Introduction
Endoscopic ultrasound-guided fine-needle aspiration
(EUS-FNA), a minimally invasive interventional diagnostic
technique, involves the insertion of a puncture needle into the
target lesion for aspiration biopsy under real-time EUS guidance to
obtain cells or tissues for pathological analysis. EUS-FNA has now
become the preferred method for obtaining diagnostic samples of
lesions of the gastrointestinal tract and its adjacent organs
(1-3)
and is routinely used for pancreatic lesions, subepithelial
lesions, abdominal lymph nodes, the liver, spleen, adrenal glands,
mediastinum and pelvis. EUS-FNA operates through the natural cavity
of the body, shortening the distance between the probe and the
lesion. It passes through the less normal tissue during puncture,
thus reducing the side injuries caused by the puncture, with an
overall complication rate of less than 1% (4,5).
Wiersema et al (6) first described the important role of
on-site cytopathologists in assessing the adequacy of puncture
specimens and several subsequent studies (7-9)
have demonstrated that rapid on-site evaluation (ROSE) is an
effective method for improving the diagnostic ability of EUS-FNA.
ROSE can assess whether cell sampling is adequate or representative
in real time. However, owing to the increased human and financial
burden associated with ROSE, it is not routinely performed in all
healthcare facilities (2,10). To improve the positivity rate of
FNA, Iwashita et al (11),
in 2015, introduced the concept of macroscopic on-site evaluation
(MOSE), which helps to determine the presence of a macroscopic
visible core (MVC), a white or yellowish strip of tissue, in
histological specimens obtained by visual inspection during
puncture. The MVC is a more accurate predictor of the presence of a
histologic core in a puncture specimen. A histologic core is a
tissue mass that is structurally intact and sufficient for
histologic evaluation and its presence often indicates good sample
adequacy (12).
Most previous studies related to MOSE (13-19)
selected 19G standard FNA needles and 22G fine needle biopsy (FNB)
needles. EUS-FNB with MOSE shows comparable accuracy to that of
EUS-FNB with three needle passes. MOSE reliably assesses sample
adequacy and reduces the number of needle passes required to obtain
a diagnosis with a 22G Franseen needle (20), whereas 22G FNA needles are
currently the most used in clinical practice. It is uncertain
whether the results of these studies are applicable to 22G standard
FNA needles and further studies are needed to determine whether 22G
standard FNA needles can improve clinical diagnostic efficacy
through MOSE in the absence of ROSE.
Materials and methods
Patients
The present retrospective study included patients
who underwent EUS-FNA for solid lesions between October 2015 and
June 2021 at the Affiliated Hospital of Nantong University. EUS-FNA
was performed using the PENTAX linear-array echoendoscope (PENTAX
EPK-i5000 and PENTAX EG-3270UK; PENTAX Medical) under anesthesia.
Patients in whom the first puncture had been performed by the same
endoscopist using a standard 22G FNA needle and those with
relatively complete clinical data, detailed records of FNA
operations and traceable follow-up information were included.
Patients operated with 19G or 25G FNA needles or FNB needles; those
with severe cardiac, cerebral and pulmonary disorders and hence
could not tolerate the operation; those with severe psychiatric
disorders who could not cooperate with the clinical team members;
those with untreated bleeding tendencies, including a platelet
count <50x109/l, an international normalized ratio
>1.5, or those on anticoagulation or antiplatelet drugs; and
those with incomplete follow-up data and unknown clinical outcomes
were excluded from the present study. All patients provided written
informed consent prior to enrolment. The present study was
conducted in accordance with the principles of the Declaration of
Helsinki and was approved by the Ethical Committee of Affiliated
Hospital of Nantong University (approval no. 2019-K055).
Endoscopic procedures
EUS was performed using the PENTAX linear scanning
video echoendoscope (PENTAX EPK-i5000 and PENTAX EG-3270UK; PENTAX
Medical) and a 22G needle (Expect™; Boston Scientific Corp.) was
used as the EUS-FNA needle in all cases. All solid lesions were
classified as pancreatic and non-pancreatic. EUS-FNA was performed
by a single endoscopist who had experience in performing the
operation under sedation with intravenous propofol, without any
specific experience in cytopathology. No on-site cytologic
pathologist was present during the puncture. The puncture site was
determined under real-time EUS guidance by avoiding blood vessels,
pancreatic ducts, bile ducts and other important organs. The
puncture needle, which was pressed against the wall of the GI
tract, was linearly hyperechoic on EUS and the ‘comet tail’ sign
produced by the metal could be observed. The puncture needle was
inserted into the target lesion, the core was removed, a
negative-pressure syringe was attached and the needle was lifted
and inserted into the lesion more than 20 times (Fig. S1). The endoscopist decided the
number of punctures and chose the appropriate puncture method, such
as using the needle core, adjusting the negative pressure and the
fan puncture technique, according to the characteristics of the
lesion, the situation while obtaining the specimen and his or her
own experience. After each puncture, the negative pressure was
released, the puncture needle was withdrawn and the specimen was
pushed into the culture dish.
MOSE technique
The specimen was carefully examined by the
endoscopist for the presence of MVC, which was defined as whitish
or yellowish pieces of tissue with an apparent bulk, not including
paste-like or liquid-like material (Fig. 1). The FNA procedure would be
terminated if the endoscopist observed MVC in the obtained
specimen. MVCs scattered throughout the sample were collected and
aligned using an injection needle, after which the total length of
the MVC was measured using a ruler. If the endoscopist could not
detect the MVC, additional punctures were performed while ensuring
procedural safety.
Final diagnosis
On the basis of the patient's preoperative
laboratory tests, imaging data and clinical presentation, the final
diagnosis was established based on the following points: i)
Pathological findings after surgical resection; ii) positive FNA
malignancy without surgical intervention and a clinical course
consistent with the FNA diagnosis; and iii) negative FNA or
puncture pathology showing benign lesions without worsening or
spontaneous lesions on imaging review after at least 6 months of
regression observed on follow-up.
