Introduction
The pathophysiology of traumatic brain injury (TBI)
consists of primary brain injury and secondary brain injury. The
primary injury results from the mechanical damage caused by the
external force, whereas the secondary injury occurs due to various
factors such as hypoxia, elevated intracranial pressure, metabolic
disorder, brain edema, inflammation, etc. Therefore, the treatment
of TBI focuses on the prevention or mitigation of the damage from
the secondary injuries.
Astrocytes maintain the pH and the concentration of
ions such as sodium and potassium in the extracellular space,
supply oxygen and glucose to the nerve cells, and reabsorb the
glutamate, a neurotransmitter released from nerve terminal.
Consequently, astrocytes are essential for maintaining nerve cell
homeostasis. On the other hand, microglia, another glial cell, is
responsible for the inflammatory response in the brain. The concept
of gliotransmission proposed in 1990 suggests that astrocytes may
be crucial in signal transduction and constitute a long-distance
signaling system in the brain (1-3).
In response to an increase in the glutamate concentration in the
extracellular space, including due to stress or insult such as
ischemia or trauma, astrocytes increase the intracellular calcium
level and release gliotransmitters, such as adenosine triphosphate
(ATP), glutamate, and D-serine, into the extracellular space. When
the ATP binds to P2 receptor on the cell surface of adjacent
astrocytes in a paracrine manner, signal transduction such as
inositol trisphosphate pathway is activated and a large amount of
calcium is released into the cell, and then these adjacent
astrocytes are also activated as a group in a chain reaction
(4). These streams are called
‘calcium waves’ (5,6), and also activate the microglia
through the ATP receptor, namely P2 receptors. The P2 receptor is
an ATP-gated ion channel receptor, classified as P2X and P2Y
receptors. P2X receptor is a ligand-gated ion channel, and P2Y
receptor is a G protein-coupled receptor. The P2X4 and/or P2X7
receptors on microglia are responsible for receiving ATP signals
that will activate microglia to initiate several inflammatory
responses such as migration to the damaged area, phagocyte brain
debris, and release cytokines (7,8).
Massive release of cytokines will cause excessive inflammatory
response that results in secondary injury (9-13).
More recently, extracellular ATP concentration was
shown to immediately increase after cortical contusion injury (CCI)
in rats using microdialysis technique (9). The source of this rapid increase is
the damaged brain cells which leak intracellular ATP and subsequent
activation of neuronal cells and astrocytes that release ATP into
the extracellular space, but is significantly attenuated by the
selective P2Y1 receptor blocker, MRS2179, or store-operated calcium
channels. Furthermore, in our previous study (14,15),
in situ administration of MRS2179 successfully suppressed
microglial activation in a rat CCI model, which can inhibit the
initiation of inflammation. In this study, we focused on the ATP
receptors, P2X4 and P2X7, which are more involved in microglial
activation, and investigated whether antagonists of P2X4 and/or
P2X7 could be beneficial for the post-injury inflammatory response
that leads to secondary injury, a prognostic aggravation factor of
TBI (16,17). As mentioned above, astrocytes are
responsible for maintaining nerve cell homeostasis, so the
receptors of microglia were targeted without suppressing these
functions of astrocytes.
Materials and methods
Ethics
All experimental procedures were conducted according
to the animal experimental protocol manuals at Nihon University
School of Medicine. For example, rats with loss of postoperative
weight to below 80% of the preoperative weight, or rats which did
not exercise, eat or drink, or showing any signs of infection were
considered to be hyperinvasive and were euthanized. However, there
were no such rats in the present study.
Injury model
Male Sprague-Dawley rats (weighing 270 to 330 g)
were used for the CCI model as previously described (18). Briefly, after induction of general
anesthesia with 4% isoflurane in oxygen at 1.5 l/min, rats were
fixed in a stereotaxic frame and anesthesia was maintained with 2%
isoflurane during surgery. The body temperatures were maintained at
37.0˚C with a thermostatically controlled heating pad
monitored by a rectal probe. Local anesthesia was applied to the
scalp using subcutaneous injection of 0.1 ml 1% lidocaine (3.3
mg/kg), then the scalp was sterilized by repeated (three times)
cleaning with Betadine (Dynarex Corp., Orangeburg, NY) followed by
70% ethanol. A midline skin incision was then made and the
pericranium was spread bilaterally. A 6.0 mm diameter circular
craniotomy was made, 3.5 mm lateral (left) and 3.0 mm posterior
from the bregma. CCI was made using a 4.0 mm diameter flap-tip
impactor with fixed magnitude such as depth (2.0 mm), velocity (3.5
m/sec), and dwell time (100 msec). This intensity is known to
induce cortical brain contusion and delayed hippocampus cell death,
but will not mechanically damage the hippocampus (15,19).
Immediately after CCI, an Alzet Brain Infusion Kit 2 (Alzet 8663;
DURECT Corp., Cupertino, CA) was implanted 2.0 mm deep from the
brain surface at the center of contusion, connected with an osmotic
pump (Alzet Mini-Osmotic Pump Model 2001; DURECT Corp.) and
implanted subcutaneously. Surgical osmotic pump implantation was
completed within 15 min in all cases, and continuous drug
administration was initiated. After surgery, rats were placed in a
heated recovery box until ambulatory and then kept in individual
cages.
Treatment
The P2X4 receptor antagonist 5-BDBD (SML0450;
Sigma-Aldrich, St. Louis, MO), P2X7 receptor antagonist AZ11645373
(A7231; Sigma-Aldrich) or dimethyl sulfoxide (DMSO) as a control
drug was administered from the implanted osmotic pump. Both 5-BDBD
and AZ11645373 were prepared to 1.0 mM in DMSO and given at 1.0
µl/h flow rate. Drugs were injected for 3 days [1 µl/h (total 72
µl) over 3 days, containing 0.085 mg/kg of 5-BDBD or 0.111 mg/kg of
AZ11645373], because cytokine releases are prominent in this period
according to the previous reports (20). AZ11645373 is a highly selective and
potent antagonist of the human P2X7 receptor, but is less active in
rats (less than 50% inhibition at 10 mM), as evaluation using HEK
cells and THP-1 monocytes (human cells derived from monocytic
leukemia that differentiate into macrophages) found lower activity
against rat macrophages (21).
Macrophages invade the central nervous system after trauma-induced
disruption of the BBB (22). The
present study focused on microglia and used AZ11645373 with lower
activity on rat macrophages.
All animals were randomly assigned to five groups:
no surgical intervention (naïve group), DMSO treatment after CCI
(CCI-control group), 5-BDBD treatment after CCI (CCI-5-BDBD group),
CCI-AZ11645373 treatment after CCI (CCI-AZ11645373 group), and
5-BDBD and AZ11645373 treatment after CCI (CCI-5-BDBD + AZ11645373
group).
Immunohistological staining
Immunostaining was performed to evaluate the
expression of microglia. Three days after CCI, rats of the naïve
group (n=2), CCI-control group (n=2), CCI-5-BDBD group (n=2),
CCI-AZ11645373 group (n=2), and CCI-5-BDBD + AZ11645373 group (n=2)
were deeply anesthetized with intraperitoneal injection of lethal
dose pentobarbital (100 mg/kg). Brains were removed after
transcardiac perfusion of 200 ml physiological saline followed by
200 ml of 4% paraformaldehyde. After fixing in the same fixative
for 24 h, brains were immersed in 10, 20, and 30% gradient sucrose
solution (24 h each) for cryoprotection. Brains were flash frozen
and sliced to 20 µm thickness by a cryostat (CM1850; Leica
Biosystems, Nussloch, Germany) from anteroposterior 1.0 to 6.0 mm
relative to bregma. After blocking with 2% goat or horse serum,
sections were reacted with 20,000 time diluted anti-Iba-1 antibody
(019-19741; Wako Pure Chemical Industries, Osaka, Japan) in 4˚C,
overnight. The secondary antibody reaction was performed using
VECTASTAIN Elite ABC-HRP Kit (PK-6101, 6102; Vector Laboratories,
Newark, CA). After staining, the tissues were placed on a coated
slide glass, dehydrated and cover slipped. Resting (ramified) and
activated (amoeboid) microglia were distinguished according to
previously reported procedures (21).
