|
1
|
Azamjah N, Soltan-Zadeh Y and Zayeri F:
Global trend of breast cancer mortality rate: A 25-year study.
Asian Pac J Cancer Prev. 20:2015–2020. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Libson S and Lippman M: A review of
clinical aspects of breast cancer. Int Rev Psychiatry. 26:4–15.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tang JL, Liu BY and Ma KW: Traditional
Chinese medicine. Lancet. 372:1938–1940. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pei S, Yang X, Wang H, Zhang H, Zhou B,
Zhang D and Lin D: Plantamajoside, a potential anti-tumor herbal
medicine inhibits breast cancer growth and pulmonary metastasis by
decreasing the activity of matrix metalloproteinase-9 and -2. BMC
Cancer. 15(965)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liew AC, Peh KK, Tan BS, Zhao W and
Tangiisuran B: Evaluation of chemotherapy-induced toxicity and
health-related quality of life amongst early-stage breast cancer
patients receiving Chinese herbal medicine in Malaysia. Support
Care Cancer. 27:4515–4524. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jung SH, Lee GB, Ryu Y, Cui L, Lee HM, Kim
J, Kim B and Won KJ: Inhibitory effects of scoparone from chestnut
inner shell on platelet-derived growth factor-BB-induced vascular
smooth muscle cell migration and vascular neointima hyperplasia. J
Sci Food Agric. 99:4397–4406. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Y, Wang M, Chen B and Shi J:
Scoparone attenuates high glucose-induced extracellular matrix
accumulation in rat mesangial cells. Eur J Pharmacol. 815:376–380.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim JK, Kim JY, Kim HJ, Park KG, Harris
RA, Cho WJ, Lee JT and Lee IK: Scoparone exerts anti-tumor activity
against DU145 prostate cancer cells via inhibition of STAT3
activity. PLoS One. 8(e80391)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kielbus M, Skalicka-Wozniak K, Grabarska
