Introduction
Intrauterine hypoxia is a relatively common
complication that can occur during pregnancy (1). It can adversely impact cardiac
myogenesis and increase the risk of heart disease in children
(1). In addition, the health
problems associated with intrauterine hypoxia (IUH) continue into
adulthood (2). Cardiac hypertrophy
is one such issue that can arise and increases the mortality rate
(3). However, the underlying
mechanism of cardiac hypertrophy development that is associated
with IHU remains unclear.
Protein kinase C β type isoform 2 (PKCβII) belongs
to the PKC family of serine- and threonine-specific protein kinases
that can be activated by calcium and the second messenger
diacylglycerol (4). PKCβII was
shown to regulate several different cellular processes, including
apoptosis induction, cell proliferation and metabolism, by
phosphorylating a wide variety of different target proteins
(5). Early growth response 1
(Egr-1) is one of these PKCβII downstream targets (6). Egr-1 is a member of the C2H2-type
zinc-finger family of proteins and functions as a transcription
regulator (7). It was demonstrated
that hypoxia/reoxygenation could induce cardiomyocyte injury
through the PKCβII/Egr-1 pathway (8). However, the potential effects of this
pathway on the induction of cardiac hypertrophy associated with IHU
remain unclear.
Astragaloside IV (AS-IV) is a bioactive, naturally
occurring compound that can be extracted from the plant
Astragalus membranaceus (8). It is applied as a traditional Chinese
medicine for the treatment of viral and bacterial infections,
inflammation and cancer (9).
Previous studies showed that AS-IV could serve a potential role in
protecting the heart against myocardial ischemia (10). The mechanism of action may involve
antioxidative and nitric oxide-inducing properties, reduction of
intracellular calcium levels and sarcoplasmic reticulum calcium
load and decreased lipid peroxidation (11). Furthermore, another previous study
indicated that AS-IV could attenuate neonatal rat myocardial
ischemia-reperfusion injury through the PKCβ/Egr-1 pathway
(12).
The present study investigated the effects of AS-IV
on the development of cardiac hypertrophy associated with IHU.
Materials and methods
Animals
AS-IV was purchased from Sigma-Aldrich (Merck KGaA)
and was dissolved in distilled water. The animal study protocol was
approved by the Medical Ethics Committee of Zhejiang Provincial
People's Hospital (approval no. 2020022). Experiments were
performed on 8-week-old male and female rats (body weight, 220-250
g) obtained from Beijing Vital River Laboratory Animal Technology
Co., Ltd. (Charles River Laboratories, Inc.). All of the animals
were kept in an environment with controlled temperature (22-25˚C)
and humidity (50-65%) and a 12-h light/dark cycle with free access
to food and water.
The adult parent rats were sacrificed by inhalation
of 4% isoflurane followed by cervical dislocation after delivering
the neonatal rats. Neonatal rats were sacrificed by inhalation of
4% isoflurane followed by cervical dislocation at the end of the
experiment.
The humane endpoints of the present study were: i)
Abnormal physical appearance, including abnormal posture, rough
coat, head tucked into the abdomen, exudate surrounding eyes and/or
nose, skin lesions and abnormal breathing; ii) ≥20% body weight
loss (as compared with the original body weight of the animal); and
iii) body condition score <2.0.
Experimental protocol
For the present study, 3-month-old male and female
rats were mated in one cage at a ratio of one male to two females.
The female rats were adjudged to be pregnant by checking if there
was a vaginal plug or if there was sperm in the vaginal secretion
smear of the female rat obtained the following morning. If a rat
was found to be pregnant at this stage, that was considered as day
0 of pregnancy.
In the hypoxia group, 10 pregnant rats were then
placed in a plexiglass chamber from day 15-21 of pregnancy with an
oxygen supply of 10% for 4 h every day. In the control group, 2
pregnant rats were placed in an atmosphere with a normal air
composition. The percentage of oxygen in the plexiglass chamber
(XBS-03; AIPU Laboratory; https://www.cn-aipu.com/laboratory.html) was monitored
using a continuous infusion of a mixture of nitrogen gas and air
with an oxygen analyzer (2KY-4F; AIPU Laboratory). During hypoxia,
arterial blood was collected every 60 min, which was immediately
used to measure oxygen partial pressure, blood oxygen saturation
and pH using a detection chip (ABL-9 blood gas analyzer; Radiometer
Medical ApS). The arterial partial pressure of oxygen in the
pregnant rats was maintained at 50-55 mmHg and the blood oxygen
saturation was maintained at 80-85% (13,14).
Subsequently, 100 µl arterial blood was collected from the tail
artery and the rats did not need to be anesthetized before this
step. By contrast, the pregnant rats in the control group were kept
at room temperature with an oxygen concentration of 21% until
natural delivery.
After birth, neonatal rats from the two treatment
groups were reared with their mothers and housed under room air
conditions. Neonatal rats born from the pregnant rats in the
control group constituted the control (Ctrl) group for the
subsequent experiments performed on neonatal rats.