Western blotting
Western blotting was performed to assess the
expression level of microglia and astrocytes. On the third day
after CCI, rats of the naïve group (n=4), CCI-control group (n=5),
CCI-5-BDBD group (n=7), CCI-AZ11645373 group (n=7), and CCI-5BDBD +
AZ11645373 group (n=6) were deeply anesthetized with 5% isoflurane
and decapitated. Initially, seven rats were assigned to each group,
but some rats died after anesthesia or after trauma (contusion),
resulting in uneven numbers of rats in each group. The numbers of
rats in the naïve group were minimized for ethical reasons. Brains
were removed and sliced to 2 mm thickness from anteroposterior 0.0
to 6.0 mm relative to bregma. Then the brains were dissected into 5
regions: peri-contusional cortex (cortex within 2 mm from the
contusion border), ipsilateral distal cortex (cortex other than
peri-contusional cortex), ipsilateral hippocampus, contralateral
cortex, and contralateral hippocampus. Total protein concentration
of the peri-contusional cortex was measured by RC DC Protein assay
Kit (5000122JA; Bio-Rad Laboratories, Hercules, CA). Samples were
prepared with Laemmli sample buffer (1610737; Bio-Rad Laboratories)
and beta-mercaptoethanol (1610710; Bio-Rad Laboratories). Then a 15
µg protein sample was loaded on to polyacrylamide gel (567-1095;
Bio-Rad Laboratories) and electrophoresis was performed at 120 V,
400 mA for 70 min. Bands were electrotransferred to polyvinylidene
fluoride membrane by iBlot Dry Blotting System (IB1001; Thermo
Fisher Scientific, Waltham, MA). Membrane was incubated overnight
in 4˚C with 1,000 time diluted anti-Iba-1 antibody (019-19741),
10,000 time diluted anti-glial fibrillary acidic protein (GFAP)
antibody (GTX108711; GeneTex, Irvine, CA), or 20,000 time diluted
anti-β-actin antibody (GTX109639; GeneTex). After anti-rabbit
immunoglobulin G secondary antibodies (AP182p; Millipore,
Burlington, MA) reaction, blotted protein bands were visualized
with using enhanced chemiluminescence. The expression levels were
measured and the results were displayed as target/β-actin.
Polymerase chain reaction (PCR)
PCR was performed to assess the levels of
interleukin-1beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis
factor alpha (TNFα). Tissue samples were collected simultaneously
with western blotting samples and quickly stored into
RNAlater stabilization solution (AM7024; Thermo Fisher
Scientific). The mRNA expressions of IL-1β, IL-6, and TNFα were
evaluated by reverse transcription PCR. RNA was purified with
RNeasy Lipid Tissue Mini Kit (74804; Qiagen, Venlo, The
Netherlands) and total RNA concentration was measured with
microvolume spectrophotometer (NanoDrop Lite; Thermo Fisher
Scientific). The mRNA was reversely transferred to cDNA using
SuperScript IV Reverse Transcriptase Kit (18090010; Thermo Fisher
Scientific) and used for PCR (T100 Thermal Cycler; Bio-Rad
Laboratories). Electrophoresis was performed with 2% agarose gel
containing Gel Red (41003; Biotium, Fremont, CA). Tracklt 50 bp DNA
Ladder (10488043; Thermo Fisher Scientific) was used as a marker.
The bands were measured and analyzed by a detector (ChemiDoc XRS;
Bio-Rad Laboratories). Glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) was used as an internal control. The following primers were
used in this experiment: IL-1β forward, 5'-GGATGATGACGACCTGC-3' and
reverse, 5'-CTTGTTGGCTTATGTTCTG-3'; IL-6 forward,
5'-AAGTCGGAGGCTTAATTACACATGT-3' and reverse,
5'-AAGTGCATCATCGTTGTTCATACA-3'; TNFα forward,
5'-CTTATCTACTCCCAGGTTCTCTTCAA-3' and reverse,
5'-GAGACTCCTCCCAGGTACATGG-3'; and GAPDH forward,
5'-AAGAAGGTGGTGAAGCAGGC-3' and reverse, 5'-TCCACCACCCTGTTGCTGTA-3'.
Annealing temperature and cycle number were: IL-1β, 54˚C, 32
cycles; IL-6, 62˚C, 32 cycles; TNFα, 57˚C, 31 cycles; GAPDH, 63˚C,
23 cycles. The expression levels are displayed as target/GAPDH.
Enzyme-linked immunosorbent assay
(ELISA)
In addition to PCR, ELISA was used for quantitative
analysis of IL-1β. ELISA with IL-1β Rat ELISA Kit (BMS630; Thermo
Fisher Scientific) was done according to the manufacturer's
protocol. After visualization, color intensity was read at 450
nm.
Behavioral tests
The following four behavioral tests were conducted
to evaluate the treatment effects (apart from the rats described
above). Rats only from the CCI-control group (n=5) and CCI-5-BDBD +
AZ11645373 group (n=6) underwent behavior testing, because the
CCI-5-BDBD + AZ11645373 group showed the most remarkable effects in
biological assay. Starting 7 days before the behavior test, rats
were handled for 15 min every day in order to get used to the
investigator. The osmotic pumps had an internal volume of 200 µl,
which allowed continuous administration for about one week. To
exclude factors such as anesthesia and brain injury associated with
removal, the pump was left in place for 28 days for the behavioral
tests.
i) Corner turn test
Corner turn test is used to detect unilateral
sensorimotor dysfunction in rodents (10,22)
and was conducted at 1, 3, 7, and 14 days after CCI. Briefly, two
boards (200 mm height and 300 mm length) were placed on a flat
floor with edge of two boards attached at a 30˚ angle. Rats are
placed between the two boards facing the corner. After entering
deep into the corner, rats will turn back to face the open side.
Naïve rats will turn either left or right with the same frequency,
but rats with hemiparesis preferentially turn toward the
non-impaired side. Trials were recorded for 10 times and the ratio
of right turns per all turns was calculated.
ii) Cylinder test
Cylinder test is another test to detect unilateral
sensorimotor dysfunction in rodents (10,23).
This test was conducted at 1, 3, 8, 15, and 28 days after CCI.
Briefly, rats placed in the transparent cylinder will explore by
rearing and touching the cylinder wall with forelimb paws for
postural support. Hemiparesis rats rely mainly on the unaffected
forelimb paw to support posture, resulting in less contact with the
affected paw. During a 5-min trial, number of touches with right,
left, or both forelimbs were counted. Asymmetry score was
calculated as: (right touch/total touch)-(left touch/total
touch).
iii) Grid walking test
Grid walking test is used to evaluate sensorimotor
coordination of four limbs in various rodent disease models
(24). This test was conducted at
1, 3, 8, and 15 days after CCI. The rat was placed on a 20x20 mm
wire mesh grid and freely walked for 2 min. Number of foot faults,
a paw slipping through an opening of the grid, was counted. The
foot fault index was calculated as: (contralateral
faults-ipsilateral faults)/(total steps). Score ‘0’ represents no
asymmetry in sensorimotor coordination and positive score
represents unilateral sensorimotor deficit due to the brain
injury.
iv) Plus maze test
Plus maze test is used to evaluate the spatial
memory function (25,26). Plus maze test was conducted at 14
and 28 days after CCI. The maze was made of four black Plexiglas
arms (120 mm height wall, 250 mm length, and 100 mm width)
positioned in a plus shape and connected to an open central space
(250 mm diameter). Rats were placed into the center of the maze and
allowed to explore freely for 20 min. Rats will normally
spontaneously alternate all arms of the maze using spatial working
memory to retain knowledge of previously entered arms of the maze.
The number of arm entries and sequence of entries into each arm
were recorded. Arms were considered ‘entered’ when both hind limbs
passed the halfway line of the arm. Spatial working memory was
evaluated by calculating the percent four/five alternation score
(25,26).
Statistical analysis
SPSS Statistics (version 21; IBM, Armonk, NY) was
used for statistical analysis. One way analysis of variance (ANOVA)
was used for comparisons between three or more groups. Subsequent
tests with the Tukey method were performed when significant
differences were found in the factors of ANOVA. All tests were
two-tailed, and P-values <0.05 were considered significant. All
data are indicated as mean ± standard deviation.
Results
Microglia
Iba-1-positive cells, indicating the microglia, were
observed in all regions of the brain including the cerebral cortex
and hippocampus in the naïve group. In contrast, Iba-1-positive
microglia were also detected in both the ipsilateral and
contralateral cortices and the hippocampus 3 days after the CCI in
the CCI-control group. However, based on the morphological features
(27), Iba-1-positive cells were
mostly activated (amoeboid) microglia (enlarged cell body and
shorter protrusions) in the ipsilateral cortex and ipsilateral
hippocampus, and mostly resting (ramified) microglia (small
cytoplasm with elongated protrusions) in the contralateral cortex.
