Introduction
Severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) was the causative agent of the Coronavirus disease
2019 (COVID-19) pandemic. A number of drug treatments such as
antiviral drugs; monoclonal antibodies; convalescent plasma and
cytokine therapy targeting the SARS-CoV-2 immunopathological
process have recently been either approved or tested to treat
COVID-19 (1,2). While a majority of the cases of
SARS-CoV-2 infection are asymptomatic or associated with mild
symptoms, 10-20% of patients with COVID-19 may encounter acute
respiratory distress syndrome (ARDS), particularly patients who are
elderly or have co-morbidities (3). Recent studies have reported a fatal
immunopathological process defined as a ‘cytokine storm’, which is
a component of the macrophage activation syndrome, also known as
secondary hemophagocytic lymphohistiocytosis that can lead to ARDS
(4,5). Several cytokines, such as: IL-1;
IL-5; IL-7; IL-9; IL-10 and TNF-α, were detected at higher
concentrations in the serum of patients with a severe case of
COVID-19, compared with those who had milder infections (6-10).
Further research assessing the cytokine profile changes and their
mechanisms are necessary to comprehend how COVID-19 infections can
become severe, in addition to providing information that could be
used to develop treatment options to control disease pathogenesis
(11).
In order for the immune system to target an invading
virus, antigen-presenting cells (APCs) process and present viral
antigens to other cells of the immune system. Viral antigens are
recognized by CD8+ cytotoxic T lymphocytes and natural
killer (NK) cells, activating both the innate and adaptive branches
of the immune system. Apoptosis is induced by NK cells and
CD8+ cytotoxic T cells to kill virus-infected cells. To
avoid the unnecessary initiation of cell death, APCs and
CD8+ cytotoxic T cells are being eliminated through
apoptosis after antigenic reactivity has ceased. However, defects
in the cytotoxic activities of lymphocytes, whether acquired or
genetic, can result in a failure of CD8+ cytotoxic T
cells and NK cells to lyse infected cells and activate APCs, which
leads to exaggerated and prolonged interactions between adaptive
and innate immune cells. High levels of serum proinflammatory
cytokines, such as IL-1, IL-6, IL-17 and TNF-α, are then released
uncontrollably, resulting in a cytokine storm. Cytokine storm,
ARDS, thrombotic tendency, disseminated intravascular coagulation,
hepatic dysfunction and multi-organ failure can result from this
immunopathologic process (5,11,12).
This life-threatening phenomenon is regarded as the leading cause
of death in patients with COVID-19 (13,14).
Metformin is an anti-diabetic medication with a
well-characterized safety profile. Previous studies have reported
that metformin may have other physiological effects in addition to
lowering blood glucose levels (15). It may influence the AMP kinase
(AMPK)/mTOR signaling pathway, which in turn regulates a variety of
proinflammatory cytokines, such as IL-1; IL-2: IL-6; IL-12 and
TNF-α (15). Metformin, an
immunomodulatory drug with high tolerability, is an add-on drug in
the treatment of certain malignant, autoimmune and aging-related
conditions, such as antiproliferative and antioxidant effects, T2D,
obesity associated inflammation, autoimmune diseases and cardio and
nephro-protection (16-23).
In patients with chronic inflammation, metformin can be used as a
protective or therapeutic option (24,25),
including conditions such as colitis-associated colon cancer
(26), otitis media (27) and airway inflammation (28). Metformin has previously been
reported to aid in the treatment of sepsis-related brain injury by
inhibiting neuroinflammation, oxidative stress and apoptosis
(29).
The aim of the present study was to evaluate the
anti-inflammatory effect of oral and intraperitoneal (IP) metformin
in a mouse model of lipopolysaccharide (LPS)-induced cytokine
storm.
Materials and methods
Animal model
The present study included 60 female wild-type
BALB/c mice aged 5-6 weeks and weighing ~25 g. The animal cages
were well ventilated, and subjected to a 12-h light/dark cycle. The
relative humidity was kept at around 45-55%. The rooms temperature
was adjusted to a range of 22-24˚C. Food and water were freely
available to the animals. After 2 weeks of acclimation, mice were
randomly assigned to one of six groups (n=10/group): i) Control;
ii) LPS model; iii) oral saline + LPS; iv) oral metformin + LPS; v)
IP saline + LPS; and vi) IP metformin + LPS. The control group
received no intervention other than the collection of blood samples
at the end of the experiment to estimate the basal levels of serum
cytokines. For 30 days, metformin or saline were administered to
the mice via either the oral or IP route. Mice were administered
metformin at a concentration of 250 mg/kg body weight diluted in
100 µl normal saline once daily via oral gavage or the IP route
(28). Saline-treated groups of
mice were administered an equivalent volume of saline (100 µl)
daily, either orally or via the IP route. The Jouf University Local
Committee of Bioethics approved all experimental procedures
(approval no. 07-08-42; Sakaka, Saudi Arabia).
