Introduction
Non-alcoholic fatty liver disease (NAFLD) is defined
as acquired metabolic stress-induced liver injury, characterized by
increased hepatic lipid accumulation in the absence of excessive
alcohol consumption (1). According
to the degree of pathological changes, NAFLD can be divided into
simple fatty liver, non-alcoholic steatohepatitis (NASH), and
NASH-associated cirrhosis.
The homeostasis of lipid metabolism in the liver
plays a major role in the development of NAFLD. The homeostasis of
intrahepatic lipid levels mainly depends on the balance between the
synthesis of intrahepatic triglycerides (TGs) and β-oxidation of
fatty acids (2,3). Glucose and free fatty acids increase
as a result of overnutrition and reduced physical activity, causing
overload in the liver and other organs of the human body. In turn,
this overload causes oxidative stress and inflammation, thus
accelerating the process of NAFLD (4,5).
Fatty acid β-oxidation is the main pathway of lipid metabolism.
Peroxisome proliferator-activated receptors (PPAR-α, PPAR-β/δ, and
PPAR-γ) are members of the nuclear receptor superfamily, acting as
ligand-inducible transcription factors that play crucial roles in
β-oxidation (6-8).
In the present study, fatty acid catabolism was impaired following
the deletion of PPAR-α in hepatocytes, resulting in hepatic lipid
accumulation in a mouse model (9).
Therefore, improving β-oxidation (by upregulating PPAR-α) may be
beneficial in ameliorating the effects of NASH. In addition,
oxidative stress can induce inflammation in NAFLD and regulate the
expression of tumor necrosis factor-alpha and interleukin-6, thus
advancing the development of hepatitis (10). Nuclear factor erythroid 2-related
factor 2 (Nrf2) has an important role in anti-oxidant stress;
during oxidative stress, Nrf2 dissociates from kelch-like
ECH-associated protein 1 and translocates into the nucleus to
increase the expression of anti-oxidant genes and improve damage
caused unto the liver by oxidative stress (11,12).
Many studies have confirmed that in NAFLD models, the regulation of
Nrf2 pathway activation is an important mechanism that plays a role
in liver protection.
SIRT6 is an NAD+-dependent class III
deacetylase that is characterized by its unique chromatin location
(13). In the liver, SIRT6
potentially interacts with PPAR-α and Nrf2 to maintain fatty acid
oxidation rate and improve oxidative stress during high-fat diet
feeding (14). Furthermore, SIRT6
is a histone H3 lysine 9 (H3K9) deacetylate on the promoters of
many genes and plays an essential role in glycolysis and lipid
metabolism (15,16). Hepatocyte-specific SIRT6 deletion
predisposes mice to NASH fatty liver; similarly, in humans with
fatty liver, SIRT6 levels are lower than those with a normal liver
(15). In conclusion, SIRT6 plays
a critical role in fat metabolism and may serve as a therapeutic
target for treating fatty liver disease (17).
Pachymic acid (Pac), an active compound isolated
from Poria cocos, is a traditional Chinese herbal drug used
to fortify the spleen and alleviate edema. Pac has remarkable
effects on phlegm and fluid retention in the body, as seen in
metabolic diseases including NAFLD and obesity (18,19).
Previously, Pac was reported to have antitumor, anti-inflammatory,
antioxidant, and hypoglycemic effects (20-22).
A 2019 study reported that Poria cocos protected mice from
hepatic steatosis by inhibiting TG accumulation (23). Although Poria cocos acid
exhibits antioxidant and anti-lipid accumulation characteristics in
many cell models, few studies on the anti-NAFLD activity of Pac
have been performed. Our study identified, for the first time, that
Pac can activate SIRT6 and protect mouse primary hepatocytes (MPHs)
against oleic acid (OA)-palmitic acid (PA)-induced NAFLD, via
SIRT6/PPAR-α and SIRT6/Nrf2 pathways.
Materials and methods
Materials
Pachymic acid (purity >97%) was purchased from
Yuanye Biological Technology Co. Ltd. (Shanghai, China).
Source of animals
Male C57BL/6J mice (6-8 weeks) were purchased from
the Model Animal Research Center of Guangzhou University of Chinese
Medicine. Hepatocytes SIRT6 deficiency mice (6-8 weeks) were kindly
provided by Professor Jinhan He (Department of Pharmacy, State Key
Laboratory of Biotherapy, West China Hospital, Sichuan University,
Chengdu, China) and Professor Yongsheng Chang (National Laboratory
of Medical Molecular Biology, Institute of Basic Medical Sciences,
Chinese Academy of Medical Sciences and Peking Union Medical
College, Beijing, China) and used as previously described (24).
Cell protocols
Male C57BL/6J mice and hepatocytes SIRT6 deficiency
mice were anesthetized by intraperitoneal injection of 1%
pentobarbital sodium 80 mg/kg and mouse hepatocytes (MPHs) were
isolated and cultured in a RPMI-1640 medium, as previously
described (7). Briefly, sterilize
the body surface of mice with 75% ethanol, cut the skin, open the
abdominal cavity, separate the inferior vena cava, place a catheter
at the distal end of the inferior vena cava, inject 1-2 ml of
heparin immediately after the blood return, and then inject 50 ml
of perfusion solution I (1,000 ml Krebs Ringer with Glucose + 2 ml
50 mM EGTA), after the injection of heparin, and cut the open vein
to bleed, and rapidly perfusion for about 3 min clarify the blood
of the mouse was removed by this procedure. During this period,
0.015 g collagenase was dissolved in 30 ml of perfusate II (1,000
ml Krebs Ringer with Glucose + 1372 µl 2 M CaCl2), mixed
well and maintained at 37˚C. Then perfusion liquid II was infused,
and the perfusion was stopped at a slow speed for about 6 min until
the liver collapsed and cracked. The whole liver was removed and
placed in a culture dish. After adding 20 ml of basic culture
medium, it was transferred to the ultraclean table for operation.
Tear the liver, filter it with a 70 µm cell net, collect the
filtrate, 800 rpm/min, centrifuge for 3 min, and discard the
supernatant. Then, Freshly prepared MPHs were suspended in
RPMI-1640 medium supplemented with 10% fetal bovine serum, and
plated in 6-well culture plates at 0.5x106 cells/well. After
attachment, MPHs were washed with PBS, and media was replaced with
RPMI-1640 medium supplemented with 10% fetal bovine serum and
penicillin-streptomycin. Then, MPHs were exposed to 200 µM OA and
100 µM PA (OA&PA), and OA&PA with different densities of
Pac.
CCK-8 assay
MPHs were seeded in 96 well plates, where five wells
were repeated. After culturing the MPHs in a cell incubator for 12
h, the cells were placed in a new medium, containing Pac. Blank and
control wells were incubated for 24 h after administration of the
Pac medium. The medium was aspirated, added, and incubated with the
pre-mixed medium containing CCK-8 (100 ml 1640 medium and 10 ml
CCK-8 solution), and the OD value was then measured at 450 nm,
using a microplate reader.
Biochemistry analysis
MPHs were harvested after a 24-h incubation and
assayed for triglycerides (TG) and total cholesterol (TC) levels
using the commercially available enzymatic assay kits (Jiancheng
Co.) according to the manufacturer's instructions.