A, Jeleniewicz W, Dmoszynska-Graniczka M, Marston A, Polberg K,
Gawda P, Klatka J and Stepulak A: 7-substituted coumarins inhibit
proliferation and migration of laryngeal cancer cells in vitro.
Anticancer Res. 33:4347–4356. 2013.PubMed/NCBI
|
|
11
|
Zhao Z, Guo Y, Liu Y, Sun L, Chen B, Wang
C, Chen T, Wang Y, Li Y, Dong Q, et al: Individualized lncRNA
differential expression profile reveals heterogeneity of breast
cancer. Oncogene. 40:4604–4614. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Venkatesh J, Wasson MD, Brown JM, Fernando
W and Marcato P: LncRNA-miRNA axes in breast cancer: Novel points
of interaction for strategic attack. Cancer Lett. 509:81–88.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Qiao K, Ning S, Wan L, Wu H, Wang Q, Zhang
X, Xu S and Pang D: LINC00673 is activated by YY1 and promotes the
proliferation of breast cancer cells via the miR-515-5p/MARK4/Hippo
signaling pathway. J Exp Clin Cancer Res. 38(418)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yuan JH, Li WX, Hu C and Zhang B:
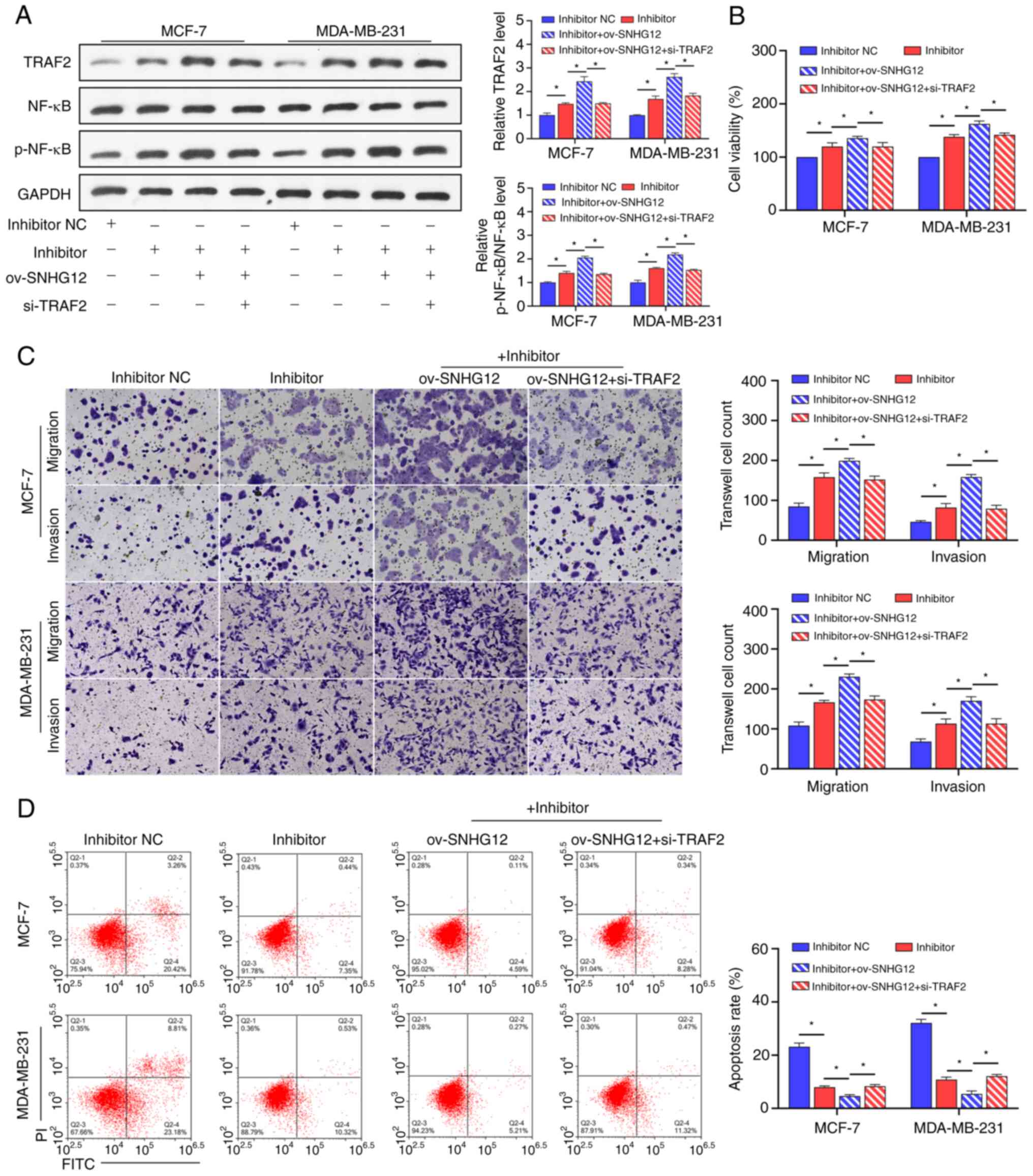
Upregulation of SNHG12 accelerates cell proliferation, migration,
invasion and restrain cell apoptosis in breast cancer by enhancing
regulating SALL4 expression via sponging miR-15a-5p. Neoplasma.
67:861–870. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhou Y, Wang B, Wang Y, Chen G, Lian Q and
Wang H: miR-140-3p inhibits breast cancer proliferation and
migration by directly regulating the expression of tripartite motif
28. Oncol Lett. 17:3835–3841. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sun LL, Wang J, Zhao ZJ, Liu N, Wang AL,
Ren HY, Yang F, Diao KX, Fu WN, Wan EH and Mi XY: Suppressive role
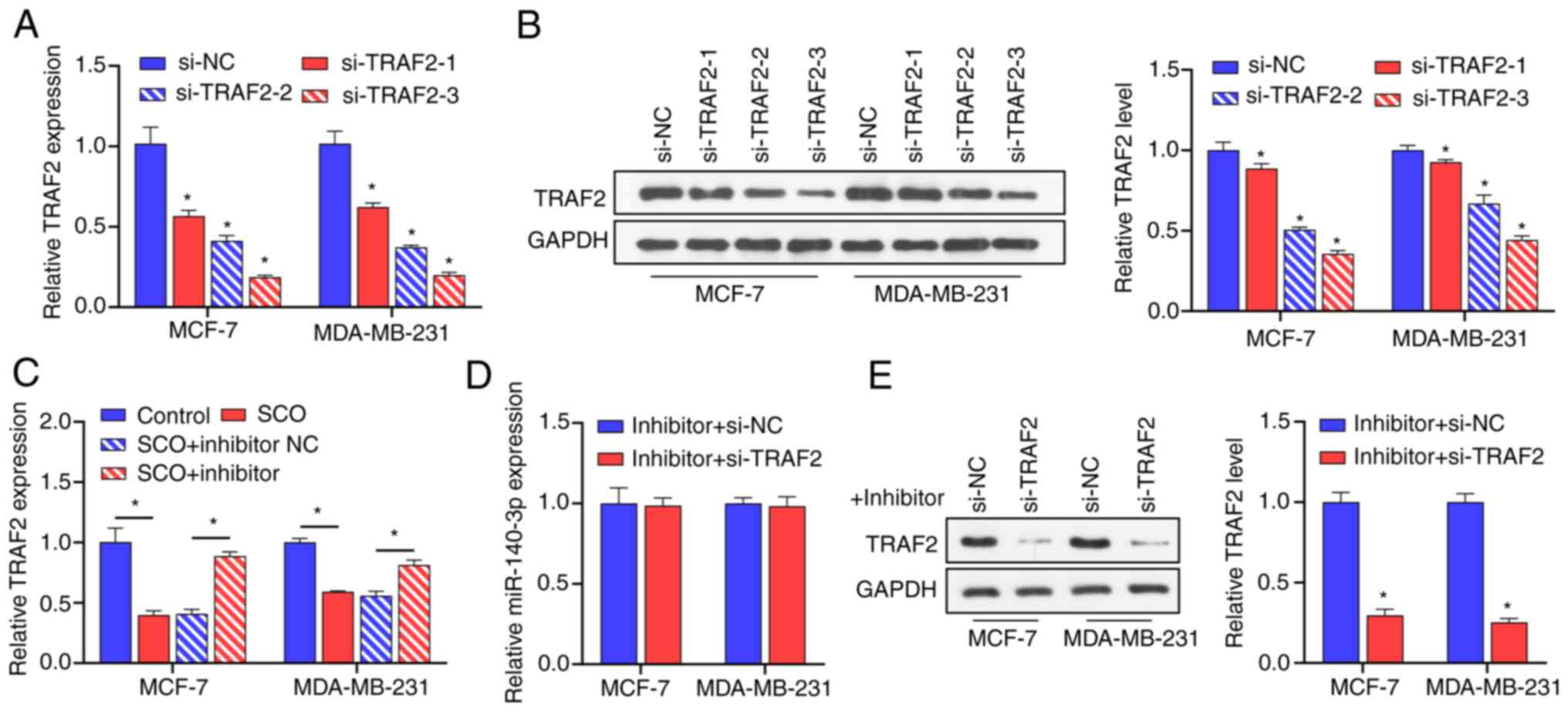
of miR-502-5p in breast cancer via downregulation of TRAF2. Oncol