Main outcome measures
The primary objectives of the present study were to
evaluate the ability of different lengths of MVC to obtain an
accurate histological diagnosis and to determine the optimal
cut-off value for MVC length and to study the effect of the
application of MOSE on the diagnostic efficacy of FNA. The
secondary objectives were to analyze the factors affecting the
accuracy of histological diagnosis and to compare any differences
between the two groups in terms of operative time, number of
punctures and the incidence of puncture-related complications.
Cases in which the nature of the lesion could be
determined by puncture, including cytological or (and) histological
pathological findings that clearly defined the histological
diagnosis of the lesion as benign or malignant, tumor cells or
cancer cells seen by puncture, were considered as FNA positive and
otherwise, as FNA negative (Fig.
S2). The accuracy of FNA was defined as the sum of cases with
true positive and true negative results divided by the total number
of cases. The operative time was defined as the difference in time
from the insertion to the exit of the endoscope.
Statistical methods
SPSS 23.0 software (IBM Corp.) was used for
statistical analysis and the results of the normality test for
continuous variables showed that they did not obey normal
distribution; hence, median (quartiles) was used for descriptive
statistics and frequency or percentage was used for descriptive
statistics for categorical variables. The Mann-Whitney U test was
used for intergroup comparisons of continuous variables and the
χ2 test or Fisher exact probability method was used to
compare the variables between the groups. The accuracy of the area
under the curve (AUC) of the receiver-operating characteristic
(ROC) curve was assessed by plotting the curve of the length of the
MVC for histopathological diagnosis, using the Youden index to
calculate the optimal cut-off value of MVC length required to
obtain an accurate histological diagnosis. Factors that may be
associated with an accurate histological diagnosis were
investigated using univariate and multivariate logistic regression
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient and lesion
characteristics
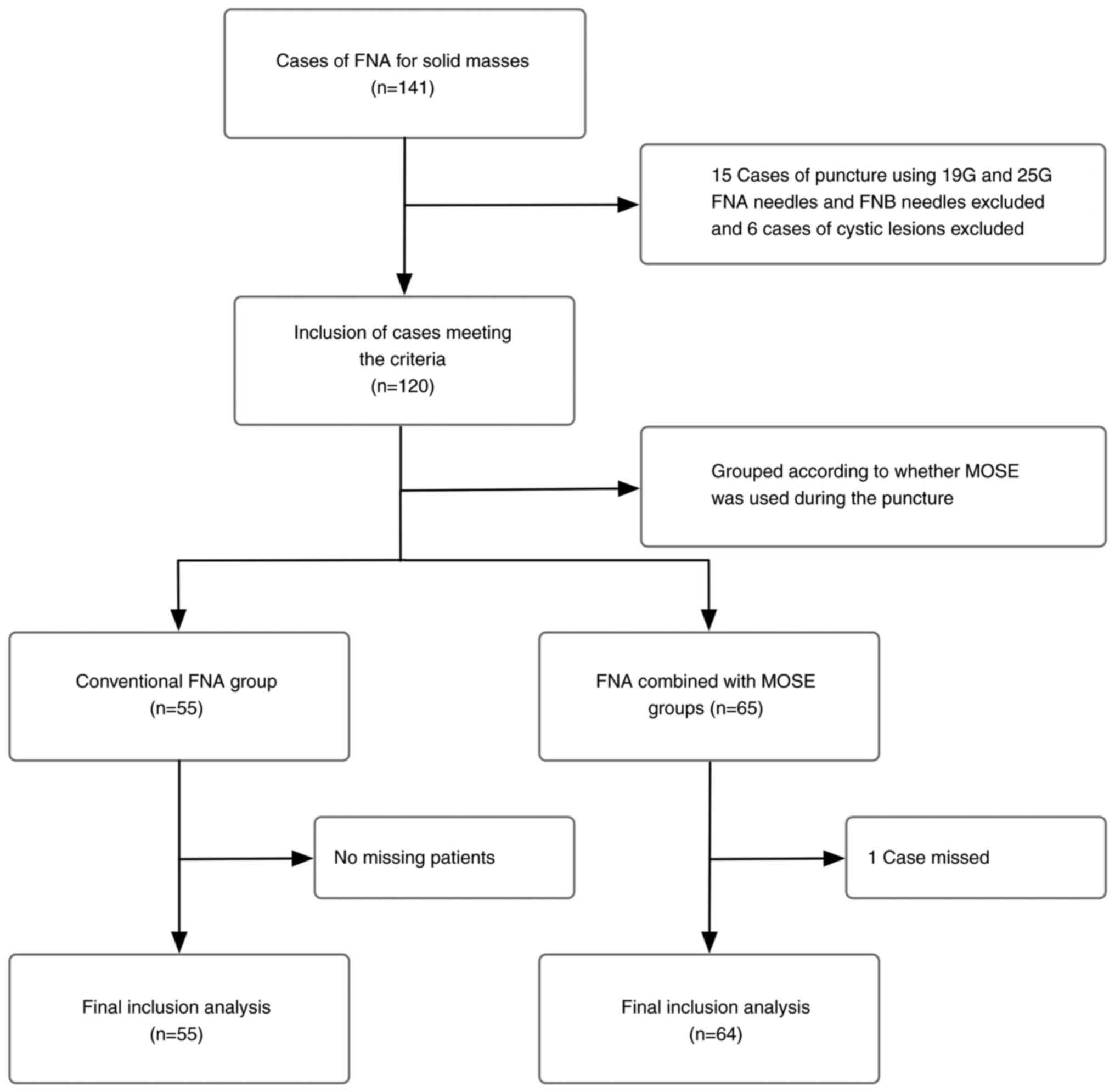
A total of 141 patients underwent FNA for occupying
lesions during the study period, of whom 22 were excluded according
to the inclusion and exclusion criteria, including 15 patients in
whom puncture was performed using other types of puncture needles
(including 19G and 25G FNA needles and FNB needles), 6 patients
with cystic lesions and 1 patient who was excluded owing to his
absence. Finally, 119 patients, namely 55 in the conventional FNA
group and 64 in the FNA combined with MOSE group, were included
(Fig. 2).