Oil Red O Staining
The MPHs were stained with Oil Red O to determine
their differentiation. After washing with phosphate buffered saline
(PBS) and fixing with 4% paraformaldehyde, for 30 min, MHPs were
then washed twice with PBS, stained with 60% saturated Oil Red O
for 10-15 min, and washed with 60% isopropanol. Finally, adipocytes
were imaged using a light microscope (Nikon x200).
Reactive oxygen species (ROS)
analysis
For Cellular ROS determination, Cells were seeded in
12-well plates and treated as above, followed by incubation with
DHE 5 µM (KeyGEN Co.) for 30 min at 37˚C in the dark. Then, cells
were washed with PBS for three times and visualized under a
fluorescence microscope (Nikon x200).
Hepatic lipid accumulation and
lipoperoxidation
MPHs were seeded in six-well plates and treated as
described above. MPHs were washed by a 1640 basic medium and
stained with BODIPY 581/591 C11 and BODIPY 493/503 (Invitrogen;
Thermo Fisher Scientific, Inc.) to investigate hepatic lipid
accumulation and lipoperoxidation, respectively. Cell nuclei were
stained with DAPI and visualized using fluorescence microscopy
(Leica x200).
Quantitative Real-Time PCR
Analysis
Total mRNA of liver tissues or MPHs was extracted
with a TRIzol reagent. Reverse transcription was performed using a
high-capacity cDNA reverse-transcription kit (Applied Biological
Materials Inc.). cDNA was subjected to qPCR analysis with the
PowerUp™ SYBRTM Green Master Mix (Abclonal Co.). All genes
expression were standardized with β-actin and specific primer
sequences were shown in Table
SI.
Western blot analysis
Total protein and nuclear protein were extracted
from cultured cells according to the manufacturer's instruction
(Beyotime Co.). The concentrations were determined by BCA assay kit
(Beyotime Co.). In total, equal amounts of the protein (20-60 µg)
were fractionated by 10% SDS-polyacrylamide gel, and separated
proteins were transferred onto PVDF membranes. The membranes were
incubated overnight at 4˚C with various primary antibodies
including anti-SIRT6 (13572-1-AP, Proteintech), anti-PPARα (A18252,
Abclonal), anti-CPT1a (A20746, Abclonal), anti-H3K9ac (A7256,
Abclonal), anti-H3K56ac (A2391, Abclonal), anti-Nrf2 (16396-1-AP,
Proteintech), anti-SOD2 (A19576, Abclonal), anti-HO-1(A19062,
Abclonal), and anti-β-ACTIN (Ac026, Abclonal) and followed by an
incubation with a secondary antibody. Finally, the blots were
observed using BIO-RAD Gel Doc XR from Science and Technology
Innovation Center of Guangzhou University of Chinese Medicine.
Molecular Modeling and Docking
Study
The processing and optimization of molecular docking
is completed by Glide module in Schrödinger Maestro software.
Protein processing uses the SIRT6 Preparation Wizard module
(25,26). The Pac structure was downloaded
from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and optimized using
the molecular mechanics program Minimize to get the most stable
structure. The three-dimensional crystal structure of the SIRT6
protein was downloaded from the RCSB PDB database (https://www.rcsb.org/structure/5MF6).
Preconditioning, optimization and minimization of receptors
(constraint minimization using OPLS3e force field). The compound
structure is prepared according to the default settings of the
LigPrep module. When screening in Glide module, the prepared
receptor is introduced. According to the protein structure original
ligand as the docking site (x=115.08, y=26.49, z=-22.42), the
docking box is set to 20x20x20Å. Finally, molecular docking and
screening were carried out by standard precision (SP) method.
Statistical analysis
The data are analyzed using GraphPad Prism (Version
8.0) and presented as means ± SEM. Statistical analysis was
performed using the one-way analysis of variance followed by post
hoc Tukey test for comparisons. Value of P<0.05 was considered
statistical significance.
Results
Pac accelerated lipid catabolism and
alleviated lipid peroxidation in MPHs
To investigate the role of Pac in abnormal lipid
metabolism, OA&PA and Pac-incubated MPHs were used. According
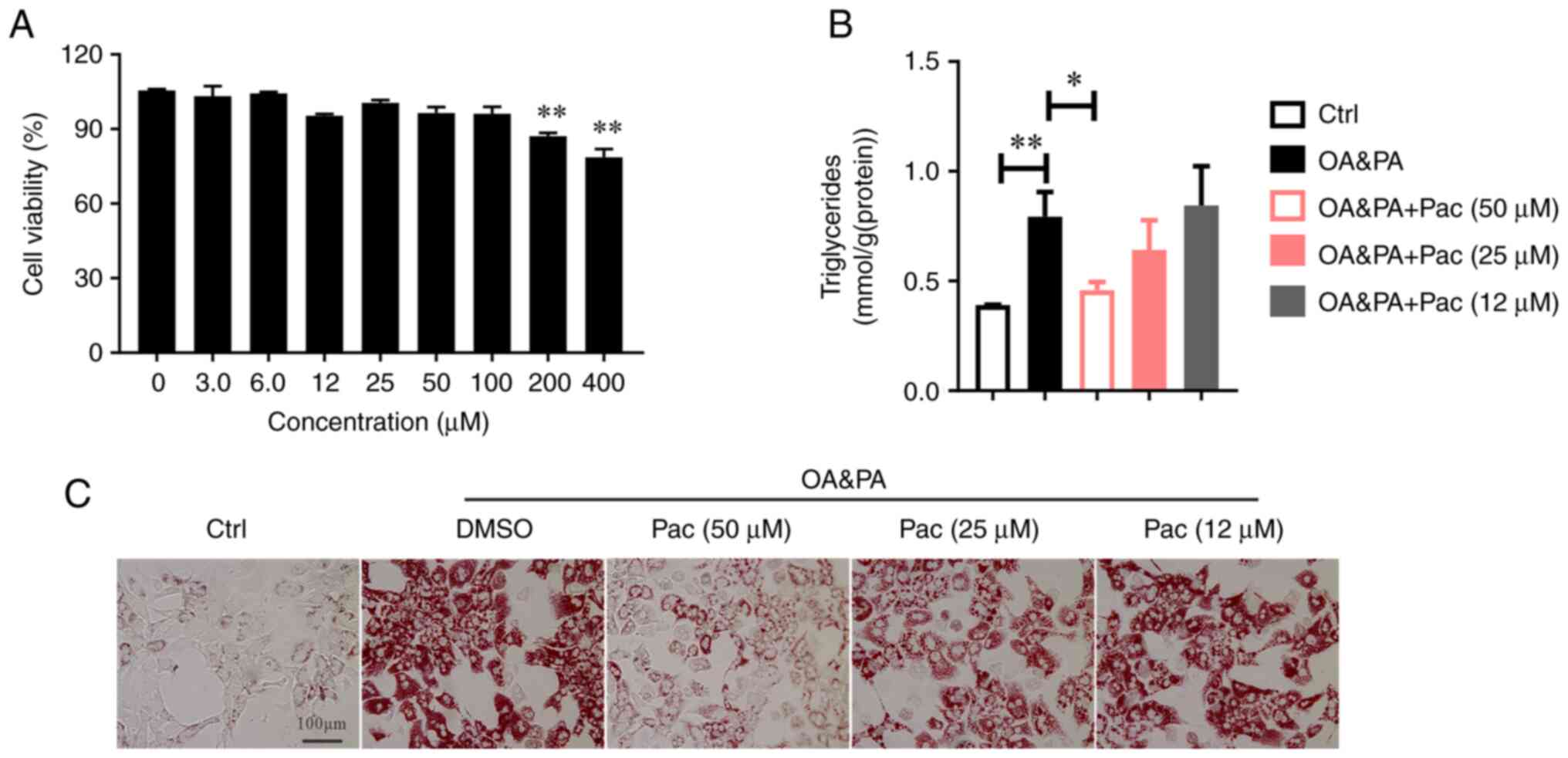
to our hypothesis, Pac displays lower cytotoxicity (Fig. 1A). In addition, Pac treatment
effectively reduced intracellular TG levels in OA&PA-incubated
MPHs, in a dose-dependent manner (P<0.05, Fig. 1B). Subsequent Oil Red O staining
revealed decreased lipid deposition in OA&PA-incubated MPHs,
following treatment with Pac (Fig.
