Introduction
Primary central nervous system lymphoma (PCNSL) is a
rare extranodal subtype of non-Hodgkin lymphoma (NHL) that affects
the brain, leptomeninges, eyes, cranial nerves, and spinal cord; it
accounts for ~3% of all primary brain tumors (1). Commonly, the underlying pathology of
PCNSL is diffuse large B-cell lymphoma (2). PCNSL is most often seen as isointense
or hypointense lesions on both T1- and T2-weighted MRI scans, and
the lesions tend to moderately enhance with intravenous contrast
administration (3).
Approximately 65% of PCNSLs present as a single
brain lesion (4). Lesions most
commonly affect the supratentorial brain and are typically found in
contact with the ventricular and meningeal surfaces, but they can
also lay deeper within the brain parenchyma (5). The disease typically does not involve
the brainstem, cerebellum, or spinal cord. PCNSL incidence
increases with age, with a peak incidence among those between 50
and 70 years old (6). Incidence
also increases with congenital and acquired immunosuppression, and
PCNSL is commonly mediated by Epstein-Barr virus (EBV), especially
in the context of inadequate cytotoxic T-cell activity (7).
EBV-associated PCNSL is a known complication of
iatrogenic immunosuppression in the posttransplant population and
among those receiving immunosuppressive therapy for immune-mediated
conditions (8). Withdrawal of the
immunosuppressive agent can even lead to PCNSL regression for some
patients (9). Though vitrectomy or
cerebrospinal fluid (CSF) cytology may be sufficient to diagnose
PCNSL if the eye is involved, the gold standard for diagnosis is a
stereotactic biopsy (4).
In this article, we report a case of EBV-induced
PCNSL caused by prolonged immunosuppression with mycophenolate
mofetil in which the patient experienced delays in diagnosis
because of atypical features in the disease presentation and
radiographic findings. This case report highlights how misdiagnosis
or delayed PCNSL diagnosis and delayed withdrawal of
immunosuppressive agents can lead to PCNSL progression. This
patient experienced remission after whole-brain radiation therapy
(WBRT).
Case report
The patient was a 68-year-old right-handed man with
stage 3 chronic kidney disease and acetylcholine receptor
antibody-positive myasthenia gravis (MG) who received mycophenolate
mofetil 500 mg twice daily for 22 years. He presented with a few
months of worsening left eye vision and left facial paresthesia and
several weeks of imbalance, vertigo, sensory changes, and vocal
changes. He initially attributed his declining left eye vision to a
herpes zoster ophthalmicus infection that occurred 3 months prior