Murine model of LPS-induced cytokine
storm
After 30 days of metformin or saline administration,
LPS was administered to the five intervention groups of mice
through IP injection at a concentration of 0.5 mg/kg body weight
(from O111:B4 Escherichia coli; MilliporeSigma) to induce a
cytokine storm. LPS was prepared in sterile PBS before use. At one
hour following LPS was administration, mice were sacrificed by
halothane at a lethal dose (5%) for 2-3 min after which animals
were confirmed to be dead based on their physical status (unable to
walk and lacking response to manipulations). Then blood was
collected from the heart (30).
Serum separator tubes were used to collect blood samples. Blood
samples were allowed to clot for 2 h at room temperature, then
centrifuged at 3.74 x g for 15 min at 4˚C, before being stored at
-80˚C.
Measurement of cytokines levels
Serum levels of IL-1β, IL-6, IL-17 and TNF-α were
determined using ELISA kits according to the manufacturer's
instructions (cat. nos. CSB-E08054m, CSB-E04639m, CSB-E04608m and
CSB-E04741m, respectively; Cusabio Technology LLC). All
experimental measurements were repeated in triplicates.
Statistical analysis
Data are presented as the mean ± standard error of
the mean and were analyzed using SPSS (v22; IBM Corp.). One-way
ANOVA followed by Tukey's post hoc test was used to determine
statistical significance between treatment groups. The correlation
among the cytokine levels within each treatment group of mice was
analyzed using Spearman's rank correlation coefficient analysis,
and the results were presented as P- and rho values. P<0.05 was
considered to indicate a statistically significant difference.
Results
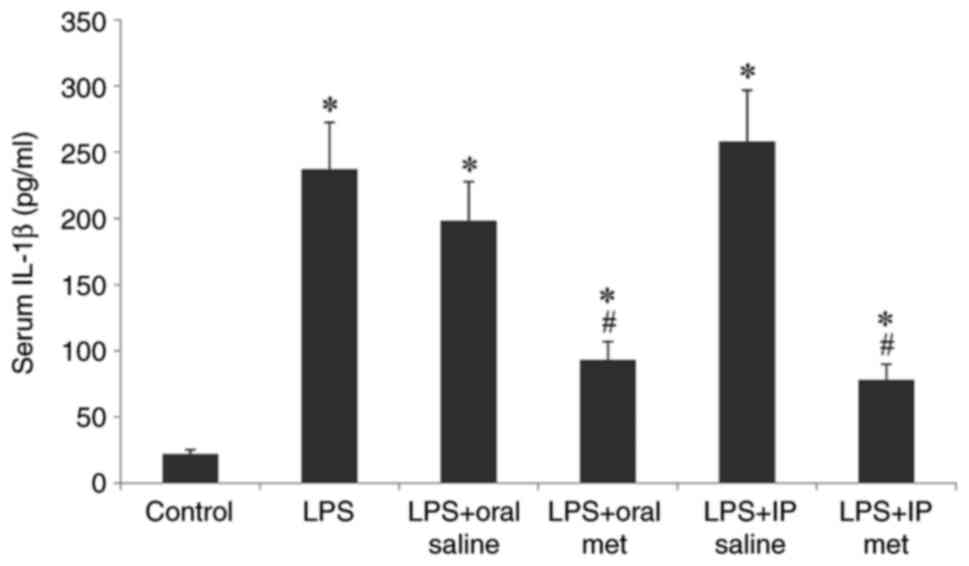
Serum IL-1β levels
There were no significant differences in the body
weight of the treatment groups of mice compared with the control
group. LPS injection significantly increased the serum levels of
IL-1β in all treatment groups compared with the control (P<0.05;
Fig. 1). Pretreatment with
metformin, either orally or IP, significantly reduced this rise in
IL-1β levels (P<0.05), with no significant difference
demonstrated between the efficacy of the two methods of metformin
administration.
