Introduction
Acute myeloid leukemia (AML) is a malignant disease
that is mainly derived from myeloid stem/progenitor cells (1). It is characterized by the aberrant
proliferation of primitive and naive myeloid cells in the bone
marrow and peripheral blood, the clinical manifestations of which
can include anemia, hemorrhage, infection and fever, organ
infiltration and/or lipid metabolic abnormalities (2). The majority of AML cases are severe
and the prognosis is unfavorable, which can become fatal if not
diagnosed and treated in the early stages (3). AML accounts for ~25% of all types of
childhood leukemias (4). To the
best of our knowledge, the pathogenesis of AML remains unclear.
Ras-related protein member RAS oncogene GTPases
(RAB) 34 is a gene that encodes a protein belonging to the RAB
family, which are small GTPases involved in intracellular protein
transport (5). Previous studies
have found that RAB34 is associated with the occurrence and
development of hepatocellular carcinoma, non-small cell lung cancer
and breast cancer through the driving of tumor cell proliferation
and migration (6-8).
Compared with that in paracancerous tissues, RAB34 expression in
human hepatocellular carcinoma tissues was found to be upregulated,
where it was associated with poorer prognosis (7). In addition, knocking down RAB34
expression was observed to inhibit hepatocellular carcinoma cell
proliferation and migration (7).
Plasmacytoma variant translocation oncogene 1 was also demonstrated
to promote the proliferation and migration of non-small cell lung
cancer by targeting the microRNA (miR)-148/RAB34 signaling axis
(6). RAB34 was found to be
overexpressed in invasive breast cancer MDA-MB-231 and BT549 cells,
where RAB34 silencing could inhibit cell migration, invasion and
adhesion (8). However, the role of
RAB34 in AML remains unclear.
E2F1 is a member of the cell cycle-related
transcription factor family (9). A
recent study has shown that long non-coding RNA (lncRNA) lncSIK1
can block the expression of E2F1 and suppress the E2F1-mediated
transcription of LC3 and DNA damage-regulated autophagy modulator
to alleviate aggressive autophagy in the myeloid leukemia Molm13
cell line, which delayed the progression of AML in vitro
(10). In another study, lncRNA
NR-104098 was previously found to effectively inhibit enhancer of
zeste homolog 2 (EZH2) transcription by directly binding to E2F1
and recruiting E2F1 to the EZH2 promoter, which ultimately
inhibited proliferation whilst inducing the differentiation of AML
cells (11). The proliferating
cell nuclear antigen clamp-associated factor was previously
demonstrated to accelerate G1/S transition in
neuroblastoma cells by activating the E2F1/pituitary
tumor-transforming gene 1 protein signaling pathway (12).
Therefore, it was hypothesized in the present study
that RAB34 is highly expressed in AML, where it can be regulated by
E2F1 and is associated with poor prognosis, such that RAB34
regulation may affect the malignant behavior of AML cells.
Materials and methods
Database
The ‘Boxplots’ module of the GEPIA database
(gepia.cancer-pku.cn) was used to analyze
the expression of RAB34 in the tissues of patients with AML and the
‘Survival Analysis’ module of the GEPIA database was to analyze the
correlation between RAB34 and the overall survival rate of patients
with AML (13). HumanTFDB
(http://bioinfo.life.hust.edu.cn/) was
used to predict the binding sites of E2F1 on the promoter of
RAB34(14).
Cell culture
Human bone marrow stromal HS-5 cells (cat. no.
BFN60808921) and AML MOLM-14 cells (cat. no. BFN60810333) were
purchased from Qingqi (Shanghai) Biotechnology Development Co.,
Ltd. AML MV4-11 (cat. no. CRL-9591) and HL-60 cells (cat. no.
CCL-240) were purchased from the American Type Culture Collection.
The AML Kasumi-1 cell line (cat. no. SCSP-5015) was purchased from
The Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. The cells were cultured in RPMI-1640 medium supplemented
with 10% FBS (both from Thermo Fisher Scientific, Inc.) and 100
IU/ml penicillin + 100 µg/ml streptomycin (Sigma-Aldrich; Merck
KGaA) at 37˚C with 5% CO2.
Reverse transcription-quantitative PCR
(RT-qPCR)
RNA samples were extracted from cells with
chloroform and a TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Total RNA was reverse transcribed to cDNA
using the HiScript II 1st Strand cDNA Synthesis Kit (cat. no.
R211-01; Vazyme Biotech Co., Ltd.). The conditions for reverse
transcription were as follows: 37˚C for 10 min, followed by 85˚C
for 5 sec and then storage at 4˚C. qPCR was subsequently performed
using the HiScript II One Step qRT-PCR SYBR Green kit (Vazyme
Biotech Co., Ltd.), 1 µl cDNA and amplification primers. The
thermocycling conditions used were as follows: Denaturation at 95˚C
for 30 sec, followed by 40 cycles at 95˚C for 5 sec and 60˚C for 30
sec. The following primer pairs sequences were designed by
Guangzhou RiboBio Co., Ltd.: RAB34 forward,
5'-GTCTCCGATTCCCCATCACC-3' and reverse, 5'-ATGCGGACAACATCCCCAAT-3';
E2F1 forward, 5'-GGGGGAGAAGTCACGCTATG-3' and reverse,
5'-AAACATCGATCGGGCCTTGT-3' and GAPDH forward,
5'-CATGAGAAGTATGACAACAGCCT-3' and reverse,
5'-AGTCCTTCCACGATACCAAAGT-3'. The mRNA expression levels were
quantified using the 2-ΔΔCq method (15) and normalized to that of the
internal reference gene GAPDH.
Western blotting
Total protein was extracted from the cells using
RIPA lysis buffer (Cell Signaling Technology, Inc.) and quantified
using a BCA kit (Beyotime Institute of Biotechnology). Total
protein (30 µg/lane) was separated by 10% SDS-PAGE and transferred
onto a PVDF membrane. Membranes were blocked with 5% milk for 1 h
at 37˚C and then incubated in the primary antibodies, against RAB34
(1:1,000; cat. no. PA5-99697; Thermo Fisher Scientific, Inc.),
Bcl-2 (1:1,000; cat. no. ab32124; Abcam), Bax (1:1,000; cat. no.