to presentation, in the left V1 distribution. For this infection,
he was previously prescribed antiviral medication.
On examination, he was found to have mild left eye
ptosis; his eyelid was above the level of his pupil, which was not
fatigable. He had decreased left eye vision (20/40); restriction of
left eye abduction and adduction; restriction of right abduction
without subjective diplopia; vertical and horizontal nystagmus;
slight asymmetry of the left lower face; decreased sensation of
soft touch in the left lower extremity; truncal and left-sided
ataxia; and left-sided hyperreflexia. On ophthalmological
examination, he was found to have punctate keratitis with potential
endotheliitis.
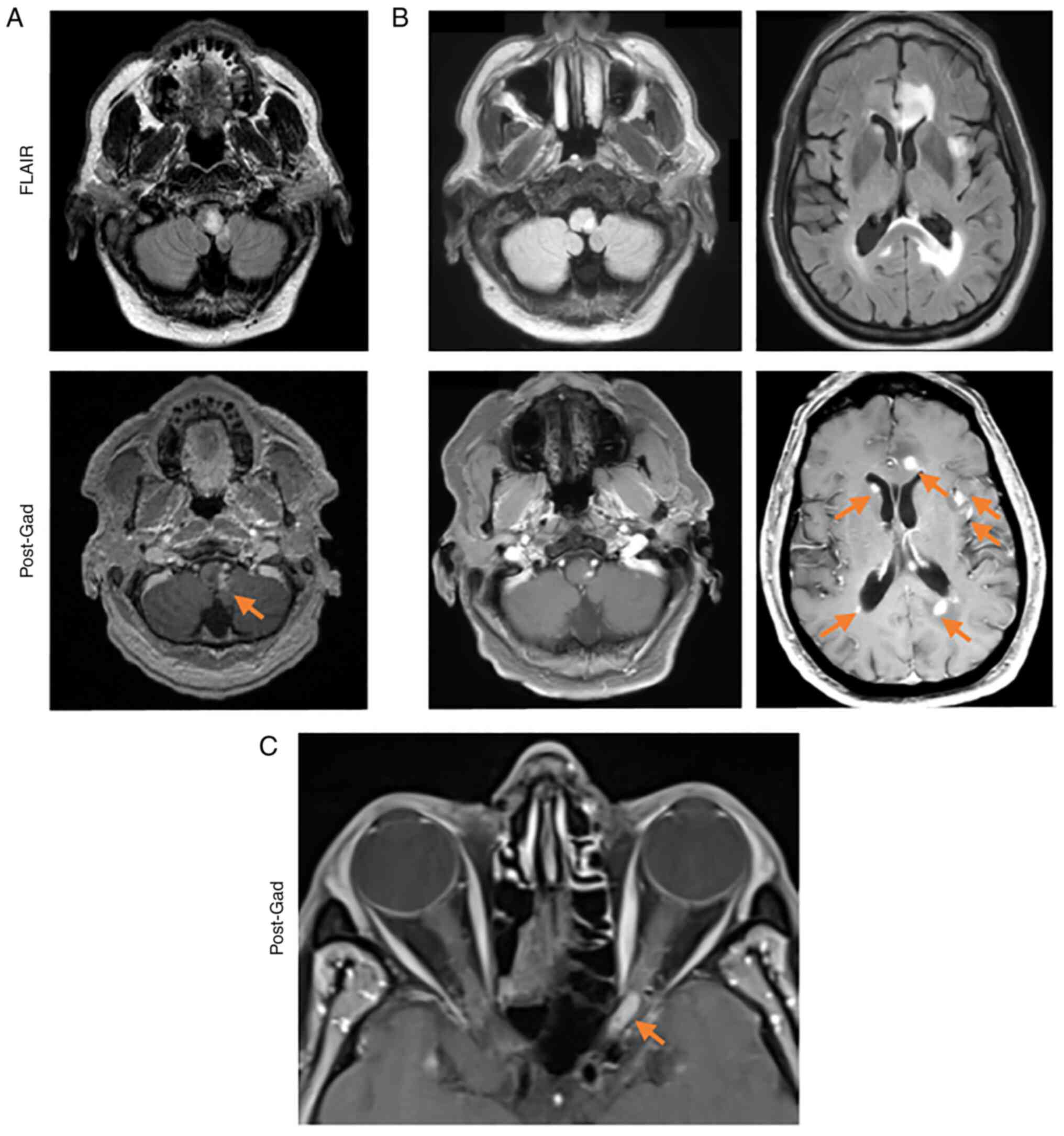
A brain MRI showed a suspicious enhancing lesion
within the left medulla that extended to the cerebellum, with no
evidence of hydrocephalus (Fig.
1A). An MRI of the spine and CT scan of the chest, abdomen, and
pelvis did not show any malignancy. A lumbar puncture (LP) showed
an opening pressure of 27 cm H2O, a nucleated cell level
of 238 cell/mm3 with 98% lymphocytes, and a total
protein level of >200 mg/dl. CSF was negative for any bacterial
or fungal cultures. CSF analysis was negative for carcinoma,
meningitis, herpes simplex virus (HSV), varicella-zoster virus
(VZV), cytomegalovirus (CMV), Toxoplasma gondii parasite, Lyme
disease, or venereal disease research laboratory (VDRL) tests;
however, the patient was positive for EBV by qualitative polymerase
chain reaction (PCR). At this point, the working diagnosis for this
patient was VZV vasculopathy. He received 750 mg of intravenous
acyclovir twice daily for 14 days and 60 mg of oral prednisone for
5 days.
Three months after his discharge, a repeat brain MRI
showed multiple new enhancing lesions that developed bilaterally
along the periventricular white matter with involvement of the
corpus callosum. There were several lesions in peripheral locations
of the cerebrum, cerebellum, and brainstem with little mass effect
(Fig. 1B) and no evidence of
restricted diffusion. He was referred to the Neuro-Oncology clinic
at Moffitt Cancer Center (MCC). During his first visit at MCC, he
complained of blurry vision and pain in the left eye, paresthesia
involving the left side of his face, poor balance, and left-sided
ataxia. An orbital MRI showed an enhancing lesion on the left optic
nerve and full-thickness enhancement (Fig. 1C). At this time, his wife also
reported that he exhibited intermittent confusion, memory decline,
and word-finding difficulty that had been more noticeable in the
last few months.