After 4 weeks, the blood pressure of the rats was
measured and the 42 neonatal rats with hypertension were randomly
divided into four groups: i) IUH; ii) IUH + AS-IV (20 mg/kg); iii)
IUH + AS-IV (40 mg/kg); and iv) IUH + AS-IV (80 mg/kg). Rats in the
Ctrl and IUH groups were administered with distilled water (1
ml/kg/day), whilst the treatment groups received AS-IV 20, 40 or 80
mg/kg/day (oral gavage) for 5 days a week (administering AS-IV
continuously for 5 days, then suspended for 2 day) for 12 weeks
(15). All rats from different
groups were kept in the same environment with controlled
temperature (22-25˚C) and humidity (50-65%) and a 12-h light/dark
cycle and free access to food and water.
LV hemodynamics experiments
Rats were anaesthetized using isoflurane, 4% for
induction and 1.5% for maintenance of anesthesia for ~15-30 min by
following the guidelines of Animal Care Committee of The University
of British Columbia (16). A 2-F
microtip pressure-volume catheter (SPR-838; Millar, Inc.) was then
inserted into the right carotid artery and advanced into the
ascending aorta. After a 5-min stabilization period, the arterial
blood pressure was recorded before the catheter was advanced into
the left ventricle under pressure control. Using a special
pressure-volume analysis machine (cat. no. BL422I; Chengdu Techman
Co., Ltd.), heart rate, systolic and diastolic blood pressures (SBP
and DBP, respectively), mean arterial pressure, LV systolic
pressure (LVSP), LV end-diastolic pressure (LVEDP), stroke volume,
ejection fraction, cardiac output, the maximal slope of the
systolic pressure increment (dP/dt maximum), the maximal slope of
the diastolic pressure decrement (dP/dt minimum) and systemic
vascular resistance were all computed and calculated. To exclude
the influence of body weight differences, cardiac output and stroke
volume were normalized to body weight, yielding the cardiac and
stroke volume indices. Ventricular relaxation was assessed using
the time constant of LV pressure decay (τ), calculated using the
Glantz method (τ-g; regression of dP/dt vs. pressure) (17). LV pressure-volume relations were
assessed by transiently compressing the inferior vena cava. The
slope Emax of the LV end-systolic pressure-volume
relationship, pre-load recruitable stroke work and the slope of
dP/dt maximum/end-diastolic volume relationship were calculated as
load-independent indices of LV contractility. The slope of the
end-diastolic pressure-volume relationship was calculated as an
index of LV stiffness (18).
Subsequently, the heart was removed and weighed.
Histological analysis
For histological analyses, heart tissues were fixed
in 4% paraformaldehyde for 24-48 h at room temperature. The tissues
were subsequently dehydrated in a graded ethanol series, cleared in
toluene, embedded in paraffin and sliced into 5-µm sections. They
were then stained with hematoxylin and eosin (H&E; C0105S;
Beyotime Institute of Biotechnology) and images were captured to
assess the overall cardiac morphology using an optical microscope
(BX53; Olympus Corporation). Dr Baomei He (co-author) and the
technicians evaluated the histological preparations.
Western blot analysis
The heart tissues were dissected on ice and
transferred into round-bottomed microcentrifuge tubes, where they
were snap-frozen by immersing in liquid nitrogen. Ice-cold lysis
buffer (1 mg tissue/50 µl; RIPA; Beyotime Institute of
Biotechnology) was then added and the tissues were homogenized
using an electric homogenizer. An additional 50 µl lysis buffer was
added during homogenization. The lysates were then agitated at 4˚C
for 2 h and centrifuged at 16,000 x g for 20 min at 4˚C before the
supernatant was collected in a fresh tube and placed on ice. Total
protein was quantified using a Bradford assay kit (Beyotime
Institute of Biotechnology) before equivalent amounts of protein
per lane (50 mg) were separated using SDS-PAGE on a 10% gel. The
separated proteins were transferred onto PVDF membranes, which were
blocked in TBS-Tween 20 (0.1%) containing 5% (w/v) skimmed milk
(Beyotime Institute of Biotechnology) at 37˚C for 1 h. The
membranes were incubated overnight at 4˚C with primary rabbit
antibodies against Egr-1 (1:1,000; cat. no. sc-515830; Santa Cruz
Biotechnology, Inc.), ERK1/2 (1:1,000; cat. no. 9102; Cell
Signaling Technology, Inc.), phosphorylated (p-)-p44/42 ERK1/2
(1:1,000; cat. no. 9101; Cell Signaling Technology, Inc.),
anti-MyHC polyclonal antibody (1:1,000; cat. no. K107673P; Solarbio
Science & Technology Co., Ltd) and α-tubulin (1:1,000; H-300,
cat. no. sc-5546; Santa Cruz Biotechnology, Inc.). After washing,
the samples were incubated with HRP-labeled goat anti-rabbit IgG
(1:1,000; cat. no. A0208; Beyotime Institute of Biotechnology) or
HRP-labeled goat anti-mouse IgG (1:1,000; cat. no. A0216; Beyotime
Institute of Biotechnology) secondary antibodies at room
temperature for 1 h. The immunoreactive proteins were then
developed using an ultrasensitive ECL luminescent solution
(Proteintech Group, Inc.) and captured using the Amersham Imager
680 (GE Healthcare). α-tubulin was used as the loading control.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from the heart tissue
samples using TRIzol™ reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocols. Total
RNA was reverse transcribed into cDNA using a reverse transcription
kit (PrimeScript™ RT Reagent Kit; cat. no. RR037A; Takara
Biotechnology Co., Ltd.). The synthetized cDNA was amplified by
RT-qPCR using the following primers: Egr-1 forward,
5'-AACAACCCTACGAGCACCTG-3' and reverse, 5'-AAAGGGGTTCAGGCCACAAA-3';
and GAPDH forward, 5'-GCATCTTCTTGTGCAGTGCC-3' and reverse,
5'-GATGGTGATGGGTTTCCCGT-3'. The following thermocycling conditions
were used for qPCR: Initial denaturation at 95˚C for 30 sec,
followed by 40 cycles of 95˚C for 5 sec and 60˚C for 30 sec, 95˚C
for 15 sec and 60˚C for 60 sec, before finishing with 95˚C for 15
sec. The target gene expression was calculated using the
2-ΔΔCq method and normalized to the internal reference
gene GAPDH (19).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism version 8.0 (GraphPad Software; Dotmatics).