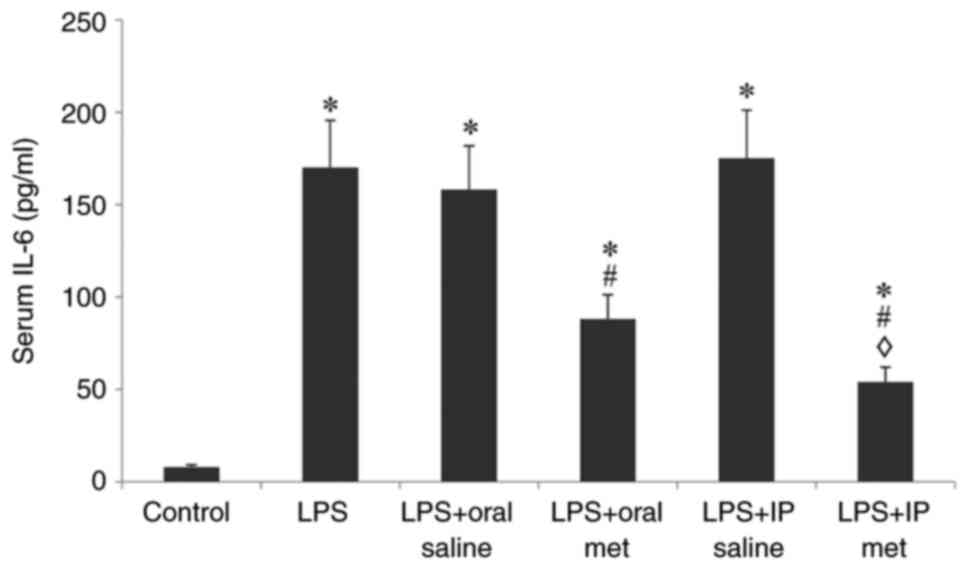
Serum IL-6 levels
Compared with the control group, injection of LPS
caused a significant elevation of IL-6 levels in all the treatment
groups (P<0.05; Fig. 2).
Pretreatment with either oral or IP metformin significantly
attenuated this increase in IL-6 levels (P<0.05). IP metformin
significantly decreased serum IL-6 levels compared with the oral
route of metformin administration (P<0.05).
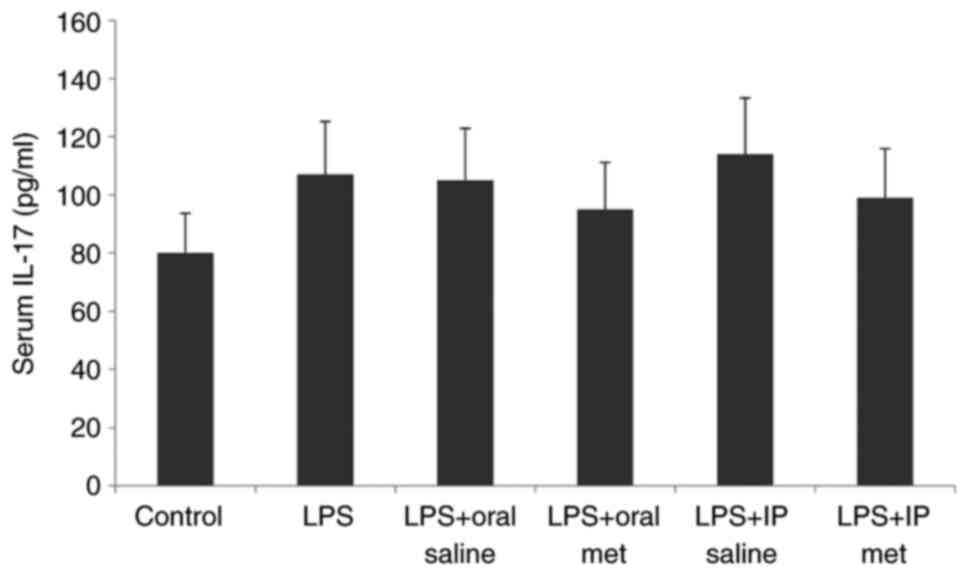
Serum IL-17 levels
Injection of LPS caused a significant increase in
serum IL-17 levels in all treatment groups compared with the
control group (Fig. 3). However,
serum levels of IL-17 were not significantly impacted by the route
of metformin administration.
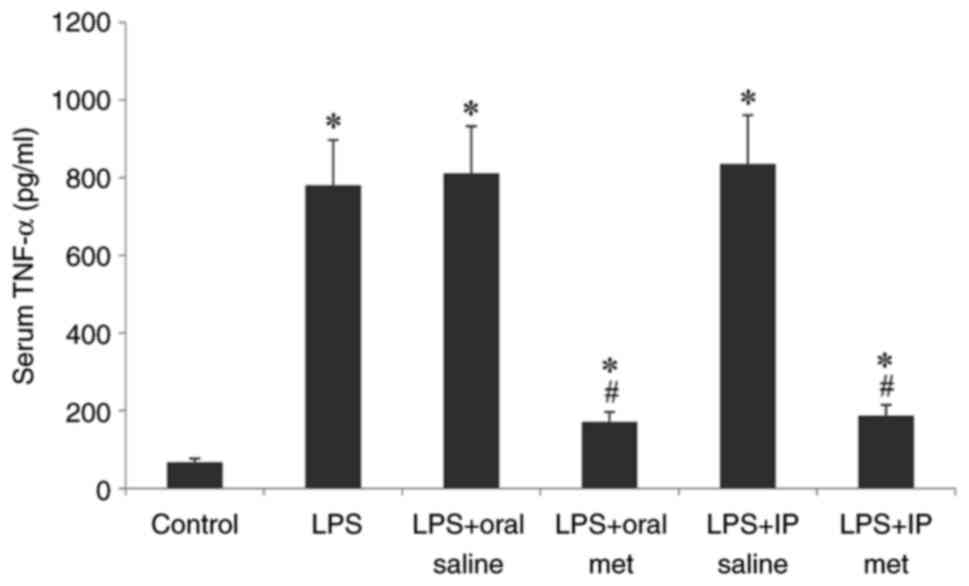
Serum TNF-α levels
Compared with the control, LPS injection caused a
significant increase in TNF-α levels across all treatment groups
(P<0.05; Fig. 4). Metformin
pretreatment significantly decreased the TNF-α levels in serum
compared with the LPS + saline groups. There was no significant
difference demonstrated by the route of metformin administration on
the reduction of serum TNF-α levels.