On the other hand, Iba-1-positive cells were mostly morphologically
resting ramified microglia in both the contralateral cortex and the
ipsilateral cortex and hippocampus in the CCI-5-BDBD,
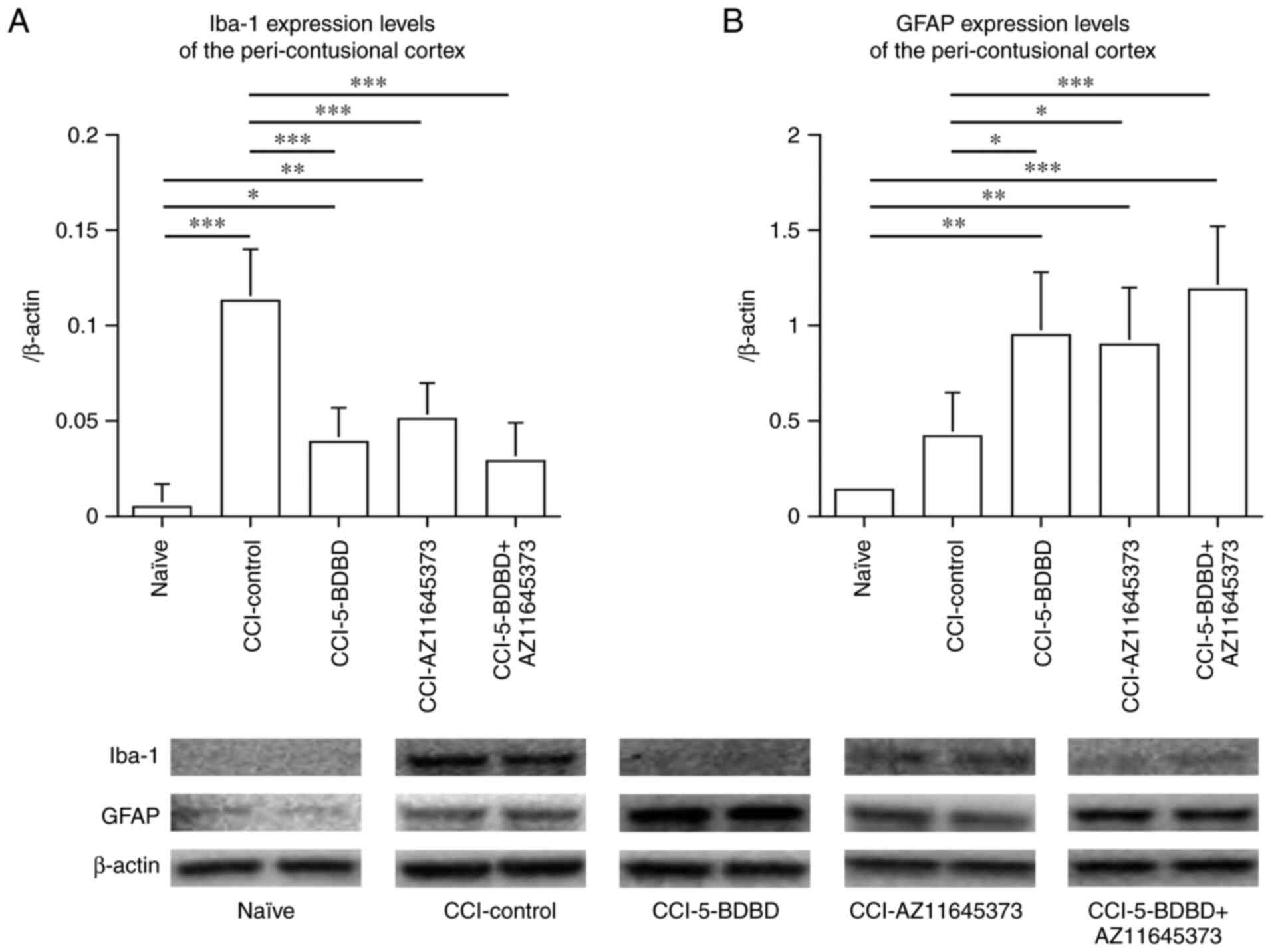
CCI-AZ11645373, and CCI-5-BDBD + AZ11645373 groups (Figs. 1 and 2). The Iba-1 expression level in the
peri-contusional cortex as quantified by western blotting was
significantly increased in the CCI-control group (0.113±0.027)
compared to the naïve groups (0.005±0.012) (P<0.001) 3 days
after the CCI. In contrast, the Iba-1 expression levels in the
peri-contusional cortex of the CCI-5-BDBD, CCI-AZ11645373, and
CCI-5-BDBD + AZ11645373 groups were 0.039±0.018, 0.051±0.019, and
0.029±0.020, respectively. These expression levels were
significantly lower compared to those in the CCI-control group
(P<0.001, P<0.001, and P<0.001, respectively) (Fig. 3A).
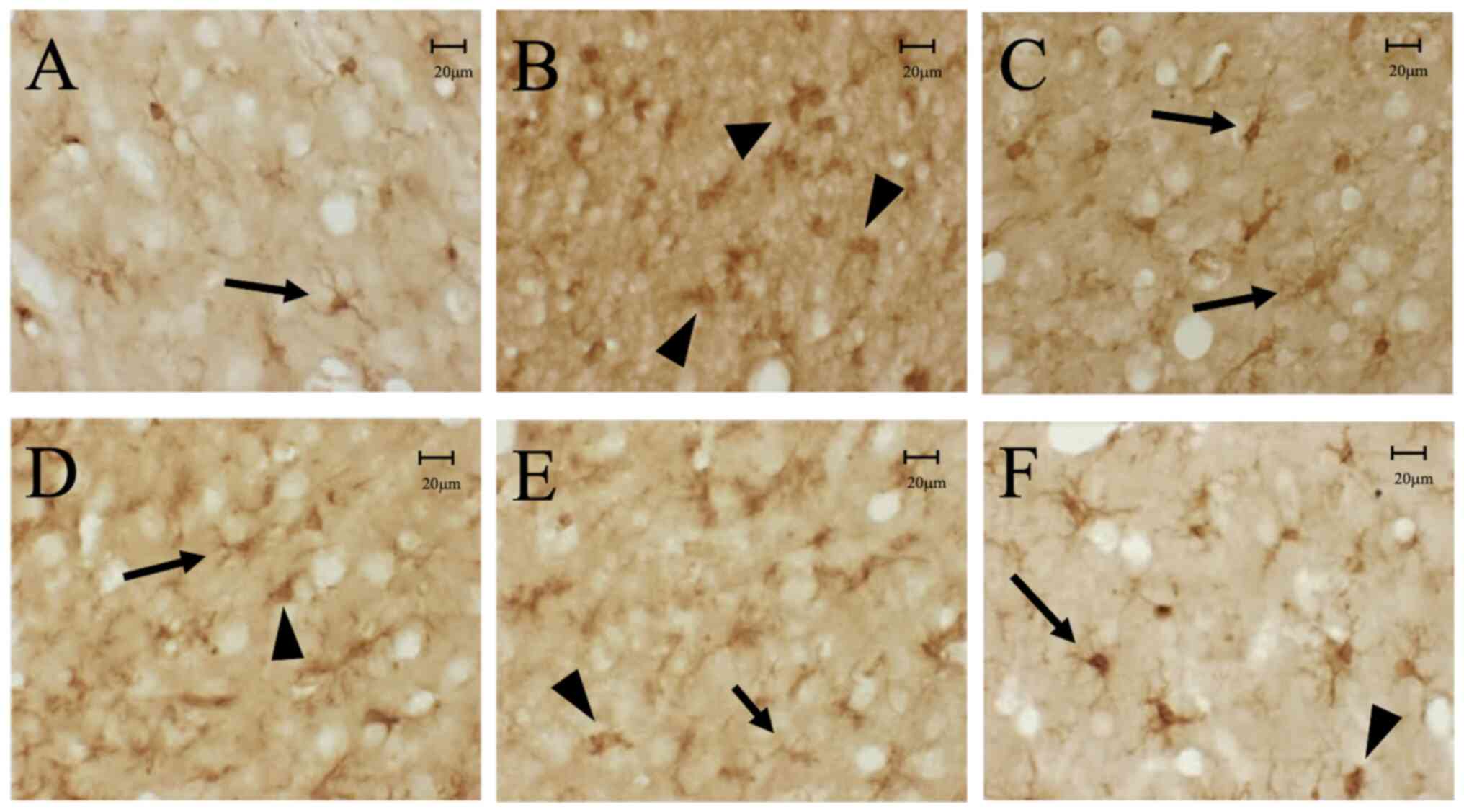 | Figure 1(A) Iba-1-positive cells were
observed throughout the brain including the cerebral cortex and
hippocampus in the naïve group, with morphology of resting
(ramified) microglia. (B) In the CCI-control group, increased
numbers of Iba-1-positive cells in the peri-contusional cortex and
ipsilateral distal cortex were observed, with morphology of
activated (amoeboid) microglia, (C) whereas in the contralateral
CCI cortex, morphology was resting (ramified) microglia 3 days
after the CCI. By contrast, in the (D) CCI-5-BDBD, (E)
CCI-AZ11645373 (F) and CCI-5-BDBD + AZ11645373 groups, fewer
Iba-1-positive cells were found in the peri-contusional cortex and
ipsilateral distal cortex with morphology of amoeboid microglia,
compared with the CCI-control group. Resting microglia are
indicated with arrows and activated microglia are indicated with
arrowheads. Scale bar=20 µm. Iba-1, ionized calcium-binding adaptor
molecule 1; CCI, cortical contusion injury. |
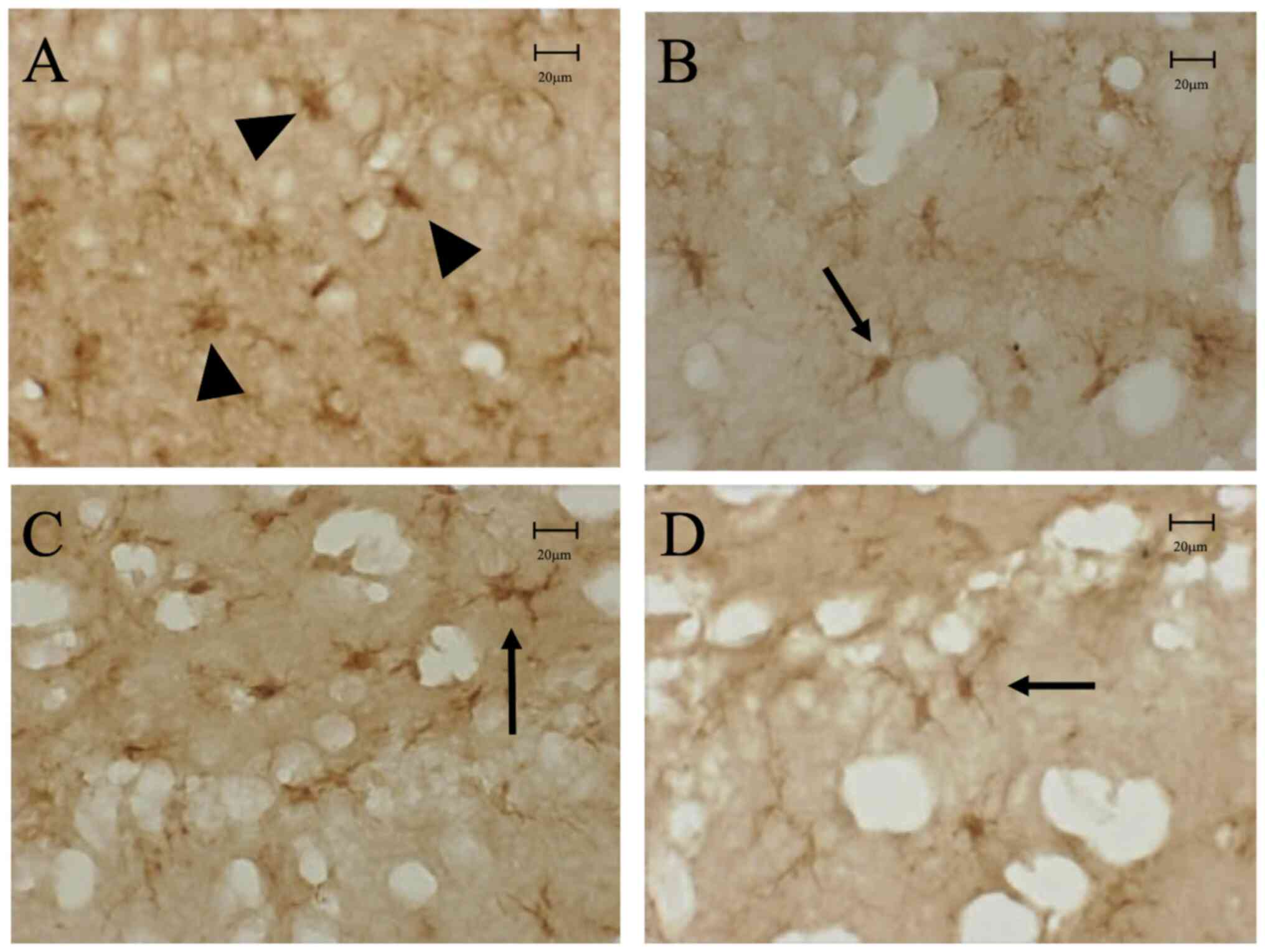 | Figure 2(A) In the ipsilateral CCI
hippocampus in the CCI-control group, despite the absence of direct
damage caused by CCI, the number of Iba-1-positive microglia had
increased with morphology of activated (amoeboid) form, not resting
(ramified) form, 3 days after the CCI. In contrast, in the (B)
CCI-5-BDBD, (C) CCI-AZ11645373, (D) CCI-5-BDBD + AZ11645373 groups,