Statistical significance was analyzed using ANOVA followed by
Tukey's post hoc test. Data are presented as the mean ± standard
error of the mean. P<0.05 was considered to indicate a
statistically significant difference.
Results
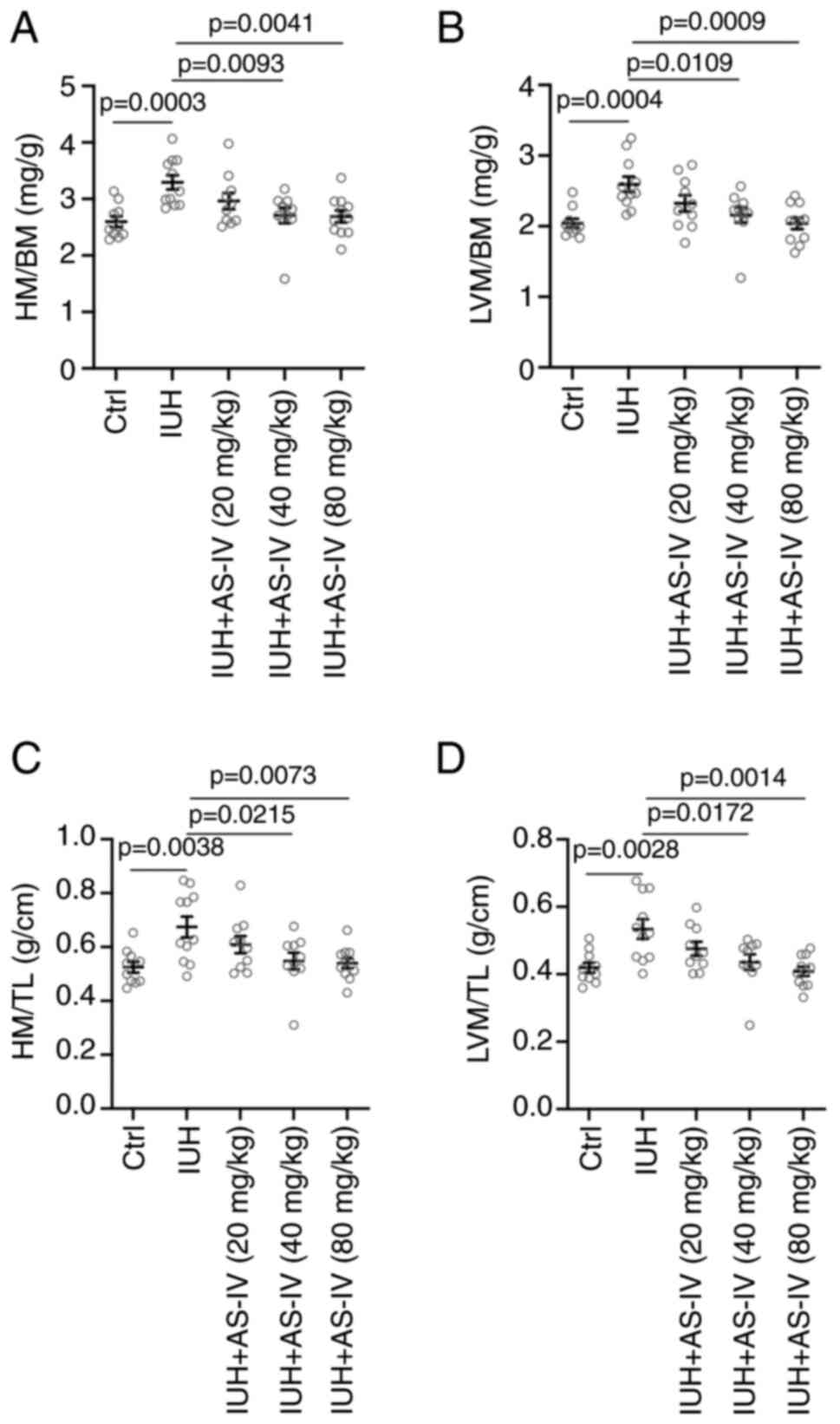
AS-IV prevents the increases in heart
mass (HM)/body weight (BW), LVM/BW, HM/TL and LVM/TL ratios in rats
born from mothers with IHU
Following the experimental procedure, the neonatal
rats were anaesthetized at 12 weeks after birth for the subsequent
tests. As shown in Fig. 1,
compared with those in the control group (where their mothers were
maintained at room temperature with an oxygen concentration of 21%
until natural delivery) rats born from mothers with IHU exhibited
the characteristics of cardiac hypertrophy, with higher HW/BW
(Fig. 1A), LVM/BM (Fig. 1B), HM/TL (Fig. 1C) and LVM/TL (Fig. 1D) ratios. However, AS-IV 40 and 80
mg/kg attenuated these characteristics of cardiac hypertrophy and
significantly decreased the HW/BW (Fig. 1A), LVM/BM (Fig. 1B), HM/TL (Fig. 1C) and LVM/TL (Fig. 1D) ratios compared with those in the
model group.
