Introduction
Hypertension (HTN) is a chronic illness with
uncertain etiology. It is a common cardiovascular risk factor that
contributes to the likelihood of cardiovascular events (1,2).
Severe myocardial injury, heart failure, chronic kidney disease,
peripheral vascular disease, stroke and derived complications
affecting organ systems, such as the brain and eyes, are known to
be associated with HTN (3-5).
According to the latest report in December 2022 conducted by the
Korea Centers for Disease Control and Prevention, HTN affects 33.2%
of the Korean population aged >30 years and 62.3% of the
population aged >65 years (6).
Similar to other vascular conditions, HTN has been characterized by
the aberrant expression of microRNAs (miRNAs/miRs), which can
induce translational repression or mRNA degradation (2,4).
Since the pathophysiology of HTN constitutes an intricate
combination of environmental and genetic factors, our understanding
of the molecular mechanism underlying this condition remains
incomplete (4).
miRNAs are a conserved class of small, non-coding
RNAs that mainly function to block the translation of or
accelerating the degradation of mRNAs by binding to their
3'-untranslated regions (3'-UTRs) (7-9).
Through this function, they have been documented to, in turn,
regulate endothelial function, the renin-angiotensin-aldosterone
system and the pathogenesis of HTN (10). Investigations into these miRNAs
have the potential to contribute to our understanding of the
pathological mechanisms underlying HTN and may lead to the
development of therapeutic medicine against this condition
(4). A previous study by Yin et
al (5) analyzed miR-128
expression in the peripheral blood of patients with HTN in China;
they found that upregulation of this miRNA was associated with the
progression of myocardial injury in the HTN group, particularly
stage III/IV HTN. Other previous studies have shown the expression
of members of the miR-29 family to be upregulated in patients with
HTN, whilst also showing a positive association with their blood
pressure (2). Zhang et al
(10) previously suggested that
miR-122 bound to the 3'-UTR of cationic amino acid transporter 1
mRNA may lead to the decreased metabolism of L-arginine and nitric
oxide in vascular endothelial cells. This can eventually lead to
the dysfunction of vascular endothelial cells, which have been
reported to be a significant cause of HTN (10). In miRNA biology, although a single
miRNA can target multiple genes, it can also be targeted by other
genes. Owing to this feature, miRNAs can potentially serve an
important role in a regulatory network controlling the pathological
mechanisms of various cardiovascular diseases, such as HTN
(4).
Previous studies have suggested that miRNA
polymorphisms can contribute to a genetic predisposition to HTN
(1,2,5,11).
Members of the miR-200 family, including miR-200b, have been
reported to serve an important role in processes associated with
hypoxia, such as angiogenic responses in microvascular endothelial
cells and the apoptosis of cardiomyocytes (12). They have also been found to have a
significant relationship with tumorigenesis, cancer metastasis and
recurrence (13). miR-200b
expression has been shown to be increased in renal tissues of
patients with hypertensive nephrosclerosis when accompanied by
expression in heart ventricles with salt-sensitive hypertension
(11). In addition, the dynamic
expression of miRNAs, a genetic regulatory mechanism for platelet
formation and activation, has been reported to be linked with the
pathogenesis of thrombotic disorders (14). A prior study examining miRNA
expression in platelets reported that the expression of miR-200b
and miR-495 may be significantly downregulated during the
maturation of megakaryocytes into platelets (15). These miRNA shave also been shown to
reduce the expression of platelet-specific functional proteins,
protein kinase cAMP-dependent type II regulatory subunit β
(PRKAR2B) and Kelch-like family member 5 (KLHL5) (16). Therefore, the target genes of
miR-200b and miR-495 may modulate platelet functions during the
activation, aggregation and proinflammatory reaction of platelets
(14).
Polymorphisms of miR-200bT>C (rs7549819) in
chromosome 1 and miR-495A>C (rs2281611) in chromosome 14 were
previously found in their corresponding promoter regions (14,17).
Their genetic variant allele frequencies in the global population
from the Allele Frequency Aggregator dataset were documented to be
0.099 for miR-200bT>C and 0.236 for miR-495A>C (17). In a study by Kim et al
(14), susceptibility to ischemic
stroke and post-stroke mortality was investigated among cohorts
with various miRNA genotypes. The study did not find a significant
difference in the distribution of miRNA single nucleotide
polymorphisms (SNPs; miR-200bT>C and miR-495A>C) between the
patient and control groups, but the miR-200b CC genotype was less
frequently found in patients with large-artery atherosclerotic
stroke (14). Furthermore, Qin
et al (18) previously
suggested that the miR-200bT>C and miR-495A>C variants were
significantly associated with genes and pathways that can regulate
ischemic stroke pathogenesis in a Chinese population. In addition,
their evidence has also supported the notion that alterations in
the miR-200b and miR-495 genetic structure were associated with the
promotion of neovascularization in ischemia and myocardial
infarction mouse models (18).
However, although miR-200b and miR-495 are likely to
be involved in several diseases, including lung, prostate, breast,
colon and endometrial cancer, to the best of our knowledge, no
studies on the association of the polymorphisms of miR-200bT>C
and miR-495A>C with HTN. Therefore, the present study aimed to
test the hypothesis of the potential association between
miR-200bT>C and/or miR-495A>C polymorphisms and
susceptibility to HTN in a Korean cohort.