182733; Abcam), CDK4 (1:1,000; cat. no. ab108357; Abcam), CDK8
(1:1,000; cat. no. ab229192; Abcam), cyclin D1 (1:1,000; cat. no.
ab16663; Abcam), E2F1 (1:1,000; cat. no. ab288369; Abcam) or GAPDH
(1:1,000; cat. no. ab9485; Abcam), overnight at 4˚C. On the next
day, membranes were incubated with the HRP-conjugated secondary
antibody (goat anti-rabbit; 1:5,000; cat. no. ab6721; Abcam) for 2
h at 37˚C. Finally, all the membranes were visualized using a
BeyoECL Plus kit (Beyotime Institute of Biotechnology) on an
Odyssey Infrared imaging system (Bio-Rad Laboratories, Inc.) and
semi-quantified using ImageJ software (version 1.42; National
Institutes of Health).
Cell transfection
Lentivirus short hairpin (sh)RNA RAB34 sequences
(shRNA-RAB34#1 and shRNA-RAB34#2) which were ligated into the
plasmid of U6/GFP/Neo and scrambled control shRNA (shRNA-NC) were
established and synthesized by Jimon Biotechnology (Shanghai) Co.,
Ltd. After 48 h of transfection, 2 µg/ml puromycin (Beyotime
Institute of Biotechnology) was added to create stably transfected
HL-60 cell lines at a multiplicity of infection of 10, followed by
maintenance with 0.5 µg/ml puromycin. The E2F1 overexpressing
lentivirus (Oe-E2F1) tagged with Flag (with the E2F1 gene inserted
into the pcDNA3.1 vector), was purchased from Jimon Biotechnology
(Shanghai) Co., Ltd. An empty vector served as the negative control
(Oe-NC), and the interim cell line used was the 293T cell line
which was purchased from the American Type Culture Collection. The
2nd generation system was used. ShRNAs (500 ng/µl) and pcDNA3.1
vectors (4 µg) were transfected into HL-60 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h at 37˚C. The sequences of two human
shRNA-RAB34 and the shRNA-NC were as follows: Sh-RAB34#1,
5'-CCGCGTAATCGTAGGAACTAT-3'; sh-RAB34#2,
5'-CGCGTAATCGTAGGAACTATC-3'; and shRNA-NC,
5'-CAACAAGATGAAGAGCACCAA-3'. After 48 h incubation at 37˚C,
transfected cells were harvested and utilized for further
experiments.
Cell counting kit-8 (CCK-8) assay
Cell viability was assessed using a CCK-8 assay kit
(Beyotime Institute of Biotechnology) following the manufacturer's
protocol. After 48 h of transfection, HL-60 cells were seeded into
96-well plates at the density of 1,000 cells/well. RPMI-1640 medium
containing 10 µl CCK-8 was then added for 4 h. The absorbance in
each well at 450 nm was then measured using a microplate reader
(Bio-Rad Laboratories, Inc.).
5-ethynyl-2'-deoxyuridine (EdU)
staining
EdU staining was used to analyze the cancer cell
proliferation according to the following protocol. Briefly, HL-60
cells were transfected and then incubated with EdU (20 mmol/l; cat.
no. KGA331-1000; Nanjing KeyGen Biotech, Co., Ltd.) at 37˚C for 2 h
to infiltrate thymine into the DNA molecule being synthesized
during DNA replication. The cells were fixed with 4%
paraformaldehyde for 20 min at room temperature. DAPI (10 µmol) was
used to stain the nucleus for 5 min at room temperature. Finally,
the images were captured with a fluorescence microscope (Olympus
Corporation; magnification, x200).
Cell cycle analysis
Flow cytometry was used to observe the cell cycle
distribution. Briefly, HL-60 cells (4x105) were
collected after 48 h of transfection and were stained with 50 µg/ml
PI (Dojindo Molecular Technologies, Inc.) for 15 min at room
temperature. Cell cycle distribution and sub-G1 DNA
content were analyzed using a BD Accuri™ C6 flow cytometer (BD
Biosciences) and the data were analyzed with ModFit 2.0 software
(Verity Software House, Inc.).
Cell apoptosis analysis
Cell apoptosis was evaluated through flow cytometry
using a cellular Annexin V-FITC/PI Kit [cat. no. 70-AP101-100;
Hangzhou Multi Sciences (Lianke) Biotech Co., Ltd.] according to
the manufacturer's protocol. Cells were plated into six-well plates
at a density of 4x105 cells/well. After 48 h of
transfection, cells (4x105 cells/well) were incubated
with 200 µl binding buffer and double stained with Annexin V-FITC
and PI at 4˚C in the dark for 20 min. The cells were then assessed
using BD FACSCalibur™ (BD Biosciences) and the data were analyzed
with ModFit 2.0 software (Verity Software House, Inc.).
Luciferase reporter assay
The pGL3 plasmid (Promega Corporation) containing
the RAB34 promoter region element was generated by site-directed
mutagenesis and subcloned into the pGL3-basic luciferase reporter
vector. A mutant type (MUT) and wild-type (WT) RAB34 promoter
vector were produced by GeneCopoeia, Inc.. The
Lipofectamine® 2000 transfection reagent was used to
co-transfect the HL-60 cells with 400 ng aforementioned plasmids
and 100 nM Oe-E2F1 or the Oe-NC plasmids. After 48 h incubation at
37˚C, the luciferase activity was assayed using a Dual-Luciferase
Reporter Assay system (Promega Corporation) according to the
manufacturer's protocol. The relative luciferase activity was
calculated by normalizing the luminescence intensity of the firefly
luciferase activity to that of the Renilla luciferase
activity.
Chromatin immunoprecipitation (ChIP)
assay
The cells were treated with 4% formaldehyde for 10
min at room temperature to generate DNA-protein cross-links. A
Bioruptor® (Diagenode, Inc.) was applied to sonicate
cell lysates (20 kHz; 4 pulses of 12 sec each, followed by 30 sec
rest on ice between each pulse) to generate chromatin fragments
with an average size of 500 bp. A total of 40 µl protein A/G
agarose beads (cat. no. sc-2003; Santa Cruz Biotechnology, Inc.)
was added to the lysates and the lysates (500 µg) were then
immunoprecipitated for 6 h with 5 µg specific antibody against E2F1
(ab245308; Abcam) at 4˚C. Normal IgG antibody (ab172730; Abcam) was
used as a control. The next day, 30 µl protein G agarose beads were
added and the precipitate was collected after incubating at 4˚C for
6 h and centrifuged at 1,000 x g at 4˚C for 3 min. The precipitate
was washed with 5X lysis buffer and resuspended in 150 µl 1X ChIP
Elution Buffer. Chromatin from the beads were eluted with gentle
vortexing (1,200 rpm) at 65˚C for 30 min. DNA was purified using
the DNA Purification kit (cat. no. D0033; Beyotime Institute of
Biotechnology). Relative enrichment was evaluated using RT-qPCR.