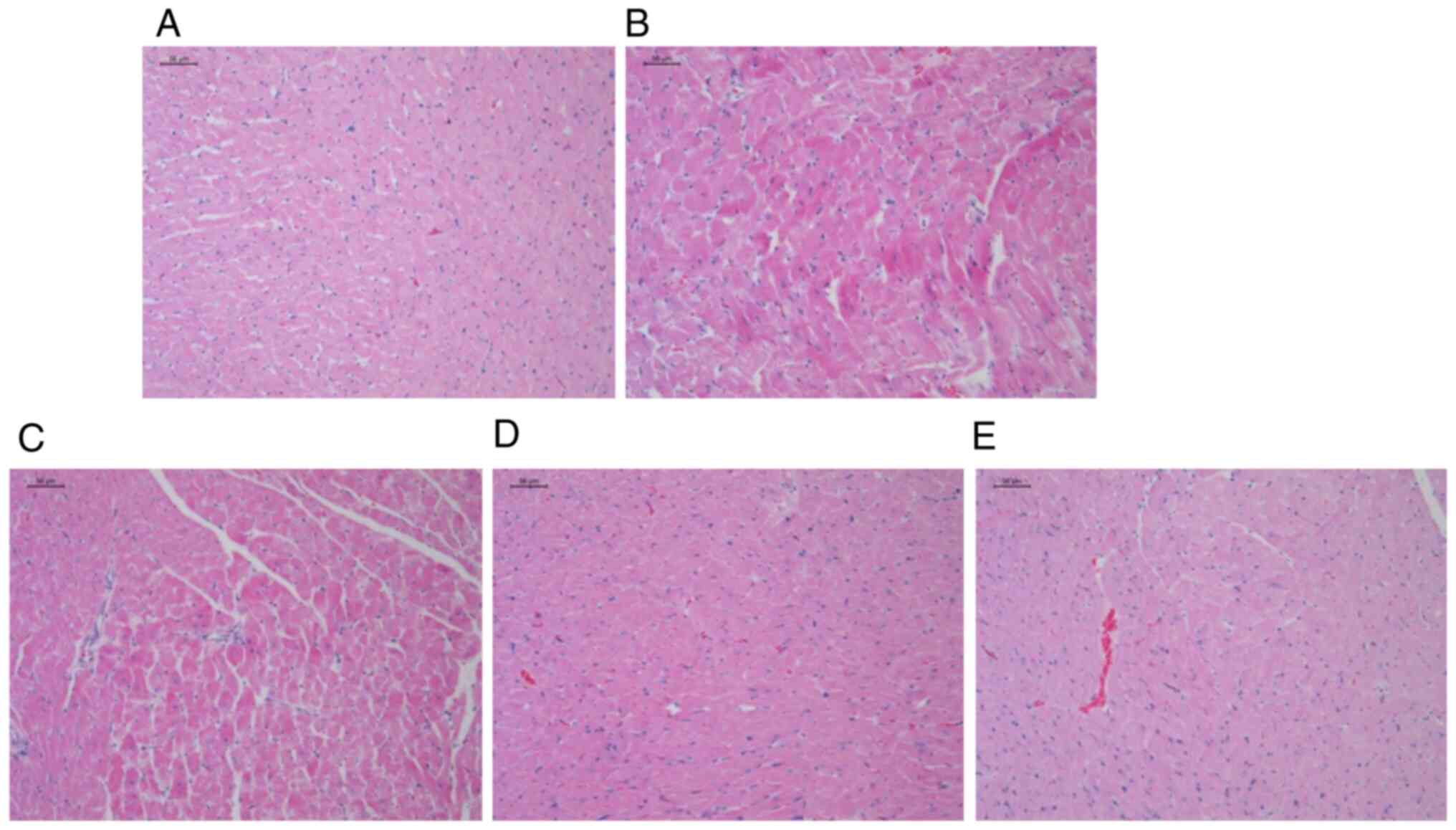
AS-IV prevents the induction of
myocardial damage in rats born from mothers with IHU
Microscopic investigation of the H&E-stained
heart tissues from the Ctrl group demonstrated typical features of
the normal endocardium and myocardium, with normal quantities and
distribution of the vascular endomysium among cardiac cells. By
contrast, heart tissues from rats in the IHU group showed focal
areas of sub-endocardium degeneration, which were mainly found in
the left ventricle. In addition, several focal areas of mononuclear
cellular infiltrations could be observed, where there was an
accumulation of fibrous tissues in the endomysium and increased
thickness of the myocardium in the left ventricle; however,
treatment with AS-IV 40 and 80 mg/kg prevented these morphometric
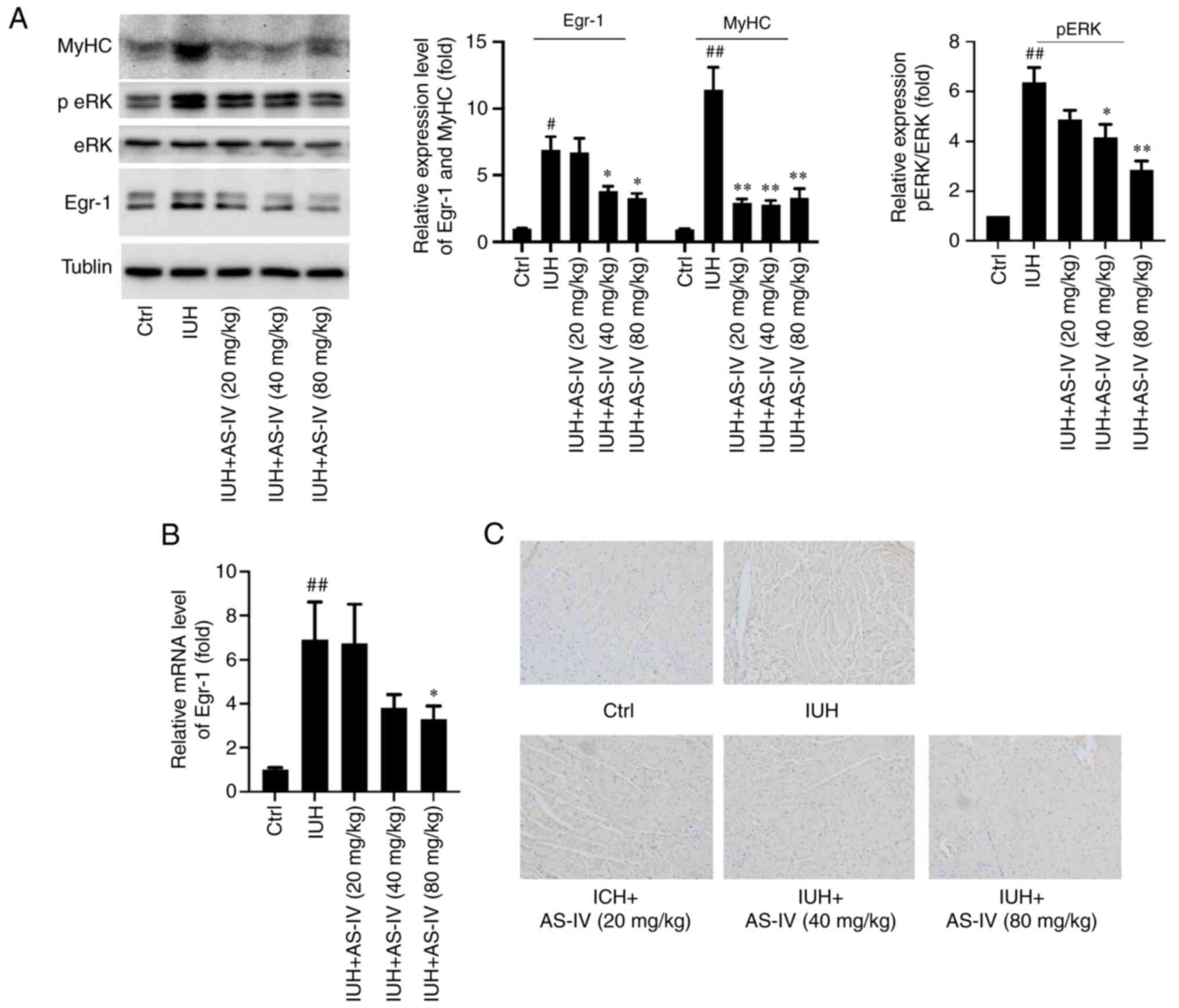
changes (Fig. 2). Meanwhile, the
marker of cardiac hypertrophy, β-myosin heavy chain (MyHC)
expression level was also increased in heart tissues from rats born
from mothers with IHU. AS-IV treatment decreased the upregulated
MyHC (Fig. 4A).
AS-IV prevents the changes in the LV
hemodynamics of rats born from mothers with IHU
Cardiac catheterization was performed at the end of
the treatment. During cardiac catheterization, increases in the
SBP, DBP, LVSP, LVEDP, dP/dt maximum and heart rate (HR) were
observed in the IHU group compared with those in the control group
(Fig. 3A-E). Treatment with 80
mg/kg AS-IV suppressed the increased SBP, DBP, LVSP, LVEDP, dP/dt
maximum and HR induced by IHU (Fig.
3A-F). However, there were no differences in all the cardiac
catheterization parameters between the AS-IV 20 mg/kg treatment
group and the IHU group. These results suggested that AS-IV could
prevent changes in the LV hemodynamics of rats born from mothers
with IHU in a dose-dependent manner.