The patient and lesion characteristics of the two
groups are listed in Table I.
There were no statistically significant differences in sex, age,
lesion site (divided into pancreatic and non-pancreatic lesions),
or lesion size between the two groups. In the conventional FNA
group, 33 patients had pancreatic lesions and 22 had non-pancreatic
lesions. Among patients with non-pancreatic lesions, five had
gastric lesions; five had hepatogastric interstitial lesions; four
had lymph node enlargement; three had hepatic lesions; and one
patient each had esophageal, rectal, hepatopancreatic interstitial,
retroperitoneal and adrenal lesions. In the FNA combined with MOSE
group, 47 patients had pancreatic lesions and 17 had non-pancreatic
lesions. Among patients with non-pancreatic lesions, seven had
gastric lesions; two had lymph node enlargement; two had
mediastinal lesions; two had hepatic lesions; and one patient each
had rectal, adrenal, parapancreatic and splenogastric interstitial
lesions.
 | Table IComparison of patient and lesion
characteristics between the two groups. |
Table I
Comparison of patient and lesion
characteristics between the two groups.
| Characteristic | Conventional FNA
group (n=55) | FNA united MOSE group
(n=64) | P-value |
|---|
| Sex, n | | | 0.800 |
|
Male | 34 | 41 | |
|
Female | 21 | 23 | |
| Median age (range),
years | 65 (60-72) | 66 (58-71) | 0.769 |
| Location of lesions,
n | | | 0.119 |
|
Pancreatic
lesions | 33 | 47 | |
|
Non-pancreatic
lesions | 22 | 17 | |
| Median lesion size
(range), mm | 33 (28-45) | 34 (25-40) | 0.602 |
Outcomes
The success rate of FNA was 100% in both groups,
with 1-5 punctures performed per lesion. The differences in the
number of punctures (a median of 3 in the conventional FNA group
and 3 in the FNA combined with MOSE group; P=0.151), operative time
(a median of 17 min in the conventional FNA group and 19 min in the
FNA combined with MOSE group; P=0.448), puncture route (P=0.353)
and complication rates (5.4% vs. 1.5%; P=0.506) in both groups were
not statistically significant. Three puncture-related complications
occurred in the conventional FNA group, namely two cases of
hyperamylasemia and one case of transient fever and one case of
self-limiting bleeding at the puncture site in the FNA combined
with MOSE group, all of which improved after symptomatic treatment,
without serious puncture-related complications (Table II).
 | Table IIComparison of puncture-related
parameters between the two groups. |
Table II
Comparison of puncture-related
parameters between the two groups.
| Puncture-related
parameter | Conventional FNA
group (n=55) | FNA combined MOSE
group (n=64) | P-value |
|---|
| Median number of
punctures (range) | 3 (2-3) | 3 (2-4) | 0.151 |
| Median operation
time (range), min | 17 (13-24) | 19 (14-23) | 0.448 |
| Puncture paths,
n | | | 0.353 |
|
Trans-duodenal | 17 | 25 | |
|
Trans-esophageal,
stomach or rectal | 38 | 39 | |
| Complications
associated with puncture, n | 3 | 1 | 0.506 |
The final diagnosis of the patient was used as the
criterion to determine the FNA results and to evaluate whether the
application of MOSE had any effect on the diagnostic ability of FNA
and the results are shown in Table
III. Compared with the conventional FNA group, the diagnostic
sensitivity (75.0% vs. 89.8%, P=0.038) and accuracy (74.5% vs.
90.6%, P=0.026) were higher in the FNA combined with MOSE group and
the differences between the groups were statistically significant,
whereas the differences in the specificity (66.7% vs. 100.0%), PPV
(97.5% vs. 100.0%) and NPV (13.3% vs. 45.5%) were not statistically
significant (P<0.05).
 | Table IIIComparison of the diagnostic ability
of FNA between the two groups. |
Table III
Comparison of the diagnostic ability
of FNA between the two groups.
| Diagnostic
ability | Conventional FNA
group | FNA combined MOSE
group | P-value |
|---|
| Sensitivity, % | 75.0
(60.8-85.5) | 89.8
(78.5-95.8) | 0.038 |
| Specificity, % | 66.7
(12.5-98.2) | 100.0
(46.3-100.0) | 0.375 |
| PPV, % | 97.5
(85.3-99.9) | 100.0
(91.6-100.0) | 0.430 |
| NPV, % | 13.3
(2.3-41.6) | 45.5
(18.1-75.4) | 0.095 |
| Accuracy, % | 75.0
(60.8-85.5) | 90.6
(80.7-96.0) | 0.026 |
The final diagnosis of the patients was established
by combining the FNA results, surgical pathology findings and
follow-up observations. Tables IV
and V show the specific
pathological types and the corresponding number of patients in each
of the two groups according to the primary site of the pancreatic
and non-pancreatic lesions. A total of 39 patients in the
conventional FNA group could be diagnosed based on FNA results
and/or post-surgical pathology. Only tumor cells or cancer cells
were seen by FNA puncture in eight patients; however, the specific
pathology was not known. FNA did not show any abnormalities and on
follow-up, two patients remained in good general condition without
receiving any special treatment; hence, they were considered as
true negatives. In the FNA combined with MOSE group, a specific
histopathological diagnosis could be established in 55 patients in
combination with FNA results or (and) surgical pathology, as shown
in Table V. FNA cytology showed
positive results in four patients; however, the histopathology was
unknown because no surgical operation was performed. The results
were confirmed as true positive on follow-up; five patients had
FNA-negative results, which were confirmed at follow-up.
 | Table IVFinal pathological results of
conventional FNA group. |
Table IV
Final pathological results of
conventional FNA group.