The primers used were as follows: RAB34 forward,
5'-GTCTCCGATTCCCCATCACC-3' and reverse,
5'-ATGCGGACAACATCCCCAAT-3'.
Statistical analysis
Data were presented as mean ± standard deviation and
analyzed using the one-way ANOVA with Tukey's post hoc test with
GraphPad Prism 5.0 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference. Each
test was repeated ≥ three times.
Results
RAB34 is highly expressed in patients
with AML and is associated with the prognosis of AML
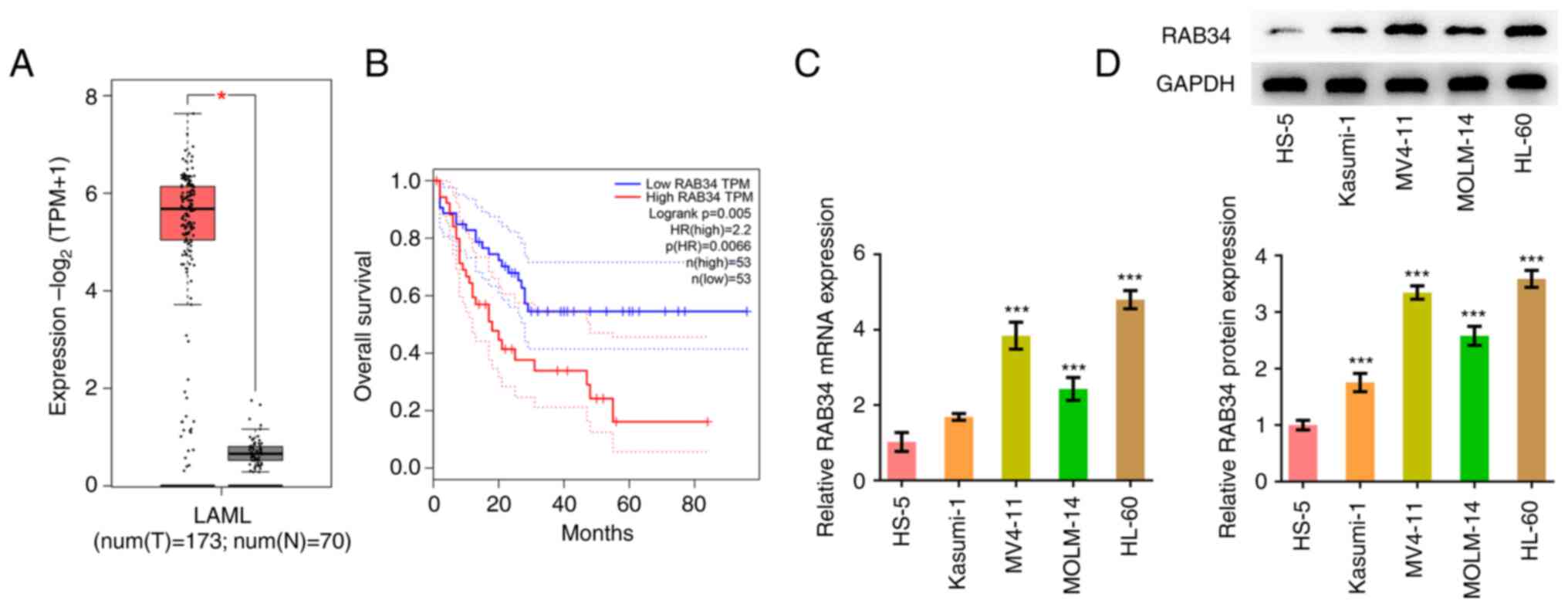
GEPIA database analysis revealed that RAB34 was
highly expressed in the tissues of patients with AML compared with
normal tissues (Fig. 1A), where
higher RAB34 expression levels were significantly associated with
poorer overall survival in patients with AML (Fig. 1B). RT-qPCR and western blotting
showed that RAB34 expression was markedly increased in AML cell
lines (Fig. 1C and D). Since the expression level of RAB34
was the highest in HL-60 cells, HL-60 cells were selected for
subsequent experiments.