Iba-1-positive microglia were present in the ipsilateral
hippocampus, with a lower number of amoeboid microglia compared
with the CCI-control group. Resting microglia are indicated with
arrows and activated microglia are indicated with arrowheads. Scale
bar=20 µm. Iba-1, ionized calcium-binding adaptor molecule 1; CCI,
cortical contusion injury. |
Astrocyte
The GFAP expression level, indicating astrocytes, of
the peri-contusional cortex was also quantified by western
blotting. The GFAP level was not significantly increased in the
CCI-control group (0.422±0.225) compared to the naïve group
(0.143±0.010). In contrast, the GFAP expression levels of the
peri-contusional cortex in the CCI-5-BDBD, CCI-AZ11645373, and
CCI-5-BDBD + AZ11645373 groups were 0.949±0.332, 0.895±0.300, and
1.185±0.339, respectively. These expression levels were
significantly higher compared to those in the CCI-control group
(P=0.029, P=0.042, and P<0.001, respectively) (Fig. 3B).
Expression of inflammatory
factors
The mRNA levels of IL-1β, IL-6, and TNFα as
inflammatory cytokines were measured by PCR in the peri-contusional
cortex, the ipsilateral distal cortex, and the ipsilateral
hippocampus 3 days after the CCI. In addition, IL-1β in the
peri-contusional cortex was quantified by ELISA 3 days after the
CCI. As shown in Fig. 4, mRNA
expressions of IL-1β (0.914±0.274), IL-6 (1.670±0.691), and TNFα
(1.001±0.030) in the peri-contusional cortex were high in the
CCI-control group compared to the naïve group (IL-1β: 0.146±0.012,
P=0.011; IL-6: 0.451±0.085, P=0.005; TNFα: 0.414±0.077,
P<0.001). In contrast, 5-BDBD treatment decreased expression of
IL-6 (0.838±0.194, P=0.010), and 5-BDBD, AZ11645373, and 5-BDBD +
AZ11645373 treatment decreased expression of TNFα (0.693±0.118,
P=0.004; 0.740±0.079, P=0.013; and 0.603±0.136, P<0.001,
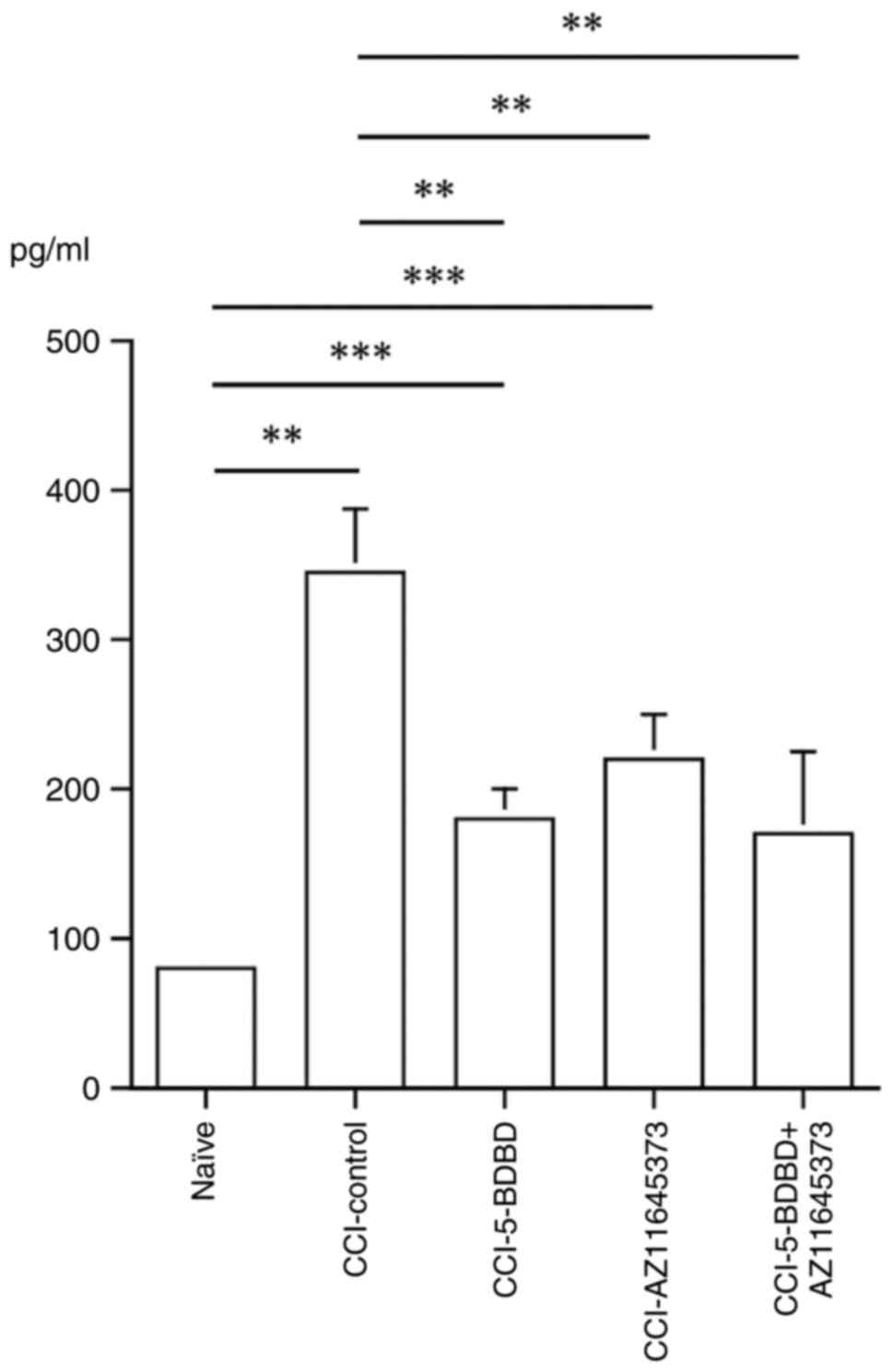
respectively). As shown in Fig. 5,
expression of IL-1β was significantly increased in the CCI-control
group (345.8±42.1 pg/ml) compared with the naïve group (80.4±7.3
pg/ml, P=0.001). Expression of IL-1β was significantly suppressed
in the CCI-5-BDBD (178.8±22.4 pg/ml, P=0.002), CCI-AZ11645373
(221.1±30.1 pg/ml, P=0.005), and CCI-5-BDBD + AZ11645373 groups
(169.4±54.9 pg/ml, P=0.001).
