benefit was long-lasting.
The present novel combined immunotherapy and anti-angiogenic
therapy paradigm merits prospective clinical trials.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW made substantial contributions to the conception
and design of the work and drafted the manuscript. YC analysed and
interpreted the data and revised the manuscript. ZY performed the
histological examination of lung biopsy material and revised the
manuscript. YW, YC and ZY confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of the Declaration of Helsinki and approved by the Ethics Committee
of the First People's Hospital of Yongkang City (approval no.
YKRM2021-LS039).
Patient consent for publication
Written informed consent was obtained from the
patient.
Competing interests
The authors declares that they have no competing
interests.
References
|
1
|
Deneka AY, Boumber Y, Beck T and Golemis
EA: Tumor-Targeted Drug Conjugates as an Emerging Novel Therapeutic
Approach in Small Cell Lung Cancer (SCLC). Cancers (Basel).
11(1297)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu L, Zhang X, Zhou L, Yang T, Qiao Y and
Jiang X: Carrelizumab combined with anlotinib in the treatment of
extensive-stage small cell lung cancer: A case report. Medicine
(Baltimore). 100(e27138)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Barrows ED, Blackburn MJ and Liu SV:
Evolving role of immunotherapy in small cell lung cancer. Semin
Cancer Biol. 86(Pt 3):868–874. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ando K, Manabe R, Kishino Y, Kusumoto S,
Yamaoka T, Tanaka A, Ohmori T, Ohnishi T and Sagara H: Comparative
Efficacy and Safety of Immunotherapeutic Regimens with PD-1/PD-L1
Inhibitors for Previously Untreated Extensive-Stage Small Cell Lung
Cancer: A Systematic Review and Network Meta-Analysis. Curr Oncol.
28:1094–1113. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
He Y and An T: Toripalimab in an advanced
non-small cell lung cancer patient with poor general condition
after multiline treatment: A case report. J Int Med Res.
49(3000605211042988)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Moya-Horno I, Viteri S, Karachaliou N and
Rosell R: Combination of immunotherapy with targeted therapies in
advanced non-small cell lung cancer (NSCLC). Ther Adv Med Oncol.
10(1758834017745012)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tian Y, Zhai X, Han A, Zhu H and Yu J:
Potential immune escape mechanisms underlying the distinct clinical
outcome of immune checkpoint blockades in small cell lung cancer. J
Hematol Oncol. 12(67)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gagnier JJ, Kienle G, Altman DG, Moher D,
Sox H and Riley D: CARE Group. The CARE guidelines: Consensus-based
clinical case reporting guideline development. Headache.
53:1541–1547. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Amin MB: American Joint Committee on
Cancer and American Cancer Society AJCC cancer staging manual. 8th
edition. Springer, Chicago IL, 2017.
|
|
10
|
Dall'Olio FG, Maggio I, Massucci M,
Mollica V, Fragomeno B and Ardizzoni A: ECOG performance status ≥2
as a prognostic factor in patients with advanced non-small cell
lung cancer treated with immune checkpoint inhibitors-a systematic
review and meta-analysis of real world data. Lung Cancer.
145:95–104. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
U.S. Department of Health and Human
Services, National Institutes of Health, National Cancer Institue:
Common Terminology Criteria for Adverse Events (CTCAE)[J/OL].
http://evs.nci.nih.gov/ftp1/CTCAE/About.html.