Correlation analysis
The results of the Spearman's correlation analysis
among measured proinflammatory cytokines within each of the
different subgroups demonstrated that certain correlations were
statistically significant (Table
I). The magnitude of the correlation coefficient indicated the
strength of the relationship between the variables, with larger
absolute values indicating stronger relationships.
 | Table ISpearman's correlation coefficient
analysis between production of serum cytokines in a mouse model of
LPS-induced cytokine storm pretreated with oral or IP
metformin. |
Table I
Spearman's correlation coefficient
analysis between production of serum cytokines in a mouse model of
LPS-induced cytokine storm pretreated with oral or IP
metformin.
| A, Control |
|---|
| Cytokine | IL-6 | IL-17 | TNF-α |
|---|
| IL-1 | 0.027
(0.633)a | 0.103 (0.438) | 0.028
(0.632)a |
| IL-6 | | 0.006
(0.774)a | 0.210 (0.287) |
| IL-17 | | | 0.067 (0.515) |
| B, LPS |
| Cytokine | IL-6 | IL-17 | TNF-α |
| IL-1 | 0.037
(0.596)a | 0.049
(0.559)a | 0.156 (0.358) |
| IL-6 | | 0.125 (0.399) | 0.021
(0.663)a |
| IL-17 | | | 0.171 (0.334) |
| C, LPS + oral
saline |
| Cytokine | IL-6 | IL-17 | TNF-α |
| IL-1 | 0.027
(0.636)a | 0.116 (0.418) | 0.077 (0.491) |
| IL-6 | | 0.033
(0.612)a | 0.214 (0.285) |
| IL-17 | | | 0.089 (0.467) |
| D, LPS + oral
metformin |
| Cytokine | IL-6 | IL-17 | TNF-α |
| IL-1 | 0.041
(0.584)a | 0.033
(0.612)a | 0.006
(0.770)a |
| IL-6 | | 0.221 (0.274) | 0.035
(0.602)a |
| IL-17 | | | 0.002
(0.830)a |
| E, LPS + IP
saline |
| Cytokine | IL-6 | IL-17 | TNF-α |
| IL-1 | 0.005
(0.782)a | 0.005
(0.782)a | 0.000
(0.915)a |
| IL-6 | | 0.008
(0.758)a | 0.015
(0.697)a |
| IL-17 | | | 0.002
(0.830)a |
| F, LPS + IP
metformin |
| Cytokine | IL-6 | IL-17 | TNF-α |
| IL-1 | 0.0014
(0.851)a | 0.0008
(0.879)a | 0.005
(0.782)a |
| IL-6 | | 0.003
(0.815)a | 0.003
(0.815)a |
| IL-17 | | | 0.003
(0.818)a |
Discussion
Effective drug treatments targeting the SARS-CoV-2
immunopathological process are of particular interest to reduce the
disease burden caused by these infections (1). Metformin, a widely available drug
treatment for diabetes, was previously reported to have a
cytokine-lowering effect in both diabetic and non-diabetic patients
(24). In patients with COVID-19,
this effect could be critical as the development of cytokine storm
can lead to very lethal outcomes such as ARDS (31). The effect of metformin on the
production of cytokines is reported to be caused by blocking the
AMPK/mTOR cytokine receptor pathway, which results in a decrease in
the expression of certain proinflammatory genes, such as IL-1α,
IL-1β, IL-2, IL-6, IL-12 and TNF-α (15). Additional studies suggest that
AMPK-independent mechanisms, such as altering the gut microbiota,
may also be involved in this process (32-34).