| Lesion site and
pathological results | n |
|---|
| Pancreatic
lesions | |
|
Adenocarcinoma | 16 |
|
Neuroendocrine
tumor | 4 |
|
Mucinous
tumor | 1 |
|
Parenchymal
pseudopapillary tumor | 1 |
| Non-pancreatic
lesions | |
|
Gastrointestinal
mesenchymal tumor | 5 |
|
Lymphoma | 3 |
|
Tuberculosis | 2 |
|
Esophageal
adenocarcinoma | 1 |
|
Gastric
squamous carcinoma | 1 |
|
Gastric
adenocarcinoma | 1 |
|
Liver
Cancer | 1 |
|
Mucinous
smooth muscle sarcoma | 1 |
|
Metastatic
cancer | 1 |
|
Hepatic
adenoma | 1 |
 | Table VFinal pathological results of FNA
combined with MOSE group. |
Table V
Final pathological results of FNA
combined with MOSE group.
| Lesion site and
pathological results | n |
|---|
| Pancreatic
lesions | |
|
Adenocarcinoma | 27 |
|
Neuroendocrine
tumor | 5 |
|
Acute
pancreatitis | 2 |
|
Chronic
pancreatitis | 2 |
|
Autoimmune
pancreatitis | 1 |
|
Parenchymal
pseudopapillary tumor | 1 |
|
Metastatic
cancer | 1 |
| Non-pancreatic
lesions | |
|
Gastrointestinal
mesenchymal tumor | 4 |
|
Lymphoma | 3 |
|
Metastatic
cancer | 3 |
|
Gastric
squamous carcinoma | 2 |
|
Mediastinal
nerve sheath tumor | 1 |
|
Gastric
adenocarcinoma | 1 |
|
Gastric
Induced cell carcinoma | 1 |
|
Abscess | 1 |
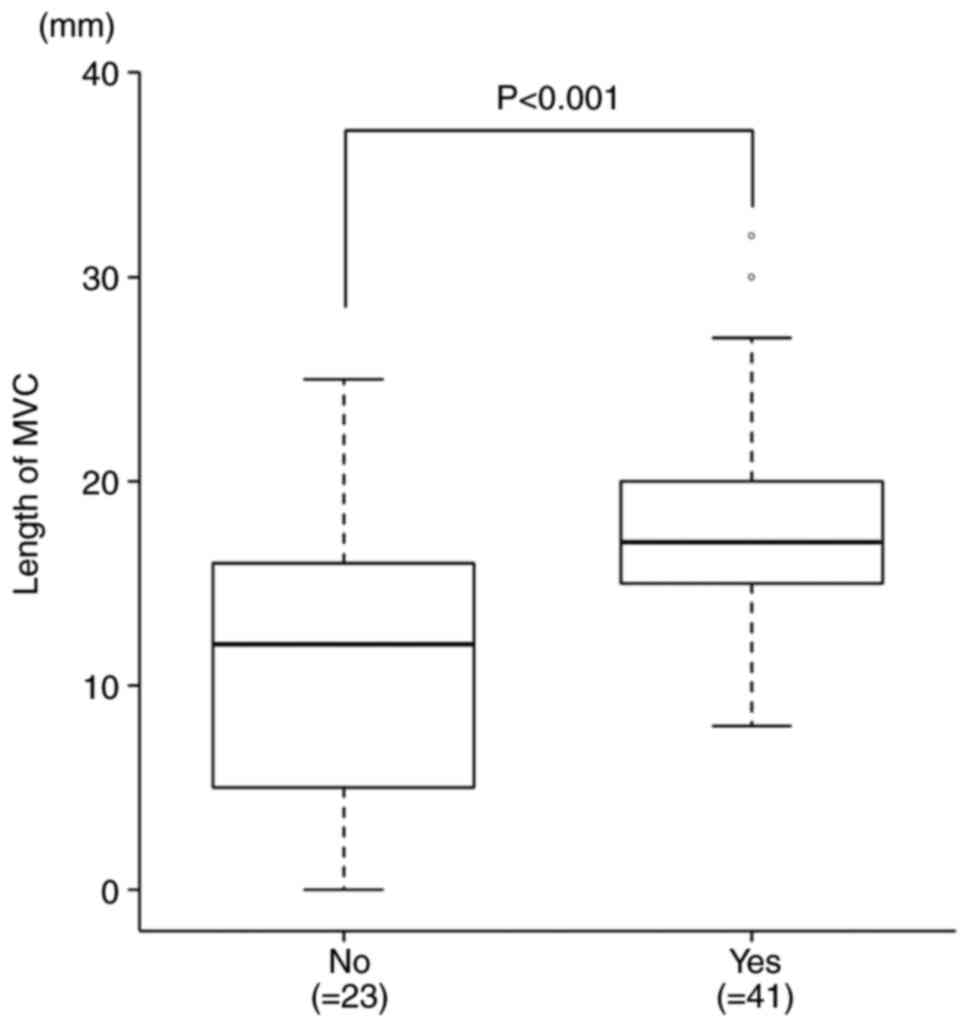
MVC was observed in 63 of 64 (98.4%) patients in the
FNA combined with MOSE group, with a median MVC length of 15
(interquartile range, 13-19) mm. Analysis of the relationship
between histological diagnosis and MVC length showed that MVC
length was greater in the group of patients in whom an accurate
histological diagnosis was obtained compared with those in whom an
accurate histological diagnosis was not obtained (median: 12 mm vs.