Two days after his visit, he presented to his local
emergency room with acutely worsening blurry vision and pain in the
left eye, nausea, and vomiting. He was prescribed 4 mg of
dexamethasone every 6 h and received 3 doses before he was
transferred to Moffitt Cancer Center. A repeat brain MRI showed
interval increases in the size of his preexisting lesions, with
interval development of central necrosis, restricted diffusion,
more indistinct margins of the enhancement, and areas of petechial
hemorrhage. A repeat LP showed an opening pressure of 15 cm
H2O, a nucleated cell level of 54 cells/mm3
with 88% lymphocytes, and a total protein level of 153 mg/dl. CSF
analysis was again negative for carcinoma and positive for EBV via
quantitative PCR, with 201,000 virus copies/cc. A repeat CT scan of
the chest, abdomen, and pelvis was negative for malignancy.
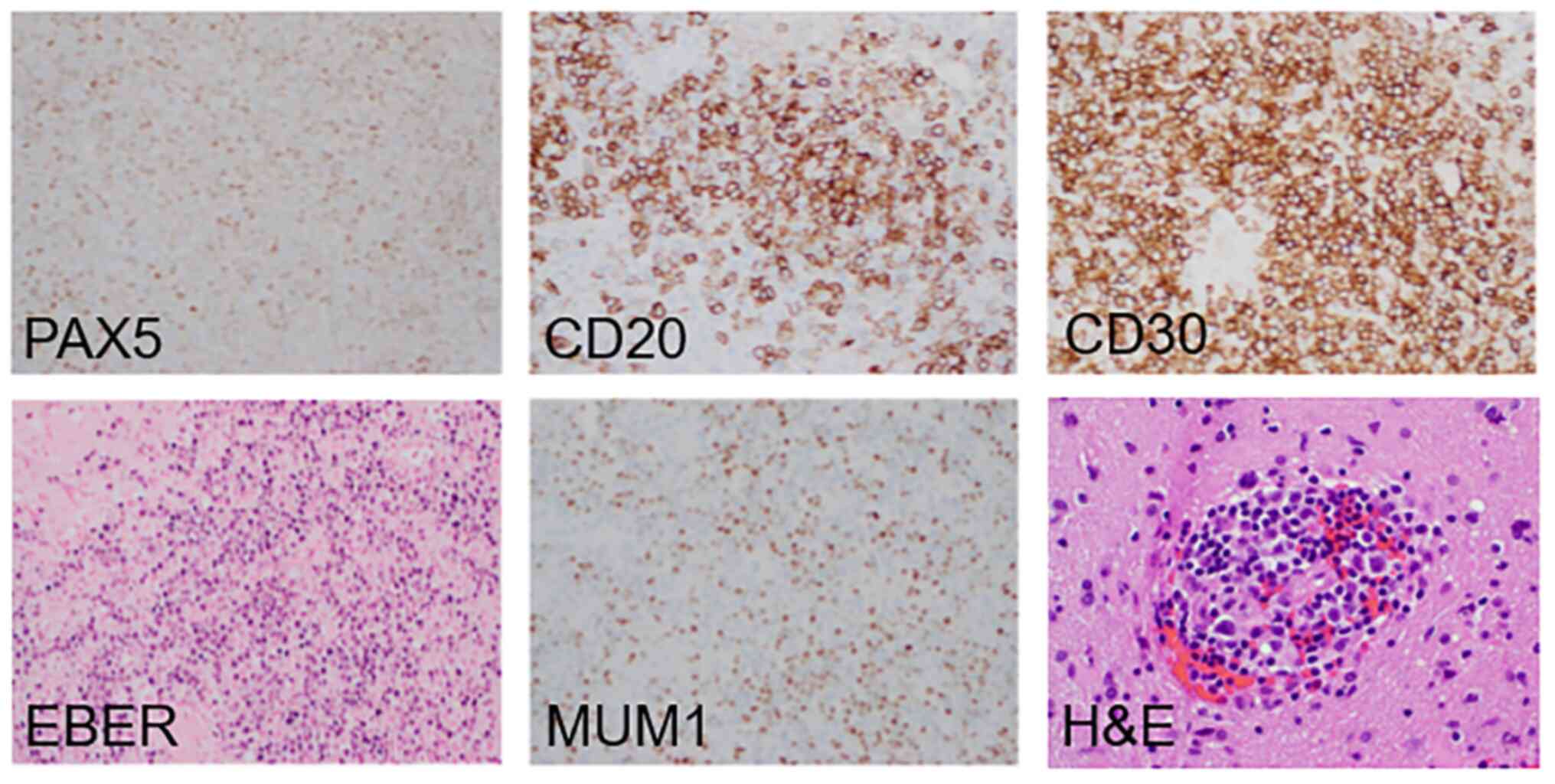
A week later, a neurosurgeon resected the left
frontal lesion, which was identified as EBV-induced diffuse large
B-cell lymphoma. The lesion showed positive expression of PAX5,
CD20, MUM1, CD30, and EBER (Fig. 2). Peripheral blood mononuclear
cells (PBMC) were prepared by Ficoll-Hypaque density gradient
separation. Automated counts (Sysmex KX-21N; Sysmex, Lincolnshire,
IL) were performed to quantify recovered cells. DNA was isolated
from PBMC (2x106 cells) and plasma (200 µl) using QIAmp
DNA blood mini reagents (Qiagen, Gaithersburg, MD). EBV qPCR was