RAB34 knockdown inhibits the
proliferation of AML cells, induces cell cycle arrest and
apoptosis
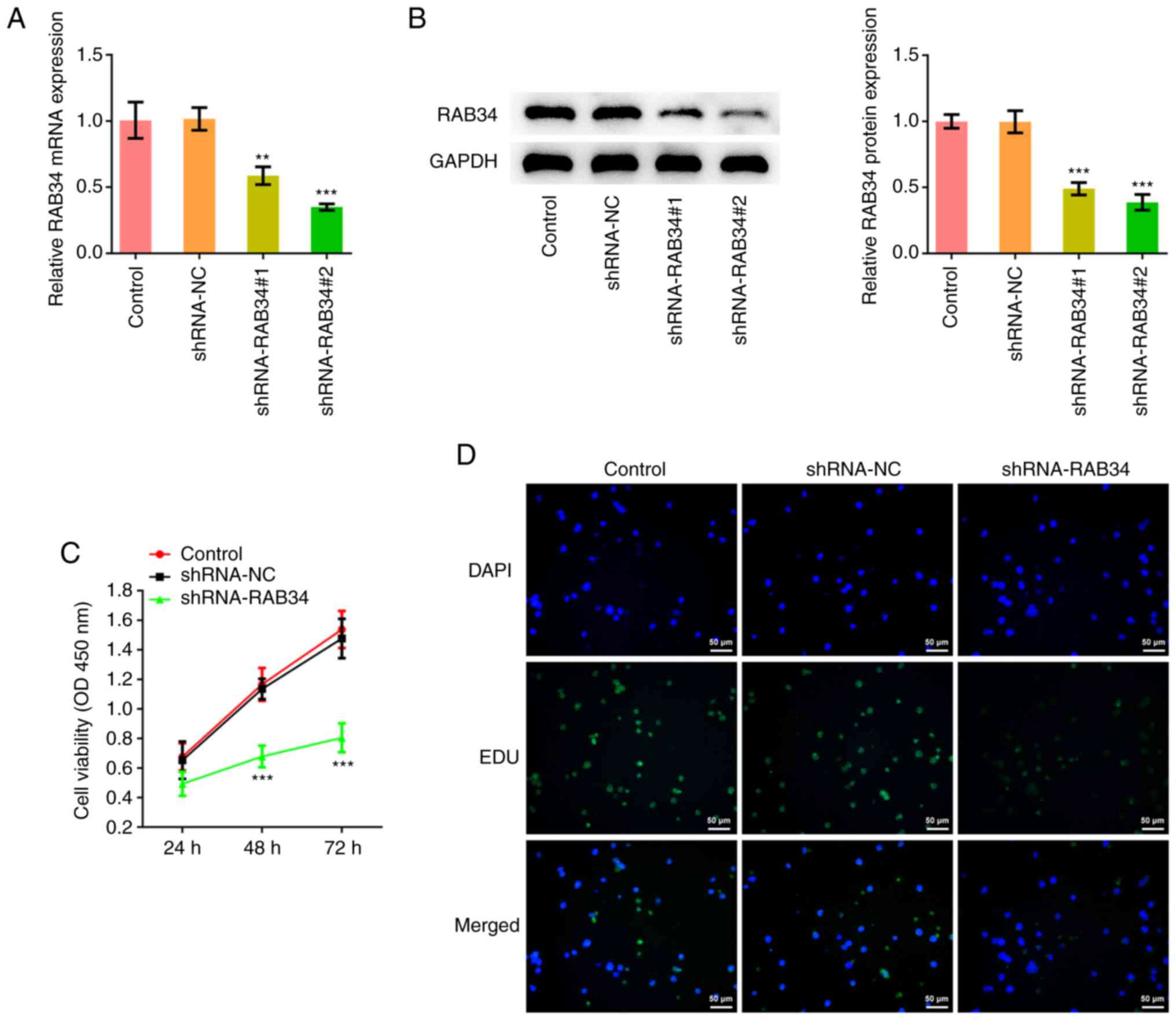
The RAB34 shRNA plasmid was constructed before the
knockdown efficiency was detected by RT-qPCR and western blotting
(Fig. 2A and B). Since the degree of knockdown
efficiency in the shRNA-RAB34#2 group was higher, shRNA-RAB34#2 was
chosen for subsequent experiments, which were divided into the
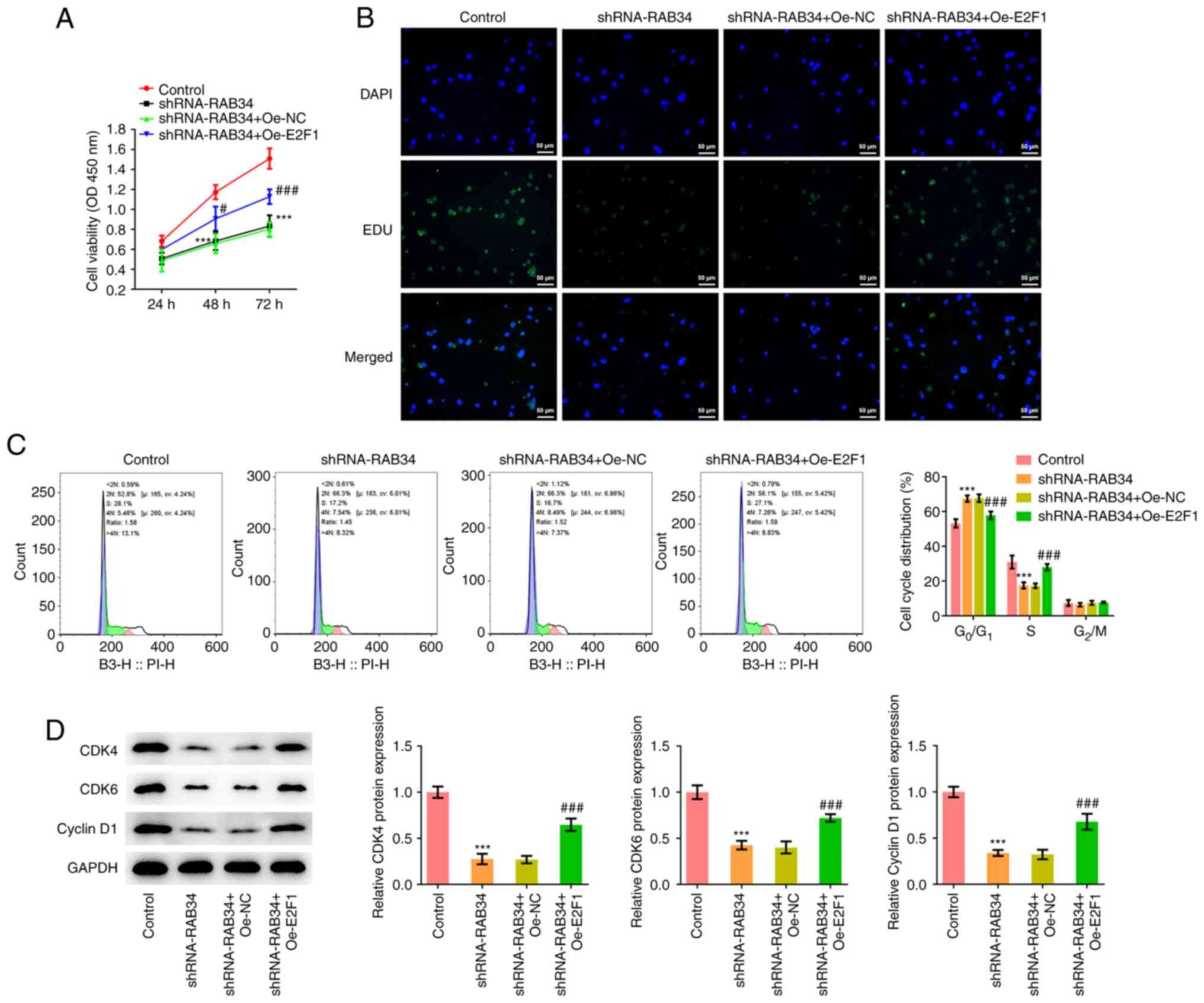
control, shRNA-NC and shRNA-RAB34 groups. Cell proliferation was
detected by CCK-8 assay and EdU staining, which showed that
compared with that in the shRNA-NC group, cell proliferation in the
shRNA-RAB34 group was markedly decreased (Fig. 2C and D).