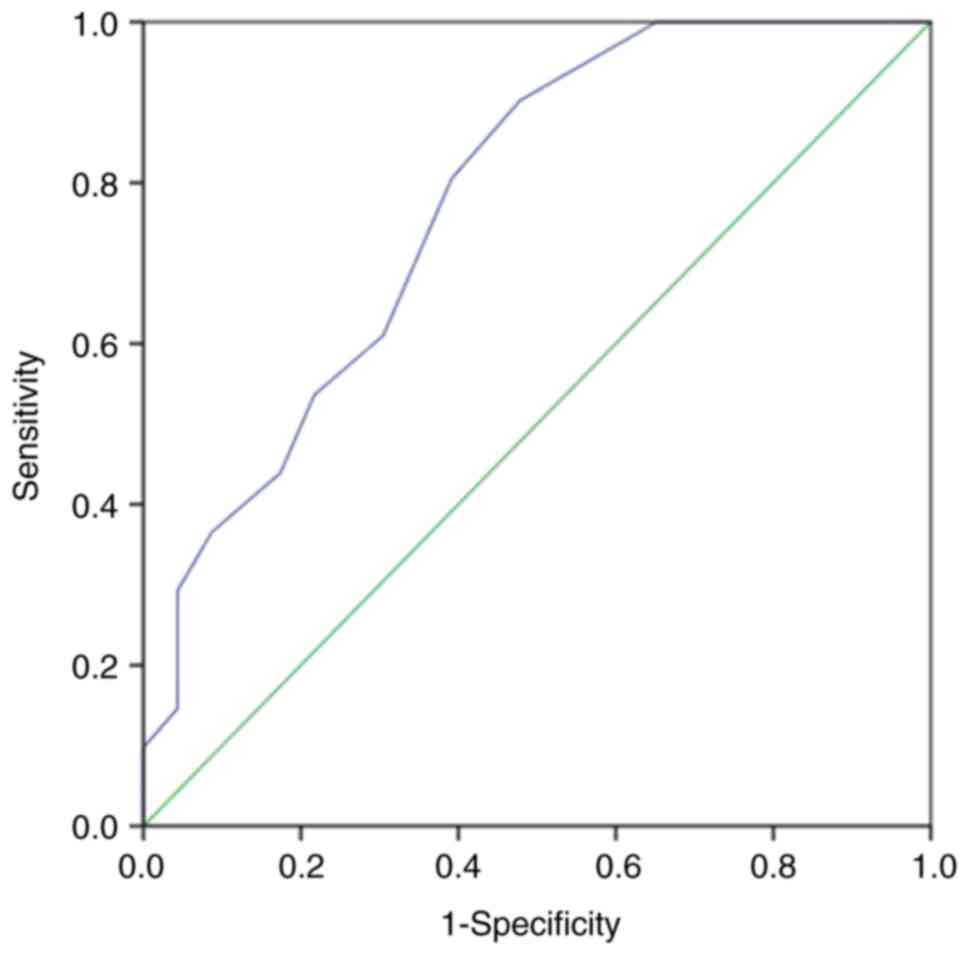
17 mm, P<0.001; Fig. 3). ROC
curves were plotted regarding the MVC length for histological
diagnosis (Fig. 4) and the optimal
MVC cutoff length required to obtain an accurate histological
diagnosis was determined using the Youden index. The results showed
that the optimal MVC length cutoff value was 13 mm and the AUC was
0.775 (95% CI 0.651-0.898), which corresponds to a diagnosis at
this MVC length cutoff value, at a sensitivity of 90.2%.
Factors that may be associated with obtaining an
accurate histological diagnosis were investigated using univariate
and multifactorial logistic regression analysis, which included
sex, age, lesion site (divided into pancreatic and non-pancreatic
lesions), lesion size, number of punctures and MVC length. The data
from the statistical analysis are shown in Table VI. Univariate logistic regression
analysis showed that histological diagnostic accuracy was
associated with the number of punctures (P=0.042) and MVC length
(P=0.001). Multifactorial logistic regression analysis showed that
only MVC length 13 mm (odds ratio=9.426, 95% CI 1.923-46.204;
P=0.006) was associated with a correct histopathological
diagnosis.
 | Table VIUnivariate and multivariate logistic
regression analysis associated with accurate histological
diagnosis. |
Table VI
Univariate and multivariate logistic
regression analysis associated with accurate histological
diagnosis.
| | Univariate | Multivariate |
|---|
| Characteristic | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Sex | | | | |
|
Male | 0.355
(0.110-1.140) | 0.082 | 0.435
(0.094-2.010) | 0.286 |
|
Female | 1.000 | | 1.000 | |
| Age, years | 1.027
(0.984-1.073) | 0.223 | 1.044
(0.982-1.110) | 0.165 |
| Location of
lesions | | | | |
|
Pancreatic
lesions | 0.453
(0.128-1.603) | 0.220 | 0.423
(0.083-2.147) | 0.299 |
|
Non-Pancreatic
lesions | 1.000 | | 1.000 | |
| Size of
lesions | 1.042
(0.999-1.087) | 0.056 | 1.053
(0.992-1.118) | 0.092 |
| Number of
punctures | 1.778
(1.022-3.094) | 0.042 | 1.688
(0.867-3.287) | 0.123 |
| Length of MVC,
mm | | | | |
|
≥13 | 10.091
(2.705-37.648) | 0.001 | 9.426
(1.923-46.204) | 0.006 |
|
<13 | 1.000 | | 1.000 | |
Discussion
ROSE is a more reliable method to improve the
diagnostic efficacy of FNA and the role of ROSE in FNA has been
confirmed by several studies (8,21-23).
The presence of an on-site cytopathologist helps to improve sample
adequacy and diagnostic positivity, while also avoiding unnecessary
repetitive FNA procedures. In addition, fewer punctures may reduce
the rate of potential procedure-related complications and improve
procedural safety. However, ROSE is not routinely performed in many
hospitals and a global survey showed that ROSE is available in only
55% of Asian institutions (24).
Moreover, ROSE requires additional time for slide staining and
pathological analysis, increasing the procedural duration (25,26).
Iwashita et al (11) reported on direct MOSE performed by
an endoscopist on the acquired specimens. They found that the
diagnostic rate was significantly higher when the MVC was 4 mm and
that MVC >4 mm could be used as an indicator of specimen
adequacy, which serves as an important reference indicator when
performing FNA in many endoscopy centers where ROSE cannot be
performed. However, as the present study used a 19G FNA needle,
this criterion may not be applicable to other needle types. A
similar study using a 22G FNB needle (Acquire™) reported that MVC
length predicted correct pathologic diagnosis and that the
diagnostic accuracy of the FNB needle was positively correlated
with MVC length, with a length of 10 mm independently influencing
correct diagnosis (16). A recent
prospective multicenter study using the same FNB needle (Acquire™)
showed that MVC length was positively correlated with the number of
samples with a score of 5 (cytology, 1-2; histology, 3-5). The
optimal cut-off value of MVC length for sample score 5 was 15 mm
and the histological diagnostic accuracy and sensitivity of
specimens with MVC >15 mm was greater than that of specimens
with MVC <15 mm. MVC length is also positively correlated with
the sensitivity of histological diagnosis (12). In a systematic review and
meta-analysis, excellent pooled diagnostic accuracy parameters were
observed in EUS-guided tissue acquisition by FNB using the MOSE
method (27).