1C).
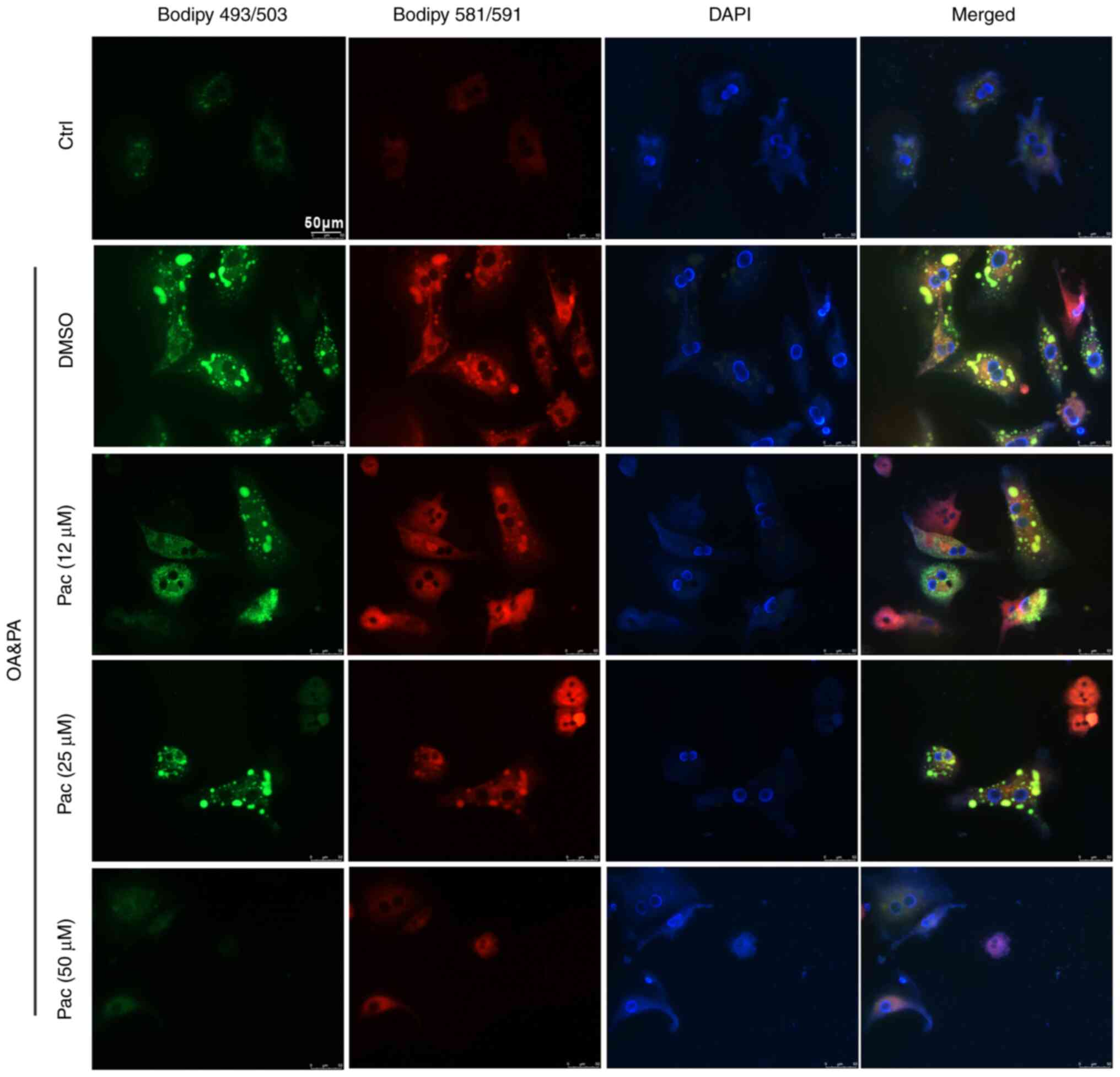
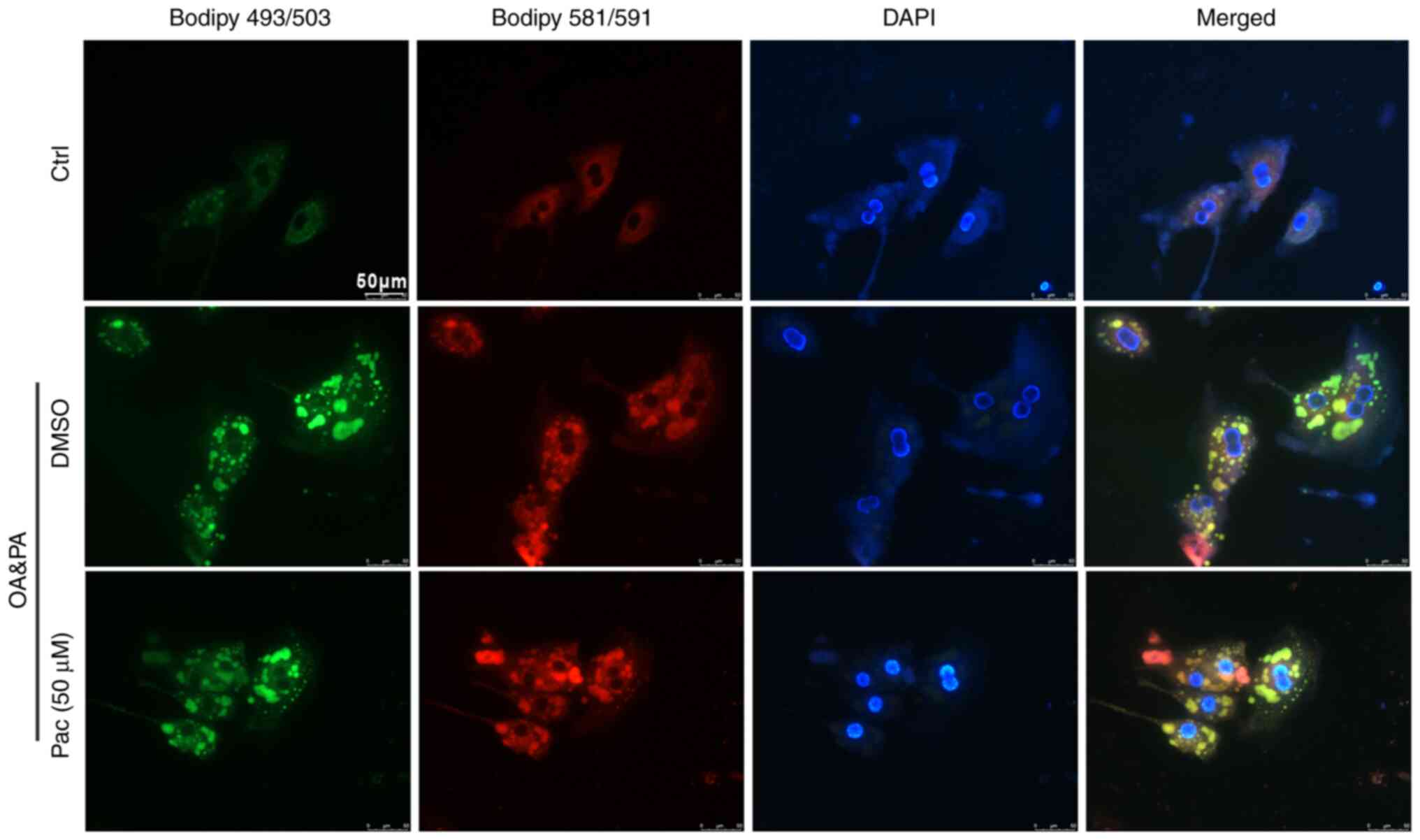
Accumulating evidence indicates that oxidative
stress damages the structure of cell membranes and leads to cell
death-important mechanisms in the development of NAFLD (27). Thus, BODIPY 581/591 C11 and BODIPY
493/503 staining were used to test the levels of lipid accumulation
and lipoperoxidation in OA&PA and Pac-incubated MPHs. Treatment
with Pac decreased intracellular oxidative stress (due to lipid
deposition) in a dose-dependent manner (Fig. 2). Collectively, these data suggest
a potential role of Pac in promoting lipid metabolism.