Cytokine storm causes severe illness in patients
with COVID-19, thereby significantly increasing the morbidity and
mortality rates by ~5% (31). The
present study sought to assess the anti-inflammatory effect of
metformin in a mouse model of LPS-induced cytokine storm. The
present results demonstrated that LPS injection caused a
significant increase in serum IL-1β levels when compared with the
control group. Oral and IP metformin significantly reduced the
elevated IL-1β levels in BALB/c mice 1 h after IP LPS injection,
with no significant difference demonstrated in efficacy between the
two routes of metformin administration. These findings support
previous reports that metformin is a potent inhibitor of the
chronic inflammatory response. For example, metformin therapy has
been reported to reduce reactive oxygen species and
hypoxia-inducible factor-1 (HIF-1) levels, which in turn reduce the
IL-1β expression levels after prolonged exposure to proinflammatory
LPS stimuli (29,35). Additionally, it has been previously
established that the IL-1β mediated inflammatory response is
involved in COVID-19 pathogenesis (36). Therefore, lowering IL-1β levels
could reduce inflammation and mortality in patients with
COVID-19(37).
The present study demonstrated that LPS injection
resulted in a significant increase in serum IL-6 levels in all
treatment groups of animals tested. Pretreatment with either oral
or IP metformin significantly reduced this increase in IL-6 levels.
IP metformin was significantly more effective at reducing IL-6
levels compared with oral metformin. Hyun et al (38) reported that metformin reduces IL-1,
IL-6 and TNF-α production at both the protein and mRNA level in a
dose-dependent manner. Similarly, Chao et al (36) reported that metformin reduces the
LPS-induced release of IL-6 in mouse livers. Additionally,
metformin inhibits the acute inflammatory response in two
macrophage-like cell lines by activating AMPK, but not HIF-1 or
IL-10(39).
TNF-α is an inflammatory cytokine produced in
response to bacterial and viral infections and can elicit tissue
damage and fibrosis (27). The
present study demonstrated that serum TNF-α levels were
significantly increased after mice were injected with LPS compared
with the controls. Oral or IP metformin were equally effective in
attenuating the significantly elevated TNF-α levels, with no
significant differences demonstrated between either route of
administration. Kim et al (30) previously reported that oral
administration of metformin to mice treated with LPS reduced the
plasma, spleen and lungs tissue levels of both TNF-α and IL-6
leading to a reduction in the effect of LPS-induced
inflammation.
Cho et al (27) investigated the anti-inflammatory
effects of metformin on LPS-induced inflammation in human middle
ear epithelial cell lines. LPS was found to elevate TNF-α and
cyclooxygenase-2 levels. However, pretreatment with metformin
reduced the production of these inflammatory factors. Metformin
also decreased the production of sepsis-induced brain injury in
mice inflammatory cytokines, such as IL-6, IL-1 β and TNF-α
(29). These findings are
consistent with the findings of the present study.
The present study demonstrated a non-significant
increase in serum IL-17 levels in mice pretreated with LPS. Neither
IP nor oral metformin had a significant effect on serum IL-17
levels. One possible explanation for these findings is that the
effect of LPS is likely to be dose- or time-dependent. Sun et
al (40) reported that IL-17A
is upregulated in LPS-induced neuroinflammation and cognitive
impairment in aged rats. Differences in the findings of the present
study regarding IL-17 levels could be due to the time frame of
sample collection following LPS injection. It could be possible
that 1 h may not be sufficient time to demonstrate a significant
increase in serum IL-17 levels. A previous study reported that
whilst IL-6 and TNF-α levels peak within 1 h of LPS injection in
mice, changes in IL-17 levels are slower and peak over 8 h
(41).
Age-related increases in serum levels of IL-17A,
IL-17F, IL-21 and IL-6 can be prevented by metformin treatment
(42). In a mouse model of
scleroderma treated with metformin, Moon et al (42) reported a decrease in the production
of the proinflammatory cytokine IL-17 in dermal tissues and
lymphocytes in a mice model of bleomycin-induced scleroderma. The
pathophysiology of IL-17 has been studied in mice challenged with
LPS, and it was reported that increased amounts of IL-17 are
associated with excessive lung injury and inflammation (43). Neutralizing increased IL-17
production using anti-IL-17 antibodies improves survival and
reduces lung injury (43).
Low-grade inflammation caused by microbiota
dysbiosis is thought to promote the occurrence of metabolic
syndrome (44,45). The gut microbiota may be involved
in the regulation of immunity, inflammation and autoinflammatory
diseases such as multiple sclerosis and osteomyelitis (46,47).
Several studies have reported that metformin can influence the
composition of the intestinal microbiota in certain clinical
situations such as dysbiosis, intestinal inflammations disorders,
and T2D (48-50).
It has been reported that the ability of metformin to alleviate
certain inflammatory diseases, such as inflammatory bowel disease
is linked to its ability to modify the diversity of gut microbiota
(51). By contrast, it has also
been proposed that the ability of metformin to reduce indices of
low-grade inflammation in metabolic syndrome is independent of its
effect on the gut microbiota (52,53).
A limitation of the present study is that further
studies are required to determine whether metformin has a direct or
indirect effect on cultured immune system cells, such as
macrophages and T cells. Investigating a potential dose-dependent
response of both LPS and metformin and longer time scales between
LPS injection and sample collection could also further elucidate
the impact of metformin in the mouse model used in the present
study.