The European Society of Gastrointestinal Endoscopy
recommends 3-4 punctures when performing FNA on target lesions when
ROSE cannot be performed (2).
Previous related studies (11,15,16)
have shown that obtaining an MVC above the truncation length may be
a useful indicator for terminating the puncture, which may be
helpful for institutions unable to perform ROSE. Using this cut-off
length to guide the puncture process is expected to improve the
rate of diagnosis by FNA, reduce the number of punctures and
subsequently reduce the occurrence of potential needle tract
metastases, which may benefit patients with surgically resectable
caudal pancreatic body tumors.
A standardized MOSE procedure has not yet been
established and evidence regarding its guidance for the puncture
procedure is limited and controversial (2,11,17,28).
By performing MOSE on specimens obtained with a 22G FNA needle, the
present study aimed to investigate the effect of MOSE on the
results of FNA guided by a standard 22G needle and to determine the
optimal cut-off value of MVC length required to perform an accurate
histological diagnosis, with the aim of using this length to guide
the subsequent FNA procedure and improve the FNA positivity rate
and safety.
The present study showed that FNA combined with MOSE
was superior to conventional FNA for the diagnosis of solid masses,
with statistical differences in diagnostic sensitivity and
accuracy, suggesting that MOSE can be used to improve the
diagnostic efficiency of FNA when ROSE cannot be performed. MVC
length may predict the correct histologic diagnosis of FNA; an MVC
length >13 mm may provide accurate histological pathology
results, corresponding to a sensitivity of 90.2% at this truncation
length, following which the FNA operation can be terminated.
Univariate and multivariate logistic regression analyses also
showed that MVC length ≥13 mm influenced accurate histopathological
diagnosis and puncturing with this cut-off length as a reference
was expected to improve the FNA positivity rate and reduce the
number of punctures required for diagnosis.
The MOSE procedure involves several steps such as
visual observation, collection and measurement of MVC length, which
may prolong the operation time; however, the results of the present
study showed that the operative time in the FNA combined with MOSE
group was not significantly different from that in the conventional
FNA group, indicating that MOSE did not significantly increase the
operative time.
The advantage of the present study is that it
selected the most widely used 22G standard FNA needle for puncture,
whereas most previous related studies used 19G standard FNA needles
and 22G FNB puncture needles. Although these needles are of higher
caliber and more likely to obtain the core tissue, their stiffness
and poor flexibility reduce the feasibility of FNA and limit the
endoscopic position, angle and forceps lifter function, increasing
the potential risk of complications (29). Compared with FNA needles, FNB
needles are characterized by their lateral beveled orifice or barb
(5), a special design that
improves tissue access; however, owing to the characteristics of
the FNB tip shape and the stiffness of the needle body, this
procedure is more difficult than that using standard FNA needles,
which is more commonly performed by less experienced endoscopists
(17). 22G standard FNA needles
are more flexible and visualization is improved under ultrasound
for obtaining adequate cytology or histology samples without
increasing the risk of operation-related complications (5). The effect of MOSE on the diagnostic
role of the 22G standard FNA needle has not been fully elucidated
and studies on the relationship between MVC length obtained with
the 22G FNA needle and histological diagnosis are lacking.
In the present study, MVC was observed in 98.4%
(63/64) of patients in the FNA combined with MOSE group, while
pathological diagnostic information was available in only 89.1%
(57/64) of patients. This indicated that the MVC did not contain
valuable diagnostic components in six patients and the white or
yellowish samples in these cases could have been necrotic material
or fibrous components. Thus, MOSE is not completely accurate for
visual inspection and it may mistake non-diagnostic components for
meaningful pathological tissue, leading to the occurrence of
false-negative diagnosis. Moreover, unlike ROSE, it cannot assess
the presence of tumor cells; hence, obtaining a large number of
samples and a longer MVC does not necessarily lead to a correct
diagnosis. In this context, the assessment in the present study of
the relationship between MVC length and histological diagnostic
accuracy is of greater clinical value, especially for pancreatic
lesions, where pancreatic adenocarcinoma often contains a large
fibrous component and less substantial tissue or cellular
components (30), which may be
mistaken for core tissue during visual assessment. A study by
Iwashita et al (11) also
showed that pancreatic lesions are important risk factors for
false-negative puncture. The use of computer analysis software to
quantify the characteristics of core tissue (including
chromaticity, transparency and hardness) and to elucidate the
criteria for good-quality core tissue may reduce the number of
false-negative cases to some extent, compared to visual assessment
(15).
The present study has several limitations. First, it
was a single-center retrospective study with a small sample size,
which may lead to a bias in the FNA diagnostic results and patient
selection. For example, the small number of GIST cases made it
difficult to group by lesion type. Therefore, the present study
attempted to minimize the selection bias through quality score
matching, such as number of functions, operation time and function
route. (Tables I and II). Further validation in multicenter
studies with larger sample sizes may be needed, considering the
differences in equipment and technical level between the various
procedures. Second, the present study only performed a preliminary
study with the 22G FNA needle and the results may vary when other
types of puncture needles are used for tissue sampling. In
addition, the amount of tissue obtained by aspiration with the 22G
FNA needle is relatively small compared with the tissue obtained
using large-bore puncture needles. Further, it is often difficult
to identify MVC in samples containing large amounts of blood-based
components, which may require a body vision microscope for the
collection and measurement of MVC. Finally, the diagnostic
expertise of different pathologists may vary and all FNA specimens
may not be judged by the same pathologist; use of blinded methods
can only minimize the relevant variability.