|
|
12
|
Rossi A, Di Maio M, Chiodini P, Rudd RM,
Okamoto H, Skarlos DV, Früh M, Qian W, Tamura T, Samantas E, et al:
Carboplatin- or cisplatin-based chemotherapy in first-line
treatment of small-cell lung cancer: The COCIS meta-analysis of
individual patient data. J Clin Oncol. 30:1692–1698.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Antonia SJ, Lopez-Martin JA, Bendell J,
Ott PA, Taylor M, Eder JP, Jäger D, Pietanza MC, Le DT, de Braud F,
et al: Nivolumab alone and nivolumab plus ipilimumab in recurrent
small-cell lung cancer (CheckMate 032): A multicentre, open-label,
phase 1/2 trial. Lancet Oncol. 17:883–895. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ott PA, Elez E, Hiret S, Kim DW, Morosky
A, Saraf S, Piperdi B and Mehnert JM: Pembrolizumab in Patients
With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase
Ib KEYNOTE-028 Study. J Clin Oncol. 35:3823–3829. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chung HC, Piha-Paul SA, Lopez-Martin J,
Schellens JHM, Kao S, Miller WH Jr, Delord JP, Gao B, Planchard D,
Gottfried M, et al: Pembrolizumab after two or more lines of
previous therapy in patients with recurrent or metastatic SCLC:
Results from the KEYNOTE-028 and KEYNOTE-158 Studies. J Thorac
Oncol. 15:618–627. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Spigel DR, Vicente D, Ciuleanu TE,
Gettinger S, Peters S, Horn L, Audigier-Valette C, Pardo Aranda N,
Juan-Vidal O, Cheng Y, et al: Second-line nivolumab in relapsed
small-cell lung cancer: CheckMate 331*. Ann Oncol.
32:631–641. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Owonikoko TK, Park K, Govindan R, Ready N,
Reck M, Peters S, Dakhil SR, Navarro A, Rodríguez-Cid J, Schenker
M, et al: Nivolumab and ipilimumab as maintenance therapy in
extensive-disease small-cell lung cancer: CheckMate 451. J Clin
Oncol. 39:1349–1359. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rudin CM, Awad MM, Navarro A, Gottfried M,
Peters S, Csőszi T, Cheema PK, Rodriguez-Abreu D, Wollner M, Yang
JC, et al: Pembrolizumab or placebo plus etoposide and platinum as
first-line therapy for extensive-stage small-cell lung cancer:
Randomized, double-blind, phase III KEYNOTE-604 study. J Clin
Oncol. 38:2369–2379. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yi M, Jiao D, Qin S, Chu Q, Wu K and Li A:
Synergistic effect of immune checkpoint blockade and
anti-angiogenesis in cancer treatment. Mol Cancer.
18(60)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
De Palma M, Biziato D and Petrova TV:
Microenvironmental regulation of tumour angiogenesis. Nat Rev
Cancer. 17:457–474. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sun Y, Niu W, Du F, Du C, Li S, Wang J, Li
L, Wang F, Hao Y, Li C and Chi Y: Safety, pharmacokinetics, and
antitumor properties of anlotinib, an oral multi-target tyrosine
kinase inhibitor, in patients with advanced refractory solid
tumors. J Hematol Oncol. 9(105)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu Y, Cheng Y, Li K, Shi J, Liu Y, Wu L,
Han B, Chen G, He J, Wang J, et al: Effect of prior thoracic
radiotherapy on prognosis in relapsed small cell lung cancer
patients treated with anlotinib: A subgroup analysis of the ALTER
1202 trial. Transl Lung Cancer Res. 10:3793–3806. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wu D, Nie J, Hu W, Dai L, Zhang J, Chen X,
Ma X, Tian G, Han J, Han S, et al: A phase II study of anlotinib in
45 patients with relapsed small cell lung cancer. Int J Cancer Dec.
15:3453–3460. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nan X, Xie C, Zhu Q, Zhang J, Fu S, Han X,
Zhang Q, Han B and Liu J: Hand-foot syndrome and survival in
patients with advanced non-small-cell lung cancer receiving
anlotinib: A subgroup analysis of data from the ALTER 0303 study.
Int J Clin Oncol. 25:1492–1498. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen
J, Li J, Shi YR, Jin F, Xu R, et al: Toripalimab or placebo plus
chemotherapy as first-line treatment in advanced nasopharyngeal
carcinoma: A multicenter randomized phase 3 trial. Nat Med.
27:1536–1543. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Duan S, Zhang X, Wang F, Shi Y, Wang J and
Zeng X: Coexistence of oral mucous membrane pemphigoid and
lichenoid drug reaction: A case of toripalimab-triggered and
pembrolizumab-aggravated oral adverse events. Oral Surg Oral Med
Oral Pathol Oral Radiol. 132:e86–e91. 2021.PubMed/NCBI View Article : Google Scholar
|