Rep. 31:2085–2092. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Taminiau A, Draime A, Tys J, Lambert B,
Vandeputte J, Nguyen N, Renard P, Geerts D and Rezsohazy R: HOXA1
binds RBCK1/HOIL-1 and TRAF2 and modulates the TNF/NF-κB pathway in
a transcription-independent manner. Nucleic Acids Res.
44:7331–7349. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jang KW, Lee KH, Kim SH, Jin T, Choi EY,
Jeon HJ, Kim E, Han YS and Chung JH: Ubiquitin ligase CHIP induces
TRAF2 proteasomal degradation and NF-κB inactivation to regulate
breast cancer cell invasion. J Cell Biochem. 112:3612–3620.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Onyeisi JOS, Greve B, Espinoza-Sanchez NA,
Kiesel L, Lopes CC and Gotte M: microRNA-140-3p modulates
invasiveness, motility, and extracellular matrix adhesion of breast
cancer cells by targeting syndecan-4. J Cell Biochem.
122:1491–1505. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dou D, Ren X, Han M, Xu X, Ge X, Gu Y,
Wang X and Zhao S: Circ_0008039 supports breast cancer cell
proliferation, migration, invasion, and glycolysis by regulating
the miR-140-3p/SKA2 axis. Mol Oncol. 15:697–709. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zamarbide Losada JN, Sulpice E, Combe S,
Almeida GS, Leach DA, Choo J, Protopapa L, Hamilton MP, McGuire S,
Gidrol X, et al: Apoptosis-modulatory miR-361-3p as a novel
treatment target in endocrine-responsive and endocrine-resistant
breast cancer. J Endocrinol. 256(e220229)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hua B, Li Y, Yang X, Niu X, Zhao Y and Zhu
X: MicroRNA-361-3p promotes human breast cancer cell viability by
inhibiting the E2F1/P73 signalling pathway. Biomed Pharmacother.
125(109994)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jin T, Zhang Y and Zhang T: MiR-524-5p
Suppresses migration, invasion, and EMT progression in breast
cancer cells through targeting FSTL1. Cancer Biother Radiopharm.