Based on the current evidence, FNB using Franseen or
Fork-tip needles is a clinically preferred scheme for solid lesions
(31). However, in cases where FNB
cannot be performed, FNA combined with MOSE may be used. When using
22G FNA for puncture sampling of solid masses, an MVC length of
>13 mm may help to achieve an accurate histologic diagnosis and
this truncation length may be used to guide the puncture procedure
and improve the diagnostic yield. MOSE helps to improve the
diagnostic ability of FNA for solid masses and may be a useful
alternative to assess the adequacy of puncture specimens in units
where ROSE cannot be performed.
Supplementary Material
Endoscopic ultrasound-guided fine
needle aspiration image.
Histopathology of the endoscopic
ultrasound-guided fine needle aspiration sample in a patient with
pancreatic cancer (H&E; magnification, x200).
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Jiangsu Provincial
Geriatric Health Research Project (grant no. LKM2022023), the
Science and Technology Program of Nantong (grant nos. JC12022001
and JC22022040), Nantong University Hospital Postdoctoral Research
Program (grant no. BSH202214), Nantong Commission of Health
Research Fund Project (grant no. MA2021004) and the Natural Science
Foundation of Jiangsu Province Youth Fund Project (grant no.
BK20200965).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CG and MW drafted the manuscript and conceived the
study. JY and ZL were responsible for the collection and analysis
of case data and literature. JZ helped to design the study and
revised the manuscript. ZM and CL performed the statistical
analysis, and confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All the patients provided written, informed consent
and the study was approved by the Ethical Committee of Affiliated
Hospital of Nantong University (approval no. 2019-K055).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Dumonceau JM, Deprez PH, Jenssen C,
Iglesias-Garcia J, Larghi A, Vanbiervliet G, Aithal GP, Arcidiacono
PG, Bastos P, Carrara S, et al: Indications, results, and clinical
impact of endoscopic ultrasound (EUS)-guided sampling in
gastroenterology: European society of gastrointestinal endoscopy
(ESGE) clinical guideline-updated january. Endoscopy. 49:695–714.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Polkowski M, Jenssen C, Kaye P, Carrara S,
Deprez P, Gines A, Fernández-Esparrach G, Eisendrath P, Aithal GP,
Arcidiacono P, et al: Technical aspects of endoscopic ultrasound
(EUS)-guided sampling in gastroenterology: European society of
gastrointestinal endoscopy (ESGE) Technical guideline-march 2017.
Endoscopy. 49:989–1006. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
ASGE Standards of Practice Committee.
Eloubeidi MA, Decker GA, Chandrasekhara V, Chathadi KV, Early DS,
Evans JA, Fanelli RD, Fisher DA, Foley K, et al: The role of
endoscopy in the evaluation and management of patients with solid
pancreatic neoplasia. Gastrointest Endosc. 83:17–28.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW,
Liao Z and Li ZS: Assessment of morbidity and mortality associated
with EUS-guided FNA: A systematic review. Gastrointest Endosc.
73:283–290. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tan Y, Tang X, Huang J and Li R: Efficacy,
feasibility, and safety of endoscopic ultrasound-guided fine-needle
biopsy for the diagnosis of gastrointestinal subepithelial lesions:
A systematic review and meta-analysis. J Clin Gastroenterol.
56:e283–e292. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wiersema MJ, Wiersema LM, Khusro Q, Cramer
HM and Tao LC: Combined endosonography and fine-needle aspiration
cytology in the evaluation of gastrointestinal lesions.
Gastrointest Endosc. 40:199–206. 1994.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Klapman JB, Logrono R, Dye CE and Waxman
I: Clinical impact of on-site cytopathology interpretation on
endoscopic ultrasound-guided fine needle aspiration. Am J
Gastroenterol. 98:1289–1294. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Iglesias-Garcia J, Dominguez-Munoz JE,
Abdulkader I, Larino-Noia J, Eugenyeva E, Lozano-Leon A and
Forteza-Vila J: Influence of on-site cytopathology evaluation on
the diagnostic accuracy of endoscopic ultrasound-guided fine needle
aspiration (EUS-FNA) of solid pancreatic masses. Am J
Gastroenterol. 106:1705–1710. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hébert-Magee S, Bae S, Varadarajulu S,
Ramesh J, Frost AR, Eloubeidi MA and Eltoum IA: The presence of a
cytopathologist increases the diagnostic accuracy of endoscopic
ultrasound-guided fine needle aspiration cytology for pancreatic
adenocarcinoma: A meta-analysis. Cytopathology. 24:159–171.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Masutani H, Okuwaki K, Kida M, Yoshida T,
Imaizumi H, Yamauchi H, Iwai T, Kaneko T, Hasegawa R, Miyata E, et
al: On-site stereomicroscope quality evaluations to estimate white
core cutoff lengths using EUS-FNA biopsy sampling with 22-gauge
needles. Gastrointest Endosc. 90:947–956. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Iwashita T, Yasuda I, Mukai T, Doi S,
Nakashima M, Uemura S, Mabuchi M, Shimizu M, Hatano Y, Hara A and
Moriwaki H: Macroscopic on-site quality evaluation of biopsy
specimens to improve the diagnostic accuracy during EUS-guided FNA
using a 19-gauge needle for solid lesions: A single-center
prospective pilot study (MOSE study). Gastrointest Endosc.
81:177–185. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kaneko J, Ishiwatari H, Sasaki K, Yasuda
I, Takahashi K, Imura J, Iwashita T, Uemura S, Hatano Y, Miyazaki
T, et al: Macroscopic visible core length can predict the
histological sample quantity in endoscopic ultrasound-guided tissue
acquisition: Multicenter prospective study. Dig Endosc. 34:622–631.
2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
So H, Seo DW, Hwang JS, Ko SW, Oh D, Song
TJ, Park DH, Lee SK and Kim MH: Macroscopic on-site evaluation
after EUS-guided fine needle biopsy may replace rapid on-site
evaluation. Endosc Ultrasound. 10:111–115. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chong CCN, Lakhtakia S, Nguyen N, Hara K,
Chan WK, Puri R, Almadi MA, Ang TL, Kwek A, Yasuda I, et al:
Endoscopic ultrasound-guided tissue acquisition with or without
macroscopic on-site evaluation: Randomized controlled trial.