Pac accelerates lipid catabolism by
upregulating β-oxidation
Mechanistic studies have revealed that free fatty
acids enter hepatocytes and provide energy through mitochondrial
β-oxidation, or TG formation stored in hepatocytes. As NAFLD
progresses, the β-oxidation of fatty acids is inhibited, leading to
excessive lipid accumulation in hepatocyte (4). Further mechanistic analyses showed
that administration of Pac (50 µM) significantly increased the
fatty acid oxidation rate in OA&PA-incubated MPHs (P<0.01,
Fig. 3B), while lipogenesis was
only slightly affected by this administration (Fig. 3A). Pac treatment significantly
upregulated the genes involved in fatty acid oxidation, along with
a slight suppression of lipogenic genes (Fig. 3C), which led to decreased lipid
deposition in OA&PA-incubated MPHs (P<0.05, P<0.01,
Fig. 3D). Western blot analyses
also indicated that Pac treatment increased the expression of
PPAR-α and Cpt1a (Fig. 3E-F).
Overall, these data suggest that Pac functions as a potent positive
regulator of fatty acid oxidation and hepatic steatosis.
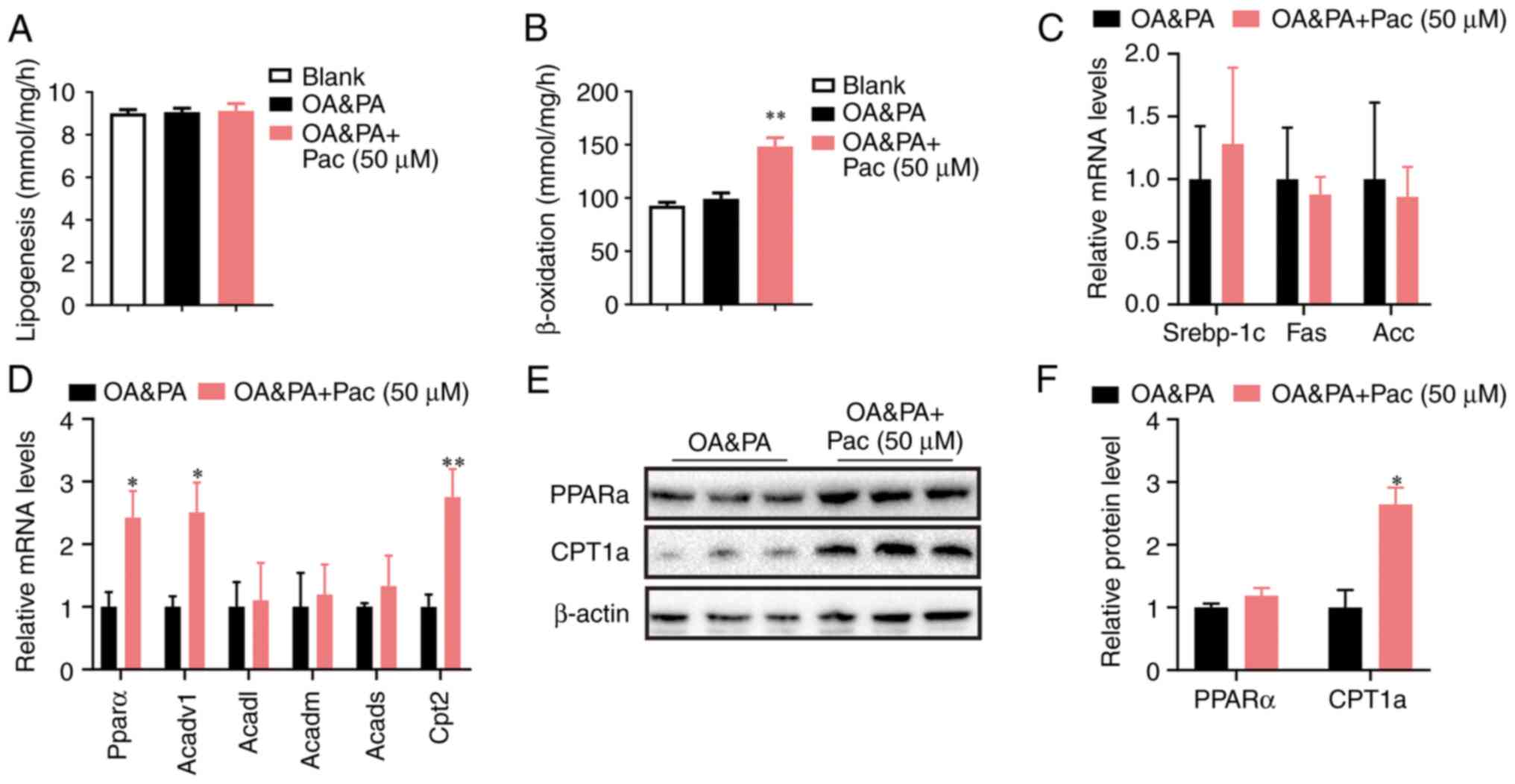 | Figure 3Pac accelerates lipid catabolism by
upregulating β-oxidation. (A) Lipogenesis in MPHs; (B) fatty acid
oxidation rate in MPHs; (C) relative expression of lipogenesis and
of (D) lipogenesis fatty acid β-oxidation; (E) Western blotting and
(F) quantification of hepatic PPAR-α and CPT1a protein in MPHs.