Materials and methods
Study population
For the present study, 232 patients with HTN,
including 190 male patients and 42 female patients (mean ± SD age,
47.35±8.23 years; age range, 31-67 years), and 247 control
individuals, including 132 men and 115 women (mean ± SD age,
48.93±9.98 years; age range, 32-80 years), were enrolled. The
patients with HTN, defined as systolic pressure >140 mmHg or
diastolic pressure >90 mmHg, were recruited from Jeju National
University Hospital (Jeju, South Korea) between January 2020 and
December 2020. Healthy control individuals had normal blood
pressure and were not on medication, and were enrolled during the
same period. Patients who were diagnosed with other chronic
diseases were excluded from the current study. The blood pressure
values of all participants were measured in a sitting position
after resting for ≥10 min. HTN would be diagnosed if three repeated
measurements of the systolic blood pressure (SBP) were >140 mmHg
and the diastolic blood pressure (DBP) were >90 mmHg. Risk
factors for hypertension were also measured. Body mass index (BMI)
was calculated from measurements of height and weight. Waist
circumference (WC) was measured using a non-stretchable fiber
measuring tape. For biochemical measurements, the levels of plasma
fasting blood glucose (FBG) (cat. no. GLU CN R1 991-18592, cat. no.
GLU CN R2 997-18692; FUJIFILM Wako Pure Chemical Corp.),
triglycerides (cat. no. TG CN R1 993-33192, cat. no. TG CN R2
999-33292; FUJIFILM Wako Pure Chemical Corp.) and high-density
lipoprotein-cholesterol (HDL-C) (cat. no. ML HDL-C S R1 55379, cat.
no. ML HDL-C S R2 55380; Minaris Medical Co., Ltd.) were measured
using commercially available enzymatic colorimetric tests in an
automated analyzer (TBA 200FR NEO; Canon Medical Systems
Corporation). Participants in the control group were randomly
selected to be subjected to a medical examination, which excluded
individuals with a history of chest pain, diabetes, and
hypertension. All enrolled subjects provided written informed
consent to participate in the present study, and ethical approval
was received from the Institutional Review Board of Jeju National
University Hospital (Republic of Korea; approval no. JEJUNUH
2020-07-005). The biospecimen and data used in the present study
were provided by the Biobank of Jeju National University Hospital,
a member of the South Korea Biobank Network supported by the
Ministry of Health and Welfare.
Genotyping
Genomic DNA was extracted from white blood cells in
3-ml blood samples taken twice 1 month after the date of HTN
diagnosis using a G-DEX blood extraction kit (iNtRON Biotechnology,
Inc.). Genotype analysis of miR-200bT>C (rs7549819) and
miR-495A>C (rs2281611) polymorphisms was conducted using PCR and
the restriction fragment length polymorphism technique, as
described previously (14).
Briefly, genomic DNA was amplified using PCR premix kits (cat. no.
STD02-P096; SolGent Co., Ltd.). PCR conditions were set at 94˚C for
5 min initial denaturation, followed by 32 cycles at 94˚C for 30
sec denaturation, 60˚C for 30 sec annealing and 72˚C for 1 min
extension, and a final step at 72˚C for 5 min. After restriction
digestions were performed at 37˚C for 17 h with AciI and
HincII restriction enzymes (New England BioLabs, Inc.), the
samples were finally subjected to electrophoresis for 40 min at 150
V on 3 or 4 % agarose gel containing ethidium bromide. The primer
sequences, PCR conditions, restriction enzymes and genotype
fragments used are summarized in Table
I. The following reference sequence shows the amplification
region for analyzing the miR-200b polymorphism (underlined bases
indicate the positions of the primers, where * is the AciI
cutting site):
5'-CTGAACCTGGCAGTGGGAGGCCGGCCCGTC*GCCGGGTGGGCTGGGAACTGTGTTTGGCCCCAGCTGCAAGGGACGAGTGCCGGGCACCTGCTGCCCTCCCCACGTGACCTGGCAGCCAGGAATGGAGCTGAAATTCAACTCTGCTCTGAACGGGAAGTCTTAGTTTCCTTAGCTCAGAAAACAGGTGAAATTGTGTTCCTGAAGCACTG-3'.
 | Table IDetails of the SNPs (miR-200bT>C
and miR-495A>C) included in the present study. |
Table I
Details of the SNPs (miR-200bT>C
and miR-495A>C) included in the present study.
| SNP | Primer
sequencea | PCR annealing
temperature (product length, bp) | Genotype (length,
bp) | Restriction
enzyme |
|---|
| miR-200bT>C | Forward primer
5'-CCTGAACCTGGCAGTGG-3'; Reverse primer
5'-CAGTGCTTCAGGAACACAATTT-3' | 60˚C (209) | TT (209); TC (209,
179, 30); CC (179, 30) | AciI |
| miR-495A>C | Forward primer
5'-GCATCAGGTAAGTTGGGTCA-3'; Reverse primer
5'-TTATCCGTGATGACTGTCCG-3' | 60˚C (94) | AA (94); AC (94, 74,
20); CC (74, 20) | HincII |
The following sequence shows the amplification
region for analyzing the miR-495 polymorphism (underlined text
indicate the positions of the primers, where * is the HincII
cutting site):
5'-GCATCAGGTAAGTTGGGTC*ACCCAGGGGAGGTGGGGGACATGCTTCGAAGGGCTGGGCTGACACTGAGCAGAGGCGGACAGTCATCACGGATAA-3'.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism version 4.0 (GraphPad Software Inc.; Dotmatics,
Inc.) and MedCalc version 12.7.1.0 (MedCalc Software Ltd.). To
compare the clinical characteristics of the study groups, the
unpaired samples t-test or Mann-Whitney test were used for
categorical variable and continuous variable analyses,
respectively. The adjusted odds ratios and 95% confidence intervals
for association with the miR-200bT>C (rs7549819) and
miR-495A>C (rs2281611) polymorphisms in terms hypertension risk
were calculated using multivariate logistic regression following
adjustment for age and sex. P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic information
The demographics of the HTN patient group and the
non-HTN group are summarized in Table
II. Compared with those in the control group, the HTN group had
significantly higher BMI, WC, SBP, DBP and FBG (all P<0.001). By
contrast, the HDL-C levels in the HTN group were significantly
lower compared with those in the control group (P<0.001).