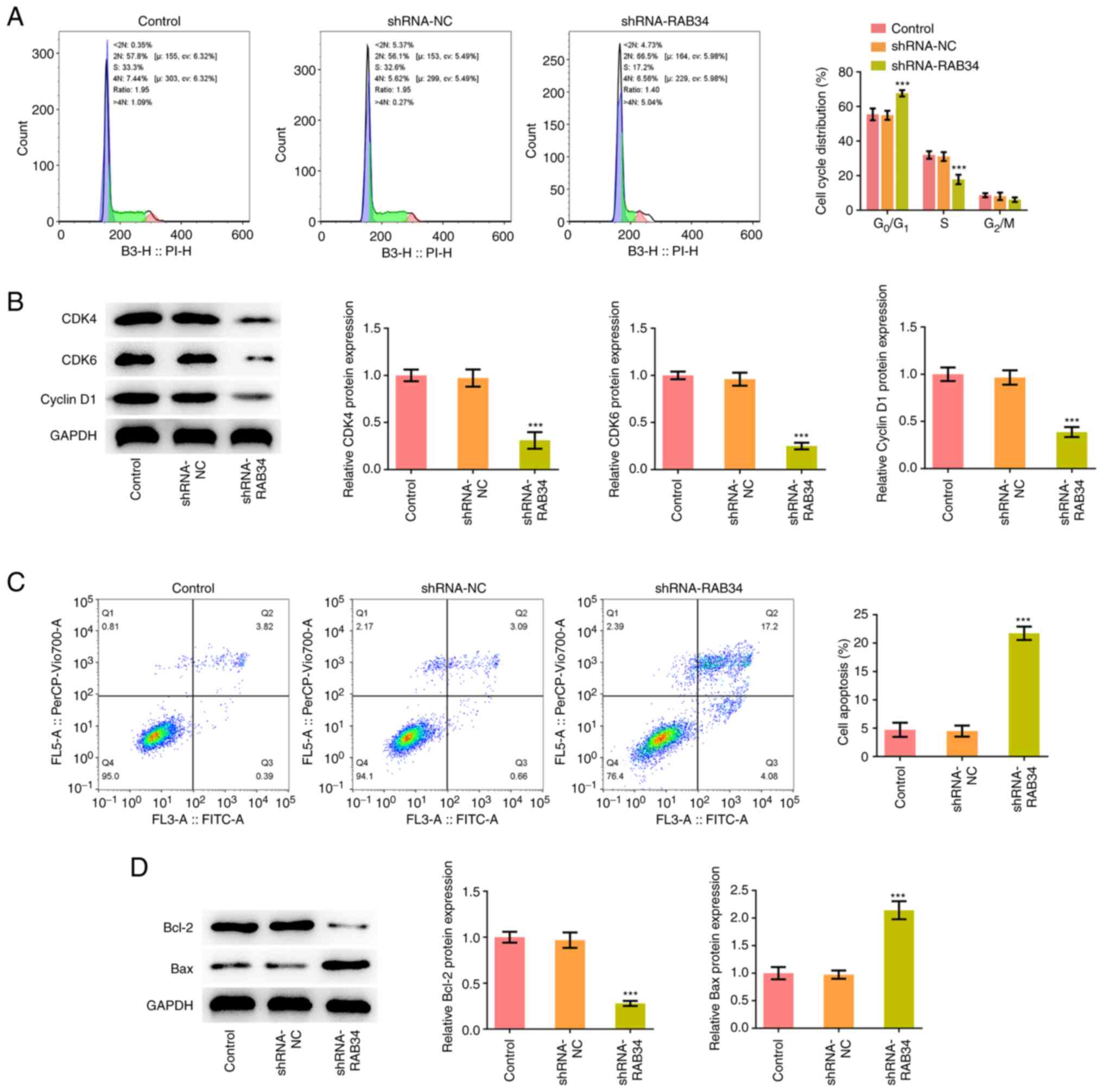
Flow cytometry analysis of the cell cycle
distribution showed that compared with that in the shRNA-NC group,
the proportion of cells in the G0/G1 phase in
the shRNA-RAB34 group was significantly increased, whilst that in
the S phase was significantly decreased (Fig. 3A). The expression of cell cycle
marker proteins CDK4, CDK6 and cyclin D1 was next detected by
western blotting, which showed that knocking down RAB34 expression
significantly inhibited the expression of CDK4, CDK6 and cyclin D1
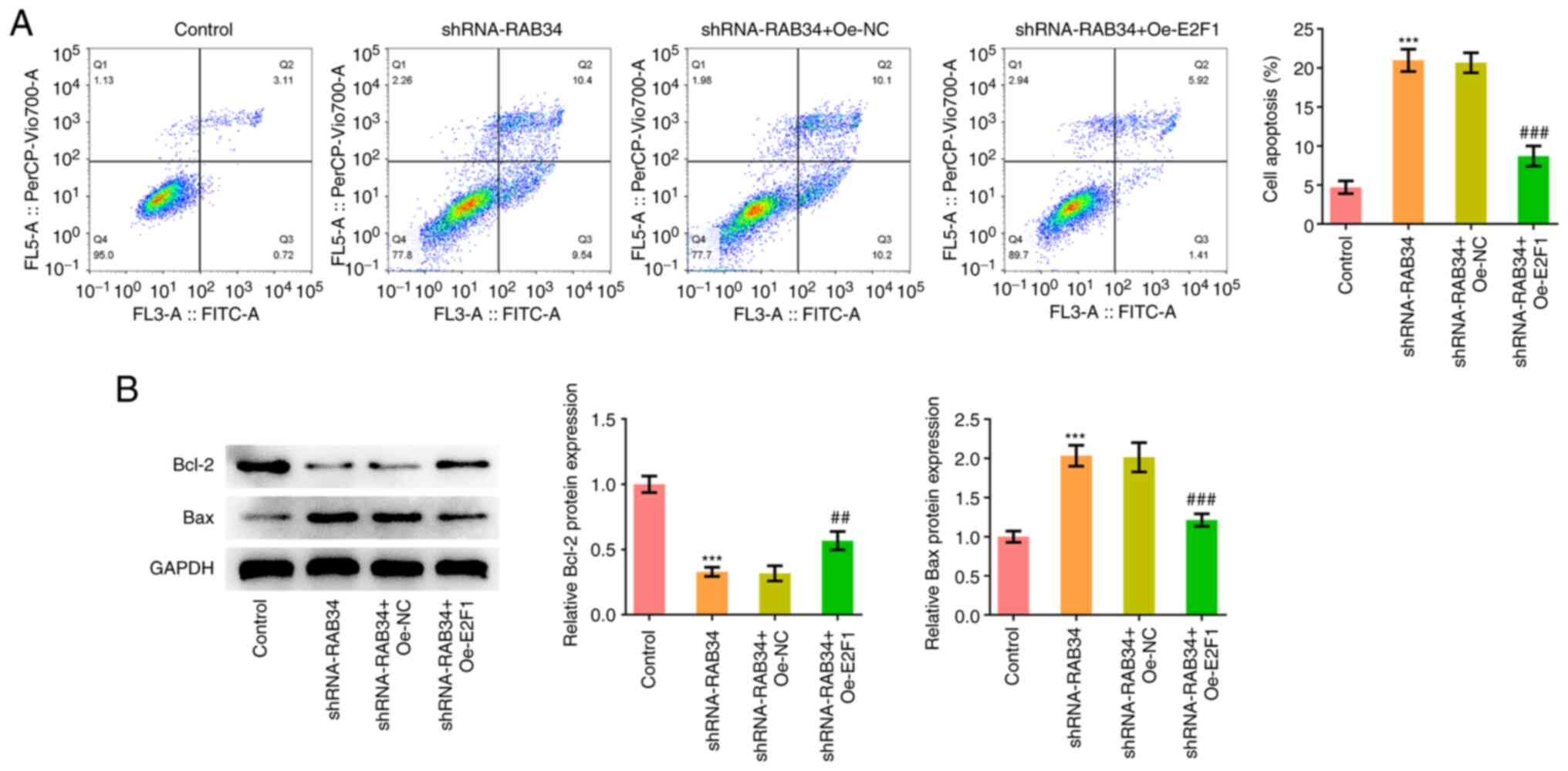
(Fig. 3B). Flow cytometry and
western blotting were subsequently used to analyze cell apoptosis
and the results showed that RAB34 knockdown significantly promoted
cell apoptosis (Fig. 3C). In
addition, significant decreases in the expression of the apoptosis
marker protein Bcl-2 and an significant increases in the expression
of Bax were observed (Fig.
3D).
E2F1 activates RAB34
transcription