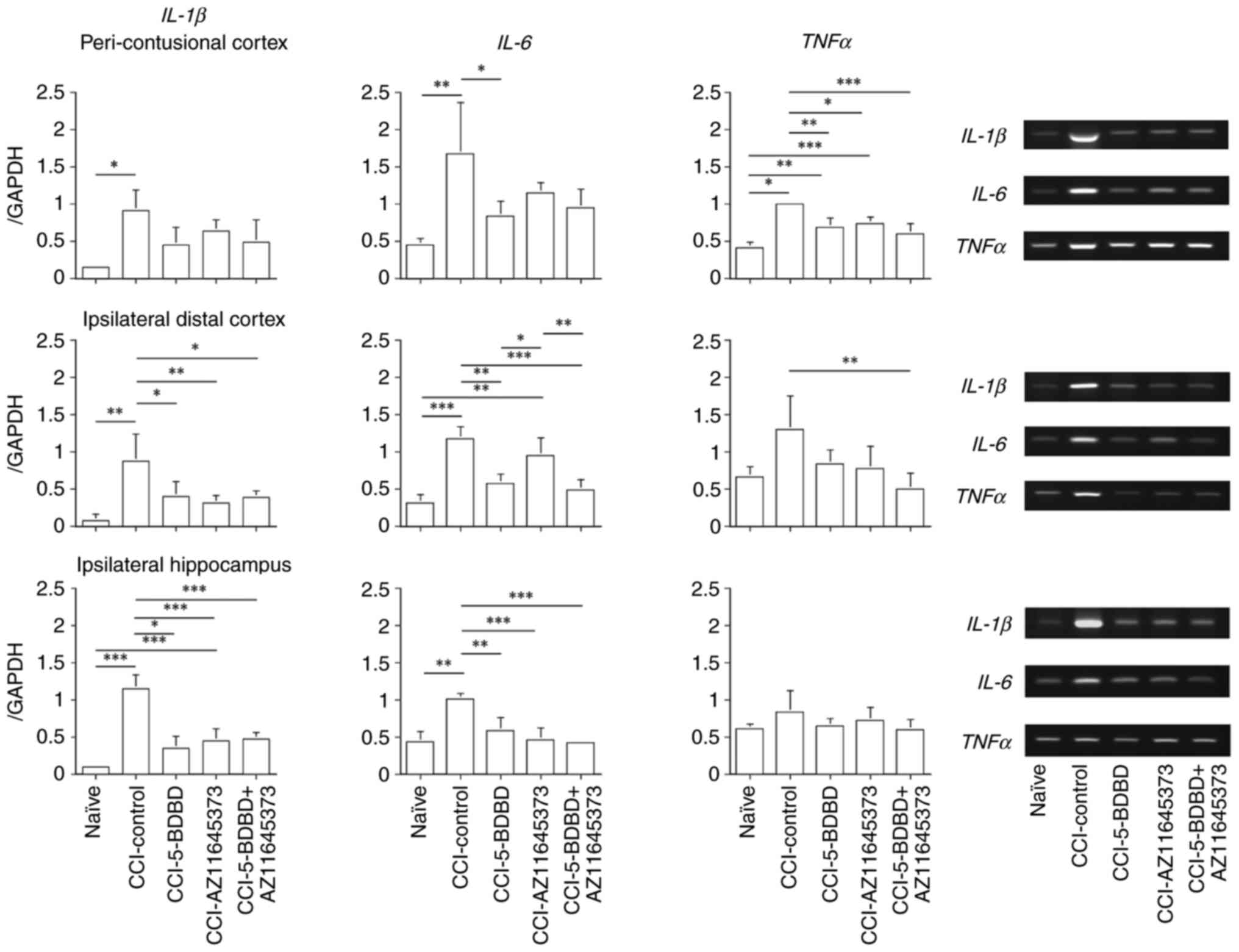 | Figure 4Expression of the cytokines measured
by PCR in the peri-contusional cortex 3 days after the CCI.
Expression levels of IL-1β, IL-6 and TNFα were increased by the
CCI. Furthermore, treatment of 5-BDBD, AZ11645373 or 5-BDBD +
AZ11645373 decreased expression of TNFα. Expression of cytokines
measured by PCR in the ipsilateral distal cortex 3 days after the
CCI. Expression levels of IL-1β and IL-6 were increased by the CCI,
but not of TNFα. Furthermore, treatment of 5-BDBD, AZ11645373 or
5-BDBD + AZ11645373 decreased expression of IL-1β, and treatment of
5-BDBD or 5-BDBD + AZ11645373 decreased expression of IL-6.
Expression of cytokines measured by PCR in the ipsilateral
hippocampus 3 days after the CCI. Expression levels of IL-1β and
IL-6 were increased by the CCI, but not of TNFα. Furthermore,
treatment of 5-BDBD, AZ11645373 or 5-BDBD + AZ11645373 decreased
expression levels of IL-1β and IL-6. *P<0.05,
**P<0.01, ***P<0.001. CCI, cortical
contusion injury; IL, interleukin; PCR, polymerase chain reaction;
TNFα, tumor necrosis factor α; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase. |
As shown in Fig. 4,
mRNA expression of IL-1β (0.877±0.361) and IL-6 (1.178±0.157) in
the ipsilateral distal cortex were high in the CCI-control group
compared to the naïve group (IL-1β: 0.074±0.085, P=0.001; IL-6:
0.315±0.110, P<0.001). However, mRNA expression of TNFα
(1.296±0.449) in the CCI-control group showed no significant
difference compared to the naïve group (0.665±0.141). In contrast,
5-BDBD, AZ11645373, and 5-BDBD + AZ11645373 treatment reduced
expression of IL-1β (0.400±0.203, P=0.012; 0.314±0.094, P=0.003;
and 0.389±0.089, P =0.013, respectively) compared to the
CCI-control group. 5-BDBD and 5-BDBD + AZ11645373 treatment reduced
expression of IL-6 (0.569±0.128, P=0.023 and 0.486±0.134, P=0.005,
respectively) compared to the CCI-control group.
As shown in Fig. 4,
the changes in inflammatory cytokines 3 days after CCI were similar
in the ipsilateral hippocampus and the ipsilateral distal cortex.
mRNA expressions of IL-1β (1.151±0.192) and IL-6 (1.007±0.079) were
high in the CCI-control group compared to the naïve group (IL-1β:
0.095±0.014, P<0.001 and IL-6: 0.433±0.138, P=0.001). mRNA
expression levels of TNFα (0.836±0.294) in the CCI-control group
showed no significant difference compared to the naïve group
(0.836±0.294). In contrast, 5-BDBD, AZ11645373, and 5-BDBD +
AZ11645373 treatment reduced expression of IL-1β (0.355±0.163,
P<0.001; 0.449±0.159, P<0.001; and 0.473±0.093, P<0.001,
respectively) and IL-6 (0.591±0.168, P=0.002; 0.459±0.165,
P<0.001; and 0.419±0.025, P<0.001, respectively) compared to
the CCI-control group.
Behavioral test
Sensorimotor dysfunction using the corner turn test
and the cylinder test, sensorimotor coordination of the four limbs
using the grid walking test, and spatial memory function using the
plus maze test were assessed in the present study. Detailed data is
not shown here, but no significant differences were found between
the CCI-control group and the CCI-5-BDBD + AZ11645373 group in the
tests other than the plus maze test.