Data are presented as means ± SEM; n=3-4. *P<0.05,
**P<0.01 vs. OA + PA. MPHs, mouse primary
hepatocytes; OA, oleic acid; PA, palmitic acid; pac, pachymic acid;
srebp-1c, sterol regulatory element-binding transcription factor 1;
Acadvl, acyl-CoA dehydrogenase very long chain; Acadm,
acyl-Coenzyme A dehydrogenase, C-4 to C-12 straight chain; Acads,
Acyl-CoA dehydrogenase, C-2 to C-3 short chain; Cpt2, carnitine
palmitoyltransferase II; CPT1a, carnitine palmitoyltransferase
1. |
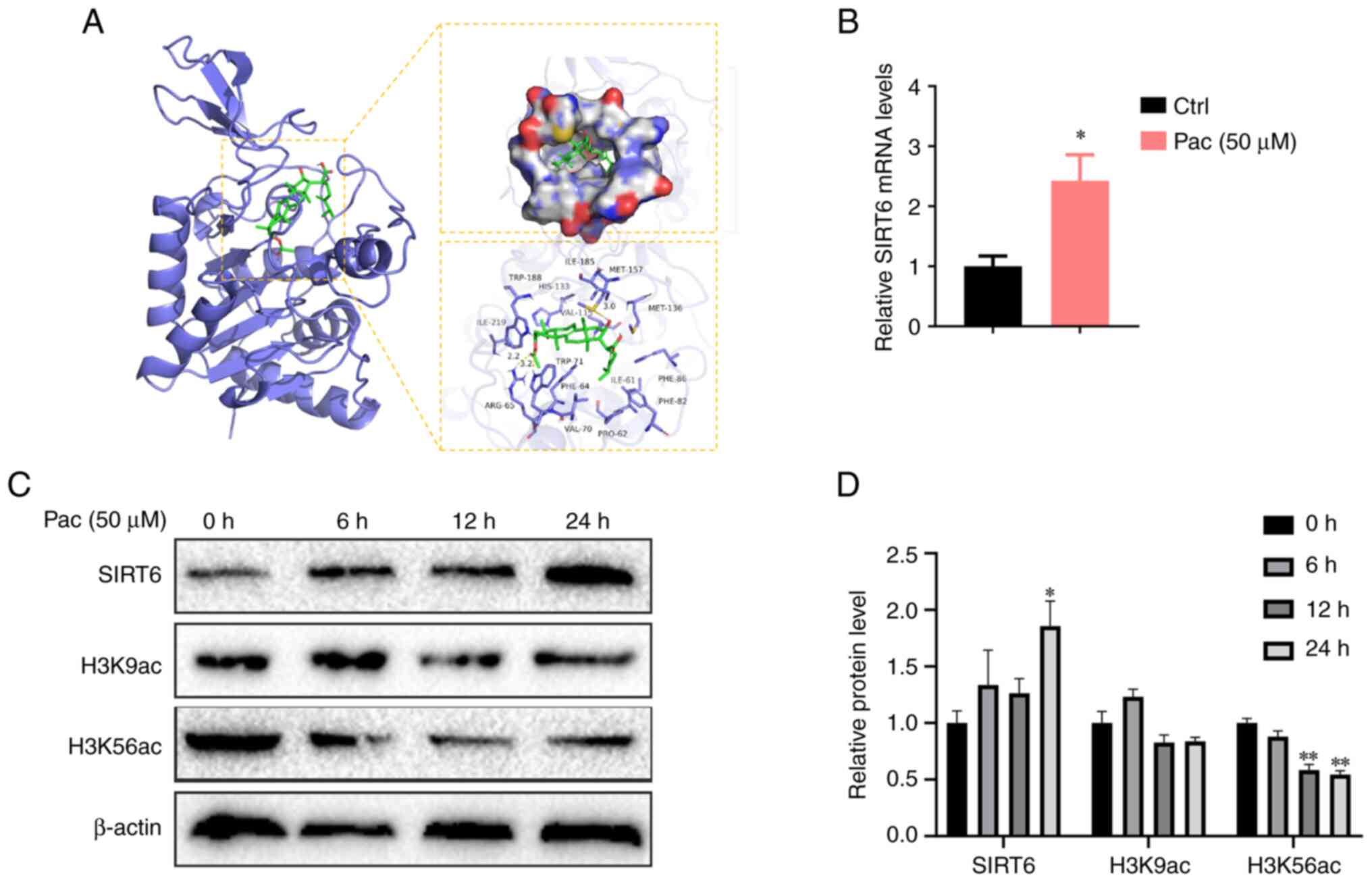
Pac can activate SIRT6
Previous studies have shown that SIRT6 can improve
the β-oxidation of fatty acids by activating PPAR-α and also
inhibit liver damage caused by ROS, where these effects are
observed when SIRT6-Nrf2 interactions increase (14,28).
To verify whether SIRT6 plays an important role in lipid
accumulation in MPHs treated with Pac, we conducted docking
analyses between Pac and SIRT6. Our data indicated notable binding
affinity via hydrophobic interactions (Fig. 4A). We further performed qPCR
analysis to confirm that Pac increased SIRT6 expression in
vitro (P<0.05, Fig.
4B).
SIRT6 normally functions as a transcriptional
repressor by deacetylating H3K9 and H3K56 on histones that bind to
gene promoters. Therefore, we tested the expression of H3K9 and
H3K56 in OA&PA and Pac-cultured MPHs-with or without Pac
treatment. As expected, Pac increased the deacetylase activity of
SIRT6 by inhibiting H3K9 and H3K56 expression (P<0.05,
P<0.01, Fig. 4C-D). Overall,
these data indicate that Pac can potentially activate SIRT6 by
altering its expression and enzyme activity. This interaction may
increase PPAR-α to mediate fatty acid oxidation and increase Nrf2,
thereby reducing lipid peroxidation.
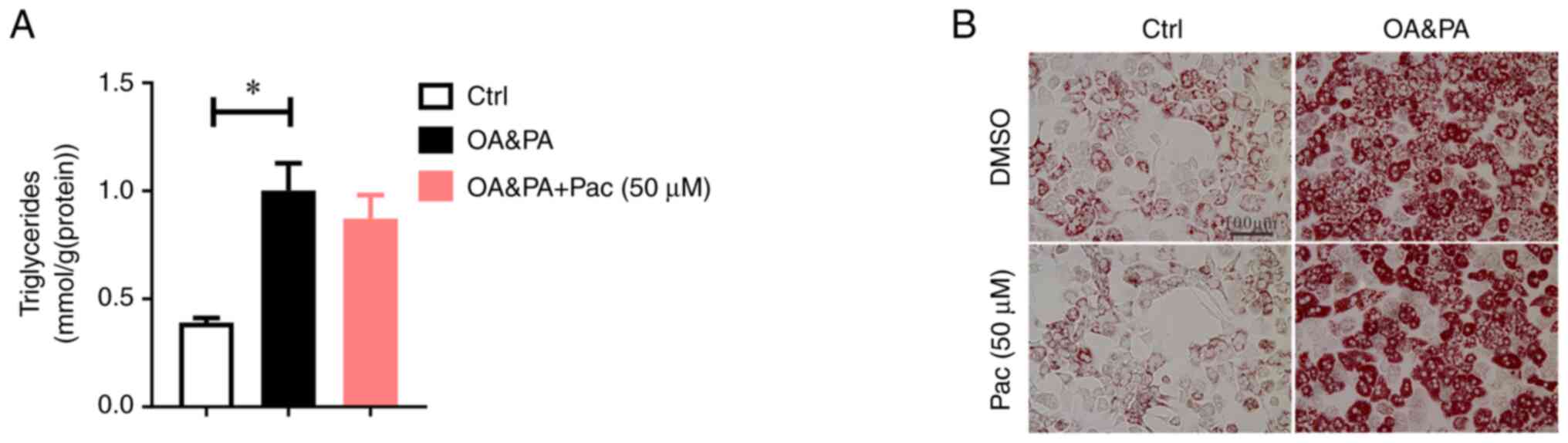
Pac failed to alter OA&PA induced
lipid deposition in Sirt6-deficient MPHs
To further confirm the effects of SIRT6 in
regulating Pac-induced therapeutic effects, MPHs isolated from
liver-specific SIRT6-deficient mice were cultured in an OA&PA
containing medium for 24 h, followed by co-treatment with Pac (50
µM) for another 24 h. Interestingly, Pac failed to reduce TG
content in MPHs with a SIRT6-deficient state (P<0.05, Fig. 5A). Moreover, SIRT6 deficiency
abrogated Pac-induced therapeutic effects on lipid accumulation, as
shown by Oil Red O staining and BODIPY 581/591 C11 and BODIPY
493/503 staining in MPHs (Figs. 5B
and 6). These data suggest that
the effects of Pac are SIRT6-dependent during OA&PA-induced
cellular damage in MPHs.