However, these two groups did not significantly differ in terms of
age.
 | Table IIBaseline characteristics of the study
cohorta. |
Table II
Baseline characteristics of the study
cohorta.
| Clinicopathological
characteristic | Control
(n=247) | Hypertension
(n=232) | P-value |
|---|
| Age, year | 48.93±9.98 | 47.35±8.23 | 0.09 |
| Body mass index,
kg/m2 | 23.94±3.45 | 26.14±3.42 | <0.0001 |
| Waist
circumference, cm | 81.33±8.65 | 87.23±9.22 | <0.0001 |
| Systolic blood
pressure, mmHg | 118.51±11.24 | 138.43±13.44 | <0.0001 |
| Diastolic blood
pressure, mmHg | 71.36±8.63 | 88.55±8.85 | <0.0001 |
| Fasting blood
glucose, mg/dl | 88.18±8.91 | 95.48±14.18 | <0.0001 |
| Triglyceride,
mg/dl | 92.15±57.38 | 133.52±96.00 | <0.0001 |
| High-density
lipoprotein-cholesterol, mg/dl | 57.45±14.25 | 52.21±11.25 | <0.0001 |
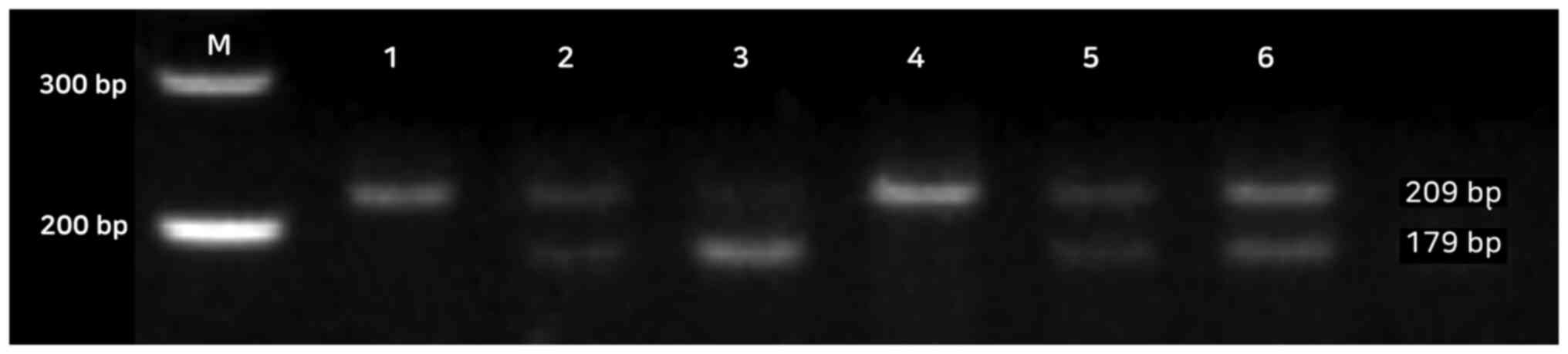
Detection of miR-200bT>C and
miR-495A>C polymorphisms
For miR-200bT>C gene polymorphism, the genotypes
were assessed as follow: A single 209 bp fragment for the wild-type
homozygous alleles (TT genotype), two fragments of 179 and 30 bp
for the mutated type (CC genotype), and three fragments of 209, 179
and 30 bp for the heterozygous alleles (TC genotype) (Fig. 1). The 30 bp fragment is not shown
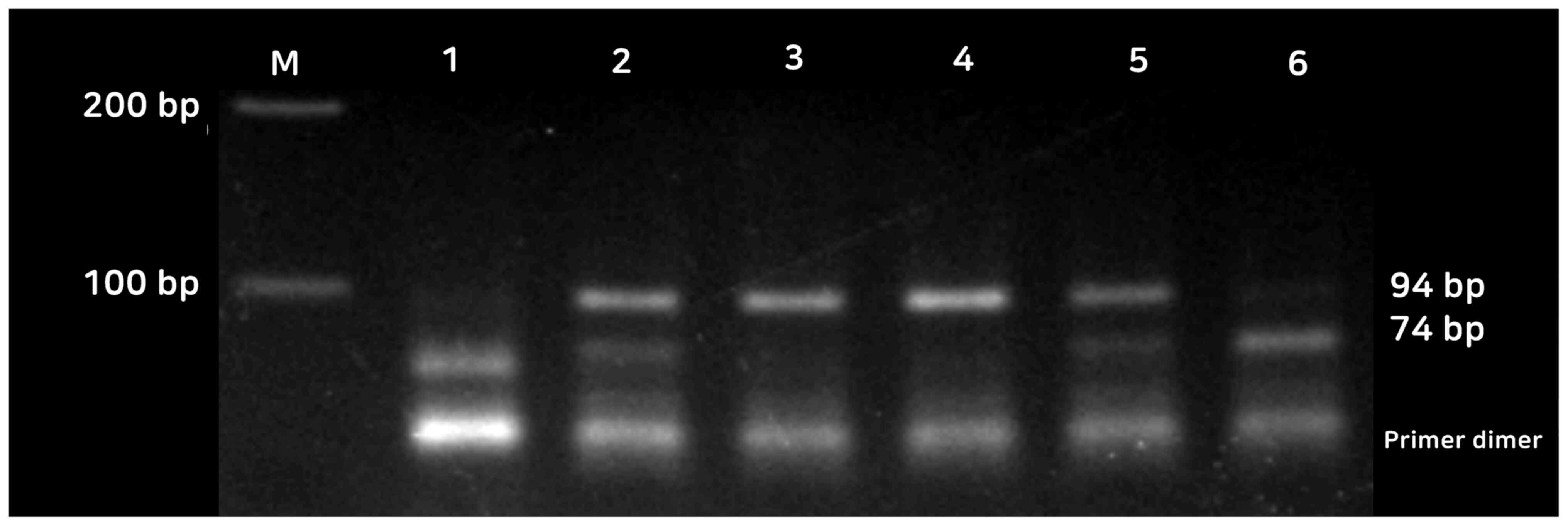
in Fig. 1. For miR-495A>C
polymorphism, homozygosity of the common allele (AA genotype)
revealed itself as a 94 bp band, while the homozygosity of the
variant allele (CC genotype) was represented by 74 and 20 bp bands.