35:789–801. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cheng S, Huang Y, Lou C, He Y, Zhang Y and
Zhang Q: FSTL1 enhances chemoresistance and maintains stemness in
breast cancer cells via integrin β3/Wnt signaling under miR-137
regulation. Cancer Biol Ther. 20:328–337. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gu M, Zhou W, Chen J, Zhao Y, Xie C and
Zhou Z: TRAF2 gene silencing induces proliferation and represses
apoptosis of nucleus pulposus cells in rats with intervertebral
disc degeneration. Life Sci. 279(119670)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yao Y, Zhao K, Yu Z, Ren H, Zhao L, Li Z,
Guo Q and Lu N: Wogonoside inhibits invasion and migration through
suppressing TRAF2/4 expression in breast cancer. J Exp Clin Cancer
Res. 36(103)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li QW, Zhang GL, Hao CX, Ma YF, Sun X,
Zhang Y, Cao KX, Li BX, Yang GW and Wang XM: SANT, a novel Chinese
herbal monomer combination, decreasing tumor growth and
angiogenesis via modulating autophagy in heparanase overexpressed
triple-negative breast cancer. J Ethnopharmacol.
266(113430)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang
Z and Han J: The advantages of using traditional Chinese medicine
as an adjunctive therapy in the whole course of cancer treatment
instead of only terminal stage of cancer. Biosci Trends. 9:16–34.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu B, Deng X, Jiang Q, Li G, Zhang J,
Zhang N, Xin S and Xu K: Scoparone improves hepatic inflammation
and autophagy in mice with nonalcoholic steatohepatitis by
regulating the ROS/P38/Nrf2 axis and PI3K/AKT/mTOR pathway in
macrophages. Biomed Pharmacother. 125(109895)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li N, Yang F, Liu DY, Guo JT, Ge N and Sun
SY: Scoparone inhibits pancreatic cancer through PI3K/Akt signaling
pathway. World J Gastrointest Oncol. 13:1164–1183. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu Y, Cheng G, Huang Z, Bao L, Liu J,
Wang C, Xiong Z, Zhou L, Xu T, Liu D, et al: Long noncoding RNA
SNHG12 promotes tumour progression and sunitinib resistance by
upregulating CDCA3 in renal cell carcinoma. Cell Death Dis.
11(515)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhou S, Yu L, Xiong M and Dai G: LncRNA
SNHG12 promotes tumorigenesis and metastasis in osteosarcoma by
upregulating Notch2 by sponging miR-195-5p. Biochem Biophys Res
Commun. 495:1822–1832. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ye Y, Ye F, Li X, Yang Q, Zhou J, Xu W,
Aschner M, Lu R and Miao S: 3,3'-diindolylmethane exerts
antiproliferation and apoptosis induction by TRAF2-p38 axis in
gastric cancer. Anticancer Drugs. 32:189–202. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wei B, Liang J, Hu J, Mi Y, Ruan J, Zhang
J, Wang Z, Hu Q, Jiang H and Ding Q: TRAF2 is a valuable prognostic
biomarker in patients with prostate cancer. Med Sci Monit.
23:4192–4204. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jiao C, Chen W, Tan X, Liang H, Li J, Yun
H, He C, Chen J, Ma X, Xie Y and Yang BB: Ganoderma lucidum spore
oil induces apoptosis of breast cancer cells in vitro and in vivo
by activating caspase-3 and caspase-9. J Ethnopharmacol.
247(112256)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Poma P: NF-κB and disease. Int J Mol Sci.
21(9181)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yu H, Lin L, Zhang Z, Zhang H and Hu H:
Targeting NF-kappaB pathway for the therapy of diseases: Mechanism
and clinical study. Signal Transduct Target Ther.
5(209)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tan Y, Sun R, Liu L, Yang D, Xiang Q, Li
L, Tang J, Qiu Z, Peng W, Wang Y, et al: Tumor suppressor DRD2
facilitates M1 macrophages and restricts NF-κB signaling to trigger
pyroptosis in breast cancer. Theranostics. 11:5214–5231.
2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jiang L, Yu L, Zhang X, Lei F, Wang L, Liu
X, Wu S, Zhu J, Wu G, Cao L, et al: miR-892b silencing activates
NF-κB and promotes aggressiveness in breast cancer. Cancer Res.
76:1101–1111. 2016.PubMed/NCBI View Article : Google Scholar
|