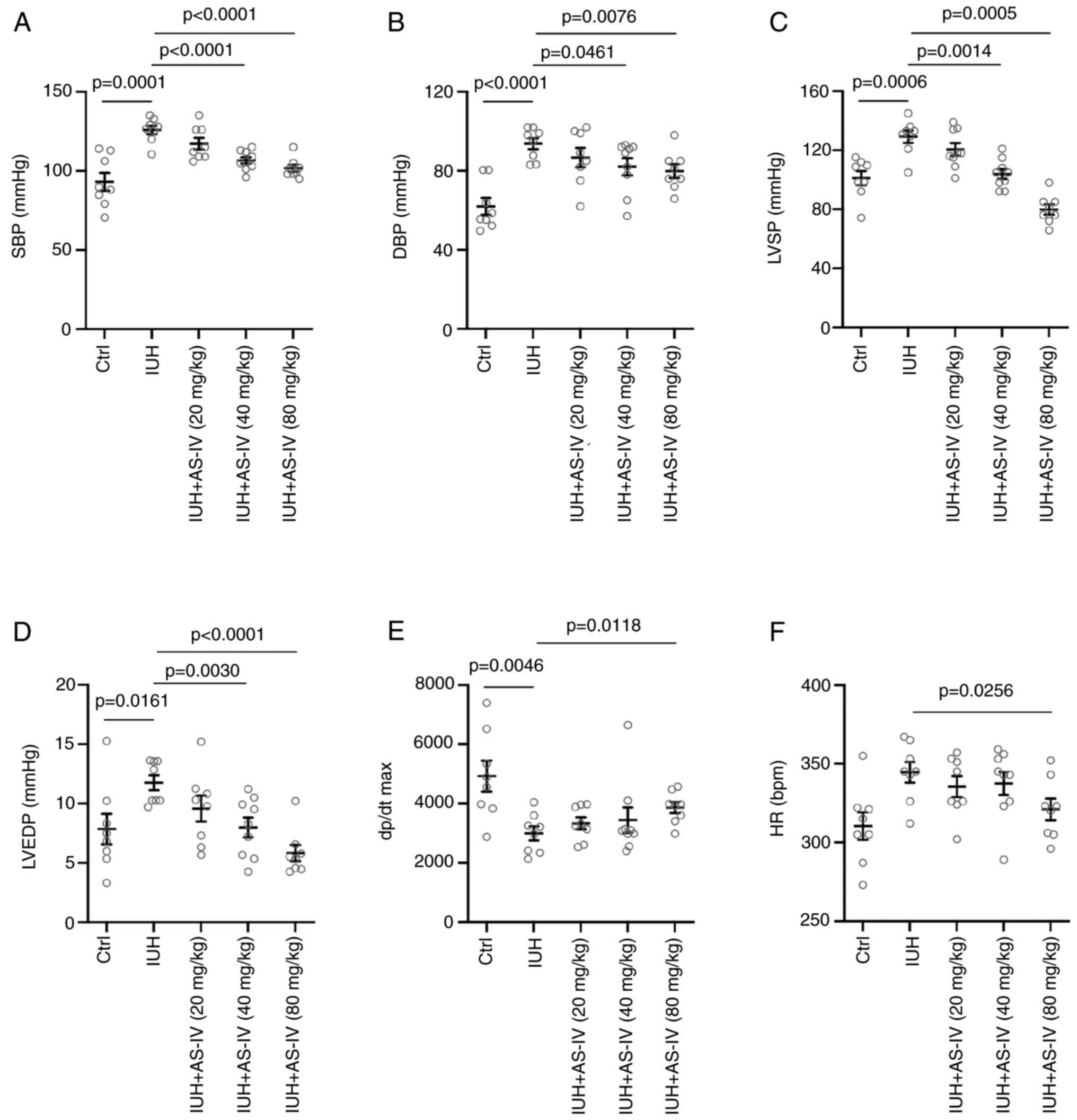 | Figure 3Astragaloside IV reverses cardiac
dysfunction associated with intrauterine hypoxia. Echocardiography
measurements of (A) Systolic blood pressure, (B) Diastolic blood
pressure, (C) Left ventricular systolic pressure, (D) Left
ventricular end-diastolic pressure, (E) Maximal slope of the
systolic pressure increment and (F) Heart rate. Statistical
analysis was performed using one-way ANOVA followed by Tukey's post
hoc test. IUH, intrauterine hypoxia; Ctrl, control group; AS-IV,
astragaloside IV; SBP, systolic blood pressure; DBP, diastolic
blood pressure; LVSP, left ventricular systolic pressure; LVEDP,
left ventricular end-diastolic pressure; dp/dt max, the maximal
slope of the systolic pressure increment; HR, heart rate. |
AS-IV prevents the activation of
PKCβII/Egr-1 signaling in rats born from mothers with IHU
PKCβII and Egr-1 were reported to be associated with
cardiomyocyte injury (20,21). Furthermore, AS-IV was shown to
exert suppressive effects on PKCβII and Egr-1 activity and mRNA
expression (12). In the present
study, ERK1/2 phosphorylation and Egr-1 protein expression were
markedly increased in rats born from mothers with IHU (Fig. 4A). Similarly, Egr-1 mRNA expression
was also significantly increased compared with that in the control
group (Fig. 4B). In addition,
using immunohistochemistry assays, p-ERK1/2 levels were increased
in the IHU group (Fig. 4C). In the
AS-IV treatment groups, these increased levels of ERK1/2
phosphorylation and Egr-1 expression were significantly decreased
compared with those in the IHU group (Fig. 4A-C).
Discussion
In the present study, treatment with 40 and 80 mg/kg
AS-IV significantly decreased the HW/BW, LVM/BM, HM/TL and LVM/TL
ratios in rats born from mothers with IHU. H&E staining
demonstrated that AS-IV 40 and 80 mg/kg prevented the pathological
changes induced by IHU. According to the LV hemodynamics
experiments, AS-IV 80 mg/kg treatment ameliorated the increased
SBP, DBP, LVSP, LVEDP, dP/dt maximum and HR induced by IHU.