The heterozygous alleles (AC genotype) were revealed by 94, 74 and
20 bp (Fig. 2). The 20 bp fragment
is not shown in Fig. 2. The lowest
bands in lanes 1-6 are primer dimers.
Association analysis
Table III
presents the comparison of genotype and allele frequencies with
regards to the miR-200bT>C and miR-495A>C polymorphisms
between the HTN and control groups. No significant difference in
the incidence in miR-200bT>C polymorphism could be observed
between the two groups. Specifically, incidence in the miR-200b TC
and CC genotypes were not significantly different, nor were the
dominant and recessive models. In addition, no significant
association could be detected between the HTN and control cohorts
in terms of the allelic distribution of this miR-200bT>C
polymorphism. By contrast, the miR-495 CC genotype (P<0.05) and
C allele (P<0.01) were found to be significantly associated with
the risk of HTN. There were no significant differences regarding
the miR-495 AC genotype or between the dominant and recessive
models.
 | Table IIIComparison of genotype and allele
frequencies for the SNPs between the HTN and control cohorts. |
Table III
Comparison of genotype and allele
frequencies for the SNPs between the HTN and control cohorts.
| A, miR-200b
T>C |
|---|
| Genotype | Control, n (%) | HTN, n (%) | Odds ratio (95%
CI)a | P-value |
|---|
| TT | 96 (38.9) | 81 (34.9) | 1.000
(reference) | |
| TC | 108 (43.7) | 114 (49.1) | 1.291
(0.850-1.960) | 0.231 |
| CC | 43 (17.4) | 37 (15.9) | 1.002
(0.575-1.748) | 0.994 |
| Dominant (TT vs.
TC+CC) | | | 1.209
(0.817-1.788) | 0.343 |
| Recessive (TT+TC
vs. CC) | | | 0.870
(0.525-1.442) | 0.589 |
| T allele | 300 (60.7) | 276 (59.5) | 1.000
(reference) | |
| C allele | 194 (39.3) | 188 (40.5) | 1.053
(0.813-1.364) | 0.694 |
| B,
miR-495A>C |
| Genotype | Control, n (%) | HTN, n (%) | Odds ratio (95%
CI)a | P-value |
| AA | 99 (40.1) | 71 (30.6) | 1.000
(reference) | |
| AC | 110 (44.5) | 106 (45.7) | 1.258
(0.814-1.946) | 0.301 |
| CC | 38 (15.4) | 55 (23.7) | 1.789
(1.033-3.098) | 0.038 |
| Dominant (AA vs. AC
+ CC) | | | 1.413
(0.945-2.111) | 0.092 |
| Recessive (AA + AC
vs. CC) | | | 1.606
(0.992-2.599) | 0.054 |
| A allele | 308 (62.3) | 248 (53.4) | 1.000
(reference) | |
| C allele | 186 (37.7) | 216 (46.6) | 1.442
(1.115-1.866) | 0.005 |
Combined genotype analysis
To investigate the association between genotype
combination and the risk of HTN, the combined genotypes of the
miR-200bT>C and miR-495A>C polymorphisms in the HTN and
control groups were then analyzed. According to the data shown
Table IV, the TC
miR-200bT>C/CC miR-495A>C (P<0.05) and CC
miR-200bT>C/CC miR-495A>C (P<0.05) combined genotypes were
found to be associated with a genetic susceptibility to HTN.
 | Table IVCombined genotype analysis for SNPs
in the HTN and control cohorts. |
Table IV
Combined genotype analysis for SNPs
in the HTN and control cohorts.
| Genotype
combination | |
|---|
| SNP 1
miR-200bT>C | SNP 2
miR-495A>C | Control, n (%) | HTN, n (%) | Odds ratio (95%
CI)a | P-value |
|---|
| TT | AA | 37 (15.0) | 21 (9.1) | 1.000
(reference) | |
| | AC | 39 (15.8) | 38 (16.4) | 1.701
(0.804-3.600) | 0.165 |
| | CC | 20 (8.1) | 22 (9.5) | 1.960
(0.808-4.756) | 0.137 |
| TC | AA | 40 (16.2) | 37 (15.9) | 1.838
(0.856-3.946) | 0.119 |
| | AC | 54 (21.9) | 53 (22.8) | 1.791
(0.865-3.709) | 0.116 |
| | CC | 14 (5.7) | 24 (10.3) | 2.772
(1.091-7.043) | 0.032a |
| CC | AA | 22 (8.9) | 13 (5.6) | 1.139
(0.395-3.290) | 0.810 |
| | AC | 17 (6.9) | 15 (6.5) | 1.504
(0.579-3.904) | 0.402 |
| | CC | 4 (1.6) | 9 (3.9) | 4.692
(1.067-20.630) | 0.041a |
To assess the combined effect of the miR-200bT>C
and miR-495A>C polymorphisms, allele combination analysis was
subsequently conducted (Table V).