Pac alleviated OA&PA induced
oxidative stress dependent on Sirt6 in MPHs
Multiple studies have indicated that long-term,
high-fat diet exposure can lead to oxidative stress and ROS
overproduction (29). Therefore,
we investigated the effects of Pac on OA&PA-induced oxidative
stress. Pac treatment significantly increased protein levels
associated with antioxidant activity (including that of SOD2, HO1,
and NRF2) in OA&PA-incubated MPHs derived from wild-type mice
(P<0.05, Fig. 7A and B). Meanwhile, Pac treatment also reduced
ROS levels in OA&PA and Pac-incubated MPHs, however, in MPHs
derived from SIRT6-deficient mice (KO) Pac treatment did not alter
ROS levels (Fig. 7C). Moreover,
Pac failed to reduce the expression of NRF2, SOD2 and HO1 in
SIRT6-deficient MPHs (Fig. 7D).
Overall, these data suggest that Pac counteracts OA&PA-induced
oxidative stress in MPHs, via the activation of SIRT6.
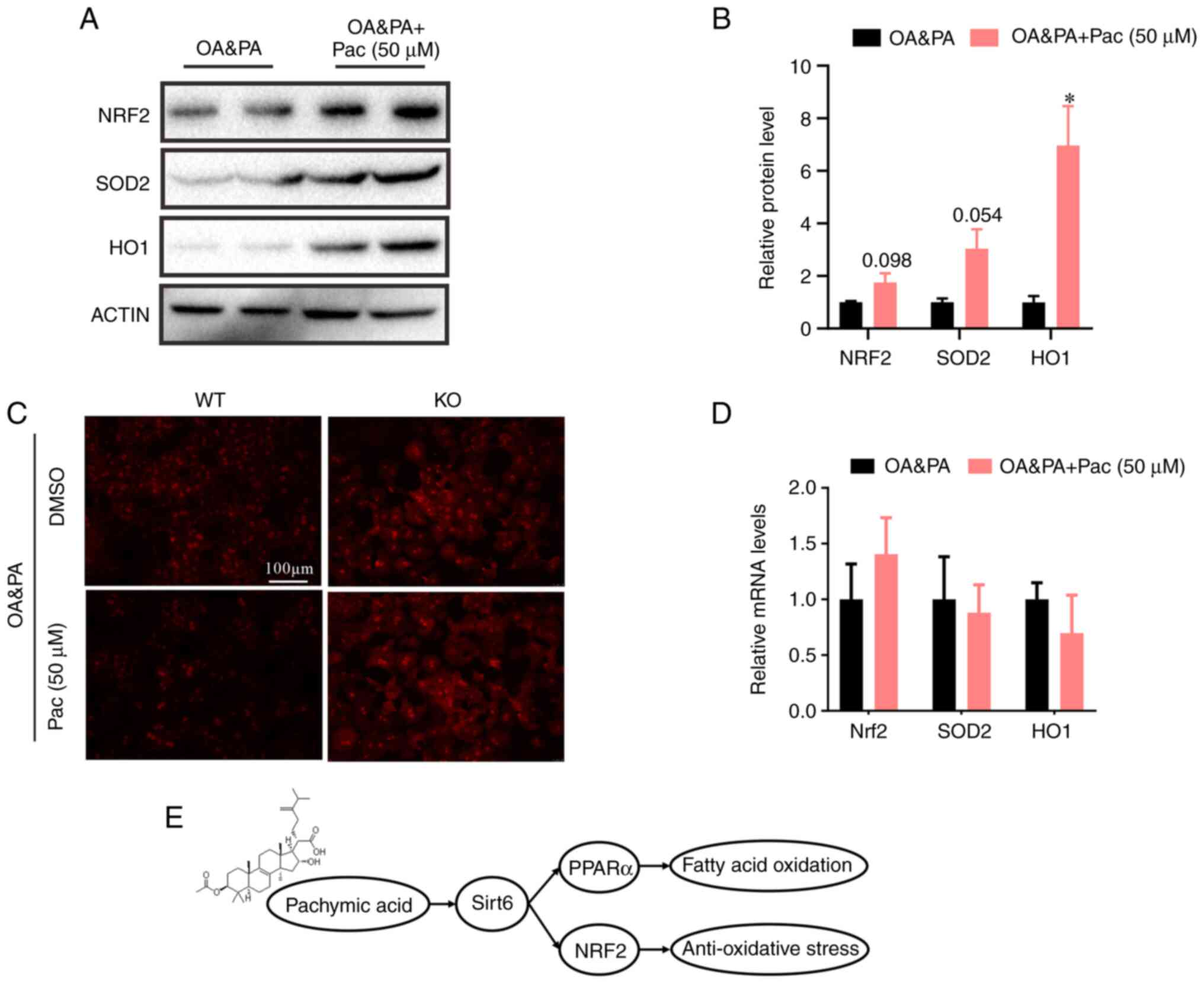 | Figure 7Pac alleviates OA + PA-induced
oxidative stress that is dependent on SIRT6 in MPHs. (A) Western
blotting and (B) quantification of hepatic NRF2, SOD2 and CPT1α
protein in MPHs; (C) Reactive oxygen species levels in WT MPHs and
SIRT6-deficient MPHs (magnification, x200). (D) Expression of Nrf2,
SOD2 and HO1 in SIRT6-deficient MPHs. (E) Schematic view of the
protective effect of Pa on OA + PA-induced NAFLD that was dependent
on the SIRT6-PPAR-α-Nrf2 axis. Data are presented as means ± SEM;
n=3-4. *P<0.05 vs. OA + PA. MPHs, mouse primary
hepatocytes; OA, oleic acid; PA, palmitic acid; pac, pachymic acid;
SIRT6, sirtuin 6; NRF2, nuclear factor erythroid 2-related factor
2; SOD2, superoxide dismutase 2; CPT1α, carnitine
palmitoyltransferase 1; WT, wild-type; KO, knockout. |
Discussion
In the current study, we demonstrated that Pac
attenuated OA&PA-induced lipid accumulation and oxidative
stress by upregulating SIRT6/PPAR-α and SIRT6/Nrf2 pathways. We
first found that Pac has a protective effect on liver TG
accumulation in the models of MPHs cells with OA&PA treatment.