performed on DNA using Qiagen/Artus EBV analyte specific reagents
(Qiagen) and 7500 Real-Time PCR System (Applied Biosystems/Thermo
Fisher Scientific, Foster City, CA), with a 97 base pair region of
EBV nuclear antigen-1 (EBNA-1) as the target. The primer used for
EBV PCR testing in CSF was EBNA-1 reaction:
5'CCGCTCCTACCTGCAATATCA 3' (forward primer) and
5'GGAAACCAGGGAGGCAAATC 3' (reverse primer);
5'VIC-TGCAGCTTTGACGATGG-MGB 3' (probe). The limit of detection by
qPCR was 50 copies per ml plasma or per 100,000 PBMCs.
Mycophenolate mofetil was discontinued. The patient received one
500 mg/m2 dose of intravenous rituximab and experienced
a dramatic improvement in his left eye vision and cognitive
deficits within 24 h. He also received one dose of high-dose
methotrexate (3.5 gm/kg) the next day but unfortunately developed
worsening kidney failure, which required hemodialysis. He received
5 more doses of rituximab on a 2-week schedule (ie, treated for a
total of 12 weeks), and a repeat brain MRI after the sixth dose
showed improvement in some lesions and progression in others. He
received WBRT (23.4 Gy) with craniospinal radiation, which resulted
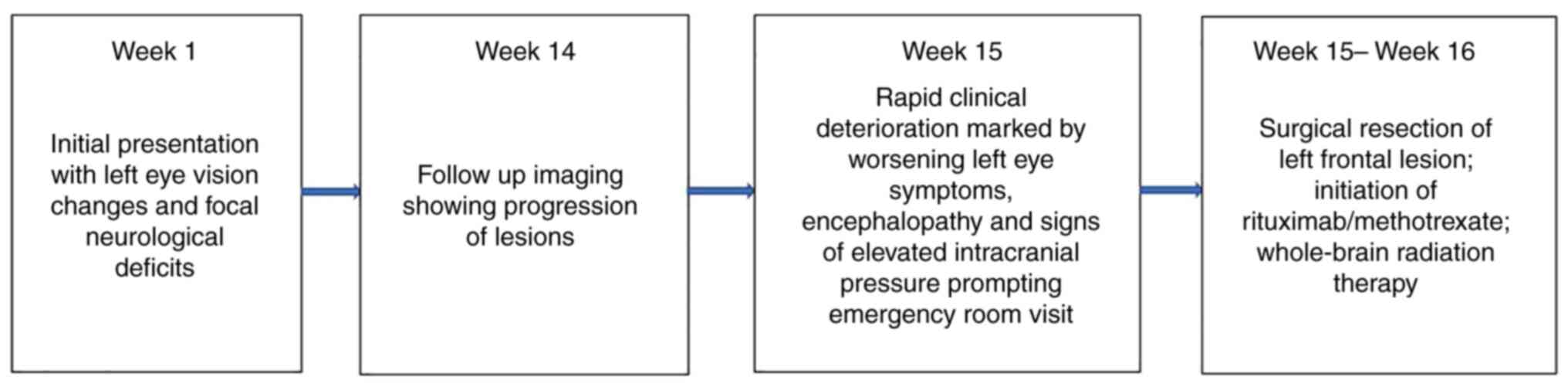
in complete remission. Please refer to Fig. 3 for a gross visual summary of the
timeline of events in the present case and to Table I for a more in-depth summary of the
timeline of clinical symptoms, findings and treatments.
 | Table IClinical summary. |
Table I
Clinical summary.