The results demonstrated that the C-A allele combination frequency
of miR-200bT>C and miR-495A>C was significantly higher in the
HTN group compared with that in the control group (P<0.05).
 | Table VComparison of allele combination
between the HTN and control cohorts. |
Table V
Comparison of allele combination
between the HTN and control cohorts.
| Haplotype
(miR-200bT>C/miR-495A>C) | Overall(Control +
HTN) | Control
(n=548) | HTN (n=464) | Odds ratio (95%
CI) | P-value |
|---|
| T-C | 0.3274 | 0.3508 | 0.3014 | 1.000
(reference) | |
| T-A | 0.2739 | 0.2565 | 0.2934 | 1.331
(0.958-1.850) | 0.088 |
| C-C | 0.2530 | 0.2727 | 0.2330 | 0.994
(0.710-1.393) | 0.974 |
| C-A | 0.1458 | 0.1200 | 0.1721 | 1.669
(1.115-2.498) | 0.012 |
According to a stratified analysis divided by the
median values of the risk factors of the HTN group (Table VI), the recessive model (TT vs.
TC+CC) of the miR-200b rs7549819 polymorphism was associated with
an increased risk in the BMI (<28.12 kg/m2;
P<0.05) and FBG (<106.26 mg/dl; P<0.05) among the possible
risk variables tested. Furthermore, the miR-495 AC+CC vs. AA of the
rs2281611 recessive model was associated with increased HTN risk in
the HDL-C (<44.29 mg/dl; P<0.05) and SBP (≥132.67 mmHg;
P<0.05).
 | Table VIStratified analysis of miR-200bT>C
and miR-495A>C polymorphisms in the HTN group according to the
HTN risk factors. |
Table VI
Stratified analysis of miR-200bT>C
and miR-495A>C polymorphisms in the HTN group according to the
HTN risk factors.
| | miR-200b TC+CC | miR-495 AC+CC |
|---|
| Variable | Odds
ratioa (95%
CI) | P-value | Odds
ratioa (95%
CI) | P-value |
|---|
| Body mass index,
kg/m2 | | | | |
|
<28.12 | 1.627
(1.009-2.623) | 0.046 | 1.289
(0.807-2.057) | 0.288 |
|
≥28.12 | 0.864
(0.528-1.416) | 0.563 | 1.418
(0.829-2.423) | 0.202 |
| Waist
circumference, cm | | | | |
|
<93.03 | 1.326
(0.833-2.113) | 0.235 | 1.452
(0.904-2.331) | 0.123 |
|
≥93.03 | 1.077
(0.652-1.777) | 0.773 | 1.211
(0.712-2.059) | 0.480 |
| Systolic blood
pressure, mmHg | | | | |
|
<132.67 | 1.243
(0.765-2.019) | 0.380 | 0.974
(0.599-1.583) | 0.915 |
|
≥132.67 | 1.181
(0.735-1.896) | 0.492 | 1.939
(1.156-3.252) | 0.012 |
| Diastolic blood
pressure, mmHg | | | | |
|
<84.21 | 1.087
(0.648-1.824) | 0.752 | 1.235
(0.725-2.102) | 0.437 |
|
≥84.21 | 1.286
(0.815-2.028) | 0.280 | 1.449
(0.904-2.323) | 0.123 |
| Fasting blood
glucose, mg/dl | | | | |
|
<106.26 | 1.595
(1.002-2.540) | 0.049 | 1.518
(0.949-2.430) | 0.082 |
|
≥106.26 | 0.829
(0.499-1.375) | 0.467 | 1.156
(0.679-1.966) | 0.594 |
| Triglyceride,
mg/dl | | | | |
|
<216.26 | 1.358
(0.873-2.114) | 0.175 | 1.332
(0.853-2.080) | 0.207 |
|
≥216.26 | 0.990
(0.575-1.704) | 0.971 | 1.390
(0.771-2.508) | 0.274 |
| High-density
lipoprotein-cholesterol, mg/dl | | | | |
|
<44.29 | 1.015
(0.637-1.617) | 0.950 | 1.855
(1.101-3.126) | 0.020 |
|
≥44.29 | 1.549
(0.929-2.581) | 0.093 | 0.937
(0.577-1.523) | 0.793 |
Discussion
Previous studies have reported that miRNAs in the
human genome act as unknown multifactorial factors in the
development of various diseases, such as ischemic stroke and
thrombotic disorders, since they work as genetic modulators
affecting target gene expression (4,14).
This is due to miRNAs having been found to be associated with
several physiological and pathological processes, such as
oncogenesis and cardiovascular diseases, including coronary artery
disease, stroke, acute myocardial infarction and heart failure
(19). In particular, circulating
miRNAs targeting other miRNAs as intercellular signaling mediators,
for instance, circulating levels of miR-181a and miR-122 were
higher in hypertensive individuals than in controls, have been
proposed to serve a role in HTN according to tissue-based studies
(1,4,10).
According to results from the present study, miRNA polymorphisms
miR-200bT>C and miR-495A>C are likely to be associated with
the development of HTN in a cohort of Korean patients. Located in
the promoters of the respective miRNAs, these miR-200b and miR-495
SNPs can influence their expression levels, which can in turn
affect the expression levels of their target genes (20).