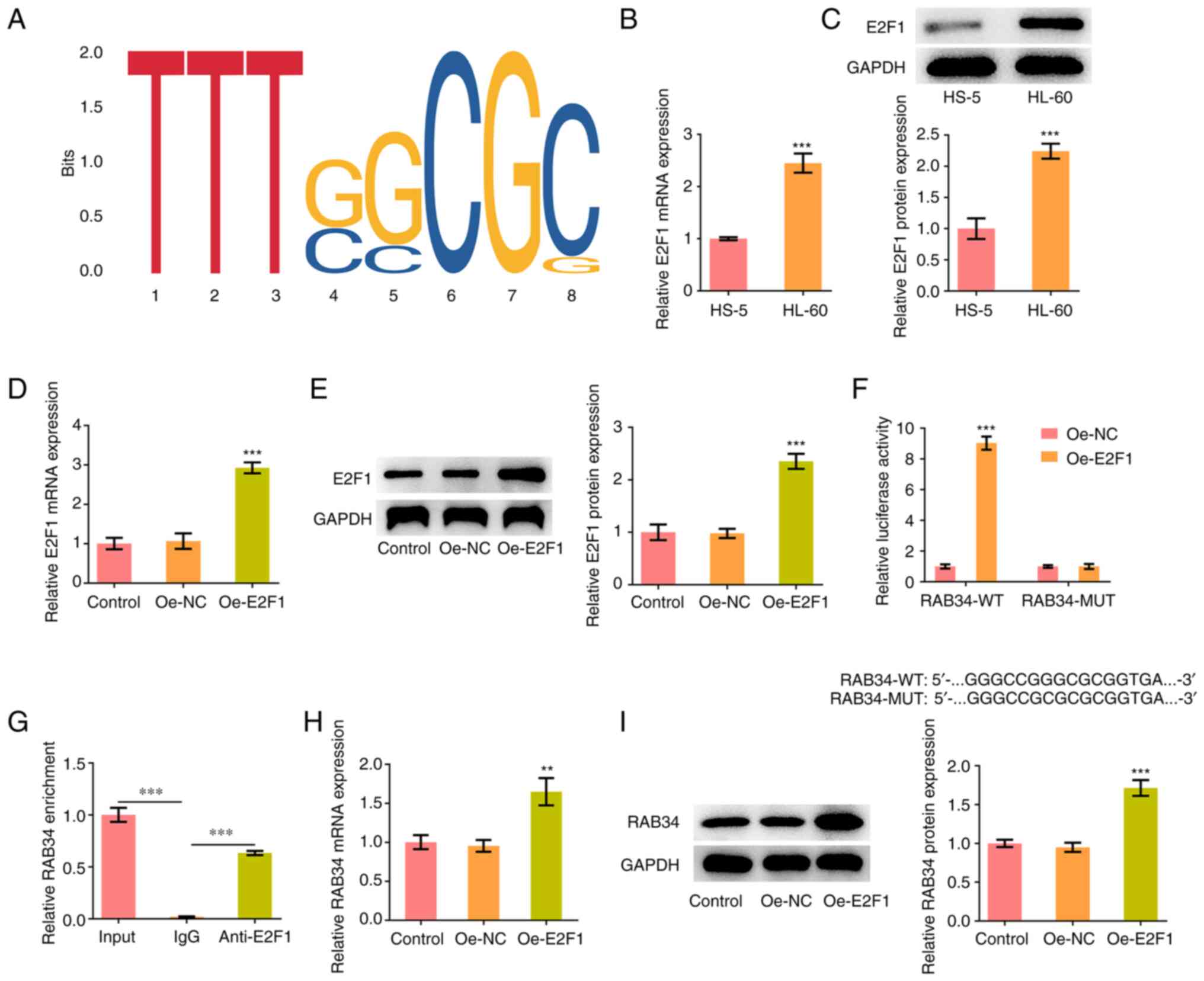
Based on the binding motif of E2F1, the HumanTFDB
website predicted the potential binding for the transcription
factor E2F1 on the RAB34 promoter (Fig. 4A). The expression of E2F1 in HL-60
cells was significantly increased compared with HS-5 cells as shown
through RT-qPCR and western blotting results (Fig. 4B and C). Subsequently, the E2F1 overexpression
plasmid was constructed and it was discovered than E2F1 expression
was significantly elevated by transduction of Oe-E2F1 plasmids,
which indicated successful transfection (Fig. 4D and E). Through the luciferase reporter assay,
it was demonstrated that the luciferase activity of RAB34-WT was
markedly enhanced after E2F1 was overexpressed (Fig. 4F). The ChIP assay also demonstrated
that the RAB34 promoter was enriched in the E2F1 antibody (Fig. 4G). In addition, RT-qPCR and western
blotting showed that RAB34 expression in HL-60 cells was
significantly increased after overexpressing E2F1 (Fig. 4H and I).