In the present study, a mouse model of LPS-induced
cytokine storm was pretreated with metformin, which was
demonstrated to suppress the release of the pro-inflammatory
cytokines IL-1β, IL-6, IL-17 and TNF-α. Certain serum cytokine
levels demonstrated a positive correlation with other cytokines in
the mouse model. These findings may potentially suggest a future
role for metformin in the treatment of human diseases associated
with the cytokine storm.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Deanship of
Scientific Research at Jouf University (grant no. DSR
2020-04-2543).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All of the authors have made substantial
contributions towards the completion of the present study. MA and
AET conceived the present study, performed the experiments, were
project administrators and prepared the draft manuscript. IAT,
EAEM, MA and AET collected the data, obtained resources, performed
data analysis and critically reviewed and edited the manuscript.
IAT and EAEM acquired funding. IAT, MA and AET supervised the
project. IAT and AET confirm the authenticity of the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Local
Committee of Bioethics of Jouf University (approval no. 07-08-42;
Sakaka, Saudi Arabia).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Catanzaro M, Fagiani F, Racchi M, Corsini
E, Govoni S and Lanni C: Immune response in COVID-19: Addressing a
pharmacological challenge by targeting pathways triggered by
SARS-CoV-2. Signal Transduct Target Ther. 5(84)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Yuan Y, Jiao B, Qu L, Yang D and Liu R:
The development of COVID-19 treatment. Front Immunol.
14(1125246)2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pfortmueller CA, Spinetti T, Urman RD,
Luedi MM and Schefold JC: COVID-19-associated acute respiratory
distress syndrome (CARDS): Current knowledge on pathophysiology and
ICU treatment-A narrative review. Best Pract Res Clin Anaesthesiol.
35:351–368. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Crayne CB, Albeituni S, Nichols KE and
Cron RQ: The immunology of macrophage activation syndrome. Front
Immunol. 10(119)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kindler E, Thiel V and Weber F:
Interaction of SARS and MERS coronaviruses with the antiviral
interferon response. Adv Virus Res. 96:219–243. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Min CK, Cheon S, Ha NY, Sohn KM, Kim Y,
Aigerim A, Shin HM, Choi JY, Inn KS, Kim JH, et al: Comparative and
kinetic analysis of viral shedding and immunological responses in
MERS patients representing a broad spectrum of disease severity.
Sci Rep. 6(25359)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Channappanavar R and Perlman S: Pathogenic
human coronavirus infections: Causes and consequences of cytokine
storm and immunopathology. Semin Immunopathol. 39:529–539.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liang Y, Wang ML, Chien CS, Yarmishyn AA,
Yang YP, Lai WY, Luo YH, Lin YT, Chen YJ, Chang PC and Chiou SH:
Highlight of immune pathogenic response and hematopathologic effect
in SARS-CoV, MERS-CoV, and SARS-Cov-2 Infection. Front Immunol.
11(1022)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yao Z, Zheng Z, Wu K and Junhua Z: Immune
environment modulation in pneumonia patients caused by coronavirus:
SARS-CoV, MERS-CoV and SARS-CoV-2. Aging (Albany NY). 12:7639–7651.
2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mehta P, McAuley DF, Brown M, Sanchez E,
Tattersall RS and Manson JJ: HLH Across Speciality Collaboration,
UK. COVID-19: Consider cytokine storm syndromes and
immunosuppression. Lancet. 395:1033–1034. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Al-Samkari H and Berliner N:
Hemophagocytic lymphohistiocytosis. Annu Rev Pathol. 13:27–49.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li X, Geng M, Peng Y, Meng L and Lu S:
Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm
Anal. 10:102–108. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sarzi-Puttini P, Giorgi V, Sirotti S,
Marotto D, Ardizzone S, Rizzardini G, Antinori S and Galli M:
COVID-19, cytokines and immunosuppression: What can we learn from
severe acute respiratory syndrome? Clin Exp Rheumatol. 38:337–342.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chung MM, Nicol CJ, Cheng YC, Lin KH, Chen
YL, Pei D, Lin CH, Shih YN, Yen CH, Chen SJ, et al: Metformin
activation of AMPK suppresses AGE-induced inflammatory response in
hNSCs. Exp Cell Res. 352:75–83. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yin Y, Choi SC, Xu Z, Perry DJ, Seay H,
Croker BP, Sobel ES, Brusko TM and Morel L: Normalization of CD4+ T
cell metabolism reverses lupus. Sci Transl Med.