Endoscopy. 52:856–863. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Okuwaki K, Masutani H, Kida M, Yamauchi H,
Iwai T, Miyata E, Hasegawa R, Kaneko T, Imaizumi H, Watanabe M, et
al: Diagnostic efficacy of white core cutoff lengths obtained by
EUS-guided fine-needle biopsy using a novel 22G franseen biopsy
needle and sample isolation processing by stereomicroscopy for
subepithelial lesions. Endosc Ultrasound. 9:187–192.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kaneko J, Ishiwatari H, Sasaki K, Satoh T,
Sato J, Matsubayashi H, Yabuuchi Y, Kishida Y, Yoshida M, Ito S, et
al: Macroscopic on-site evaluation of biopsy specimens for accurate
pathological diagnosis during EUS-guided fine needle biopsy using
22-G Franseen needle. Endosc Ultrasound. 9:385–391. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ishiwatari H, Sato J, Fujie S, Sasaki K,
Kaneko J, Satoh T, Matsubayashi H, Kishida Y, Yoshida M, Ito S, et
al: Gross visual inspection by endosonographers during endoscopic
ultrasound-guided fine needle aspiration. Pancreatology.
19:191–195. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Stigliano S, Balassone V, Biasutto D,
Covotta F, Signoretti M and Di Matteo FM: Accuracy of visual
on-site evaluation (Vose) in predicting the adequacy of Eus-guided
fine needle biopsy: A single center prospective study.
Pancreatology. 21:312–317. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mangiavillano B, Frazzoni L, Togliani T,
Fabbri C, Tarantino I, De Luca L, Staiano T, Binda C, Signoretti M,
Eusebi LH, et al: Macroscopic on-site evaluation (MOSE) of
specimens from solid lesions acquired during EUS-FNB: multicenter
study and comparison between needle gauges. Endosc Int Open.
9:E901–E906. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mangiavillano B, Crinò SF, Facciorusso A,
Di Matteo F, Barbera C, Larghi A, Rizzatti G, Carrara S, Spadaccini
M, Auriemma F, et al: Endoscopic ultrasound-guided fine-needle
biopsy with or without macroscopic on-site evaluation: A randomized
controlled noninferiority trial. Endoscopy. 55:129–137.
2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Collins BT, Murad FM, Wang JF and Bernadt
CT: Rapid on-site evaluation for endoscopic ultrasound-guided
fine-needle biopsy of the pancreas decreases the incidence of
repeat biopsy procedures. Cancer Cytopathol. 121:518–524.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wani S, Mullady D, Early DS, Rastogi A,
Collins B, Wang JF, Marshall C, Sams SB, Yen R, Rizeq M, et al: The
clinical impact of immediate on-site cytopathology evaluation
during endoscopic ultrasound-guided fine needle aspiration of
pancreatic masses: A prospective multicenter randomized controlled
trial. Am J Gastroenterol. 110:1429–1439. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Matynia AP, Schmidt RL, Barraza G,
Layfield LJ, Siddiqui AA and Adler DG: Impact of rapid on-site
evaluation on the adequacy of endoscopic-ultrasound guided
fine-needle aspiration of solid pancreatic lesions: A systematic
review and meta-analysis. J Gastroenterol Hepatol. 29:697–705.
2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
van Riet PA, Cahen DL, Poley JW and Bruno
MJ: Mapping international practice patterns in EUS-guided tissue
sampling: outcome of a global survey. Endosc Int Open. 4:E360–E370.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Khan MA, Grimm IS, Ali B, Nollan R,
Tombazzi C, Ismail MK and Baron TH: A meta-analysis of endoscopic
ultrasound-fine-needle aspiration compared to endoscopic
ultrasound-fine-needle biopsy: Diagnostic yield and the value of
onsite cytopathological assessment. Endosc Int Open. 5:E363–E375.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hayashi T, Ishiwatari H, Yoshida M, Ono M,
Sato T, Miyanishi K, Sato Y, Kobune M, Takimoto R, Mitsuhashi T, et
al: Rapid on-site evaluation by endosonographer during endoscopic
ultrasound-guided fine needle aspiration for pancreatic solid
masses. J Gastroenterol Hepatol. 28:656–663. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mohan BP, Madhu D, Reddy N, Chara BS, Khan
SR, Garg G, Kassab LL, Muthusamy AK, Singh A, Chandan S, et al:
Diagnostic accuracy of endoscopic ultrasound (EUS) guided fine
needle biopsy (FNB) by macroscopic on-site evaluation (MOSE): A
systematic review and meta-analysis. Gastrointest Endosc.
96:909–917.e11. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nguyen YP, Maple JT, Zhang Q, Ylagan LR,
Zhai J, Kohlmeier C, Jonnalagadda S, Early DS, Edmundowicz SA and
Azar RR: Reliability of gross visual assessment of specimen
adequacy during EUS-guided FNA of pancreatic masses. Gastrointest
Endosc. 69:1264–1270. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vilmann P, Săftoiu A, Hollerbach S, Skov
BG, Linnemann D, Popescu CF, Wellmann A, Gorunescu F, Clementsen P,
Freund U, et al: Multicenter randomized controlled trial comparing
the performance of 22 gauge versus 25 gauge EUS-FNA needles in
solid masses. Scand J Gastroenterol. 48:877–883. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dougan SK: The pancreatic cancer
microenvironment. Cancer J. 23:321–325. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gkolfakis P, Crinò SF, Tziatzios G, Ramai
D, Papaefthymiou A, Papanikolaou IS, Triantafyllou K, Arvanitakis
M, Lisotti A, Fusaroli P, et al: Comparative diagnostic performance
of end-cutting fine-needle biopsy needles for endoscopic ultrasound
tissue sampling of solid pancreatic masses: A network
meta-analysis. Gastrointestinal Endoscopy. 95:1067–1077.e15.
2022.PubMed/NCBI View Article : Google Scholar
|