Introduction
Intraosseous lipoma is an extremely rare neoplasm
that constitutes no more than 0.1% of all the bone tumors (1). The first case of intraosseous lipoma
was described in 1955, with Milgram having published the largest
series of these lesions (2,3).
Lipoma is common in adults, being slightly more common in males
(4). The recurrence rate of lipoma
is <5%. Because it is benign, the mortality rate is 0%. The
treatment strategy is based on complete resection. Although pain is
the major symptom reported, more than 30% of the cases have been
found incidentally through imaging studies performed for other
reasons (3,5). Intraosseous lipomas occur mainly in
the lower limbs with calcaneus and long tubular bones as common
lesion sites. Nevertheless, these lesions may occur anywhere in the
skeleton (3,5,6)
making them difficult to diagnose. Intraosseous lipoma of the
scapula is extremely rare and, so far, only case has been reported
(7). Here, we report the case of
an aggressive intraosseous lipoma that extended outside of the
scapula.
Case report
The patient was a 79-year-old woman with no previous
medical history. The patient had no complaints of localized pain in
the scapula prior to the visit. When the patient underwent chest
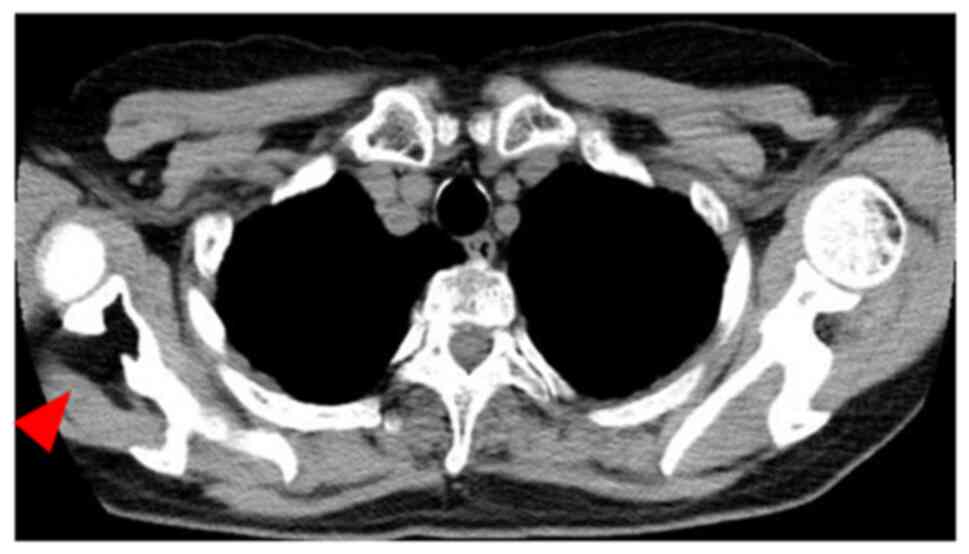
radiography and computed tomography (CT) scan at the Department of
General Medicine in our hospital (Kushimoto Hospital) in January
2019, a lytic lesion was noticed in the right scapula (Fig. 1). The pulmonologist advised the
patient to undergo a detailed examination and further tests.
However, owing to personal reasons, the patient did not visit the
hospital for 2 years. Additionally, no significant changes were
observed in blood test parameters (Table I). The inflammatory response was
also negative (CRP=0.06 mg/dl, WBC=5300/µl).
 | Table IBlood test results. |
Table I
Blood test results.