The results of the present study suggested an
association of the miR-495 CC genotype and C allele with an
increased risk of HTN. However, the risk of HTN was not associated
with the miR-200b TC and CC genotypes, dominant and recessive
models or the miR-200b variant T and C allele. miR-495 has been
previously shown to be associated with susceptibility to
blood-related diseases, such as pre-eclampsia (21), ischemic stroke and post-stroke
prognosis (14), by inhibiting
cell proliferation, migration, invasion and angiogenesis. Hu et
al (22) previously reported
that miR-495 leads to the pathogenesis of type 2 diabetes since it
could increase the blood glucose content and insulin resistance by
a targeted negative regulatory effect on fat mass and the
obesity-associated gene FTO, which encodings the
α-ketoglutarate-dependent dioxygenase FTO protein. Data from a
previous genome-wide study has shown that the expression of miR-495
targets KLHL5 expression, and is positively associated with changes
and subsequent organization involving platelet activation and
aggregation in platelet microtubules and cytoskeleton in humans
(14). In addition, several
reports have suggested that inhibiting the expression of miR-495
can improve hemodynamics and angiogenesis in hypertensive
conditions. Fu et al (23)
demonstrated that the inhibition of miR-495 can promote
hemodynamics and vascular remodeling, whose effects were involved
in increasing angiogenic transcription factor (vascular endothelial
zinc finger 1) and marked upregulation of angiogenic genes in
pulmonary hypertension according to the analysis on the biological
function of miR-495 in cultured pulmonary arterial endothelial
cells under hypoxic conditions. Furthermore, attenuation of miR-495
has been documented to promote PTEN expression to protect against
cardiomyocyte hypertrophy (24).
It has also been reported that myocardial infarction-associated
transcript, also known as retinal non-coding RNA 2 or Gomafu, can
promote the progression of acute myeloid leukemia through
suppression of miR-495 target genes (PBX3 and MEIS1) by not only
promoting cell proliferation and cell cycle progression, but also
decreasing apoptosis (25). In a
study investigating the regulatory role of miR-200b in cor
pulmonale model mice, miR-200b has been previously shown to
potentially suppress protein kinase C (PKC)-α expression, which can
modulate the proliferation and contraction of vascular smooth
muscle cells evoked by the expression of endothelin 1 through the
activation of protein kinases and transcription factors (11). The suppression of PKC-α can
attenuate pathological fibrosis and restore cardiac function
(26), indicating an association
between miR-200b and protection against heart failure.
Analysis of the genotype combination for SNPs in the
present study revealed that the TC/CC and CC/CC combined genotypes
of miR-200bT>C and miR-495A>C polymorphisms were associated
with susceptibility to HTN. Several previous studies have reported
that members of the miR-200 family can promote the pathogenesis of
atherosclerosis by targeting ZEB2 and RPS6KB1 gene expression to
promote foam-cell formation (18,27,28).
Although the present study did not cover the experimental evidence
at the cellular level, Piperigkou et al (28) demonstrated that the difference in
miRNA distributions implicated that miR-200b from five miRNA
sequences of the miR-200 family (miR-200a, miR-200b, miR-200c,
miR-141, and miR-429) might be involved in the regulation of
epithelial-to-mesenchymal transition and cell proliferation, which
can affect growth deformity and in turn the development of vascular
diseases. A previous study into the role of miR-200b in the
mechanism underlying stroke has reported that it can promote lipid
accumulation whilst inhibiting cholesterol efflux by downregulating
the expression of ATP binding cassette subfamily A member 1, an
integrated membrane protein (29).
When the expression of miR-200b was physiologically changed during
hypoxia through manipulation of the miRNA level using miR-200b
mimics and antagomirs, the expression of endothelial nitric oxide
synthase (eNOS) was reduced, which limited the bioavailability of
nitric oxide (NO) (18).
According to the haplotype results in the present
study, the allele combination of miR-200bT>C and miR-495A>C
in the haplotype of C-A significantly differed between the HTN and
control groups. The regulatory miRNA SNPs in the promoter regions
of miR-200b and miR-495 can affect the expression of their
corresponding mature miRNAs, which can cause a significant impact
on the expression of their target genes (14). The present study found interactive
effects between miR-200b and miR-495 according to the SNP-SNP
interaction analysis in a cohort of Korean patients. Through a
combinatorial gene-environment analysis, Kim et al (20) previously reported that the
combination of miR-495 CC genotype and low plasma folate
contributed to an increased risk of rectal cancer according to a
gene-environment combinatorial analysis. Using miRNA-miRNA
co-expression profiling, Gatsiou et al (30) also found that there were possible
associations between miRNA expression in platelets and their
reactivity. Knockdown experiments revealed that miR-200b and
miR-495 can target the expression of PRKAR2B and KLHL5,
respectively. The regulatory subunit encoded by PRKAR2B is
considered to control protein kinase A activity to regulate the
activation and aggregation of platelets. When cAMP concentration
increases, the catalytic subunit dissociates from the regulatory
complex, to phosphorylate its target substrates, which suppresses
platelet activation (31,32). The role of KLHL5, encoding a
Kelch-like protein that binds actin and serves as a signaling
molecule scaffold, in platelet function is reported to involve
cytoskeletal reorganization and subsequent cell shape changes,
which is an essential feature of platelet activation (16). According to these previous
findings, it is possible that both miR-200b and miR-495 are
involved in the activation of platelets, which have been reported
to robustly influence the pathophysiology of coronary artery
disease (33).