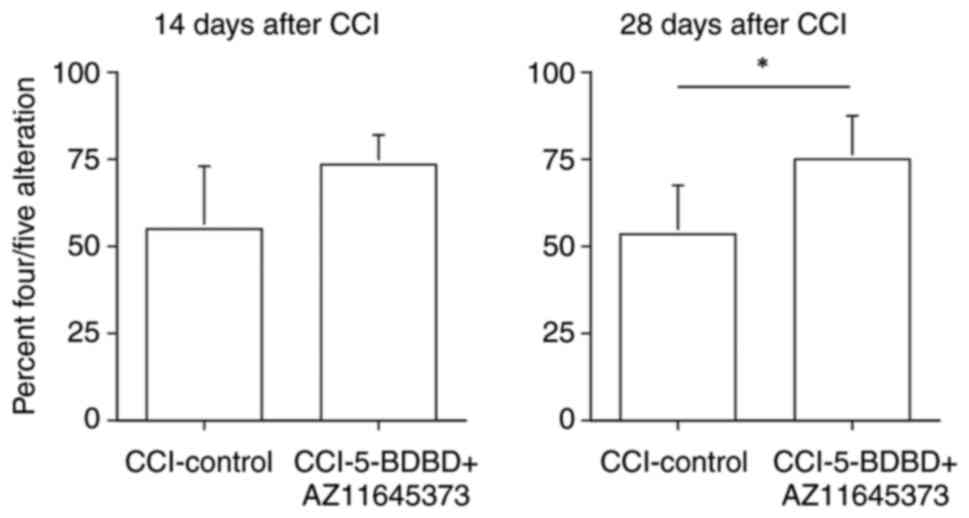
In the plus maze test, the criterion of 4/5
alternation 14 days after CCI in the CCI-control group and the
CCI-5-BDBD + AZ11645373 group was 55.18±17.79 and 73.59±8.28,
respectively (P=0.077). The criterion of the 4/5 alternation 28
days after CCI in the CCI-control group and the
CCI-5-BDBD+AZ11645373 group was 53.53±14.18 and 74.89±12.44,
respectively (P=0.047). No significant differences were found in
the 4/5 alternation criterion 14 days after CCI, but significant
increase occurred 28 days after CCI (Fig. 6).
Discussion
Increased extracellular ATP level immediately after
the insult has been demonstrated in several experimental models
including TBI (9,28-30).
The source of the rapid ATP increase is considered to be
intracellular ATP leakage from damaged cells and subsequent
cellular release from activated brain cells. The released ATP binds
to the purinergic receptors on microglia and activates these cells.
Activated microglia initiates inflammatory responses such as
migration to the damaged area and phagocyte debris (2,31),
engulfing or stripping of synapses (3,32),
and release of inflammatory cytokines (33-35).
Strong inflammatory response is well known to occur following TBI
(11,36), but excess inflammatory responses
including the release of enormous amounts of inflammatory cytokines
can be harmful to the brain (7,37)
(Fig. 7). Stimulation of P2Y6
receptors (P2 receptor is an ATP-gated ion channel receptor) is
mandatory for microglia phagocytosis (2). Other previous studies have
demonstrated that treatment with P2Y1 receptor antagonist decreases
extracellular ATP levels via restriction of cellular ATP signal
propagation in various experimental models (13,38-40).
In our previous study, in situ administration of a competitive P2Y1
receptor antagonist MRS2179 significantly reduced the extracellular
ATP level both before and after CCI in a rat model (9). Other studies reported that
administration of MRS2179 in a TBI model reduced inflammatory
responses (15).
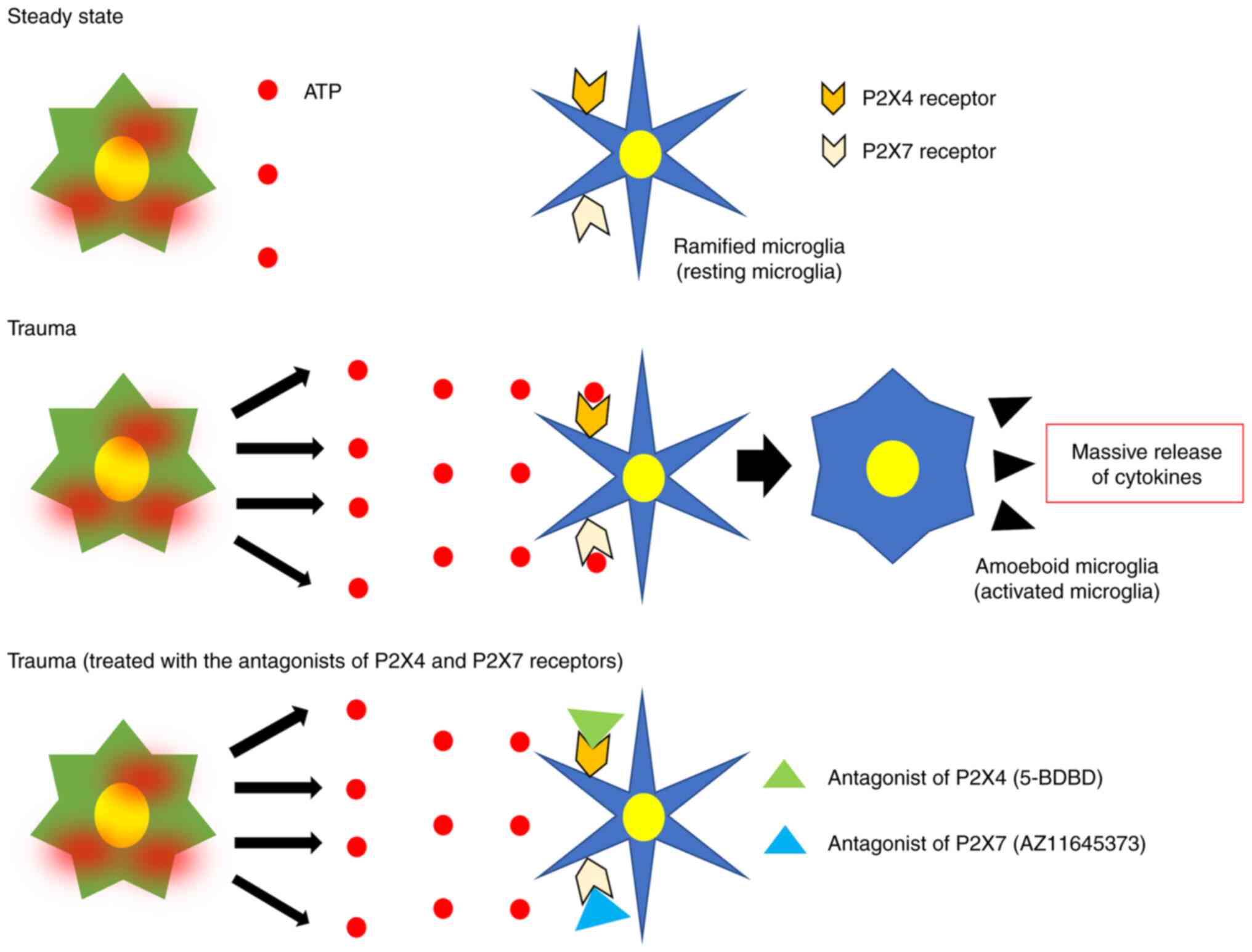 | Figure 7Release of a large amount of ATP,
gliotransmitter, into the extracellular space by TBI activates the
microglia through the ATP receptor, namely P2X and P2Y receptors.
Activated microglia retract their processes, enlarge their cell
bodies and release excessive amounts of cytokines, which can cause
excessive inflammatory response inducing secondary brain injury.
5-BDBD, an antagonist of the P2X7 receptor, and AZ11645373, an
antagonist of the P2X4 receptor, bind to these receptors and block
ATP binding, which can reduce microglial activation and thus
suppress excessive cytokine release. ATP, adenosine triphosphate;
TBI, traumatic brain injury. |
In the present study, we hypothesized that the
blockade of P2X4 and/or P2X7 receptors after TBI may potentiate an
anti-inflammatory effect through suppression of microglial
activation, and may provide therapeutic benefits. Several
astrocytic functions are essential to maintain homeostasis in the
injured brain, so we hypothesized that blocking the activation of
microglia without influencing the activation of astrocytes might be
the ideal approach to treat damage in the brain. The ATP leakage
from damaged cells (primary injury) caused by TBI cannot be
prevented, even with MRS2179 administration. Therefore, the present
study focused on blocking the receptors on microglia that receive
ATP signals by 5-BDBD and/or AZ11645373, an antagonist of P2X4 and
P2X7 receptors. In the CCI-control group, very high numbers of
activated microglia were histologically observed in the ipsilateral
cortex and hippocampus compared to the contralateral cortex and
hippocampus. In contrast, in the CCI-5-BDBD, CCI-AZ11645373, and
CCI-5-BDBD + AZ11645373 groups, smaller numbers of activated
microglia were observed in both the ipsilateral cortex and
hippocampus. Western blotting with Iba-1 antibody confirmed that
administration of CCI-5-BDBD and/or CCI-AZ11645373 significantly
suppressed the expression of microglia 3 days after CCI. Therefore,
blockade of P2X4 and P2X7 receptors was effective to regulate the
activation of microglia in the present injury model. Unlike
treating systemic inflammation, anti-inflammation therapy for brain
lesions is more complicated, but we showed that the present method
is a viable option to control the activity of microglia.