Mechanistically, ERK1/2 phosphorylation and Egr-1 protein
expression levels were increased in rats born from mothers with
IHU, which were reversed by AS-IV treatment.
Intrauterine hypoxia was shown to occur when the
fetus is deprived of an adequate supply of oxygen, which may be due
to a variety of reasons, such as prolapse or occlusion of the
umbilical cord, placental infarction, maternal diabetes
(prepregnancy or gestational diabetes) and maternal smoking
(22). Although the role of oxygen
in the development of the fetus remains controversial, it was
proposed that the lack of oxygen in utero may be responsible
for increasing the risk of cardiovascular disorders in the
offspring (23). Data from the
present study indicated that after placing the pregnant rats in a
plexiglass chamber with a maternal oxygen supply of 10%, their
offspring exhibited pathological features of cardiac hypertrophy
when growing up. This suggested that IHU could increase the risk of
cardiac hypertrophy development in children and young adults.
AS-IV is the most abundant saponin and a marker
compound found in Astragali Radix, a Chinese herb prescribed
in compound formulas for medicinal purposes or dietary purposes as
a functional food in China and other eastern Asian countries
(19). Its use was associated with
its reported potent immune-promoting and anti-aging effects
(24). A recent pharmacological
study investigated the properties of AS-IV, including its
cardioprotective, angiogenic, hepatoprotective, neuroprotective,
anti-inflammatory and immunoregulatory effects (25). The present study revealed that
AS-IV could prevent the increases in the heart function indices, LV
hemodynamics parameters, pathological changes and MyHC levels
induced by IHU. Therefore, AS-IV may be a potential compound for
preventing IHU-mediated cardiac hypertrophy. However, the present
study had certain limitations, since it did not include the
echocardiography analysis of rat cardiac function and the analysis
of other markers of cardiac hypertrophy, such as atrial natriuretic
and brain natriuretic peptides; therefore, further research is
needed.
PKCβII isoform overexpression was shown to lead to
LV hypertrophy in mice (26,27).
The present immunochemistry assay showed that AS-IV treatment
reversed the increases in PKCβII expression in the heart tissues
mediated by IHU. It is also noteworthy that ERK1/2 and Egr-1 were
indicated as downstream targets of PKCβII (28). The present western blotting and
RT-qPCR results suggested that the p-ERK1/2 protein levels and
Egr-1 protein and mRNA levels were increased upon IHU induction. By
contrast, the AS-IV treatment groups reversed the increased ERK1/2
phosphorylation and Egr-1 expression. These results suggested that
the regulatory mechanism downstream of AS-IV may be mediated by
PKCβII/Egr-1 signaling.
In conclusion, the present study demonstrated that
AS-IV could attenuate cardiac hypertrophy in rats born from mothers
with IHU through the PKCβII/Egr-1 pathway, with the underlying
mechanism requiring further study.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by Zhejiang Provincial Natural
Science Foundation of China (grant no. LGD22H040004) and the
Traditional Chinese Medicine Technology Project of Zhejiang
Province (grant no. 2021ZA015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and HL performed data analysis and interpretation
and wrote the manuscript. YZ, MW, YD and BH conducted the
experiments. All authors have read and approved the final
manuscript. YZ and HL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The animal study protocol was approved by the
Medical Ethics Committee of Zhejiang Provincial People's Hospital
(approval no. 2020022).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Giussani DA: Breath of Life: Heart disease
link to developmental hypoxia. Circulation. 144:1429–1443.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rueda-Clausen CF, Morton JS and Davidge
ST: Effects of hypoxia-induced intrauterine growth restriction on
cardiopulmonary structure and function during adulthood. Cardiovasc
Res. 81:713–722. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang P, Ke J, Li Y, Huang L, Chen Z,
Huang X, Zhang L and Xiao D: Long-term exposure to high altitude
hypoxia during pregnancy increases fetal heart susceptibility to
ischemia/reperfusion injury and cardiac dysfunction. Int J Cardiol.
274:7–15. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kamp TJ and Hell JW: Regulation of cardiac
L-type calcium channels by protein kinase A and protein kinase C.
Circ Res. 87:1095–1102. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang HL, Hu BX, Li ZL, Du T, Shan JL, Ye
ZP, Peng XD, Li X, Huang Y, Zhu XY, et al: PKCβII phosphorylates
ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell
Biol. 24:88–98. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yan SF, Lu J, Zou YS, Kisiel W, Mackman N,
Leitges M, Steinberg S, Pinsky D and Stern D: Protein kinase C-beta
and oxygen deprivation. A novel Egr-1-dependent pathway for fibrin
deposition in hypoxemic vasculature. J Biol Chem. 275:11921–11928.
2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ramadas N, Rajaraman B, Kuppuswamy AA and
Vedantham S: Early growth response-1 (EGR-1)-a key player in
myocardial cell injury. Cardiovasc Hematol Agents Med Chem.
12:66–71. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang JZ, Cai CY, Zhang YM, Zheng JH, Chen
YC, Li WQ and Shi GG: N-n-Butyl haloperidol iodide protects against
hypoxia/reoxygenation-induced cardiomyocyte injury by modulating
protein kinase C activity. Biochem Pharmacol. 79:1428–1436.