Furthermore, we found that the inhibitory effect of Pac on
hepatocyte TG accumulation was related to β-oxidation of fatty
acids rather than inhibition of lipogenesis. Pac can also inhibit
oxidative stress by increasing the expression of antioxidant genes,
such as NRF2, HO1, and SOD2. In addition, docking analyses
indicated notable binding affinity between Pac and SIRT6, via
hydrophobic interactions. To further confirm the effects of SIRT6
in regulating Pac-induced therapeutic effects, MPHs isolated from
liver-specific SIRT6-deficient mice were cultured in an
alcohol-containing medium. Interestingly, SIRT6 deficiency can
abrogate Pac-induced therapeutic effects on lipid accumulation in
hepatocellular carcinoma. In addition, in our study, the relieving
effect of Pac on oxidative stress (due to lipid accumulation) was
abolished in SIRT6-deficient MPHs. Collectively, Pac can alleviate
lipid accumulation and oxidative stress in hepatocellular cells
through SIRT6/PPAR-α and SIRT6/Nrf2 pathways, proving that Pac may
be a promising agent for the treatment of OA-induced lipid
metabolism disorders.
Under the influence of OA&PA, the inhibition of
mitochondrial β-oxidation and generation of oxidative stress
(caused by lipid accumulation) aggravates lipid metabolism
disorders in MPHs, forming a loop that contributes to the main
pathogenesis of NAFLD. Recent studies have proposed that the
‘multiple hit model’, which includes insulin resistance, hormone
secretion from fat tissue, nutritional factors, altered intestinal
flora along with genetic and epigenetic factors, may lead to NAFLD
(30,31). The multiple hit model indicates
that fatty acid accumulation is a key factor in NAFLD development.
Lipid accumulation in the liver causes an imbalance between lipid
acquisition and decomposition. Lipid acquisition pertains to diet
and the ingestion of circulating lipids, while lipid decomposition
mainly includes the oxidation of free fatty acids, ultimately
leading to oxidative stress and liver damage (32). Therefore, disorders involving the
oxidation and synthesis of fatty acids play an important role in
the pathogenesis of NAFLD (33).
Reducing fatty acid accumulation and oxidative stress may thus be
an effective way to treat NAFLD. As ample evidence suggests, Pac is
a therapeutic agent since it protects against liver function damage
through its relevant antioxidant and anti-lipid accumulation
characteristics in many cell models (20,21).
However, we must realize that our knowledge of the mechanisms Pac
operates in NAFLD is still inadequate.
SIRT6 is an important nuclear deacetylate and plays
an important role in lipid metabolism and oxidative stress
(28,34,35).
Liver-specific SIRT6-deficient mice spontaneously develop
hypoglycemia and show increased TG synthesis, fatty liver formation
and oxidative stress. Studies have shown that mice overexpressing
SIRT6 have reduced accumulation of visceral fat; improved blood
lipid levels, glucose tolerance, and insulin secretion; and
increased expression of selective PPAR regulatory genes, where
these traits affect the steady state of lipids (14). And a recent study demonstrated that
Nrf2 and SIRT6 protein-protein interactions confer an antioxidant
function in APAP-induced hepatotoxicity (28). From this observation it might be
inferred that Pac's protection against NAFLD might at least in part
depend on its regulation of the SIRT6. As we expected, docking
analysis indicated a good binding affinity of Pac and Sirt6 via
hydrophobic interaction and qPCR analyses further confirmed Pac
could increase expression of SIRT6 in vitro.
Moreover, the activation of SIRT6/PPAR-α (in
promoting fatty acid oxidation) and antioxidant effect of
SIRT6/Nrf2 interactions could be a new target for the treatment of
NAFLD. In this work, through the results of western blot and of
qPCR analyses we also showed that the treatment with Pac increased
SIRT6's anti-lipid accumulation and anti-oxidative stress action.
However, this effect was abolished by SIRT6's deficiency and
meanwhile the genes of β-oxidation (PPAR-α) and antioxidant stress
(Nrf2) have no significant changes. Taken together, the
hepatoprotective mechanisms Pac operates are tightly associated
with the regulation of the SIRT6/PPAR-α and SIRT6/NRF2 signaling
pathway.
A growing body of researches showed that Pac plays
an important role in the treatment of many diseases, however, it
has not yet been applied to clinical treatment of diseases in any
way. Although we have verified that Pac could modulate
SSIRT6/PPAR-α and SIRT6/NRF2 pathway to alleviate hepatocyte lipid
metabolism disorders firstly, our experiments lacked validation of
clinical case or animal samples and just verified on cells. Thus,
in the future works, we will continue to verify at the animal level
and further study how sirt6 regulates NRF2 and PPAR-α at the
molecular level.
In conclusion, we demonstrated that Pac prevents
hepatic lipid metabolism disorders. Furthermore, we found that Pac
could effectively ameliorate hepatocyte lipid metabolism disorders
by targeting the activation of SIRT6/PPAR-α, promoting fatty acid
oxidation and SIRT6/NRF2 antioxidant activity. This study suggests
that Pac is a potential agent for the treatment of NAFLD and
related diseases.
Supplementary Material
Primer information for gene
amplification.
Acknowledgements
Not applicable.
Funding
Funding: This work was financially supported by the National
Natural Science Foundation of China (grant no. 82160891), China
Postdoctoral Science Foundation (grant no. 2023A1515012618),
Science and Technology Program of Guangzhou (grant no.
202002020032).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CHL, XYX and CPS contributed to the conception of
the work. ZSP, YLC, KJT, ZZL, JLL and YHG assisted with
experimental preparation and data collection. ZSP and YLC fed the
animals. ZSP and KJT drafted the manuscript. All authors
contributed to manuscript revision, and have read and approved the
final manuscript. CHL and CPS confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
All animal experiments were conducted under
protocols approved by and in accordance with the guidelines of the
Animal Ethics Committee of Guangzhou University of Chinese Medicine
(approval no. 20220805003).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Juanola O, Martinez-Lopez S, Frances R and
Gomez-Hurtado I: Non-Alcoholic fatty liver disease: Metabolic,
genetic, epigenetic and environmental risk factors. Int J Environ
Res Public Health. 18(5227)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Byun S, Seok S, Kim YC, Zhang Y, Yau P,
Iwamori N, Xu HE, Ma J, Kemper B and Kemper JK: Fasting-induced
FGF21 signaling activates hepatic autophagy and lipid degradation
via JMJD3 histone demethylase. Nat Commun. 11(807)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Alves-Bezerra M and Cohen DE: Triglyceride
metabolism in the liver. Compr Physiol. 8:1–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Secor JD, Fligor SC, Tsikis ST, Yu LJ and
Puder M: Free fatty acid receptors as mediators and therapeutic
targets in liver disease. Front Physiol. 12(656441)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jiang G, Chen D, Li W, Liu C, Liu J and
Guo Y: Effects of wogonoside on the inflammatory response and
oxidative stress in mice with nonalcoholic fatty liver disease.