7(274ra18)2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee SY, Moon SJ, Kim EK, Seo HB, Yang EJ,
Son HJ, Kim JK, Min JK, Park SH and Cho ML: Metformin suppresses
systemic autoimmunity in roquin san/san mice through
inhibiting B cell differentiation into plasma cells via regulation
of AMPK/mTOR/STAT3. J Immunol. 198:2661–2670. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jing Y, Wu F, Li D, Yang L, Li Q and Li R:
Metformin improves obesity-associated inflammation by altering
macrophages polarization. Mol Cell Endocrinol. 461:256–264.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim EK, Lee SH, Lee SY, Kim JK, Jhun JY,
Na HS, Kim SY, Choi JY, Yang CW, Park SH and Cho ML: Metformin
ameliorates experimental-obesity-associated autoimmune arthritis by
inducing FGF21 expression and brown adipocyte differentiation. Exp
Mol Med. 50(e432)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Schuiveling M, Vazirpanah N, Radstake
TRDJ, Zimmermann M and Broen JCA: Metformin, a new era for an old
drug in the treatment of immune mediated disease? Curr Drug
Targets. 19:945–959. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ba W, Xu Y, Yin G, Yang J, Wang R, Chi S,
Wang Y and Li C: Metformin inhibits pro-inflammatory responses via
targeting nuclear factor-κB in HaCaT cells. Cell Biochem Funct.
37:4–10. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Jang SG, Lee J, Hong SM, Kwok SK, Cho ML
and Park SH: Metformin enhances the immunomodulatory potential of
adipose-derived mesenchymal stem cells through STAT1 in an animal
model of lupus. Rheumatology (Oxford). 59:1426–1438.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sciannimanico S, Grimaldi F, Vescini F, De
Pergola G, Iacoviello M, Licchelli B, Guastamacchia E, Giagulli VA
and Triggiani V: Metformin: Up to date. Endocr Metab Immune Disord
Drug Targets. 20:172–181. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Saisho Y: Metformin and inflammation: Its
potential beyond glucose lowering effect. Endocr Metab Immune
Disord Drug Targets. 15:196–205. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ismaiel AA, Espinosa-Oliva AM, Santiago M,
García-Quintanilla A, Oliva-Martín MJ, Herrera AJ, Venero JL and de
Pablos RM: Metformin, besides exhibiting strong in vivo
anti-inflammatory properties, increases mptp-induced damage to the
nigrostriatal dopaminergic system. Toxicol Apple Pharmacol.
298:19–30. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Koh SJ, Kim JM, Kim IK, Ko SH and Kim JS:
Anti-inflammatory mechanism of metformin and its effects in
intestinal inflammation and colitis-associated colon cancer. J
Gastroenterol Hepatol. 29:502–510. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cho JG, Song JJ, Choi J, Im GJ, Jung HH
and Chae SW: The suppressive effects of metformin on inflammatory
response of otitis media model in human middle ear epithelial
cells. Int J Pediatr Otorhinolaryngol. 89:28–32. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Park CS, Bang BR, Kwon HS, Moon KA, Kim
TB, Lee KY, Moon HB and Cho YS: Metformin reduces airway
inflammation and remodeling via activation of AMP-activated protein
kinase. Biochem Pharmacol. 84:1660–1670. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tang G, Yang H, Chen J, Shi M, Ge L, Ge X
and Zhu G: Metformin ameliorates sepsis-induced brain injury by
inhibiting apoptosis, oxidative stress and neuroinflammation via
the PI3K/Akt signaling pathway. Oncotarget. 8:97977–97989.
2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim J, Kwak HJ, Cha JY, Jeong YS, Rhee SD,
Kim KR and Cheon HG: Metformin suppresses lipopolysaccharide
(LPS)-induced inflammatory response in murine macrophages via
activating transcription factor-3 (ATF-3) induction. J Biol Chem.
289:23246–23255. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Montazersaheb S, Hosseiniyan Khatibi SM,
Hejazi MS, Tarhriz V, Farjami A, Ghasemian Sorbeni F, Farahzadi R
and Ghasemnejad T: COVID-19 infection: An overview on cytokine
storm and related interventions. Virol J. 19(92)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhao X, Cao F, Liu Q, Li X, Xu G, Liu G,
Zhang Y, Yang X, Yi S, Xu F, et al: Behavioral, inflammatory and
neurochemical disturbances in LPS and UCMS-induced mouse models of
depression. Behav Brain Res. 364:494–502. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kelly B, Tannahill GM, Murphy MP and
O'Neill LA: Metformin inhibits the production of reactive oxygen
species from NADH: Ubiquinone oxidoreductase to limit induction of
interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in
lipopolysaccharide (LPS)-activated macrophages. J Biol Chem.
290:20348–20359. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Dehkordi EH, Sattari F, Khoshdel A and
Kasiri K: Effect of folic acid and metformin on insulin resistance
and inflammatory factors of obese children and adolescents. J Res
Med Sci. 21(71)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Dias SSG, Soares VC, Ferreira AC,
Sacramento CQ, Fintelman-Rodrigues N, Temerozo JR, Teixeira L,
Nunes da Silva MA, Barreto E, Mattos M, et al: Lipid droplets fuel
SARS-CoV-2 replication and production of inflammatory mediators.