| Characteristics | Case |
|---|
| Clinical features at
initial presentation | Left eye vision
changes and left facial paresthesias for a few months with several
weeks of imbalance, vertigo, sensory changes and vocal changes |
| MRI results at
initial presentation | Abnormal
T2-weighted-FLAIR contrast-enhancing lesion within the left medulla
extending to the cerebellum without evidence of hydrocephalus |
| CSF results at
initial presentation | Nucleated cells: 238
cell/mm3 with 98% lymphocytes; total protein level:
>200 mg/dl; negative bacterial and fungal cultures, herpes
simplex virus DNA by qualitative PCR, varicella-zoster virus DNA by
PCR, cytomegalovirus DNA by PCR, Toxoplasma gondii DNA by PCR, Lyme
disease DNA by PCR, venereal disease research laboratory test;
cytology was negative for the presence of atypical/malignant cells;
positive for EBV by PCR |
| Therapies given at
initial presentation | 750 mg of intravenous
acyclovir twice daily for 14 days and oral prednisone 60 mg daily
for 5 days |
| MRI results at
3-months follow up | Multiple new
T2-weighted FLAIR contrast-enhancing lesions bilaterally along the
periventricular white matter with involvement of the corpus
callosum and several lesions in peripheral locations of the
cerebrum, cerebellum and brainstem with little mass effect |
| Clinical features at
second presentation | Encephalopathy with
acutely worsening blurry vision and pain in the left eye, nausea,
and vomiting |
| CSF results at second
presentation | Nucleated cells: 54
cells/mm3 with 88% lymphocytes; total protein level: 153
mg/dl. Negative bacterial and fungal cultures; cytology was
negative for the presence of atypical/malignant cells; positive for
EBV by PCR with qualitative analysis revealing 201,000 virus
copy/cc |
| Interventions and
therapies given at second presentation | Gross total resection
of the left frontal lesion which showed EBV-induced diffuse large
B-cell lymphoma (see Fig. 2);
intravenous dexamethasone; 3 cycles of 500 mg/m2
intravenous rituximab; whole-brain radiation therapy with
craniospinal radiation |
| Response to
treatment | Dramatic improvement
in his left eye vision and cognitive deficits followed by complete
remission |
Discussion
This case report highlights how delays in PCNSL
diagnosis due to atypical imaging features can lead to PCNSL
progression. It also emphasizes the importance of recognizing
diagnostic clues, such as positive EBV PCR, the patient's
immunosuppression, presentation with progressive neurological
symptoms, and presence of a new brain lesion. Early diagnosis and
treatment of PCNSL among patients receiving immunosuppressive
therapy is crucial; in this case, a heightened index of suspicion
for PCNSL based on these factors would have warranted a brain
biopsy during the patient's first hospitalization. However, the
patient had only a medullary lesion, which was thought to be due to
VZV vasculitis at presentation, so a brain biopsy was not
performed. An earlier diagnosis would have resulted in immediate
treatment of the isolated brain lesion and, subsequently, reduced
neurological burden.
This case had limitations that complicated the
diagnostic process. Firstly, a notable limitation of this patient's
diagnosis was his recent co-occurring zoster infection at the time
of his initial presentation. It was thought that his brain lesion
in the medulla was due to VZV vasculopathy. Secondly, PCNSL is
known to be notably responsive to steroids, so the patient's
dexamethasone treatment may have masked some of his neurological
symptoms and normalized the cellular examination of the CSF.
Regarding the CSF studies, it is also notable that we did not check
CSF interleukin-10 for this patient which is a known helpful tool
to distinguish between an infection and B cell lymphoma. Thirdly,
there is a lack of standardized association between EBV-induced
PCNSL and neurological decline among immunosuppressed patients.
However, there are extensive case reports and series that highlight
presence of EBV-induced PCNSL in patients with immune disorders who
are receiving immunosuppressive agents (8-15).
Medications such as mycophenolate mofetil causes further
immunosuppression, which can increase the risk of viral infections,
including EBV, which then increases the risk of developing PCNSL.
Still, the causal relationship between prolonged exposure to
mycophenolate mofetil and PCNSL is not well established. Our
patient had multiple risk factors for EBV-induced PCNSL, including
advanced age, immunosuppressive medications, and presence of
MG.
Future research studies could be conducted in with
patients with EBV-induced PCNSL to systematically correlate the
presence and type of immunosuppressive therapy these patients have
received. This will help prevent delays in the identification of
EBV-induced PCNSL in both primary and acute care services. It will
also reinforce the need to expeditiously escalate diagnostic workup
to a stereotactic brain biopsy if clinical suspicion for PCNSL is
high. Instituting early treatment with rituximab and methotrexate
with or without WBRT may improve patients' survival, morbidity, and
quality of life.