| Item | Value (normal
value) |
|---|
| Total protein,
g/dl | 6.5 (6.5-7.9) |
| Albumin, g/dl | 3.8 (3.8-4.5) |
| BUN, mg/dl | 20.1 (8.0-20.0) |
| Creatinine,
mg/dl | 0.59 (0.5-0.9) |
| eGFR, ml/min/1.73
m2 | 73 (>60) |
| Urea acid, mg/dl | 4.5 (2.5-5.8) |
| Total cholesterol,
mg/dl | 208 (140-199) |
| Total bilirubin,
mg/dl | 0.7 (0.2-1.2) |
| AST, U/l | 22 (7-23) |
| ALT, U/l | 25 (7-23) |
| ALP, U/l | 209 (38-113) |
| CK, U/l | 90 (20-150) |
| Na, mEq/l | 143 (135-150) |
| Cl, mEq/l | 107 (101-108) |
| K, mEq/l | 4.4 (3.5-5.0) |
| Ca, mg/dl | 8.9 (8.8-10.4) |
| CRP, mg/dl | 0.06 (<0.03) |
| WBC, /µl | 5,300
(4,000-8,000) |
| Hb, g/dl | 12 (11.4-14.6) |
| PLT, /µl | 21.4x104
(25x104-40x104) |
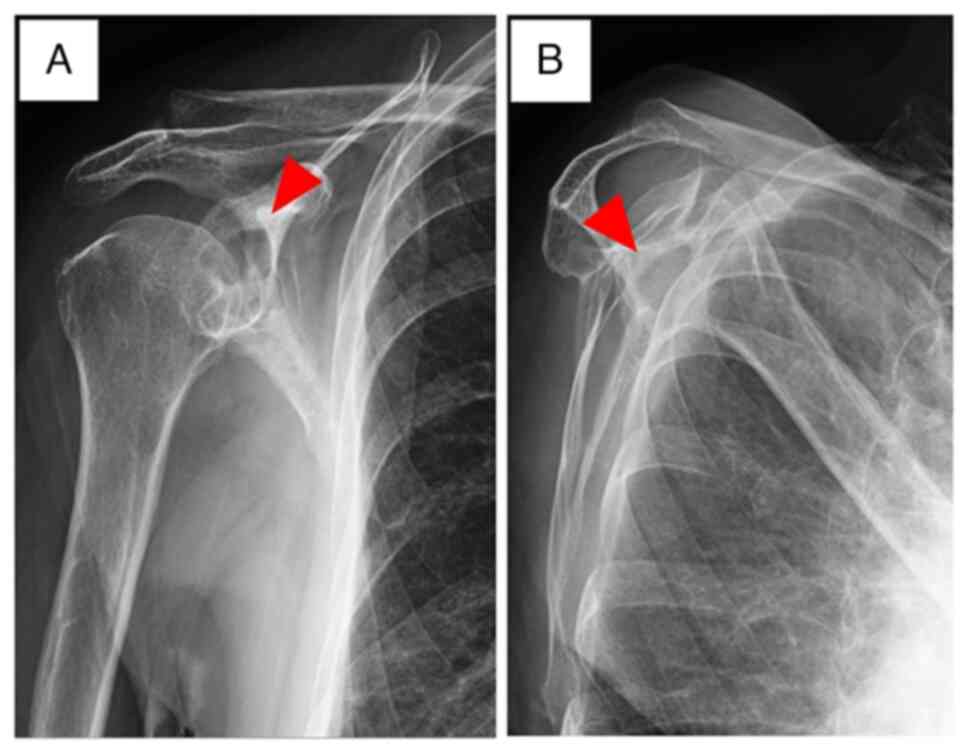
The patient visited the hospital again and was
referred to our clinic for further examination in January 2021.
Radiographs of the right scapula showed a lytic lesion in the