PLoS Pathog. 16(e1009127)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chao L, Hui-Jie G, Fang Y, Ni K, Bao-Kub W
and Teng-yuan Z: Anti-inflammatory effect of metformin LPS-induced
inflammation in mice. Basic Clin Med.
39(1001-6325-1248-04)2019.
|
|
37
|
Cavalli G, Larcher A, Tomelleri A,
Campochiaro C, Della-Torre E, De Luca G, Farina N, Boffini N,
Ruggeri A, Poli A, et al: Interleukin-1 and interleukin-6
inhibition compared with standard management in patients with
COVID19 and hyperinfammation: A cohort study. Lancet Rheumatol.
3:E253–E261. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hyun B, Shin S, Lee A, Lee S, Song Y, Ha
NJ, Cho KH and Kim K: Metformin Down-regulates TNF-α secretion via
suppression of scavenger receptors in macrophages. Immune Netw.
13:123–132. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Postler TS, Peng V, Bhatt DM and Ghosh S:
Metformin selectively dampens the acute inflammatory response
through an AMPK-dependent mechanism. Sci Rep.
11(18721)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sun J, Zhang S, Zhang X, Zhang X, Dong H
and Qian Y: IL-17A is implicated in lipopolysaccharide-induced
neuroinflammation and cognitive impairment in aged rats via
microglial activation. J Neuroinflammation. 12(165)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Furuya S, Kono H, Hara M, Hirayama K, Sun
C and Fujii H: Interleukin 17A plays a role in
lipopolysaccharide/D-galactosamine-induced fulminant hepatic injury
in mice. J surg Res. 199:487–493. 2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Moon J, Lee SY, Choi JW, Lee AR, Yoo JH,
Moon SJ, Park SH and Cho ML: Metformin ameliorates scleroderma via
inhibiting Th17 cells and reducing mTOR-STAT3 signaling in skin
fibroblasts. J Transl Med. 19(192)2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li Q, Gu Y, Tu Q, Wang K, Gu X and Ren T:
Blockade of Interleukin-17 restrains the development of acute lung
injury. Scand J Immunol. 83:203–211. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Carvalho FA, Aitken JD, Vijay-Kumar M and
Gewirtz AT: Toll-like receptor-gut microbiota interactions: Perturb
at your own risk! Annu Rev. Physiol. 74:177–198. 2012.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Rodriguez J, Hiel S and Delzenne NM:
Metformin: Old friend, new ways of action-implication of the gut
microbiome? Curr Opin Clin Nutr Metab Care. 21:294–301.
2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lee YK, Menezes JS, Umesaki Y and
Mazmanian SK: Proinflammatory T-cell responses to gut microbiota
promote experimental autoimmune encephalomyelitis. Proc Natl Acad
Sci USA. 108 (Suppl 1):S4615–S4622. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lukens JR, Gurung P, Vogel P, Johnson GR,
Carter RA, McGoldrick DJ, Bandi SR, Calabrese CR, Vande Walle L,
Lamkanfi M and Kanneganti TD: Dietary modulation of the microbiome
affects autoinflammatory disease. Nature. 516:246–249.
2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Elbere I, Kalnina I, Silamikelis I,
Konrade I, Zaharenko L, Sekace K, Radovica-Spalvina I, Fridmanis D,
Gudra D, Pirags V and Klovins J: Association of metformin
administration with gut microbiome dysbiosis in healthy volunteers.
PLoS One. 13(e0204317)2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhang W, Xu JH, Yu T and Chen QK: Effects
of berberine and metformin on intestinal inflammation and gut
microbiome composition in db/db mice. Biomed Pharmacother.
118(109131)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhang Q and Hu N: Effects of metformin on
the gut microbiota in obesity and type 2 diabetes mellitus.
Diabetes Metab Cinder Obes. 13:5003–5014. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Liu Z, Liao W, Zhang Z, Sun R, Luo Y, Chen
Q, L X, Lu R and Ying Y: Metformin affects gut microbiota
composition and diversity associated with amelioration of dextran
sulfate sodium-induced colitis in mice. Front Pharmacol.
12(640347)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Adeshirlarijaney A, Zou J, Tran HQ,
Chassaing B and Gewirtz AT: Amelioration of metabolic syndrome by
metformin associates with reduced indices of low-grade inflammation
independently of the gut microbiota. Am J Physiol Endocrinol Metab.
317:E1121–E1130. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Petrakis D, Margină D, Tsarouhas K, Tekos
F, Stan M, Nikitovic D, Kouretas D, Spandidos DA and Tsatsakis A:
Obesity a risk factor for increased COVID19 prevalence, severity
and lethality (Review). Mol Med Rep. 22:9–19. 2020.PubMed/NCBI View Article : Google Scholar
|