Cytokines are involved in both improvement and
exacerbation depending on the timing after injury (41). However, the influence of
inflammatory cytokines after TBI is not fully understood. IL-1β or
TNFα receptor blockers suppressed neural cell death and
improved motor coordination function in a rat TBI model (35). In addition, administration of IL-1β
neutralizing antibodies improved cognitive function (42) but only inhibition of cytokine
secretion could not provide anti-inflammatory effects (43). In this study, administration of
5-BDBD and/or AZ11645373 reduced the IL-1β level and improved
behavioral outcome. We note that cytokine secretion can be
controlled by inhibiting purinergic signaling which represents
another potential therapeutic approach for investigation in the
future. Further types of cytokines might be simultaneously
suppressed by this approach. However, we found obvious differences
in cytokine secretion levels between 5-BDBD and AZ11645373. The
P2X4 receptor seems to be more involved in cytokine secretion and
no synergistic effect was seen with the combination of 5-BDBD and
AZ11645373. Our study confirmed suppression of cytokine release by
the administration of 5-BDBD and/or AZ11645373, and our results
have established a highly significant new perspective on treatment
methods mediated through gliotransmission control.
Gliosis is well known to occur after TBI (42). Increased expression levels of GFAP
in the acute phase are considered to reflect an increase in
reactive astrocytes which are believed to partly remain as gliosis
in the chronic phase (21).
Reactive astrocytes may also protect against oxidative stress
(44) and the blood-brain barrier
(45), and reduce glutamate
toxicity (46), brain edema in
stroke (22), and the inflammatory
response in various conditions (47-49).
However, gliosis also potentiates adverse effects especially in the
chronic phase such as inhibiting nerve regeneration (40) and can act as an epileptic focus
(50). In the present study, GFAP
expression increased after CCI, reflecting the increase of reactive
astrocytes in the acute phase, as previously reported (51). However, interestingly, treatment
with 5-BDBD and/or AZ11645373 further increased GFAP expression.
These findings suggest that 5-BDBD and/or AZ11645373 suppressed
microglial activation, but not reactive astrocytes. No other
studies show similar results in a TBI model, but this trend was
similar to a study using an ischemia model in a P2X7 receptor
knockout mice (52). Astrocyte
response to P2 receptor antagonists is important and we plan a
detailed study (including GFAP staining) in the near future.
The corner turn test, the cylinder test, and the
grid walking test, which evaluate unilateral sensorimotor
dysfunction and sensorimotor coordination of the four limbs, did
not show any significant changes after administration of 5-BDBD
and/or AZ11645373, whereas the plus maze test, which reflects
spatial memory function and involves the hippocampal function,
showed significant improvement. Since the brain contusion induced
in this study was not intense enough to cause direct (primary)
damage on both sides of the hippocampus and the contralateral
cortex, the improved performance in the plus maze test was
considered to indicate the effect of blocking P2X4 and/or P2X7
receptors on the hippocampus despite the absence of observed
primary damage, which implies that the ameliorative effect reduced
secondary damage to the hippocampus. Cytokine expressions
contributed to both improved and worsened outcomes in a rat trauma
model (35,42,43,53).
Therefore, we considered that secondary brain injury could be
effectively treated by suppressing microglial activation and
subsequent secretion of cytokines.
In conclusion, secondary brain injury after cerebral
contusion is therapeutically important, and has been investigated
by various studies. However, no effective treatment has been
established. We confirmed that blocking the P2X4 and P2X7
receptors, which are ATP receptors central in gliotransmission,
suppressed microglial activation and subsequent cytokine expression
after brain injury. These findings indicate the potential as an
effective treatment for reducing secondary brain injury.
Acknowledgements
The authors would like to thank Dr Yuto Furukawa
(Department of Rehabilitation, Tums Urayasu Hospital, Urayasu,
Japan) for technical assistance with the experiments.
Funding
Funding: This work was supported in part by Grants-in-Aid for
Scientific Research from the Japan Society for the Promotion of
Science (grant no. 25861292).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MK and NM designed all the experiments. MK, NM, and
TK performed the experiments. AY, KS, TM, and HO helped with data
analysis and draft writing. MK, NM, and AY reviewed the manuscript.
All authors read and approved the final manuscript. MK, NM and AY
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by the animal care and use
committee at Nihon University School of Medicine (approval no.
AP16M046-1). All experiments were conducted according to the animal
experimental protocol manuals at Nihon University School of
Medicine. Efforts were made to avoid pain and distress to the
animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Davalos D, Grutzendler J, Yang G, Kim JV,
Zuo Y, Jung S, Littman DR, Dustin ML and Gan WB: ATP mediates rapid
microglial response to local brain injury in vivo. Nat Neurosci.
8:752–758. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Koizumi S, Shigemoto-Mogami Y, Nasu-Tada
K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S
and Inoue K: UDP acting at P2Y6 receptors is a mediator of
microglial phagocytosis. Nature. 446:1091–1095. 2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Wyatt SK, Witt T, Barbaro NM, Cohen-Gadol
AA and Brewster AL: Enhanced classical complement pathway
activation and altered phagocytosis signaling molecules in human
epilepsy. Exp Neurol. 295:184–193. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Haydon PG and Carmignoto G: Astrocyte
control of synaptic transmission and neurovascular coupling.
Physiol Rev. 86:1009–1031. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cornell-Bell AH, Finkbeiner SM, Cooper MS
and Smith SJ: Glutamate induces calcium waves in cultured
astrocytes: Long-range glial signaling. Science. 247:470–473.
1990.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Guthrie PB, Knappenberger J, Segal M,
Bennett MV, Charles AC and Kater SB: ATP released from astrocytes
mediates glial calcium waves. J Neurosci. 19:520–528.
1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Taib T, Leconte C, Van Steenwinckel J, Cho
AH, Palmier B, Torsello E, Lai Kuen R, Onyeomah S, Ecomard K,
Benedetto C, et al: Neuroinflammation, myelin and behavior:
Temporal patterns following mild traumatic brain injury in mice.
PLoS One. 12(e0184811)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Miller WJ, Leventhal I, Scarsella D,
Haydon PG, Janmey P and Meaney DF: Mechanically induced reactive
gliosis causes ATP-mediated alterations in astrocyte stiffness. J
Neurotrauma. 26:789–797. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Moro N, Ghavim SS and Sutton RL: Massive
efflux of adenosine triphosphate into the extracellular space
immediately after experimental traumatic brain injury. Exp Ther
Med. 21(575)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hua Y, Schallert T, Keep RF, Wu J, Hoff JT
and Xi G: Behavioral tests after intracerebral hemorrhage in the
rat. Stroke. 33:2478–2484. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Thelin EP, Frostell A, Mulder J, Mitsios
N, Damberg P, Aski SN, Risling M, Svensson M, Morganti-Kossmann MC
and Bellander BM: Lesion size is exacerbated in hypoxic rats
whereas hypoxia-inducible factor-1 alpha and vascular endothelial
growth factor increase in injured normoxic rats: A prospective
cohort study of secondary hypoxia in focal traumatic brain injury.
Front Neurol. 7(23)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu L, He D and Bai Y: Microglia-mediated
inflammation and neurodegenerative disease. Mol Neurobiol.
53:6709–6715. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Delekate A, Füchtemeier M, Schumacher T,
Ulbrich C, Foddis M and Petzold GC: Metabotropic P2Y1 receptor
signalling mediates astrocytic hyperactivity in vivo in an
Alzheimer's disease mouse model. Nat Commun. 5(5422)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Choo AM, Miller WJ, Chen YC, Nibley P,
Patel TP, Goletiani C, Morrison B III, Kutzing MK, Firestein BL,
Sul JY, et al: Antagonism of purinergic signalling improves
recovery from traumatic brain injury. Brain. 136:65–80.
2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kumagawa T, Moro N, Maeda T, Kobayashi M,
Furukawa Y, Shijo K and Yoshino A: Anti-inflammatory effect of P2Y1
receptor blocker MRS2179 in a rat model of traumatic brain injury.
Brain Res Bull. 181:46–54. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Filiano AJ, Gadani SP and Kipnis J:
Interactions of innate and adaptive immunity in brain development
and function. Brain Res. 1617:18–27. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sordillo PP, Sordillo LA and Helson L:
Bifunctional role of pro-inflammatory cytokines after traumatic
brain injury. Brain Inj. 30:1043–1053. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Moro N, Ghavim SS, Harris NG, Hovda DA and
Sutton RL: Pyruvate treatment attenuates cerebral metabolic
depression and neuronal loss after experimental traumatic brain
injury. Brain Res. 1642:270–277. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Goodman JC, Cherian L, Bryan RM Jr and
Robertson CS: Lateral cortical impact injury in rats: Pathologic
effects of varying cortical compression and impact velocity. J
Neurotrauma. 11:587–597. 1994.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dalgard CL, Cole JT, Kean WS, Lucky JJ,
Sukumar G, McMullen DC, Pollard HB and Watson WD: The cytokine
temporal profile in rat cortex after controlled cortical impact.