glenoid fossa (Fig. 2). Later, in
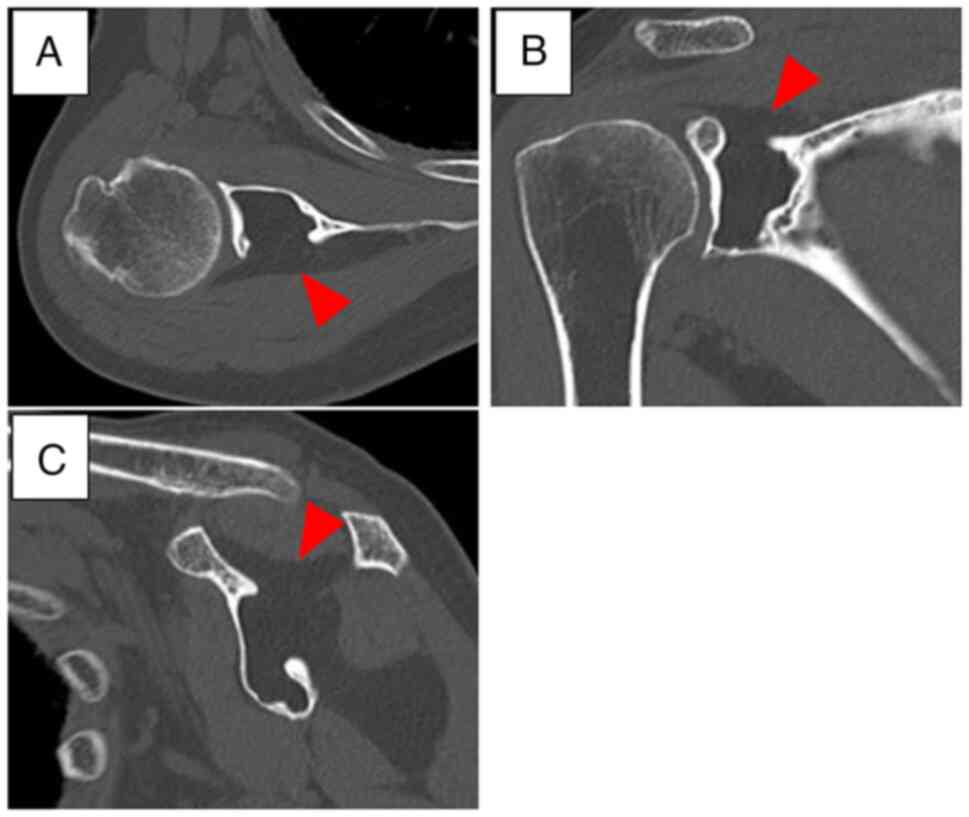
February 2021, a CT scan of the right scapula also revealed a
low-density, homogeneous lytic lesion in the same region (Fig. 3). Specifically, the destruction of
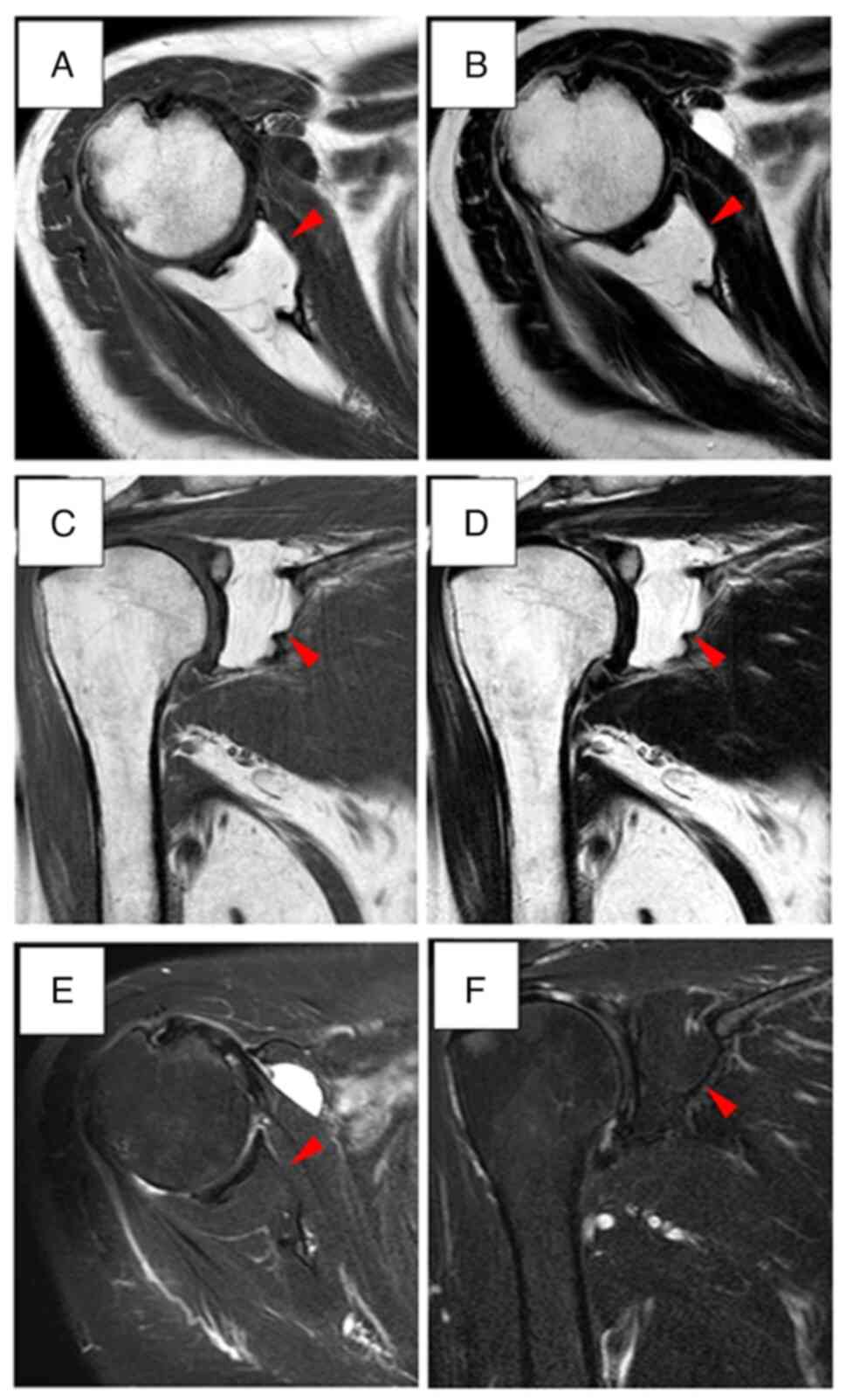
dorsal cortical bone of the glenoid fossa was observed. Magnetic
resonance imaging (MRI) also indicated a high-intensity mass on
both T1 and T2-weighted images in February 2021 (Fig. 4). No septa were detected inside the
tumor mass. Since the lesion was deep and had spread outside the
skeleton, the possibility of malignancy was considered. Therefore,
in March 2021, we performed a tumor resection. The final
observational visit took place in June 2023 and no recurrence was
observed.
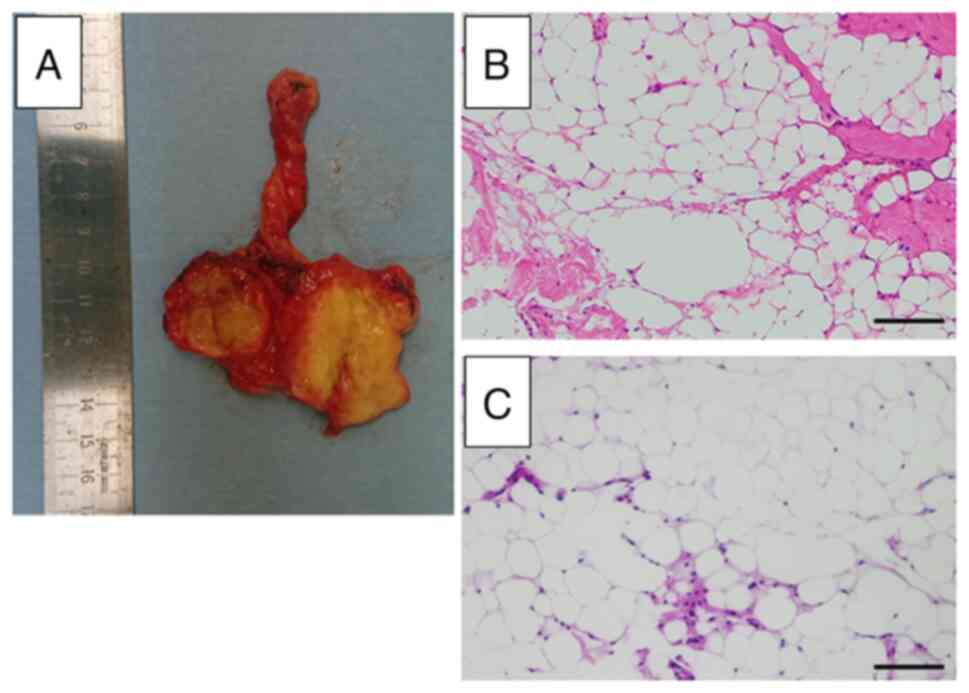
The tumor appeared yellow and shiny. Hematoxylin and
eosin staining was performed by our laboratory technician (M.T.)
per standard protocol. Tumor tissue samples were fixed in 4%
paraformaldehyde at 4˚C for 12 h. After washing with
phosphate-buffered saline (PBS), the samples were decalcified in
10% ethylenediaminetetraacetic acid solution at 4˚C for 2 weeks and
then embedded in paraffin. Coronary sections (5-µm) were cut and
mounted onto slides. The sections were deparaffinized in xylene and
dehydrated using an ethanol gradient, and then immersed in
hematoxylin solution (Agilent Technologies) for 10 min. The
sections were washed in PBS for 5 min, and then immersed in eosin
solution (Agilent Technologies) for 5 min. The histopathological
examination by our pathologist (T.I.) revealed that the tumor was a
lipoma (Fig. 5). No lipoblasts
were observed. The patient was followed up for 2 years. Patients
with lipoma are followed up postoperatively every 6 months with CT
examination. Neither recurrence nor shoulder joint range of motion
restriction were seen thereafter.