Based on the stratified analysis of the
miR-200bT>C and miR-495A>C polymorphisms according to the HTN
risk factors, the miR-200b TC+CC was associated with the BMI of
<28.12 kg/m2 and FBG levels of <106.26 mg/dl. By
contrast, the miR-495 AA+CC was associated with HDL-C levels
<44.29 mg/dl, whilst also being associated with SBP levels of
≥132.67 mmHg. These findings are similar to those of Motawi et
al (21), who previously
conducted a study on a cohort of Egyptian patients with
pre-eclampsia and found a significant positive association between
miR-495 expression and the parameters of systolic and diastolic
blood pressure, and cholesterol level. In addition, a negative
association was found with high density lipoprotein level and
miR-495 expression (21).
The present study has several limitations. The study
population was confined to a cohort of middle-aged Korean patients
who visited the Jeju National University Hospital. The subjects,
therefore, may not be representative of the general population from
various contexts including age, ethnicity, place of residence and
sampling period. For additional verification, it will be necessary
to conduct further studies on larger sample sizes containing more
diverse patient cohorts. The functional mechanisms regarding how
the miRNA polymorphisms may affect HTN remains unclear. Since the
present study did not assess the expression levels of the miRNAs of
interest on either molecular and functional levels, it is likely
that the miR-200bT>C and miR-495A>C polymorphisms are
associated with HTN risk by targeting the expression of genes
involved in HTN induction. However, this requires experimental
confirmation.
In conclusion, the present study investigated the
relationship between HTN susceptibility and miR-200bT>C and
miR-495A>C polymorphisms. It was found that the miR-495A>C
polymorphism may be associated with HTN susceptibility. Although
there have been studies describing the relationship between the
miR-200 family and the incidence of tumorigenesis and cancer, the
present study specifically demonstrated the association between the
miR-200bT>C and miR-495A>C polymorphisms with HTN risk; to
the best of our knowledge, this has not yet been reported.
Therefore, results of the present study may provide evidence that
miR-495A>C polymorphism is a potential candidate as a biomarker
for HTN diagnosis and prevention in Korean populations.
Acknowledgements
The authors would like to thank Dr Young Ree Kim
(Department of Laboratory Medicine, Jeju National University
Hospital, Jeju, Republic of Korea) for assistance in collecting
samples.
Funding
Funding: The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education, Science and Technology (grant
no. NRF-2017R1D1A3B03027985).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SHH designed the study, collected the medical data
and performed the genetic analysis. YMC was a major contributor in
analyzing and interpreting the data, and in writing the manuscript.
All authors read and approved the final manuscript. YMC and SHH
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
All enrolled subjects provided written informed
consent to participate in the present study. The study was approved
by the Institutional Review Board (approval no. JEJUNUH
2020-07-005) of Jeju National University Hospital (Jeju, Republic
of Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oh J, Matkovich SJ, Riek AE, Bindom SM,
Shao JS, Head RD, Barve RA, Sands MS, Carmeliet G, Osei-Owusu P, et
al: Macrophage secretion of miR-106b-5p causes renin-dependent
hypertension. Nat Commun. 11(4798)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sun L, Zhang J and Li Y: Chronic central
miR-29b antagonism alleviates angiotensin II-induced hypertension
and vascular endothelial dysfunction. Life Sci.
253(116862)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lionakis N, Mendrinos D, Sanidas E,
Favatas G and Georgopoulou M: Hypertension in the elderly. World J
Cardiol. 4:135–147. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Romaine SPR, Charchar FJ, Samani NJ and
Tomaszewski M: Circulating microRNAs and hypertension-from new
insights into blood pressure regulation to biomarkers of
cardiovascular risk. Curr Opin Pharmacol. 27:1–7. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yin J, Liu H, Huan L, Song S, Han L, Ren
F, Zhang Z, Zang Z, Zhang J and Wang S: Role of miR-128 in
hypertension-induced myocardial injury. Exp Ther Med. 14:2751–2756.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Korea Centers for Disease Control and
Prevention: Korea Health Statistics 2021: Korea national health and
nutrition examination survey (KNHANES Ⅷ-3). Korea Centers for
Disease Control and Prevention, Chungcheongbuk-do, 2022. https://knhanes.kdca.go.kr/knhanes/sub04/sub04_04_01.do
(In Korean).
|
|
7
|
Aquino-Jarquin G: Emerging role of
CRISPR/Cas9 technology for microRNAs editing in cancer research.
Cancer Res. 77:6812–6817. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cao Q, Lu K, Dai S, Hu Y and Fan W:
Clinicopathological and prognostic implications of the miR-200
family in patients with epithelial ovarian cancer. Int J Clin Exp
Pathol. 7:2392–2401. 2014.PubMed/NCBI
|
|
9
|
Zhao H, Guo Y, Sun Y, Zhang N and Wang X:
miR-181a/b-5p ameliorates inflammatory response in
monocrotaline-induced pulmonary arterial hypertension by targeting
endocan. J Cell Physiol. 235:4422–4433. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang HG, Zhang QJ, Li BW, Li LH, Song XH,
Xiong CM, Zou YB, Liu BY, Han JQ and Xiu RJ: The circulating level
of miR-122 is a potential risk factor for endothelial dysfunction
in young patients with essential hypertension. Hypertens Res.
43:511–517. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qian X, Zhao H and Feng Q: Invovlement of
miR-200b-PKCα signalling in pulmonary hypertension in cor
pulmonale model. Clin Exp Pharmacol Physiol. 4:478–484.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang C, Zhang X, Yin H, Du Z and Yang Z:
MiR-429/200a/200b negatively regulate Notch1 signaling pathway to
suppress CoCl2-induced apoptosis in PC12 cells. Toxicol
In Vitro. 65(104787)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Saadat YR, Poursief MM, Vahed SZ,
Barzegari A, Omidi Y and Barar J: Modulatory role of
vaginal-isolated lactococcus lactis on the expression of miR-21,
miR-200b, and TLR-4 in CAOV-4 cells and in silico revalidation.