Front Mol Neurosci. 5(6)2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sofroniew MV and Vinters HV: Astrocytes:
Biology and pathology. Acta Neuropathol. 119:7–35. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang L, Schallert T, Zhang ZG, Jiang Q,
Arniego P, Li Q, Lu M and Chopp M: A test for detecting long-term
sensorimotor dysfunction in the mouse after focal cerebral
ischemia. J Neurosci Methods. 117:207–214. 2002.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gharbawie OA, Whishaw PA and Whishaw IQ:
The topography of three-dimensional exploration: A new
quantification of vertical and horizontal exploration, postural
support, and exploratory bouts in the cylinder test. Behav Brain
Res. 151:125–135. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chao OY, Pum ME, Li JS and Huston JP: The
grid-walking test: Assessment of sensorimotor deficits after
moderate or severe dopamine depletion by 6-hydroxydopamine lesions
in the dorsal striatum and medial forebrain bundle. Neuroscience.
202:318–325. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
McNay EC, Fries TM and Gold PE: Decreases
in rat extracellular hippocampal glucose concentration associated
with cognitive demand during a spatial task. Proc Natl Acad Sci
USA. 97:2881–2885. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Taylor AN, Rahman SU, Sanders NC, Tio DL,
Prolo P and Sutton RL: Injury severity differentially affects
short- and long-term neuroendocrine outcomes of traumatic brain
injury. J Neurotrauma. 25:311–323. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tan J, Town T, Paris D, Mori T, Suo Z,
Crawford F, Mattson MP, Flavell RA and Mullan M: Microglial
activation resulting from CD40-CD40L interaction after beta-amyloid
stimulation. Science. 286:2352–2355. 1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Faroqi AH, Lim MJ, Kee EC, Lee JH, Burgess
JD, Chen R, Di Virgilio F, Delenclos M and McLean PJ: In vivo
detection of extracellular adenosine triphosphate in a mouse model
of traumatic brain injury. J Neurotrauma. 38:655–664.
2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Melani A, Turchi D, Vannucchi MG, Cipriani
S, Gianfriddo M and Pedata F: ATP extracellular concentrations are
increased in the rat striatum during in vivo ischemia. Neurochem
Int. 47:442–448. 2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Peng J, Liu Y, Umpierre AD, Xie M, Tian
DS, Richardson JR and Wu LJ: Microglial P2Y12 receptor regulates
ventral hippocampal CA1 neuronal excitability and innate fear in
mice. Mol Brain. 12(71)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Abiega O, Beccari S, Diaz-Aparicio I,
Nadjar A, Layé S, Leyrolle Q, Gómez-Nicola D, Domercq M,
Pérez-Samartín A, Sánchez-Zafra V, et al: Neuronal hyperactivity
disturbs ATP microgradients, impairs microglial motility, and
reduces phagocytic receptor expression triggering
apoptosis/microglial phagocytosis uncoupling. PLoS Biol.
14(e1002466)2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Trapp BD, Bö L, Mörk S and Chang A:
Pathogenesis of tissue injury in MS lesions. J Neuroimmunol.
98:49–56. 1999.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Aboud O, Mrak RE, Boop F and Griffin ST:
Apolipoprotein epsilon 3 alleles are associated with indicators of
neuronal resilience. BMC Med. 10(35)2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Osipova ED, Semyachkina-Glushkovskaya OV,
Morgun AV, Pisareva NV, Malinovskaya NA, Boitsova EB, Pozhilenkova
EA, Belova OA, Salmin VV, Taranushenko TE, et al: Gliotransmitters
and cytokines in the control of blood-brain barrier permeability.
Rev Neurosci. 29:567–591. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Perez-Polo JR, Rea HC, Johnson KM, Parsley
MA, Unabia GC, Xu GY, Prough D, DeWitt DS, Paulucci-Holthauzen AA,
Werrbach-Perez K and Hulsebosch CE: Inflammatory cytokine receptor
blockade in a rodent model of mild traumatic brain injury. J
Neurosci Res. 94:27–38. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jassam YN, Izzy S, Whalen M, McGavern DB
and El Khoury J: Neuroimmunology of traumatic brain injury: Time
for a paradigm shift. Neuron. 95:1246–1265. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xu H, Wang Z, Li J, Wu H, Peng Y, Fan L,
Chen J, Gu C, Yan F, Wang L and Chen G: The polarization states of
microglia in TBI: A new paradigm for pharmacological intervention.
Neural Plast. 2017(5405104)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Menzel L, Kleber L, Friedrich C, Hummel R,
Dangel L, Winter J, Schmitz K, Tegeder I and Schäfer MK:
Progranulin protects against exaggerated axonal injury and
astrogliosis following traumatic brain injury. Glia. 65:278–292.
2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nikolic L, Shen W, Nobili P, Virenque A,
Ulmann L and Audinat E: Blocking TNFα-driven astrocyte purinergic
signaling restores normal synaptic activity during epileptogenesis.
Glia. 66:2673–2683. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Silver J and Miller JH: Regeneration
beyond the glial scar. Nat Rev Neurosci. 5:146–156. 2004.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kaiser M, Penk A, Franke H, Krügel U,
Nörenberg W, Huster D and Schaefer M: Lack of functional P2X7
receptor aggravates brain edema development after middle cerebral
artery occlusion. Purinergic Signal. 12:453–463. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ekmark-Lewén S, Flygt J, Fridgeirsdottir
GA, Kiwanuka O, Hånell A, Meyerson BJ, Mir AK, Gram H, Lewén A,
Clausen F, et al: Diffuse traumatic axonal injury in mice induces
complex behavioural alterations that are normalized by
neutralization of interleukin-1β. Eur J Neurosci. 43:1016–1033.
2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Helmy A, Guilfoyle MR, Carpenter KLH,
Pickard JD, Menon DK and Hutchinson PJ: Recombinant human
interleukin-1 receptor antagonist promotes M1 microglia biased
cytokines and chemokines following human traumatic brain injury. J
Cereb Blood Flow Metab. 36:1434–1448. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Fehily B and Fitzgerald M: Repeated mild
traumatic brain injury: Potential mechanisms of damage. Cell
Transplant. 26:1131–1155. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Shih AY, Johnson DA, Wong G, Kraft AD,
Jiang L, Erb H, Johnson JA and Murphy TH: Coordinate regulation of
glutathione biosynthesis and release by Nrf2-expressing glia
potently protects neurons from oxidative stress. J Neurosci.
23:3394–3406. 2003.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bush TG, Puvanachandra N, Horner CH,
Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH and
Sofroniew MV: Leukocyte infiltration, neuronal degeneration, and
neurite outgrowth after ablation of scar-forming, reactive
astrocytes in adult transgenic mice. Neuron. 23:297–308.
1999.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Rothstein JD, Dykes-Hoberg M, Pardo CA,
Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke
JP and Welty DF: Knockout of glutamate transporters reveals a major
role for astroglial transport in excitotoxicity and clearance of
glutamate. Neuron. 16:675–686. 1996.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Li L, Lundkvist A, Andersson D,
Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Ståhlberg A, Aprico K,
Larsson K, et al: Protective role of reactive astrocytes in brain
ischemia. J Cereb Blood Flow Metab. 28:468–481. 2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Okada S, Nakamura M, Katoh H, Miyao T,
Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y
and Okano H: Conditional ablation of Stat3 or Socs3 discloses a
dual role for reactive astrocytes after spinal cord injury. Nat
Med. 12:829–834. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
50
|
Voskuhl RR, Peterson RS, Song B, Ao Y,
Morales LB, Tiwari-Woodruff S and Sofroniew MV: Reactive astrocytes
form scar-like perivascular barriers to leukocytes during adaptive
immune inflammation of the CNS. J Neurosci. 29:11511–11522.
2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Oberheim NA, Tian GF, Han X, Peng W,
Takano T, Ransom B and Nedergaard M: Loss of astrocytic domain
organization in the epileptic brain. J Neurosci. 28:3264–3276.
2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Norden DM, Trojanowski PJ, Villanueva E,
Navarro E and Godbout JP: Sequential activation of microglia and
astrocyte cytokine expression precedes increased Iba-1 or GFAP
immunoreactivity following systemic immune challenge. Glia.
64:300–316. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Scherbel U, Raghupathi R, Nakamura M,
Saatman KE, Trojanowski JQ, Neugebauer E, Marino MW and McIntosh
TK: Differential acute and chronic responses of tumor necrosis
factor-deficient mice to experimental brain injury. Proc Natl Acad
Sci USA. 96:8721–8726. 1999.PubMed/NCBI View Article : Google Scholar
|