The patient provided written informed consent for
the publication of this information. Ethical approval was not
necessary because the patient was conventionally treated.
Discussion
Intraosseous lipoma is one of the rarest tumors with
an incidence of 0.1-2.5% (1,8,9).
Most intraosseous lipomas occur in the lower extremities (71%). The
most common site is the calcaneus (32%), followed by the
subtrochanteric femur, proximal tibia, distal femur, and proximal
and distal fibula (10). Lesions
of the upper extremities usually involve the proximal and distal
humerus and the radial shaft, although they have also been observed
in the mandible, pelvis, and ribs (11). To date, only one case of
intraosseous lipoma (early phase) has been reported in a
20-year-old man, but without any sign of destruction in the body of
the scapula (7). Compared to this
previously reported case, the present case is characterized by the
absence of pain. Moreover, for the present case, a relatively
detailed treatment course has also been provided.
Intraosseous lipomas are highly prevalent in the
calcaneus and femur intertrochanteric regions, where trabecular
bone is scarce. This has led to the hypothesis that these lipomas
are an ‘overshoot’ phenomenon that occurs during the transition
from hematopoietic to fatty bone marrow (12). Further, it was suggested that the
intraosseous lipomas developing in these regions can likely be
considered hematomas rather than neoplasms (1,8). In
the current case, the tumor was present in the scapula, which is a
flat bone. In addition, the patient did not face any trauma in the
past according to her medical record. Therefore, the tumor cannot
be considered a hematoma.
In general, intraosseous lipomas are painless
(3,5,11).
If painful, they are thought to be due to coexisting bone
expansion, remodeling, and ischemic changes in the bone. Also,
pathological fractures are uncommon (1,3,5). The
current case is an extremely rare one in which the patient,
inexplicably, did not complain of any pain, despite pathological
fracture. However, it is important to keep in mind that lipomas in
the scapula can cause pathological fractures.
Milgram classified intraosseous lipomas into three
types. In the first type, the tumor contains viable fat cells; in
the second type, the viable fat cells are partially replaced by
necrosis and calcification; and in the third type, necrosis,
calcification, and lipid cyst formation are seen (3). The current case is a rare and
aggressive case of extraosseous extension in spite of
classification I. Furthermore, the differential diagnosis, in this
case, included fibrous dysplasia, inflammation, hematoma, malignant
soft tissue tumor, and bone tumor. MRI findings of a typical acute
hematoma include a hypointense mass on T1-weighted images and a
hyperintense mass on T2-weighted images (13). The presence of fresh and
preexisting hematomas may present as a mosaic pattern (13). In this case, there were no MRI
findings suggestive of hematoma. Histopathology was performed for
the excised specimen to confirm the diagnosis. Notably, the
intraosseous lipoma lesions may be indistinguishable from normal
adipose tissue in the xanthoma, making the pathological
interpretation difficult (1,8,9).
It is seldom possible to remove an intraosseous
lipoma completely. Usually, these lipomas do not show any symptoms,
have a slow course, and can be managed with a ‘wait and see’
approach (1,5). The exception to this is when the
lipoma results in a pathological fracture and extraskeletal
extension (11). In the present
case, surgical resection was performed because of extraskeletal
extension and the possibility of malignancy. Intraosseous lipomas
have a favorable prognosis with no recurrences following lesion
curettage and grafting (6,7) and complete involution (14,15).
These tumors are considered benign and patients are usually
asymptomatic. These facts have prompted other authors to propose
clinical and radiological observations only, rather than active
treatment (15,16). Thus, the prognosis of intraosseous
lipoma has been reported to be excellent; however, a careful
follow-up is necessary for intraosseous lipoma particularly in
cases with extraosseous extension.
The current case presentation has a limitation. We
failed to demonstrate negative immunohistochemical findings for
MDM2 or CDK4, which are specific for liposarcoma (17). However, we were able to confirm the
benign nature of the tumor by demonstrating the absence of nuclear
atypia on hematoxylin and eosin imaging. Additional immunostaining
for MDM2 and CDK4 should be performed in future similar cases.