Overexpression of E2F1 reverses the
effect of RAB34 knockdown on the proliferation and apoptosis of AML
cells
The regulatory mechanism of RAB34 on the
proliferation and apoptosis of AML cells was next investigated.
Cells were divided into the control (shRNA-NC), shRNA-RAB34,
shRNA-RAB34 + Oe-NC and shRNA-RAB34 + Oe-E2F1 groups. CCK-8 and EdU
staining showed that compared with that in the shRNA-RAB34 + Oe-NC
group, cell proliferation of the shRNA-RAB34 + OE-E2F1 group was
markedly increased (Fig. 5A and
B). Flow cytometry showed that
compared with that in the shRNA-RAB34 + Oe-NC group, the proportion
of cells in the G0/G1 phase in the
shRNA-RAB34 + Oe-E2F1 group was significantly decreased, whilst
that in the S-phase in the shRNA-RAB34 + Oe-E2F1 group was
significantly increased (Fig. 5C).
Western blotting showed that E2F1 overexpression significantly
reversed the inhibitory effects of RAB34 knockdown on CDK4, CDK6
and cyclin D1 protein expression (Fig.
5D).
Subsequently, analysis of apoptosis showed that
compared with that in the shRNA-RAB34 + Oe-NC group, the
shRNA-RAB34 + Oe-E2F1 group showed a significant decrease in the
levels of apoptosis (Fig. 6A).
Furthermore, shRNA-RAB34 + Oe-E2F1 co-transfection significantly
increased Bcl-2 expression whilst decreasing Bax expression,
compared with those in the shRNA-RAB34 + Oe-NC group (Fig. 6B).
Discussion
The molecular mechanism of cancer occurrence and
development has been widely concerned and discussed. Identifying
novel biomarkers and potential drug targets in cancer will greatly
promote and enrich the early diagnosis and treatment of cancer
(16). At present, delaying cancer
onset and prevention of further metastasis by cancer cells may be
the more urgent aim to be achieved instead of the complete
eradication of cancer.
RAB34 is an important regulator of vesicle
transport, which exerts specific physiological functions by binding
to GTP or GDP since guanine nucleoside releasing protein on the
donor membrane recognizes the specific Rab protein in the cytosol,
which induces the release of GDP and binding to GTP, thus changing
the configuration of Rab protein (17). Because vesicle transport is an
important part of the normal physiological activities of cells,
abnormal expression of RAB proteins frequently leads to the
occurrence of cancer (18). A
previous study has shown that the expression of RAB34 is associated
with the gradual progression of glioma, where the prognosis of
patients with high-grade glioma is poor (19). In addition, the expression of miR-9
was found to be downregulated in gastric cancer, where its target
was the tumor-associated protein RAB34(20). These results suggest that RAB34
serves a role as an oncogene in tumor development. Through GEPIA
database analysis, the present study found that RAB34 expression
was significantly increased in patients with AML, where high RAB34
expression was significantly associated with poorer overall
survival. Subsequently, AML cell lines were selected and the
expression of RAB34 was measured. It was found that RAB34
expression was also markedly increased in the AML cell lines. To
further determine the regulatory effect of RAB34 on the
proliferation, cell cycle progression and apoptosis of AML cell
lines, the expression of RAB34 was knocked down in AML cells. It
was found that the viability and proliferation of AML cells were
decreased, cell cycle arrest occurred and the levels of cell
apoptosis were significantly increased. These results suggest that
RAB34 can also serve as an oncogene in AML.
By using the HumanTFDB database, the present study
identified the potential binding motif of E2F1 with the RAB34
promoter. It was also demonstrated that E2F1 could
transcriptionally regulate RAB34 expression in AML cells. E2F is an
important family of transcription factors that can regulate gene
expression (21). E2F1 is a member
of the E2F family, which is a central factor involved in the cell
cycle progression and apoptosis (22). Its regulatory role in cell
progression has been widely studied and reported. Previous studies
have found that E2F1 is highly expressed in a variety of tumor
cells, such as lung (23),
prostate (24) and breast cancer
(25). E2F1 is a pro-oncogene and
serves an important role in the occurrence and development of
tumors (23-25).
In addition, E2F1 and miR-223 have been reported to form an
autoregulatory negative feedback loop in AML (26). The present study found that the
expression of E2F1 in the AML cell line HL-60 was significantly
increased. In addition, overexpression of transcription factor E2F1
reversed the effects of RAB34 knockdown on the proliferation and
apoptosis of AML cells.
The present study has certain limitations.
Experiments were performed only in cell lines according to the
database conclusions. In the future, these findings should be
further explored in animal models and clinical human tissue
samples. In addition, the effect of RAB34 on the sensitivity of AML
cells to chemotherapeutic drugs was not considered in the present
study. This aspect should also be explored further in future
experiments.