2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang J, Wu C, Gao L, Du G and Qin X:
Astragaloside IV derived from Astragalus membranaceus: A research
review on the pharmacological effects. Adv Pharmacol. 87:89–112.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zheng Q, Zhu JZ, Bao XY, Zhu PC, Tong Q,
Huang YY, Zhang QH, Zhang KJ, Zheng GQ and Wang Y: A preclinical
systematic review and meta-analysis of astragaloside IV for
myocardial ischemia/reperfusion injury. Front Physiol.
9(795)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zang Y, Wan J, Zhang Z, Huang S, Liu X and
Zhang W: An updated role of astragaloside IV in heart failure.
Biomed Pharmacother. 126(110012)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang P, Zhou Y, Xia Q, Yao L and Chang X:
Astragaloside IV Regulates the PI3K/Akt/HO-1 signaling pathway and
inhibits H9c2 cardiomyocyte injury induced by
hypoxia-reoxygenation. Biol Pharm Bull. 42:721–727. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sun Y, Li L, Song J, Mao W, Xiao K and
Jiang C: Intrauterine hypoxia changed the colonization of the gut
microbiota in newborn rats. Front Pediatr. 9(675022)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chai N, Zhang H, Li L, Yu X, Liu Y, Lin Y,
Wang L, Yan J, Nikolaevna SE and Zhao Y: Spermidine prevents heart
injury in neonatal rats exposed to intrauterine hypoxia by
inhibiting oxidative stress and mitochondrial fragmentation. Oxid
Med Cell Longev. 2019(5406468)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tuerxun D, Aierken R, Zhang YM, Huang Y,
Sui S, Li XY, Abulikemu Z and Dilixiati N: Astragaloside IV
alleviates lipopolysaccharide-induced preeclampsia-like phenotypes
via suppressing the inflammatory responses. Kaohsiung J Med Sci.
37:236–244. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dai W, Shi J, Carreno J and Kloner RA:
Different effects of volatile and nonvolatile anesthetic agents on
long-term survival in an experimental model of hemorrhagic shock. J
Cardiovasc Pharmacol Ther. 25:346–353. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kalkhoran S and Glantz SA: E-cigarettes
and smoking cessation in real-world and clinical settings: A
systematic review and meta-analysis. Lancet Respir Med. 4:116–128.
2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Korkmaz-Icöz S, Lehner A, Li S, Vater A,
Radovits T, Brune M, Ruppert M, Sun X, Brlecic P, Zorn M, et al:
Left ventricular pressure-volume measurements and myocardial gene
expression profile in type 2 diabetic Goto-Kakizaki rats. Am J
Physiol Heart Circ Physiol. 311:H958–H971. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang S, Wang W, Li L, Wang T, Zhao Y, Lin
Y, Huang W, Wang Y and Huang Z: P2X7 receptor deficiency
ameliorates STZ-induced cardiac damage and remodeling through PKCβ
and ERK. Front Cell Dev Biol. 9(692028)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhou J, Yao Y, Zhang J, Wang Z, Zheng T,
Lu Y, Kong W and Zhao J: JNK-dependent phosphorylation and nuclear
translocation of EGR-1 promotes cardiomyocyte apoptosis. Apoptosis.
27:246–260. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tarvonen M, Hovi P, Sainio S, Vuorela P,
Andersson S and Teramo K: Intrapartal cardiotocographic patterns
and hypoxia-related perinatal outcomes in pregnancies complicated
by gestational diabetes mellitus. Acta Diabetol. 58:1563–1573.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Siu SC and Colman JM: Heart disease and
pregnancy. Heart. 85:710–715. 2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhou RN, Song YL, Ruan JQ, Wang YT and Yan
R: Pharmacokinetic evidence on the contribution of intestinal
bacterial conversion to beneficial effects of astragaloside IV, a
marker compound of astragali radix, in traditional oral use of the
herb. Drug Metab Pharmacokinet. 27:586–597. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Chen C, Yu LT, Cheng BR, Xu JL, Cai Y, Jin
JL, Feng RL, Xie L, Qu XY, Li D, et al: Promising therapeutic
candidate for myocardial ischemia/reperfusion injury: What are the
possible mechanisms and roles of phytochemicals? Front Cardiovasc
Med. 8(792592)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Xia Z, Kuo KH, Nagareddy PR, Wang F, Guo
Z, Guo T, Jiang J and McNeill JH: N-acetylcysteine attenuates
PKCbeta2 overexpression and myocardial hypertrophy in
streptozotocin-induced diabetic rats. Cardiovasc Res. 73:770–782.
2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wakasaki H, Koya D, Schoen FJ, Jirousek
MR, Ways DK, Hoit BD, Walsh RA and King GL: Targeted overexpression
of protein kinase C beta2 isoform in myocardium causes
cardiomyopathy. Proc Natl Acad Sci USA. 94:9320–9325.
1997.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Heo KS, Kim DU, Kim L, Nam M, Baek ST,
Park SK, Park Y, Myung CS, Hwang SO and Hoe KL: Activation of
PKCbeta(II) and PKCtheta is essential for LDL-induced cell
proliferation of human aortic smooth muscle cells via Gi-mediated
Erk1/2 activation and Egr-1 upregulation. Biochem Biophys Res
Commun. 368:126–131. 2008.PubMed/NCBI View Article : Google Scholar
|