Acknowledgements
Editorial assistance was provided by the Moffitt
Cancer Center's Office of Scientific Publishing (Moffitt Cancer
Center, Tampa, USA) by Mrs Daley White.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
OB, SSK and SM designed and conceptualized the
study, acquired and analyzed the data, and drafted the manuscript
for intellectual content. GW, DI, TR, AE and YP interpreted the
data and revised the manuscript for intellectual content. RM
reviewed the brain biopsy pathology and provided the pathology
slides. All authors read and approved the final manuscript. SM and
OB confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This publication is considered exempt from
Institutional Review Board review at Moffitt Cancer Center, as the
retrospective review of records for publication of a single-patient
case report is not considered to be research involving human
subjects.
Patient consent for publication
Written informed consent was obtained from the
patient/patient representative for publication of this case report
and accompanying images. A copy of the written consent is available
for review by the Editor-in-Chief of this journal. Institutional
approval was not required to publish the case details.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Villano JL, Koshy M, Shaikh H, Dolecek TA
and McCarthy BJ: Age, gender, and racial differences in incidence
and survival in primary CNS lymphoma. Br J Cancer. 105:1414–1418.
2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chiavazza C, Pellerino A, Ferrio F,
Cistaro A, Soffietti R and Ruda R: Primary CNS lymphomas:
Challenges in diagnosis and monitoring. Biomed Res Int.
2018(3606970)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mansour A, Qandeel M, Abdel-Razeq H and
Abu Ali HA: MR imaging features of intracranial primary CNS
lymphoma in immune competent patients. Cancer Imaging.
14(22)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Grommes C and DeAngelis LM: Primary CNS
lymphoma. J Clin Oncol. 35:2410–2418. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mohile NA and Abrey LE: Primary central
nervous system lymphoma. Neurol Clin. 25:1193–1207. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Green K and Hogg JP: Central nervous
system lymphoma. Feb 12, 2022.
|
|
7
|
Bashir R, McManus B, Cunningham C,
Weisenburger D and Hochberg F: Detection of Eber-1 RNA in primary
brain lymphomas in immunocompetent and immunocompromised patients.
J Neurooncol. 20:47–53. 1994.PubMed/NCBI View Article : Google Scholar
|
|
8
|
O'Neill BP, Vernino S, Dogan A and
Giannini C: EBV-associated lymphoproliferative disorder of CNS
associated with the use of mycophenolate mofetil. Neuro Oncol.
9:364–369. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kleinschmidt-DeMasters BK, Damek DM,
Lillehei KO, Dogan A and Giannini C: Epstein Barr virus-associated
primary CNS lymphomas in elderly patients on immunosuppressive
medications. J Neuropathol Exp Neurol. 67:1103–1111.
2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chatterjee S, Angelov L, Ahluwalia MS and
Yeaney GA: Epstein-Barr virus-associated primary central nervous
system lymphoma in a patient with diffuse cutaneous systemic
sclerosis on long-term mycophenolate mofetil. Joint Bone Spine.
87:163–166. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Finelli PF, Naik K, DiGiuseppe JA and
Prasad A: Primary lymphoma of CNS, mycophenolate mofetil and lupus.
Lupus. 15:886–888. 2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gandhi MK, Hoang T, Law SC, Brosda S,
O'Rourke K, Tobin JWD, Vari F, Murigneux V, Fink L, Gunawardana J,
et al: EBV-associated primary CNS lymphoma occurring after
immunosuppression is a distinct immunobiological entity. Blood.
137:1468–1477. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Migita K, Miyashita T, Mijin T, Sakito S,
Kurohama H, Ito M, Toda K, Tsustumi K, Baba H, Izumi Y, et al:
Epstein-Barr virus and methotrexate-related CNS lymphoma in a
patient with rheumatoid arthritis. Mod Rheumatol. 23:832–836.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tsang HH, Trendell-Smith NJ, Wu AK and Mok
MY: Diffuse large B-cell lymphoma of the central nervous system in
mycophenolate mofetil-treated patients with systemic lupus
erythematosus. Lupus. 19:330–333. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vernino S, Salomao DR, Habermann TM and
O'Neill BP: Primary CNS lymphoma complicating treatment of
myasthenia gravis with mycophenolate mofetil. Neurology.
65:639–641. 2005.PubMed/NCBI View Article : Google Scholar
|