Probiotics Antimicrob Proteins. 12:1083–1096. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim J, Choi GH, Ko KH, Kim JO, Oh SH, Park
YS, Kim OJ and Kim NK: Association of the single nucleotide
polymorphisms in mircoRNAs 130b, 200b, and 495 with ischemic stroke
susceptibility and post-stroke mortality. PLoS One.
11(e0162519)2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Opalinska JB, Bersenev A, Zhang Z,
Schmaier AA, Choi J, Yao Y, D'Souza J, Tong W and Weiss MJ:
MircoRNA expression in maturing murine megakaryocytes. Blood.
116:e128–e138. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nagalla S, Shaw C, Kong K, Kondkar AA,
Edelstein LC, Ma L, Chen J, McKnight GS, López JA, Yang L, et al:
Platelet microRNA-mRNA coexpression profiles correlate with
platelet reactivity. Blood. 117:5189–5197. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
National Library of Medicine, National
Center for Biotechnology Information: dbSNP, Short genetic
variations. National Library of Medicine, Bethesda, MD, 2022.
http://www.ncbi.nlm.nih.gov/SNP.
|
|
18
|
Qin S, Shen C, Tang W, Wang M, Lin Y, Wang
Z, Li Y, Zhang Z and Liu X: Impact of miR-200b and miR-495 variants
on the risk of large-artery atherosclerosis stroke. Metab Brain
Dis. 38:631–639. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Choi GH, Ko KH, Kim JO, Kim J, Oh SH, Han
IB, Cho KG, Kim OJ, Bae J and Kim NK: Association of miR-34a,
miR-130a, miR-150 and miR-155 polymorphisms with the risk of
ischemic stroke. Int J Mol Med. 38:345–356. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim EG, Kim JO, Park HS, Ryu CS, Oh J, Jun
HH, Kim JW and Kim NK: Genetic associations between the miRNA
polymorphisms miR-130b (rs373001), miR-200b (rs7549819), and
miR-495 (rs2281611) and colorectal cancer susceptibility. BMC
Cancer. 19(480)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Motawi TM, Sabry D, Maurice NW and Rizk
SM: Role of mesenchymal stem cells exosomes derived mircoRNAs;
miR-136. miR-494 and miR-495 in pre-eclampsia diagnosis and
evaluation. Arch Biochem Biophys. 659:13–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hu F, Tong J, Deng B, Zheng J and Lu C:
MiR-495 regulates macrophage M1/M2 polarization and insulin
resistance in high-fat diet-fed mice via targeting FTO. Pflugers
Arch. 471:1529–1537. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fu J, Bai P, Chen W, Yu T and Li F:
Inhibition of miR-495 improves both vascular remodeling and
angiogenesis in pulmonary hypertension. J Vasc Res. 56:97–106.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fu J, Chen Y and Li F: Attenuation of
microRNA-495 derepressed PTEN to effectively protect rat
cardiomyocytes from hypertrophy. Cardiology. 139:245–254.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang G, Li X, Song L, Pan H, Jiang J and
Sun L: Long noncoding RNA MIAT promotes the progression of acute
myeloid leukemia by negatively regulating miR-495. Leuk Res.
87(106265)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Boyle A, Kelly D, Zhang Y, Cox AJ, Gow RM,
Way K, Itescu S, Krum H and Gilbert RE: Inhibition of protein
kinase C reduces left ventricular fibrosis and dysfunction
following myocardial infarction. J Mol Cell Cardiol. 39:213–221.
2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jin HF, Wang JF, Song TT, Zhang J and Wang
L: MiR-200b inhibits tumor growth and chemoresistance via targeting
p70S6K1 in lung cancer. Front Oncol. 10(643)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Piperigkou Z, Franchi M, Riethmüller C,
Götte M and Karamanos NK: miR-200b restrains EMT and aggressiveness
and regulates matrix composition depending on ER status and
signaling in mammary cancer. Matrix Biol Plus.
6-7(100024)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wu YT, Li JB, Lin HQ, Zhang GX, Hong CM,
Li M, Guo ZJ and Yang YB: Inhibition of miR-200b-3p alleviates
lipid accumulation and promotes cholesterol efflux by targeting
ABCA1 in macrophage-derived foam cells. Exp Ther Med.
22(831)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gatsiou A, Boeckel JN, Randriamboavonjy V
and Stellos K: MicroRNAs in platelet biogenesis and function:
Implication in vascular homeostasis and inflammation. Currt Vasc
Pharmacol. 10:524–531. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gambaryan S, Kobsar A, Rukoyatkina N,
Herterich S, Geiger J, Smolenski A, Lohmann SM and Walter U:
Thrombin and collagen induce a feedback inhibitory signaling
pathway in platelets involving dissociation of the catalytic
subunit of protein kinase A from an NFκBNFkappaB-IkappaB complex. J
Biol Chem. 285:18352–18363. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Goode BL, Drubin DG and Barnes G:
Functional cooperation between the microtubule and actin
cytoskeletons. Curr Opin Cell Biol. 12:63–71. 2000.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pedersen OB, Hvas AM, Grove EL, Larsen SB,
Pasalic L, Kristensen SD and Nissen PH: Association of whole blood
microRNA expression with platelet function and turnover in patients
with coronary artery disease. Thromb Res. 211:98–105.
2022.PubMed/NCBI View Article : Google Scholar
|