Pharm Biol. 58:1177–1183. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kersten S and Stienstra R: The role and
regulation of the peroxisome proliferator activated receptor alpha
in human liver. Biochimie. 136:75–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sun N, Shen C, Zhang L, Wu X, Yu Y, Yang
X, Yang C, Zhong C, Gao Z, Miao W, et al: Hepatic Kruppel-like
factor 16 (KLF16) targets PPARα to improve steatohepatitis and
insulin resistance. Gut. 70:2183–2195. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wahli W and Michalik L: PPARs at the
crossroads of lipid signaling and inflammation. Trends Endocrinol
Metab. 23:351–363. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Montagner A, Polizzi A, Fouche E, Ducheix
S, Lippi Y, Lasserre F, Barquissau V, Régnier M, Lukowicz C,
Benhamed F, et al: Liver PPARα is crucial for whole-body fatty acid
homeostasis and is protective against NAFLD. Gut. 65:1202–1214.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang DK and Jo DG: Mulberry fruit extract
ameliorates nonalcoholic fatty liver disease (NAFLD) through
inhibition of mitochondrial oxidative stress in rats. Evid Based
Complement Alternat Med. 2018(8165716)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu W, Peng G, Yang F, Zhang Y, Mu Z and
Han X: Sulforaphane has a therapeutic effect in an atopic
dermatitis murine model and activates the Nrf2/HO1 axis. Mol Med
Rep. 20:1761–1771. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yan C, Sun W, Wang X, Long J, Liu X, Feng
Z and Liu J: Punicalagin attenuates palmitate-induced lipotoxicity
in HepG2 cells by activating the Keap1-Nrf2 antioxidant defense
system. Mol Nutr Food Res. 60:1139–1149. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Van Gool F, Galli M, Gueydan C, Kruys V,
Prevot PP, Bedalov A, Mostoslavsky R, Alt FW, De Smedt T and Leo O:
Intracellular NAD levels regulate tumor necrosis factor protein
synthesis in a sirtuin-dependent manner. Nat Med. 15:206–210.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Naiman S, Huynh FK, Gil R, Glick Y, Shahar
Y, Touitou N, Nahum L, Avivi MY, Roichman A, Kanfi Y, et al: SIRT6
promotes hepatic beta-oxidation via activation of PPARα. Cell Rep.
29:4127–4143 e8. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim HS, Xiao C, Wang RH, Lahusen T, Xu X,
Vassilopoulos A, Vazquez-Ortiz G, Jeong WI, Park O, Ki SH, et al:
Hepatic-specific disruption of SIRT6 in mice results in fatty liver
formation due to enhanced glycolysis and triglyceride synthesis.
Cell Metab. 12:224–236. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kuang J, Chen L, Tang Q, Zhang J, Li Y and
He J: The role of Sirt6 in obesity and diabetes. Front Physiol.
9(135)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kanfi Y, Peshti V, Gil R, Naiman S, Nahum
L, Levin E, Kronfeld-Schor N and Cohen HY: SIRT6 protects against
pathological damage caused by diet-induced obesity. Aging Cell.
9:162–173. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ding B, Ji X, Sun X, Zhang T and Mu S: In
vitro effect of pachymic acid on the activity of Cytochrome P450
enzymes. Xenobiotica. 50:913–918. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Akihisa T, Nakamura Y, Tokuda H, Uchiyama
E, Suzuki T, Kimura Y, Uchikura K and Nishino H: Triterpene acids
from Poria cocos and their anti-tumor-promoting effects. J Nat
Prod. 70:948–953. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim TG, Lee YH, Lee NH, Bhattarai G, Lee
IK, Yun BS and Yi HK: The antioxidant property of pachymic acid
improves bone disturbance against AH plus-induced inflammation in
MC-3T3 E1 cells. J Endod. 39:461–466. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lee YH, Lee NH, Bhattarai G, Kim GE, Lee
IK, Yun BS, Hwang PH and Yi HK: Anti-inflammatory effect of
pachymic acid promotes odontoblastic differentiation via HO-1 in
dental pulp cells. Oral Dis. 19:193–199. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lu C, Ma J and Cai D: Pachymic acid
inhibits the tumorigenicity of gastric cancer cells by the
mitochondrial pathway. Anticancer Drugs. 28:170–179.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim JH, Sim HA, Jung DY, Lim EY, Kim YT,
Kim BJ and Jung MH: Poria cocus Wolf extract ameliorates hepatic
steatosis through regulation of lipid metabolism, inhibition of ER
stress, and activation of autophagy via AMPK activation. Int J Mol
Sci. 20(4801)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen L, Liu Q, Tang Q, Kuang J, Li H, Pu
S, Wu T, Yang X, Li R, Zhang J, et al: Hepatocyte-specific Sirt6
deficiency impairs ketogenesis. J Biol Chem. 294:1579–1589.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Friesner RA, Banks JL, Murphy RB, Halgren
TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK,
et al: Glide: A new approach for rapid, accurate docking and
scoring. 1. Method and assessment of docking accuracy. J Med Chem.
47:1739–1749. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fazi R, Tintori C, Brai A, Botta L,
Selvaraj M, Garbelli A, Maga G and Botta M: Homology model-based
virtual screening for the identification of human helicase DDX3
inhibitors. J Chem Inf Model. 55:2443–2454. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen Z, Tian R, She Z, Cai J and Li H:
Role of oxidative stress in the pathogenesis of nonalcoholic fatty
liver disease. Free Radic Biol Med. 152:116–141. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhou Y, Fan X, Jiao T, Li W, Chen P, Jiang
Y, Sun J, Chen Y, Chen P, Guan L, et al: SIRT6 as a key event
linking P53 and NRF2 counteracts APAP-induced hepatotoxicity
through inhibiting oxidative stress and promoting hepatocyte
proliferation. Acta Pharm Sin B. 11:89–99. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rives C, Fougerat A, Ellero-Simatos S,
Loiseau N, Guillou H, Gamet-Payrastre L and Wahli W: Oxidative
stress in NAFLD: Role of nutrients and food contaminants.
Biomolecules. 10(1702)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Fang YL, Chen H, Wang CL and Liang L:
Pathogenesis of non-alcoholic fatty liver disease in children and
adolescence: From ‘two hit theory’ to ‘multiple hit model’. World J
Gastroenterol. 24:2974–2983. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Buzzetti E, Pinzani M and Tsochatzis EA:
The multiple-hit pathogenesis of non-alcoholic fatty liver disease
(NAFLD). Metabolism. 65:1038–1048. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pawlak M, Lefebvre P and Staels B:
Molecular mechanism of PPARalpha action and its impact on lipid
metabolism, inflammation and fibrosis in non-alcoholic fatty liver
disease. J Hepatol. 62:720–733. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ferramosca A and Zara V: Modulation of
hepatic steatosis by dietary fatty acids. World J Gastroenterol.
20:1746–1755. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kawahara TL, Michishita E, Adler AS,
Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang
HY and Chua KF: SIRT6 links histone H3 lysine 9 deacetylation to
NF-kappaB-dependent gene expression and organismal life Span. Cell.
136:62–74. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li Z, Xu K, Guo Y, Ping L, Gao Y, Qiu Y,
Ni J, Liu Q and Wang Z: A high-fat diet reverses metabolic
disorders and premature aging by modulating insulin and IGF1
signaling in SIRT6 knockout mice. Aging Cell.
19(e13104)2020.PubMed/NCBI View Article : Google Scholar
|