In conclusion, the present results demonstrated that
E2F1-mediated RAB34 upregulation may regulate the proliferation,
cell cycle progression and apoptosis of AML. This provides a
theoretical basis for understanding the pathogenesis of AML and
designing the targeted therapy of AML.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XX conceived and designed the study. XX and GJ
performed the experiments and wrote the manuscript. All authors
read and approved the final manuscript. XX and GJ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khwaja A, Bjorkholm M, Gale RE, Levine RL,
Jordan CT, Ehninger G, Bloomfield CD, Estey E, Burnett A,
Cornelissen JJ, et al: Acute myeloid leukaemia. Nat Rev Dis
Primers. 2(16010)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pelcovits A and Niroula R: Acute myeloid
leukemia: A review. R I Med J. 103:38–40. 2020.PubMed/NCBI
|
|
3
|
De Kouchkovsky I and Abdul-Hay M: ‘Acute
myeloid leukemia: A comprehensive review and 2016 update’. Blood
Cancer J. 6(e441)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lonetti A, Pession A and Masetti R:
Targeted therapies for pediatric AML: Gaps and perspective. Front
Pediatr. 7(463)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stuck MW, Chong WM, Liao JC and Pazour GJ:
Rab34 is necessary for early stages of intracellular ciliogenesis.
Curr Biol. 31:2887–2894.e4. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Xi Y, Shen W, Jin C, Wang L and Yu B: PVT1
promotes the proliferation and migration of non-small cell lung
cancer via regulating miR-148/RAB34 signal axis. Onco Targets Ther.
13:1819–1832. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu J, Lu Y, Qin A, Qiao Z and Jiang X:
Overexpression of RAB34 correlates with poor prognosis and tumor
progression in hepatocellular carcinoma. Oncol Rep. 38:2967–2974.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun L, Xu X, Chen Y, Zhou Y, Tan R, Qiu H,
Jin L, Zhang W, Fan R, Hong W and Wang T: Rab34 regulates adhesion,
migration, and invasion of breast cancer cells. Oncogene.
37:3698–3714. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Denechaud PD, Fajas L and Giralt A: E2F1,
a novel regulator of metabolism. Front Endocrinol (Lausanne).
8(311)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang K, Liu JD, Deng G, Ou ZY, Li SF, Xu
XL, Zhang MJ, Peng XQ and Chen FH: LncSIK1 enhanced the sensitivity
of AML cells to retinoic acid by the E2F1/autophagy pathway. Cell
Prolif. 55(e13185)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Feng Y, Hu S, Li L, Zhang S, Liu J, Xu X,
Zhang M, Du T, Du Y, Peng X and Chen F: LncRNA NR-104098 inhibits
AML proliferation and induces differentiation through repressing
EZH2 transcription by interacting with E2F1. Front Cell Dev Biol.
8(142)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu X, Cai Y, Cheng C, Gu Y, Hu X, Chen K,
Wu Y and Wu Z: PCLAF promotes neuroblastoma G1/S cell cycle
progression via the E2F1/PTTG1 axis. Cell Death Dis.
13(178)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li C, Tang Z, Zhang W, Ye Z and Liu F:
GEPIA2021: Integrating multiple deconvolution-based analysis into
GEPIA. Nucleic Acids Res. 49(W1):W242–W246. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hu H, Miao YR, Jia LH, Yu QY, Zhang Q and
Guo AY: AnimalTFDB 3.0: A comprehensive resource for annotation and
prediction of animal transcription factors. Nucleic Acids Res.
47(D1):D33–D38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen T: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Costa-Pinheiro P, Montezuma D, Henrique R
and Jerónimo C: Diagnostic and prognostic epigenetic biomarkers in
cancer. Epigenomics. 7:1003–1015. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ganga AK, Kennedy MC, Oguchi ME, Gray S,
Oliver KE, Knight TA, De La Cruz EM, Homma Y, Fukuda M and Breslow
DK: Rab34 GTPase mediates ciliary membrane formation in the
intracellular ciliogenesis pathway. Curr Biol. 31:2895–2905.e7.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tzeng HT and Wang YC: Rab-mediated vesicle
trafficking in cancer. J Biomed Sci. 23(70)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang HJ, Gao Y, Chen L, Li YL and Jiang
CL: RAB34 was a progression- and prognosis-associated biomarker in
gliomas. Tumour Biol. 36:1573–1578. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Luo H, Zhang H, Zhang Z, Zhang X, Ning B,
Guo J, Nie N, Liu B and Wu X: Down-regulated miR-9 and miR-433 in
human gastric carcinoma. J Exp Clin Cancer Res.
28(82)2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kent LN and Leone G: The broken cycle: E2F
dysfunction in cancer. Nat Rev Cancer. 19:326–338. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fouad S, Hauton D and D'Angiolella V:
E2F1: Cause and consequence of DNA replication stress. Front Mol
Biosci. 7(599332)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Malaney P, Palumbo E, Semidey-Hurtado J,
Hardee J, Stanford K, Kathiriya JJ, Patel D, Tian Z, Allen-Gipson D
and Davé V: PTEN Physically Interacts With And Regulates
E2F1-mediated transcription in lung cancer. Cell Cycle. 17:947–962.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Rodriguez-Bravo V, Pippa R, Song WM,
Carceles-Cordon M, Dominguez-Andres A, Fujiwara N, Woo J, Koh AP,
Ertel A, Lokareddy RK, et al: Nuclear pores promote lethal prostate
cancer by increasing POM121-Driven E2F1, MYC, and AR nuclear
import. Cell. 174:1200–1215.e20. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ma J, He Z, Zhang H, Zhang W, Gao S and Ni
X: SEC61G promotes breast cancer development and metastasis via
modulating glycolysis and is transcriptionally regulated by E2F1.
Cell Death Dis. 12(550)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pulikkan JA, Dengler V, Peramangalam PS,
Peer Zada AA, Müller-Tidow C, Bohlander SK, Tenen DG and Behre G:
Cell-cycle regulator E2F1 and microRNA-223 comprise an
autoregulatory negative feedback loop in acute myeloid leukemia.
Blood. 115:1768–1778. 2010.PubMed/NCBI View Article : Google Scholar
|