Oncologists must consider that intraosseous lipoma
might occur in the scapula with cortical bone destruction.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NO, YS, SN, MA and KH participated in data
collection, analysis and manuscript writing. YS, SN and MA
participated in the study design. NO, KH, YS, SN and MA confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The case studies were conducted in accordance with
the Declaration of Helsinki. Ethical approval was not necessary
because the patient was conventionally treated.
Patient consent for publication
Written informed consent for publication was
obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Murphey MD, Carroll JF, Flemming DJ, Pope
TL, Gannon FH and Kransdorf MJ: From the archives of the AFIP:
Benign musculoskeletal lipomatous lesions. Radiographics.
24:1433–1466. 2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Eroglu A, Gundogdu C, Turkyilmaz A and
Karaoglanoglu N: Intraosseous lipoma of the rib. J Thorac
Cardiovasc Surg. 130:1468–1469. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Milgram JW: Intraosseous lipomas. A
clinicopathologic study of 66 cases. Clin Orthop Relat Res.
231:277–302. 1988.PubMed/NCBI
|
|
4
|
Fletcher CD, Lazar AJ, Baldini EH, Messiou
C, Blay JY, Pollock RE, et al: WHO classification of tumors of soft
tissue and bone. Fritchie KJ, Goldblum JR and Mertens F (eds). 5th
edition. IARC Publications, Lyon, pp13, 2020.
|
|
5
|
Campbell RS, Grainger AJ, Mangham DC,
Beggs I, Teh J and Davies AM: Intraosseous lipoma: Report of 35 new
cases and a review of the literature. Skelet Radiol. 32:209–222.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Radl R, Leithner A, Machacek F, Cetin E,
Koehler W, Koppany B, Dominkus M and Windhager R: Intraosseous
lipoma: Retrospective analysis of 29 patients. Int Orthop.
28:374–378. 2004.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kang HS, Kim T, Oh S, Park S and Chung SH:
Intraosseous lipoma: 18 years of experience at a single
institution. Clin Orthop Surg. 10:234–239. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Palczewski P, Świątkowski J, Gołębiowski M
and Błasińska-Przerwa K: Intraosseous lipomas: A report of six
cases and a review of literature. Pol J Radiol. 76:52–59.
2011.PubMed/NCBI
|
|
9
|
Propeck T, Bullard MA, Lin J, Doi K and
Martel W: Radiologic-pathologic correlation of intraosseous
lipomas. AJR Am J Roentgenol. 175:673–678. 2000.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim JT, Han YM, Chung DS and Park YS:
Intraosseous lipoma of the lumbar spine. J Korean Neurosurg Soc.
35:220–222. 2004.
|
|
11
|
Hashimoto K, Nishimura S, Kakinoki R and
Akagi M: Aggressive intraosseous lipoma of the intermediate
phalanges of the thumb. Mol Clin Oncol. 9:62–65. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lanisnik B and Didanovic V: Sphenoclival
intraosseus lipoma: Case report and literature review. Skull Base.
17:211–214. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Akata S, Ohkubo Y, Jinho P, Saito K,
Yamagishi T, Yoshimura M, Kotake F, Kakizaki D and Abe K: MR
features of a case of chronic expanding hematoma. Clin Imaging.
24:44–46. 2000.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mannem RR, Mautz AP, Baynes KE, Zambrano
EV and King DM: AIRP best cases in radiologic-pathologic
correlation: Intraosseous lipoma. Radiographics. 32:1523–1528.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Goto T, Kojima T, Iijima T, Yokokura S,
Motoi T, Kawano H, Yamamoto A and Matsuda K: Intraosseous lipoma: A
clinical study of 12 patients. J Orthop Sci. 7:274–280.
2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bagatur AE, Yalcinkaya M, Dogan A, Gur S,
Mumcuoglu E and Albayrak M: Surgery is not always necessary in
intraosseous lipoma. Orthopedics. 12(33)2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kammerer-Jacquet SF, Thierry S, Cabillic
F, Lannes M, Burtin F, Henno S, Dugay F, Bouzillé G, Rioux-Leclercq
N, Belaud-Rotureau MA and Stock N: Differential diagnosis of
atypical lipomatous tumor/well-differentiated liposarcoma and
dedifferentiated liposarcoma: utility of p16 in combination with
MDM2 and CDK4 immunohistochemistry. Hum Pathol. 59:34–40.
2017.PubMed/NCBI View Article : Google Scholar
|